Comparison of Corrosion Resistance of the AA2524-T3 and the AA2024-T3
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.Q.; Pan, S.P.; Zhou, M.Z.; Yi, D.Q.; Xu, D.Z.; Xu, Y.F. Effects of inclusions, grain boundaries and grain orientations on the fatigue crack initiation and propagation behavior of 2524-T3 Al alloy. Mater. Sci. Eng. A 2013, 580, 150–158. [Google Scholar] [CrossRef]
- Golden, P. A comparison of fatigue crack formation at holes in 2024-T3 and 2524-T3 aluminum alloy specimens. Int. J. Fatigue 1999, 21, 211–219. [Google Scholar] [CrossRef]
- Bonzom, R.; Oltra, R. Droplet cell investigation of intergranular corrosion on AA2024. Electrochem. Commun. 2017, 81, 84–87. [Google Scholar] [CrossRef]
- Moreto, J.A.; Broday, E.E.; Rossino, L.S.; Fernandes, J.C.S.; Bose Filho, W.W. Effect of localized corrosion on fatigue–crack growth in 2524-T3 and 2198-T851 aluminum alloys used as aircraft materials. J. Mater. Eng. Perform. 2018, 27, 1917–1926. [Google Scholar] [CrossRef]
- Queiroz, F.M.; Donatus, U.; Prada Ramirez, O.M.; de Sousa Araujo, J.V.; Gonçalves de Viveiros, B.V.; Lamaka, S.; Zheludkevich, M.; Masoumi, M.; Vivier, V.; Costa, I.; et al. Effect of unequal levels of deformation and fragmentation on the electrochemical response of friction stir welded AA2024-T3 alloy. Electrochim. Acta 2019, 313, 271–281. [Google Scholar] [CrossRef]
- Cabrini, M.; Bocchi, S.; D’Urso, G.; Giardini, C.; Lorenzi, S.; Testa, C.; Pastore, T. Stress corrosion cracking of friction stir-welded AA-2024 T3 alloy. Materials 2020, 13, 2610. [Google Scholar] [CrossRef] [PubMed]
- Nisancioglu, K. Corrosion of aluminium alloys. In Proceedings of the 3rd International Conference on Aluminium Alloys, Trondheim, Norway, 25–29 October 1992; p. 239. [Google Scholar]
- Milagre, M.X.; Donatus, U.; Machado, C.S.C.; Araujo, J.V.S.; da Silva, R.M.P.; de Viveiros, B.V.G.; Astarita, A.; Costa, I. Comparison of the corrosion resistance of an Al–Cu alloy and an Al–Cu–Li alloy. Corros. Eng. Sci. Technol. 2019, 54, 402–412. [Google Scholar] [CrossRef]
- Queiroz, F.M.; Magnani, M.; Costa, I.; de Melo, H.G. Investigation of the corrosion behaviour of AA 2024-T3 in low concentrated chloride media. Corros. Sci. 2008, 50, 2646–2657. [Google Scholar] [CrossRef]
- Queiroz, F.M. Estudo Do Comportamento de Corrosão Dos Intermetálicos Presentes Na Liga Aa2024-T3, Por Meio De Técnicas De Microscopia Associadas A Técnicas Eletroquímicas. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2008. [Google Scholar]
- Murer, N.; Buchheit, R.G. Stochastic modeling of pitting corrosion in aluminum alloys. Corros. Sci. 2013, 69, 139–148. [Google Scholar] [CrossRef]
- Birbilis, N.; Cavanaugh, M.K.; Buchheit, R.G. Electrochemical behavior and localized corrosion associated with Al7Cu2Fe particles in aluminum alloy 7075-T651. Corros. Sci. 2006, 48, 4202–4215. [Google Scholar] [CrossRef]
- Bousquet, E. Durabilité des Assemblages Soudès par Friction Stir Welding (FSW). Ph.D. Thesis, L’Université de Bordeaux, Bordeaux, France, 2011. [Google Scholar]
- Jariyaboon, M.; Davenport, A.J.; Ambat, R.; Connolly, B.J.; Williams, S.W.; Price, D.A. The effect of welding parameters on the corrosion behaviour of friction stir welded AA2024-T351. Corros. Sci. 2007, 49, 877–909. [Google Scholar] [CrossRef]
- Paglia, C.S.; Buchheit, R.G. A look in the corrosion of aluminum alloy friction stir welds. Scr. Mater. 2008, 58, 383–387. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Hashimoto, T.; Hughes, A.E.; Thompson, G.E. Study of localized corrosion in AA2024 aluminium alloy using electron tomography. Corros. Sci. 2012, 58, 299–306. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Grant, R.P.; Hlava, P.F.; Mckenzie, B.; Zender, G.L. Local dissolution phenomena associated with s phase (al2cumg) particles in aluminum alloy 2024-T3. J. Electrochem. Soc. 1997, 144, 2621–2628. [Google Scholar] [CrossRef]
- Li, J.F.; Ziqiao, Z.; Na, J.; Chengyu, T. Localized corrosion mechanism of 2×××-series Al alloy containing S(Al2CuMg) and θ′(Al2Cu) precipitates in 4.0% NaCl solution at pH 6.1. Mater. Chem. Phys. 2005, 91, 325–329. [Google Scholar] [CrossRef]
- Moreto, J.; Gamboni, O.; Rocha, L. Corrosion behavior of Al and Al-Li alloys used as aircraft materials. Corrosão Protecção Mater. 2012, 31, 60–64. [Google Scholar]
- Bugarin, A.F.S.; De Abreu, C..; Terada, M.; De Melo, H.G.; Costa, I. Effect of friction stir welding (FSW) on the electrochemical behavior and galvanic coupling of AA2024-T3 and AA7475-T651. Mater. Today Commun. 2020, 25, 101591. [Google Scholar] [CrossRef]
- Buchheit, R.G. A compilation of corrosion potentials reported for intermetallic phases in aluminum alloys. J. Electrochem. Soc. 1995, 142, 3994–3996. [Google Scholar] [CrossRef]
- Buchheit, R.; Boger, R.; Carroll, M.; Leard, R.; Paglia, C.; Searles, J. The electrochemistry of intermetallic particles and localized corrosion in Al alloys. JOM 2001, 53, 29–33. [Google Scholar] [CrossRef]
- Blanc, C.; Lavelle, B.; Mankowski, G. The role of precipitates enriched with copper on the susceptibility to pitting corrosion of the 2024 aluminium alloy. Corros. Sci. 1997, 39, 495–510. [Google Scholar] [CrossRef]
- Blanc, C.; Gastaud, S.; Mankowski, G. Mechanistic studies of the corrosion of 2024 aluminum alloy in nitrate solutions. J. Electrochem. Soc. 2003, 150, B396. [Google Scholar] [CrossRef]
- Campestrini, P.; Terryn, H.; Hovestad, A.; de Wit, J.H.W. Formation of a cerium-based conversion coating on AA2024: Relationship with the microstructure. Surf. Coat. Technol. 2004, 176, 365–381. [Google Scholar] [CrossRef]
- Leard, R.R.; Buchheit, R.G. Electrochemical characterization of copper-bearing intermetallic compounds and localized corrosion of Al-Cu-Mg-Mn alloy 2024. Mater. Sci. Forum 2002, 396–402, 1491–1496. [Google Scholar] [CrossRef]
- Obispo, H.M.; Murr, L.E.; Arrowood, R.M.; Trillo, E.A. Copper deposition during the corrosion of aluminum alloy 2024 in sodium chloride solutions. J. Mater. Sci. 2000, 35, 3479–3495. [Google Scholar] [CrossRef]
- Zhu, D.; van Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl) propyl]tetrasulfide in sodium chloride solution. Part 2: Mechanism for corrosion protection. Corros. Sci. 2003, 45, 2177–2197. [Google Scholar] [CrossRef]
- Schneider, O.; Ilevbare, G.O.; Scully, J.R.; Kelly, R.G. In situ confocal laser scanning microscopy of aa 2024-t3 corrosion metrology. J. Electrochem. Soc. 2004, 151, B465. [Google Scholar] [CrossRef]
- Leblanc, P.; Frankel, G.S. A study of corrosion and pitting initiation of AA2024-T3 using atomic force microscopy. J. Electrochem. Soc. 2002, 149, B239. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Characterization of AA2024-T3 by scanning kelvin probe force microscopy. J. Electrochem. Soc. 1998, 145, 2285–2295. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Corrosion study of AA2024-T3 by scanning kelvin probe force microscopy and in situ atomic force microscopy scratching. J. Electrochem. Soc. 1998, 145, 2295–2306. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.J. Feature recognition of corrosion pit for pre-corroded AA 2524 and statistical analysis. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 906, pp. 263–267. [Google Scholar]
- Costenaro, H.; Lanzutti, A.; Paint, Y.; Fedrizzi, L.; Terada, M.; de Melo, H.G.; Olivier, M.-G. Corrosion resistance of 2524 Al alloy anodized in tartaric-sulphuric acid at different voltages and protected with a TEOS-GPTMS hybrid sol-gel coating. Surf. Coat. Technol. 2017, 324. [Google Scholar] [CrossRef]
- Guadagnin, H.C. Corrosion Resistance Study of AA2524 Anodized in Sulphuric-Tartaric Acid and Sealed with Hybrid Coatings. Ph.D. Thesis, Universidade de São Paulo, State of São Paulo, Brazil, 2017. [Google Scholar]
- Liu, C.; Liu, Y.; Li, S.; Ma, L.; Zhao, X.; Wang, Q. Effect of creep aging forming on the fatigue crack growth of an AA2524 alloy. Mater. Sci. Eng. A 2018, 725, 375–381. [Google Scholar] [CrossRef]
- Terada, M.; Queiroz, F.M.; Costenaro, H.; Olivier, M.; Costa, I.; Melo, H.G. The Effect of Cerium ( III ) on the Corrosion Protection Properties of the Film Formed on the Aa2524-T3 Alloy by Hydrothermal Treatments. In Proceedings of the European Corrosion Congress, Montpellier, France, 11–15 September 2016. [Google Scholar]
- Yang, B.; Yan, J.; Sutton, M.A.; Reynolds, A.P. Banded microstructure in AA2024-T351 and AA2524-T351 aluminum friction stir welds. Mater. Sci. Eng. A 2004, 364, 55–65. [Google Scholar] [CrossRef]
- The Aluminium Association. International Alloy. Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys With Support for On-Line Access From:s Aluminum Extruders Council Use of the Information; The Aluminium Association: Arlington County, VA, USA, 2015. [Google Scholar]
- ASM Handbook Committee. ASM Handbook Volume 2 Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Cleveland, OH, USA, 1993. [Google Scholar]
- Fontana, Á. Utilização de Polianilina Como Revestimento Protetor Contra Corrosão Das Ligas de Alumínio 2014 F, 2024 T3 e 7075 O. Master’s Degree, Universidade de São Paulo, São Paulo, Brazil, 2008. [Google Scholar]
- Moreto, J.A.; Gambonf, O.; Rucherf, C.O.F.T.; Romagnoli, F.; Moreira, M.F.; Beneduce, F.; Filho, W.W.B. Corrosion and fatigue behavior of new Al alloys. Procedia Eng. 2011, 10, 1521–1526. [Google Scholar] [CrossRef]
- Moreto, J.A. Estudo da Corrosão e Corrosão-Fadiga em Ligas de Al e Al–Li de Alta Resistência para Aplicação Aeronáutica. Ph.D. Thesis, Universidade de São Paulo, State of São Paulo, Brazil, 2012. [Google Scholar]
- Moreto, J.A.; dos Santos, M.S.; Ferreira, M.O.A.; Carvalho, G.S.; Gelamo, R.V.; Aoki, I.V.; Taryba, M.; Bose Filho, W.W.; Fernandes, J.C.S. Corrosion and corrosion-fatigue synergism on the base metal and nugget zone of the 2524-T3 Al alloy joined by FSW process. Corros. Sci. 2021, 182, 109253. [Google Scholar] [CrossRef]
- Moreto, J.A.; Rossino, L.S.; Filho, W.W.B.; Marino, C.E.B.; Da Conceição Ferreira, M.; Taryba, M.; Fernandes, J.C.S. On the global and localised corrosion behaviour of the AA2524-T3 aluminium alloy used as aircraft fuselage skin. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Queiroz, F.M.; Bugarin, A.F.S.; Hammel, N.P.; Capelossi, V.R.; Terada, M.; Costa, I. EIS behavior of anodized and primer coated AA2198–T851 compared to AA2024–T3 exposed to salt spray CASS test. Surf. Interface Anal. 2016, 48. [Google Scholar] [CrossRef]
- Campestrini, P.; Van Westing, E.P.M.; De Wit, J.H.W. Influence of surface preparation on performance of chromate conversion coatings on Alclad 2024 aluminium alloy–Part II: EIS investigation. Electrochim. Acta 2001, 46, 2631–2647. [Google Scholar] [CrossRef]
- Capelossi, V.R.; Poelman, M.; Recloux, I.; Hernandez, R.P.B.; de Melo, H.G.; Olivier, M.G. Corrosion protection of clad 2024 aluminum alloy anodized in tartaric-sulfuric acid bath and protected with hybrid sol–gel coating. Electrochim. Acta 2014, 124, 69–79. [Google Scholar] [CrossRef]
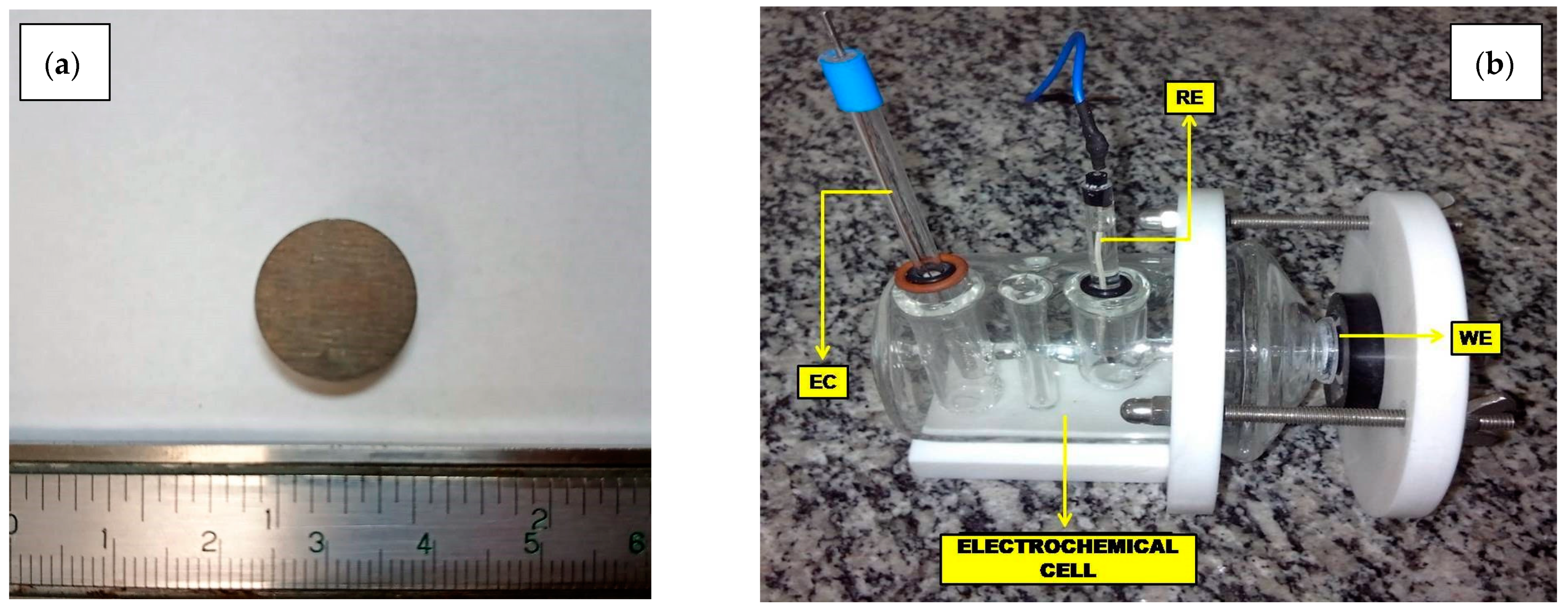
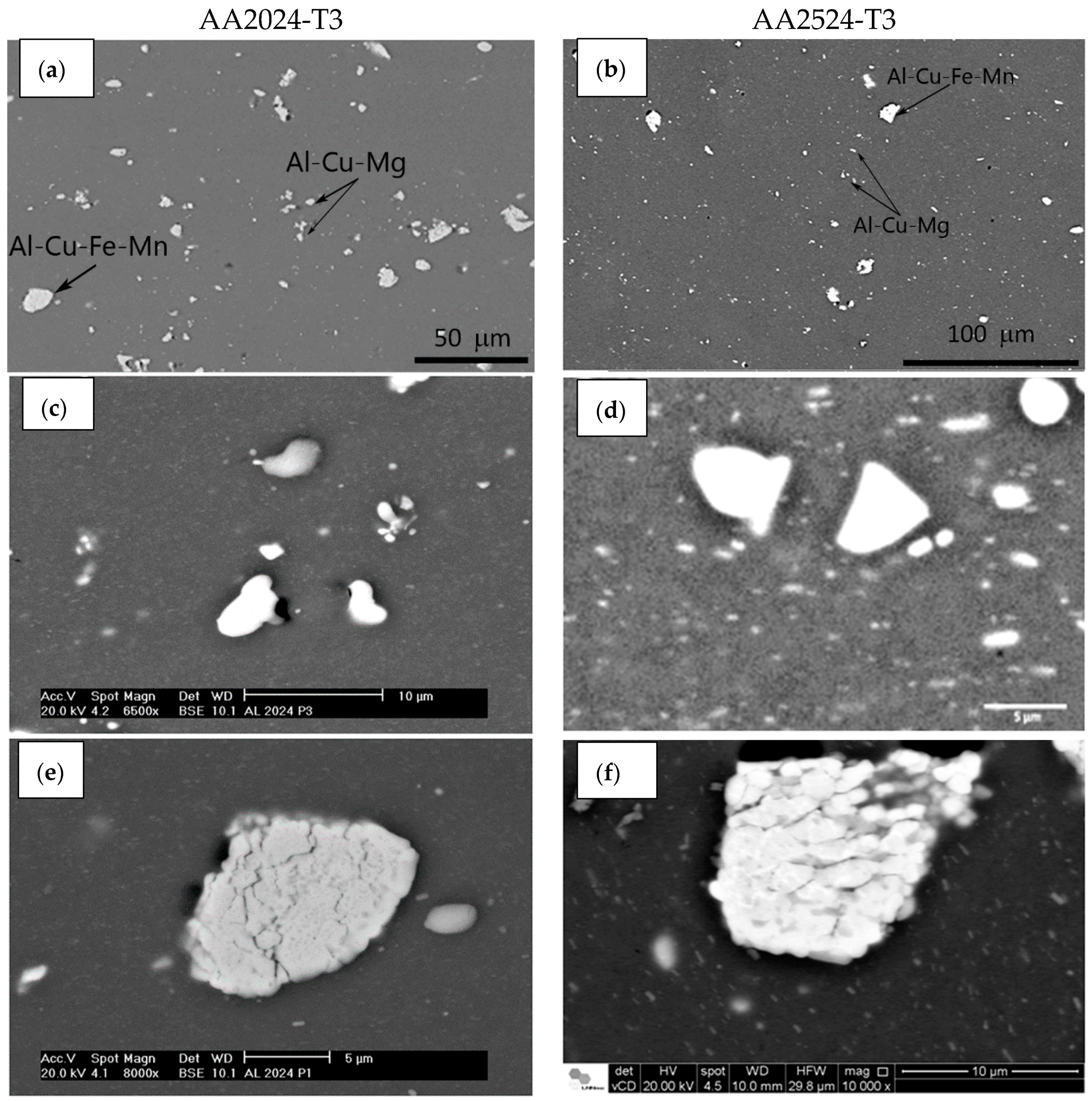

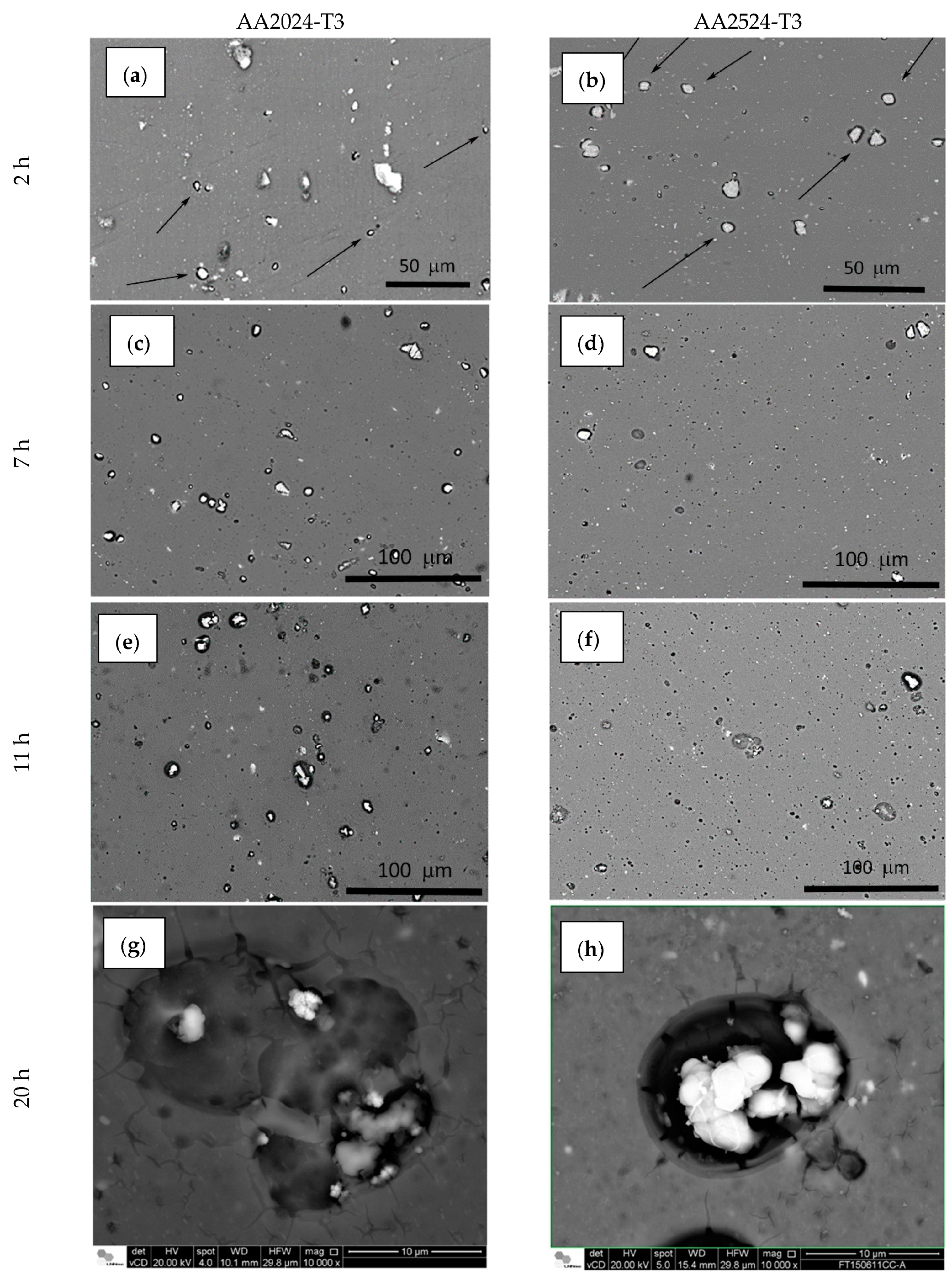
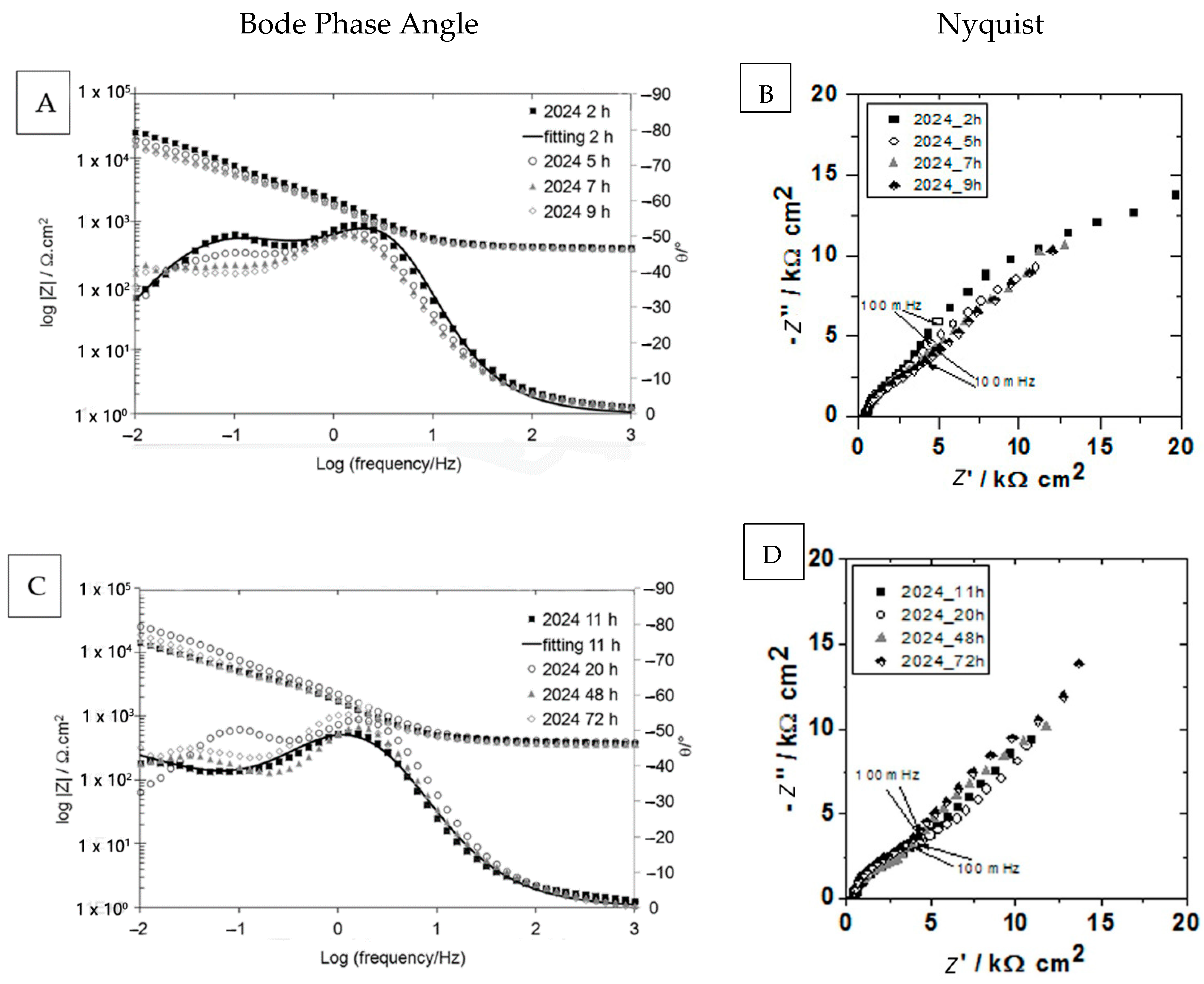
| Elements | AA2024-T3 | AA2524-T3 |
|---|---|---|
| Cu | 4.06 (3.8–4.9) | 3.84 (4.0–4.5) |
| Mg | 1.77 (1.2–1.8) | 1.31 (1.2–1.6) |
| Mn | 0.63 (0.3–0.9) | 0.56 (0.45–0.7) |
| Si | 0.11 (0.5 max) | 0.04 (0.06 max) |
| Fe | 0.14 (0.5 max) | 0.06 (0.12 max) |
| Ti | −(0.15 max) | 0.029 (0.10 max) |
| Zn | 0.02 (0.25 max) | 0.01 (0.15 máx) |
| Al | Balance | Balance |
| Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | Electrical Conductivity % IACS | Open Circuit Potential (VSCE) | |
|---|---|---|---|---|---|
| AA2024 [40] | 435 | 290 | 10–15 | 30 | −0.722 [41] |
| AA2524 [42] | 445.4 | 340.3 | 19 | 34 | −0.590 [43] |
| Material | IMCs Area Fraction (%) |
|---|---|
| AA2024-T3 | 2.52 ± 0.42 |
| AA2524-T3 | 1.64 ± 0.14 |
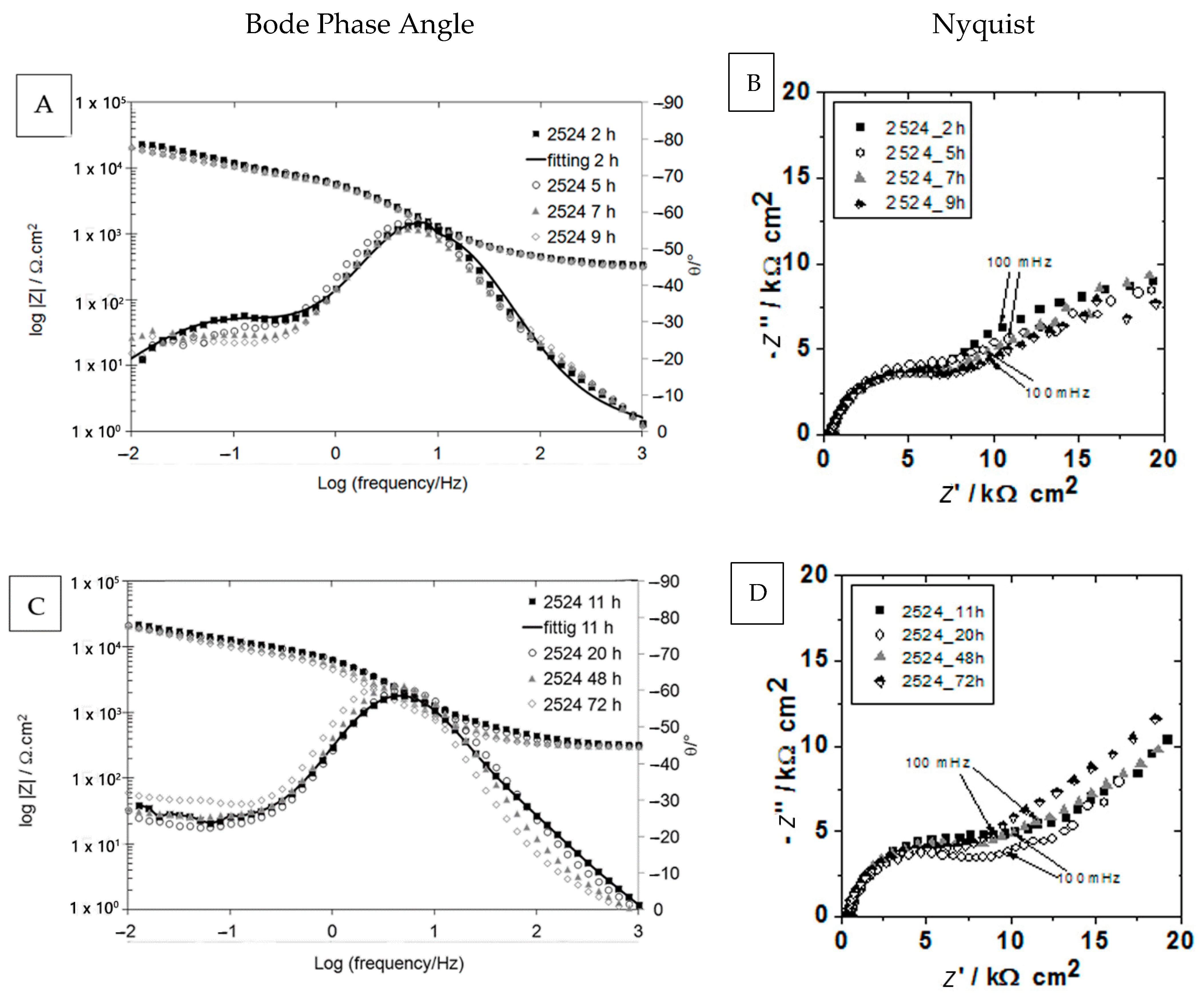
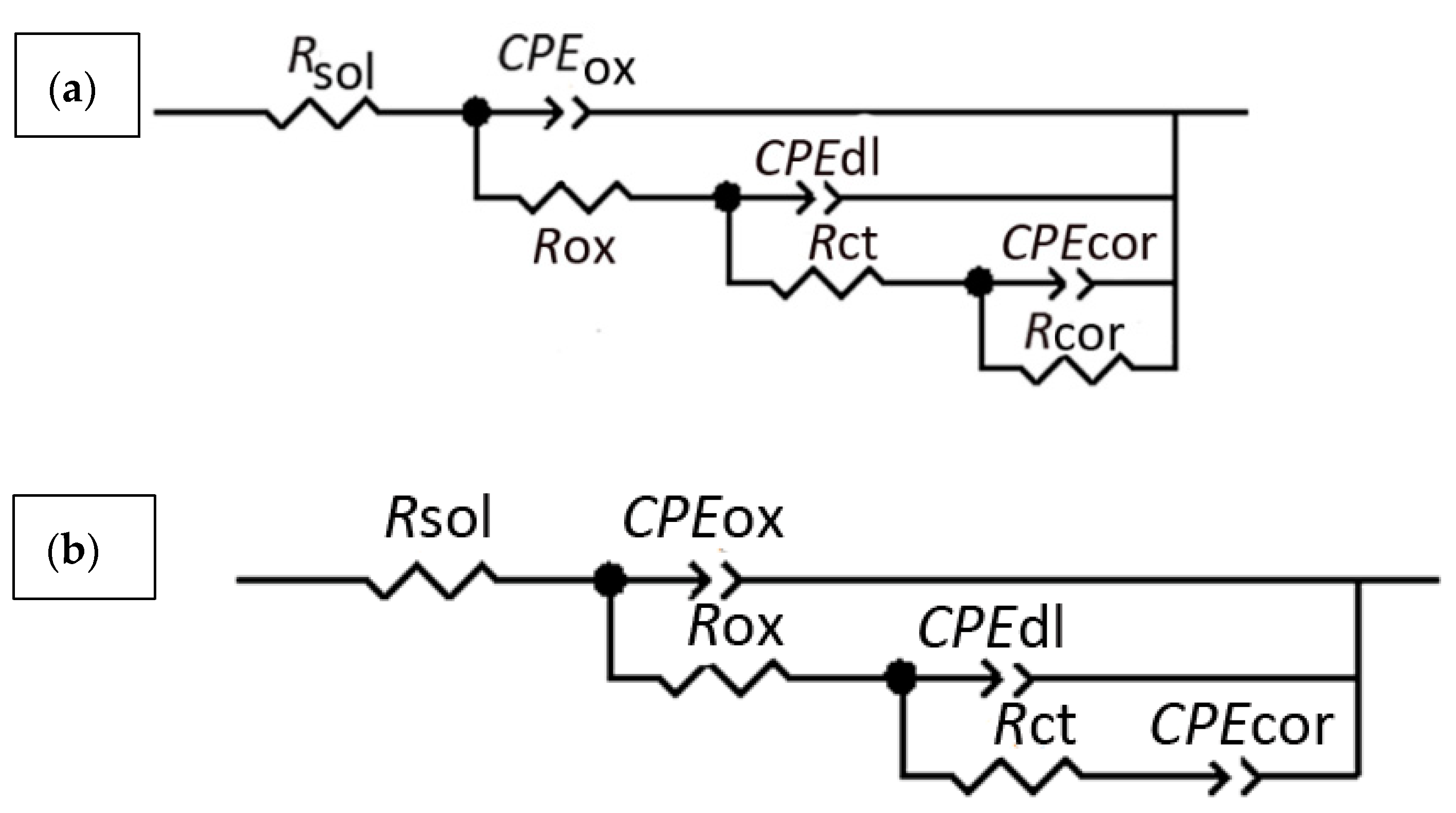
| 2 h | 5 h | 7 h | 9 h | 11 h | 20 h | 48 h | 72 h | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2524 | 2024 | 2524 | 2024 | 2524 | 2024 | 2524 | 2024 | 2524 | 2024 | 2524 | 2024 | 2524 | 2024 | 2524 | 2024 | |
| Rsol Ω·cm2 | 346.40 | 394.20 | 342.10 | 388.70 | 334.90 | 382.80 | 330 | 380.40 | 328.40 | 381.20 | 325.10 | 373.80 | 316.90 | 362.30 | 318.80 | 376.90 |
| Error (%) | 0.40 | 0.27 | 0.50 | 0.22 | 0.40 | 0.16 | 0.32 | 0.16 | 0.34 | 0.27 | 0.34 | 0.25 | 0.37 | 0.37 | 0.32 | 0.30 |
| CPEox µF·cm−2·s(α−1) | 6.27 | 16.60 | 5.99 | 16.30 | 6.07 | 14.90 | 6.39 | 15.30 | 6.84 | 19.20 | 8.58 | 20.80 | 13.80 | 34.70 | 18.00 | 43.30 |
| Error (%) | 2.44 | 8.14 | 2.36 | 5.84 | 1.75 | 3.88 | 1.28 | 3.52 | 1.29 | 4.57 | 1.27 | 3.41 | 1.39 | 4.28 | 1.44 | 3.63 |
| Rox Ω·cm2 | 327.10 | 57.15 | 351.60 | 56.04 | 413.10 | 57.71 | 479.10 | 64.06 | 541.80 | 79.83 | 752.30 | 107 | 954.50 | 210.20 | 761.40 | 239.70 |
| Error (%) | 6.18 | 9.32 | 4.53 | 5.17 | 3.78 | 3.02 | 3.15 | 2.77 | 3.37 | 3.78 | 5.21 | 3.33 | 7.58 | 9.46 | 7.78 | 10.02 |
| CPEdL µF·cm−2·s(α−1) | 14.80 | 58.90 | 16.40 | 75.40 | 16.60 | 89.30 | 16.10 | 91.50 | 14.40 | 83.50 | 11.50 | 80.80 | 8.85 | 44.00 | 13.10 | 50.20 |
| Error (%) | 3.23 | 3.39 | 3.26 | 2.19 | 3.13 | 1.40 | 2.53 | 1.35 | 2.84 | 2.06 | 4.65 | 2.14 | 6.20 | 6.93 | 4.73 | 6.19 |
| α dL | 0.86 | 0.89 | 0.88 | 0.88 | 0.88 | 0.87 | 0.87 | 0.87 | 0.89 | 0.89 | 0.90 | 0.90 | 0.97 | 0.99 | 0.94 | 0.96 |
| Error (%) | 0.81 | 0.66 | 0.81 | 0.49 | 0.83 | 0.35 | 0.71 | 0.36 | 0.78 | 0.50 | 1.33 | 0.57 | 1.74 | 1.79 | 1.40 | 1.69 |
| Rct kΩ·cm2 | 7.37 | 4.66 | 7.73 | 4.37 | 6.98 | 4.28 | 7.27 | 4.06 | 7.88 | 3.16 | 6.26 | 3.48 | 5.43 | 2.47 | 6.54 | 3.50 |
| Error (%) | 2.09 | 2.94 | 2.89 | 2.78 | 2.61 | 2.20 | 2.01 | 2.11 | 2.88 | 2.61 | 3.79 | 3.43 | 4.30 | 5.09 | 3.03 | 5.48 |
| CPEcor µF·cm−2·s(α−1) | 174.00 | 180.04 | 187.70 | 241.00 | 239.59 | 313.00 | 274.00 | 358.00 | 204.00 | 341.00 | 283.00 | 327.00 | 199.00 | 352.41 | 275.00 | 295.00 |
| Error (%) | 1.95 | 1.00 | 3.31 | 1.28 | 3.05 | 1.38 | 2.88 | 1.44 | 3.07 | 1.16 | 4.46 | 1.87 | 2.65 | 1.75 | 2.71 | 1.98 |
| α cor | 0.76 | 0.81 | 0.63 | 0.74 | 0.65 | 0.69 | 0.64 | 0.64 | 0.45 | 0.54 | 0.46 | 0.50 | 0.42 | 0.59 | 0.57 | 0.60 |
| Error (%) | 1.77 | 1.00 | 2.78 | 1.42 | 3.26 | 1.51 | 2.72 | 1.43 | 2.25 | 0.79 | 3.84 | 1.26 | 1.70 | 1.33 | 2.16 | 1.56 |
| Rcor kΩ·cm2 | 22.46 | 30.15 | 27.51 | 27.98 | 27.91 | 33.66 | 26.06 | 54.26 | ||||||||
| Error (%) | 3.36 | 1.93 | 5.35 | 3.70 | 8.76 | 5.49 | 6.94 | 7.53 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, F.M.; Terada, M.; Bugarin, A.F.S.; de Melo, H.G.; Costa, I. Comparison of Corrosion Resistance of the AA2524-T3 and the AA2024-T3. Metals 2021, 11, 980. https://doi.org/10.3390/met11060980
Queiroz FM, Terada M, Bugarin AFS, de Melo HG, Costa I. Comparison of Corrosion Resistance of the AA2524-T3 and the AA2024-T3. Metals. 2021; 11(6):980. https://doi.org/10.3390/met11060980
Chicago/Turabian StyleQueiroz, Fernanda Martins, Maysa Terada, Aline F. Santos Bugarin, Hercílio Gomes de Melo, and Isolda Costa. 2021. "Comparison of Corrosion Resistance of the AA2524-T3 and the AA2024-T3" Metals 11, no. 6: 980. https://doi.org/10.3390/met11060980
APA StyleQueiroz, F. M., Terada, M., Bugarin, A. F. S., de Melo, H. G., & Costa, I. (2021). Comparison of Corrosion Resistance of the AA2524-T3 and the AA2024-T3. Metals, 11(6), 980. https://doi.org/10.3390/met11060980





