In Vitro Physical-Chemical Behaviour Assessment of 3D-Printed CoCrMo Alloy for Orthopaedic Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Method for Producing the CoCrMo Alloy
2.1.1. 3D Manufacturing
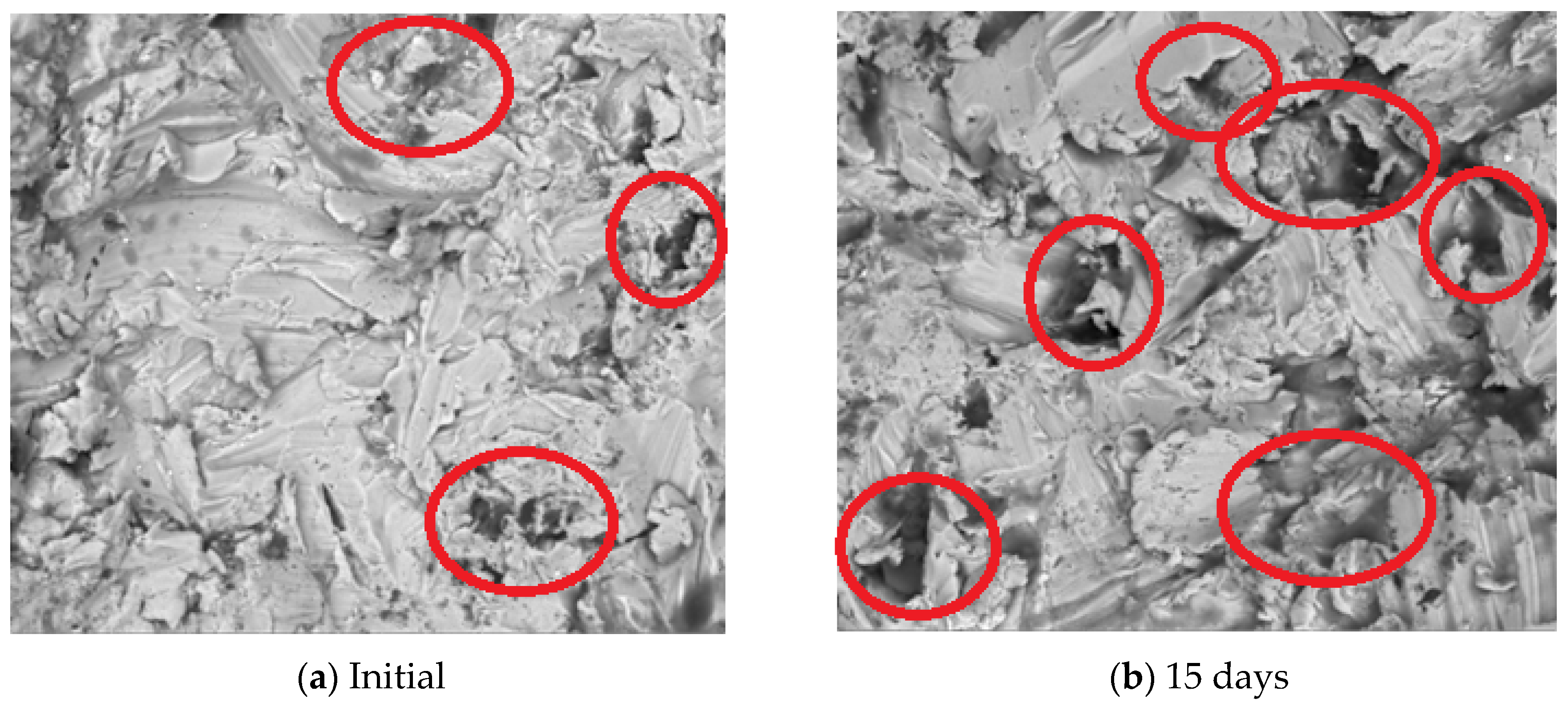
2.1.2. SEM Assessment
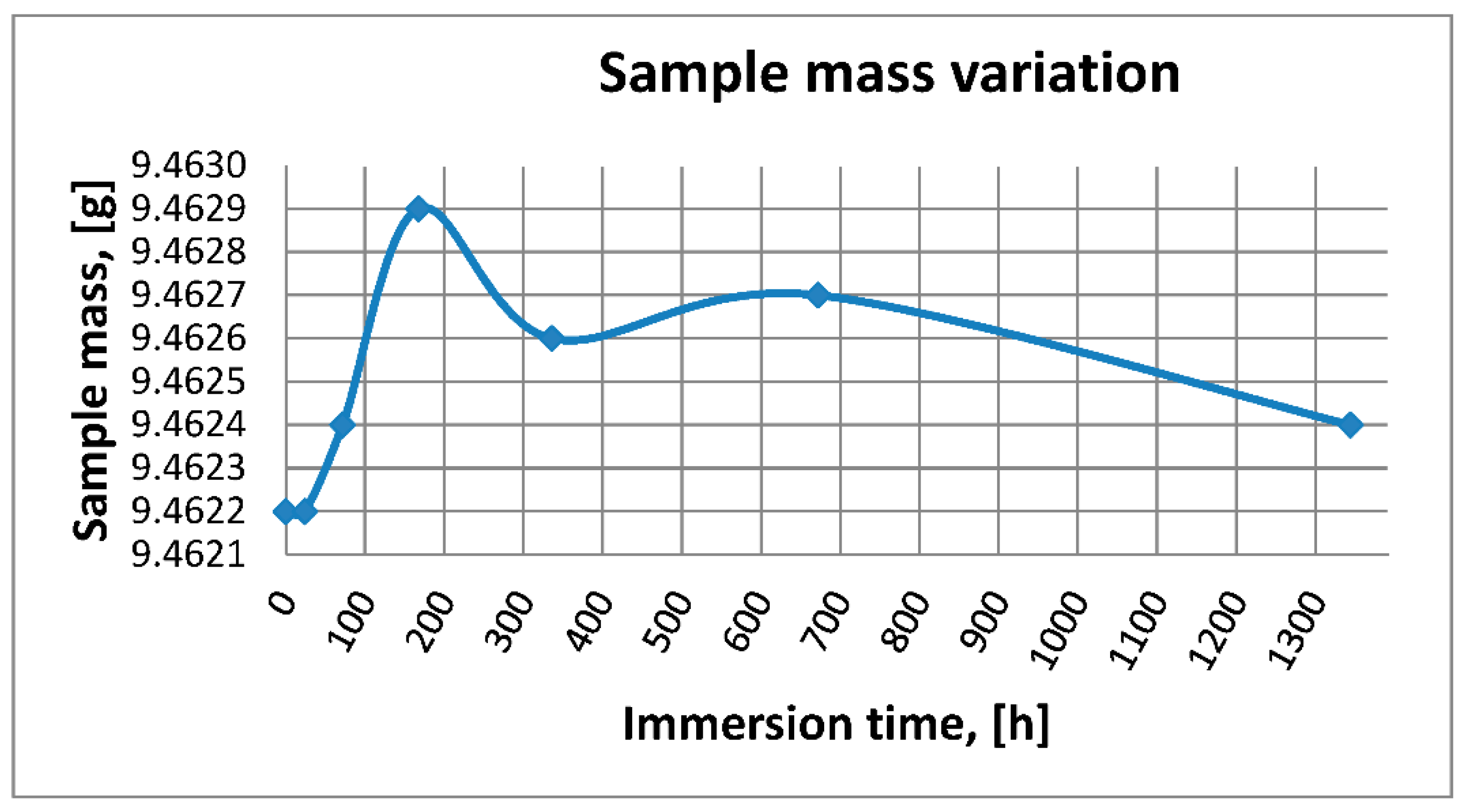
2.1.3. Mass Variation Assessment
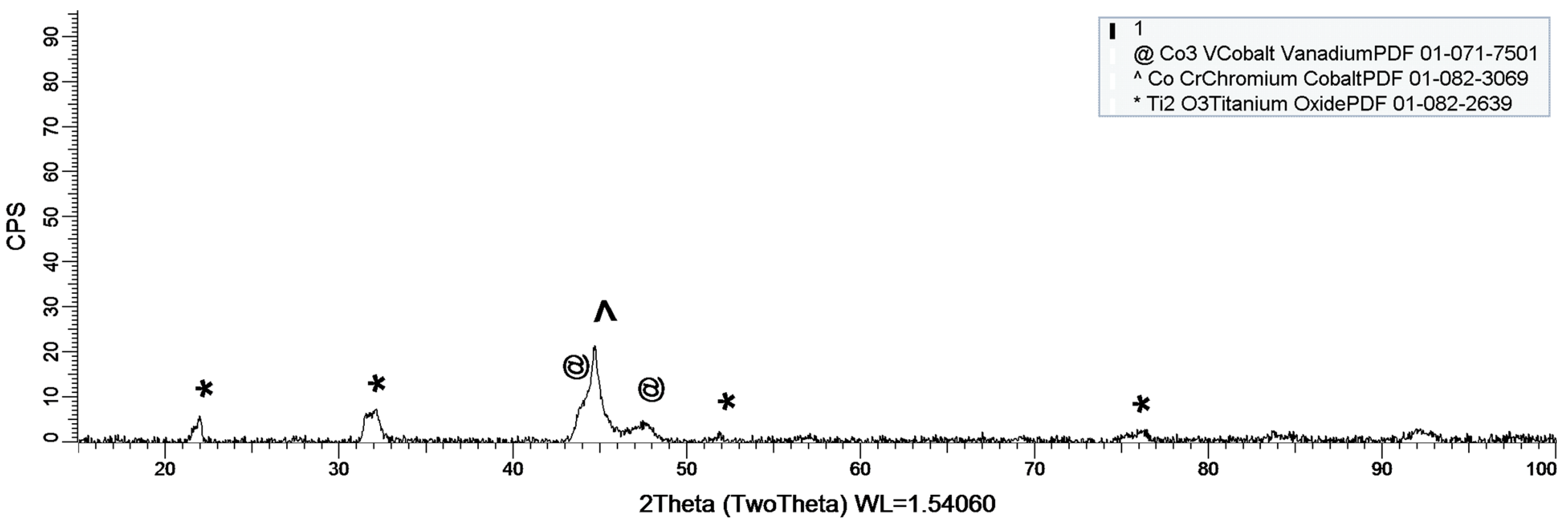
2.1.4. XRD Assessment
2.2. Method for Preparing the SBF
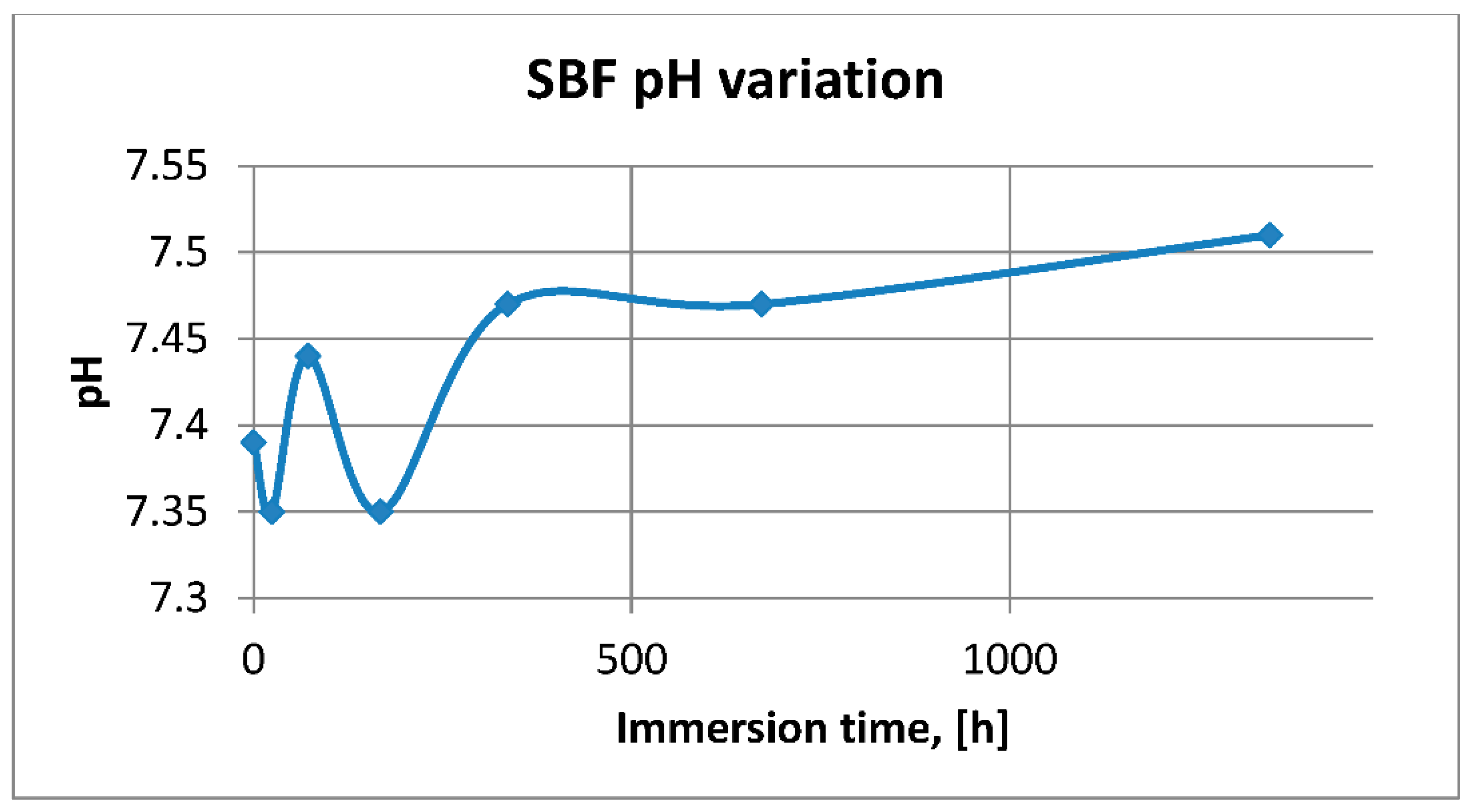
2.2.1. PH and Electrical Conductivity Assessment
2.2.2. ICP-MS Assessment
3. Results and Discussions
3.1. Material Characterization
3.1.1. SEM Assessment
3.1.2. XRD Assessment
3.1.3. Mass Variation Assessment
3.2. SBF Characterization
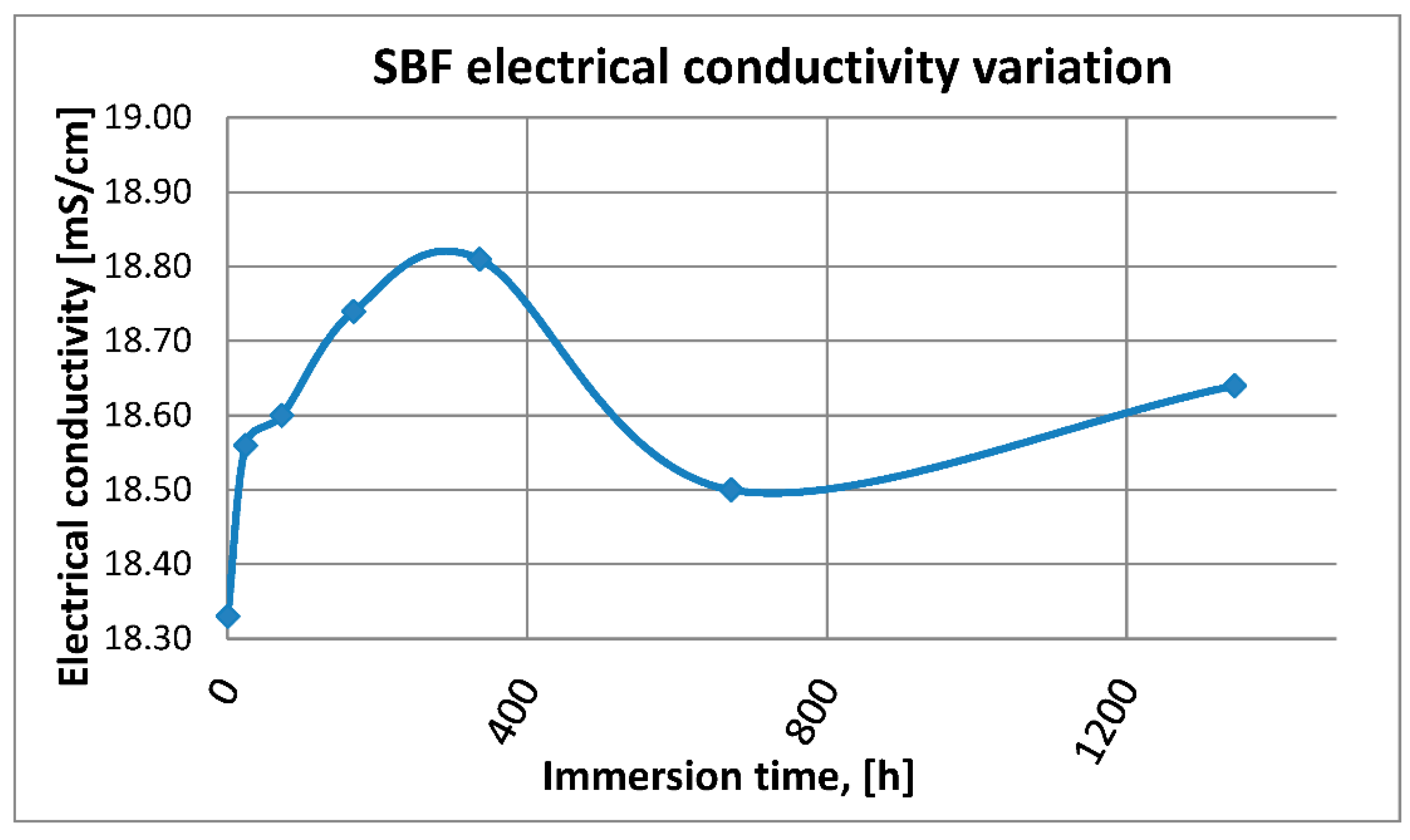
3.2.1. SBF pH and Electrical Conductivity Assessment
3.2.2. Metal Ion Release Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baht, S.V. Biomaterials; Narosa Publishing House: New Delhi, India, 2002. [Google Scholar]
- Benmassauod, M.M.; Kohama, C.; Kim, T.W.B.; Kadlowec, J.A.; Foltiny, B.; Mercurio, T.; Ranganathan, S.I. Efficiency of eluted antibiotics trough 3D printed femoral implants. Biomed. Microdevices 2019, 21, 1–10. [Google Scholar]
- El-Hajje, A.; Kolos, E.C.; Wang, J.K.; Malekaseedi, S.; He, Z.; Wiria, F.E.; Choong, C.; Ruys, A.J. Physical and mechanical characterization of 3D-printed porous titanium for biomedical applications. J. Mater. Sci. 2014, 25, 2471–2481. [Google Scholar]
- Attarilar, S.; Ebrahimi, M.; Djavanroodi, F.; Fu, Y.; Wang, L.; Yang, J. 3D printing technologies in metallic implants: A thematic review of the techniques and procedures. Int. J. Bioprinting 2021, 7, 21–46. [Google Scholar] [CrossRef]
- ASTM Standard F2792-12a Standard Terminology for Additive Manufacturing Technologies; ASTM International: West Conshohocken, PA, USA, 2012.
- Sing, S.L.; An j Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic imlants: A review of processes, materials and design. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear characteristics of metallic biomaterials: A review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Popescu, D.; Ene, R.; Popescu, A.; Cîrstoiu, M.; Sinescu, R.; Cîrstoiu, C. Total Hip Joint Replacement in Young Male Patient With Osteoporosis, Secondary To Hypogonadotropic Hypogonadism. Acta Endocrinol. 2015, 11, 109–113. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fredrick, L.; Spoor, J.; Synes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current chalanges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Gawlik, M.M.; Wiese, B.; Desharnis, V.; Ebel, T.; Willumeit-Romer, R. The effect of surface treatments on the degradation of biomedical Mg alloys—A review. Materials 2018, 11, 2561. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Bidhendi, H.R.A.; Pouranvari, M. Corrosion study o metallic biomaterials in simulated body fluid. Metalurjia-MJoM 2011, 17, 13–22. [Google Scholar] [CrossRef]
- Baino, F.; Yamaguchi, S. The use of simulated body fluid (SBF) for assessing material bioactivity in the context of tissue engineering: Review and chalanges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef]
- Petkovic, D.S.; Mandrino, D.; Sarler, B.; Horky, J.; Ojdanic, A.; Zehetbauer, M.J.; Orlov, D. Surface analysis of biodegradable Mg-alloys after immersion in simulated body fluid. Materials 2020, 13, 1740. [Google Scholar]
- Diomidis, N.; Mischler, S.; More, N.S.; Manish, R. Tribo-electrochemical characterization of metallic biomaterials for total joint replacement. Acta Biomater. 2012, 8, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.; Demetrescu, I.; Vasilescu, E.; Drob, P.; Ionita, D.; Vasilescu, C. Corrosion Resistance of Some Thermo-mechanically Treated Titanium Bioalloys Depending on pH of Ringer Solution. Rev. Roum. Chim. 2009, 60, 241–247. [Google Scholar]
- Nica, M.; Cretu, B.; Ene, D.; Antoniac, I.; Gheorghita, D.; Ene, R. Failure Analysis of Retrieved Osteosynthesis Implants. Materials 2020, 13, 1201. [Google Scholar] [CrossRef]
- Wong, K.-C.; Scheinemann, P. Additive manufactured metallic implants for orthopaedic applications, Adv. In Metallic Biomaterials. Sci. China Mater. 2018, 61, 440–454. [Google Scholar] [CrossRef]
- Available online: https://www.mee-inc.com/hamm/scanning-electron-microscopy-sem/ (accessed on 22 January 2021).
- Marques, M.R.C.; Loedberg, R.; Almukainzi, M. Simulated biological fluids with possible applicaation in dissolution testing. Technologies 2011, 18, 15–26. [Google Scholar]
- Mirea, R.; Ceatra, L.; Cucuruz, A.T.; Ene, R.; Popescu, E.; Biris, I.; Cretu, M. Advanced experimental investigation of used metallic biomaterials. Rom. J. Mater. 2019, 59, 138–145. [Google Scholar]
- Dickinson, C.; Zhou, W.; Hodgkins, R.P.; Shi, Y.; Zhao, D.; He, H. Formation mechanism of porous single-crystal Cr2O3 and Co3O4 templated by mesoporous silica. Chem, Matter. 2006, 18, 3088–3095. [Google Scholar] [CrossRef]
- Gorji, N.E.; O’Connor, R.; Brabazon, D. XPS, XRD and SEM characterization of the virgin and recycled powders for 3D printing applications. IOP Conf. Ser. Mater. Sci. Eng. 2019, 591, 012016. [Google Scholar] [CrossRef]
- Aksakal, B.; Yildirim, Ö.S.; Gul, H. Metallurgical failure analysis of various implant materials used in orthopedic applications. J. Fail. Anal. Prev. 2004, 4, 17–23. [Google Scholar] [CrossRef]

| Element | Co | Cr | W | Mo | Other: C, Fe, Mn, N, Si |
|---|---|---|---|---|---|
| wt.% | 59 | 25 | 9.5 | 3.5 | Less than 1.5 |
| Reagent | Amount for 1 L of SBF |
|---|---|
| Sodium chloride | 8.035 g |
| Sodium bicarbonate | 0.355 g |
| Potassium chloride | 0.225 g |
| Potassium phosphate dibasic tri-hydrate | 0.231 g |
| Magnesium chloride hexahydrate | 0.311 g |
| 1M hydrochloric acid | 39 mL |
| Calcium chloride | 0.292 g |
| Sodium sulphate | 0.072 g |
| Tris(hydroxyl-methyl) amino methane | 6.118 g |
| Element | Co (µg/L) | Cr (µg/L) | Mo (µg/L) | Mn (µg/L) |
|---|---|---|---|---|
| Time | ||||
| 0 h—Initial | 0 | 0 | 0 | 0 |
| 24 h | 5.346 | 7.39 | 0.453 | 1.374 |
| 72 h | 9.771 | 6.852 | 0.729 | 1.824 |
| 168 h | 14.646 | 6.801 | 1.108 | 2.147 |
| 336 h | 20.32 | 6.58 | 1.611 | 2.295 |
| 672 h | 30.058 | 6.48 | 2.413 | 2.551 |
| 1334 h | 30.325 | 5.149 | 2.598 | 2.145 |
| Sample | Corrosion Rate {g/(mm2 Week)} | Ion Release ({mg/L}/(mm2 Week)) | ||||
|---|---|---|---|---|---|---|
| Co | Cr | Mo | Mn | W | ||
| 3D-printed CoCrMo | 6.87 e−5 | 1.4 e−5 | 5.13 e−5 | 1.5 e−5 | 14.9 e−5 | 10.3 e−5 |
| Metal | Effect |
|---|---|
| Nickel (Ni) | It is the main cause of contact dermatitis. The main biological parameter is the amount of metal released on the skin during direct contact and exposure to human sweat. The limit is 0.5 mg/cm2 x week, of which an insignificant part of Ni sensitive subjects will react. It has a toxic effect by creating cellular lesions and large cellular cultures. It is dangerous for bones and tissues, although less dangerous than Co or V, and it has cancer potency. The normal level of Ni in the blood is 5 mg/L [1]. |
| Cobalt (Co) | Its function limits the role of vitamin B12 [1], by diminishing the adsorption of Fe in the blood stream [24]. The normal concentration of Co in human fluids is 1.5 mg/L. |
| Chromium (Cr) | It causes ulceration and central nerve system disorders [24]. The maximal concentration in the blood stream should be 28 mg/L. Its compounds are adsorbed only after oral ingestion. Cr (III) is usually deposited in reticular systems in the cell, while Cr (IV) can penetrate cellular membrane in both directions [1]. |
| Aluminium (Al) | It provokes epileptic episodes and Alzheimer’s disease [24]. The maximal concentration in the blood stream should be 30 mg/L. |
| Vanadium (V) | It is very toxic in its elementary state [24], therefore, the maximum concentration should not exceed 0.5 µg/L. |
| Molybdenum (Mo) | It is an essential element use by specific enzymes. It is easily adsorbed through the intestines, and its normal concentration in the blood stream should be 1–3 ppm. It is very toxic and sometimes lethal in large doses, regular symptoms are diarrhoea, coma, heart failure, and inhibitor for some essential enzymes. In addition, large concentration of Mo can interfere with Ca and P metabolism [1]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirea, R.; Biris, I.M.; Ceatra, L.C.; Ene, R.; Paraschiv, A.; Cucuruz, A.T.; Sbarcea, G.; Popescu, E.; Badea, T. In Vitro Physical-Chemical Behaviour Assessment of 3D-Printed CoCrMo Alloy for Orthopaedic Implants. Metals 2021, 11, 857. https://doi.org/10.3390/met11060857
Mirea R, Biris IM, Ceatra LC, Ene R, Paraschiv A, Cucuruz AT, Sbarcea G, Popescu E, Badea T. In Vitro Physical-Chemical Behaviour Assessment of 3D-Printed CoCrMo Alloy for Orthopaedic Implants. Metals. 2021; 11(6):857. https://doi.org/10.3390/met11060857
Chicago/Turabian StyleMirea, Radu, Iuliana Manuela Biris, Laurentiu Constantin Ceatra, Razvan Ene, Alexandru Paraschiv, Andrei Tiberiu Cucuruz, Gabriela Sbarcea, Elisa Popescu, and Teodor Badea. 2021. "In Vitro Physical-Chemical Behaviour Assessment of 3D-Printed CoCrMo Alloy for Orthopaedic Implants" Metals 11, no. 6: 857. https://doi.org/10.3390/met11060857
APA StyleMirea, R., Biris, I. M., Ceatra, L. C., Ene, R., Paraschiv, A., Cucuruz, A. T., Sbarcea, G., Popescu, E., & Badea, T. (2021). In Vitro Physical-Chemical Behaviour Assessment of 3D-Printed CoCrMo Alloy for Orthopaedic Implants. Metals, 11(6), 857. https://doi.org/10.3390/met11060857






