Investigation on the Reaction Behaviour of Partially Reduced Iron under Blast Furnace Conditions
Abstract
1. Introduction
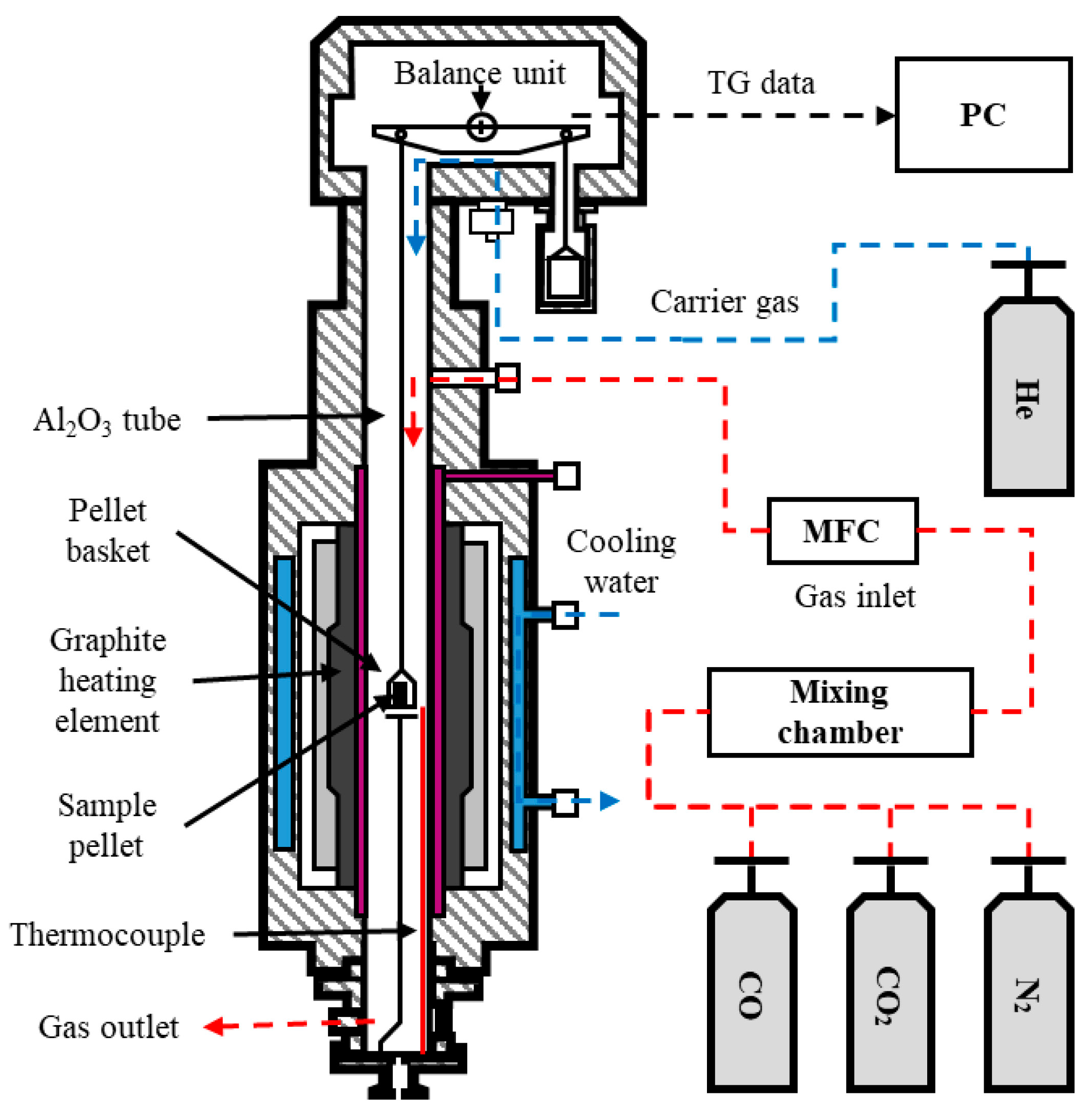
2. Materials and Methods
2.1. Sample Preparation
2.2. Experiments
3. Results and Discussion
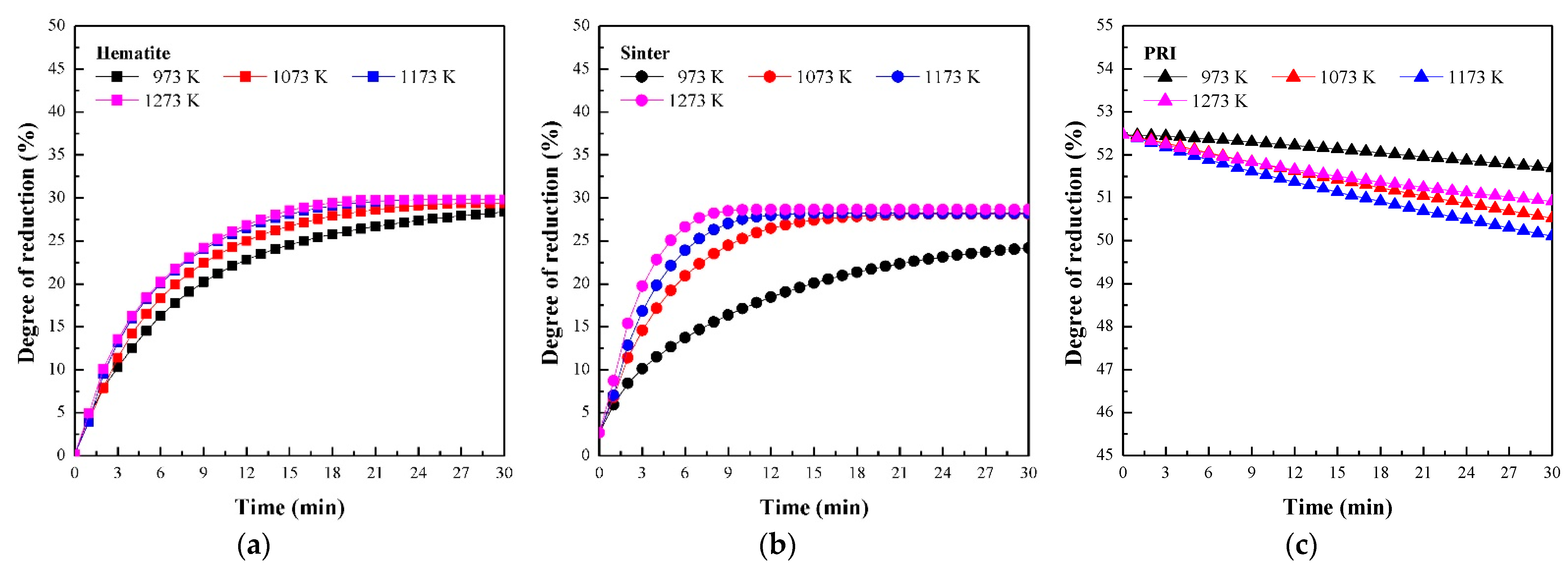
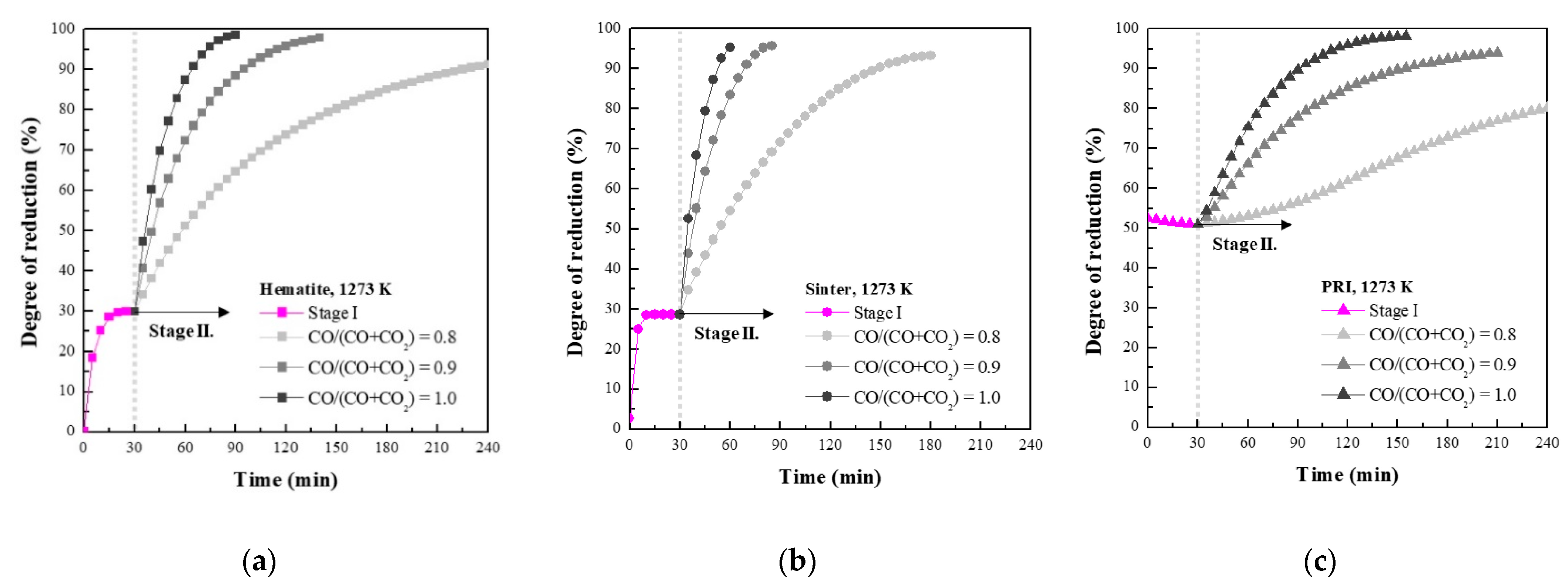
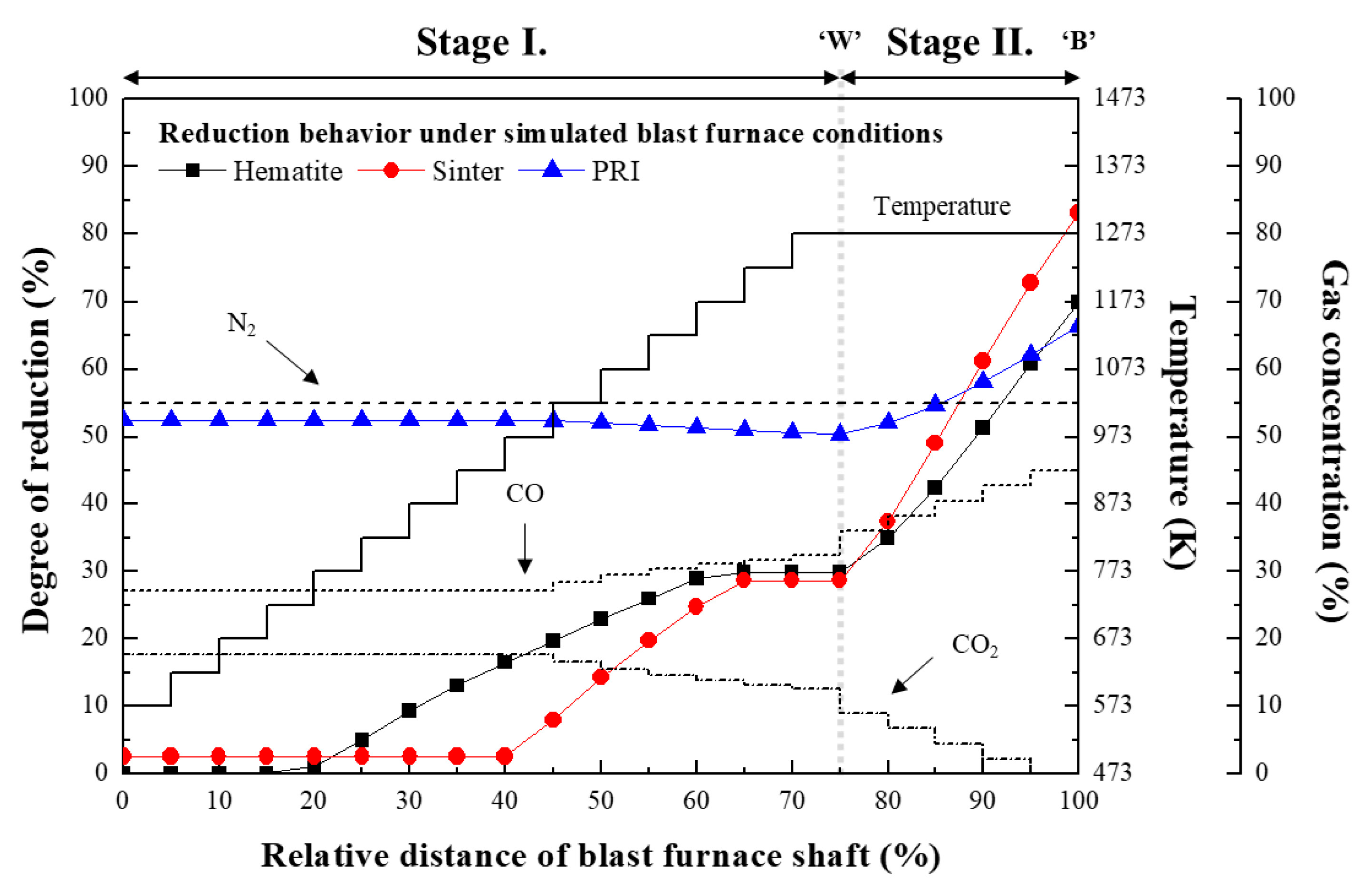
3.1. Reaction Behaviour of Iron Oxide in Shaft Zone of Blast Furnace
- (a)
- Interfacial chemical reaction:
- (b)
- Gaseous mass transport through the product layer (internal diffusion):
- (c)
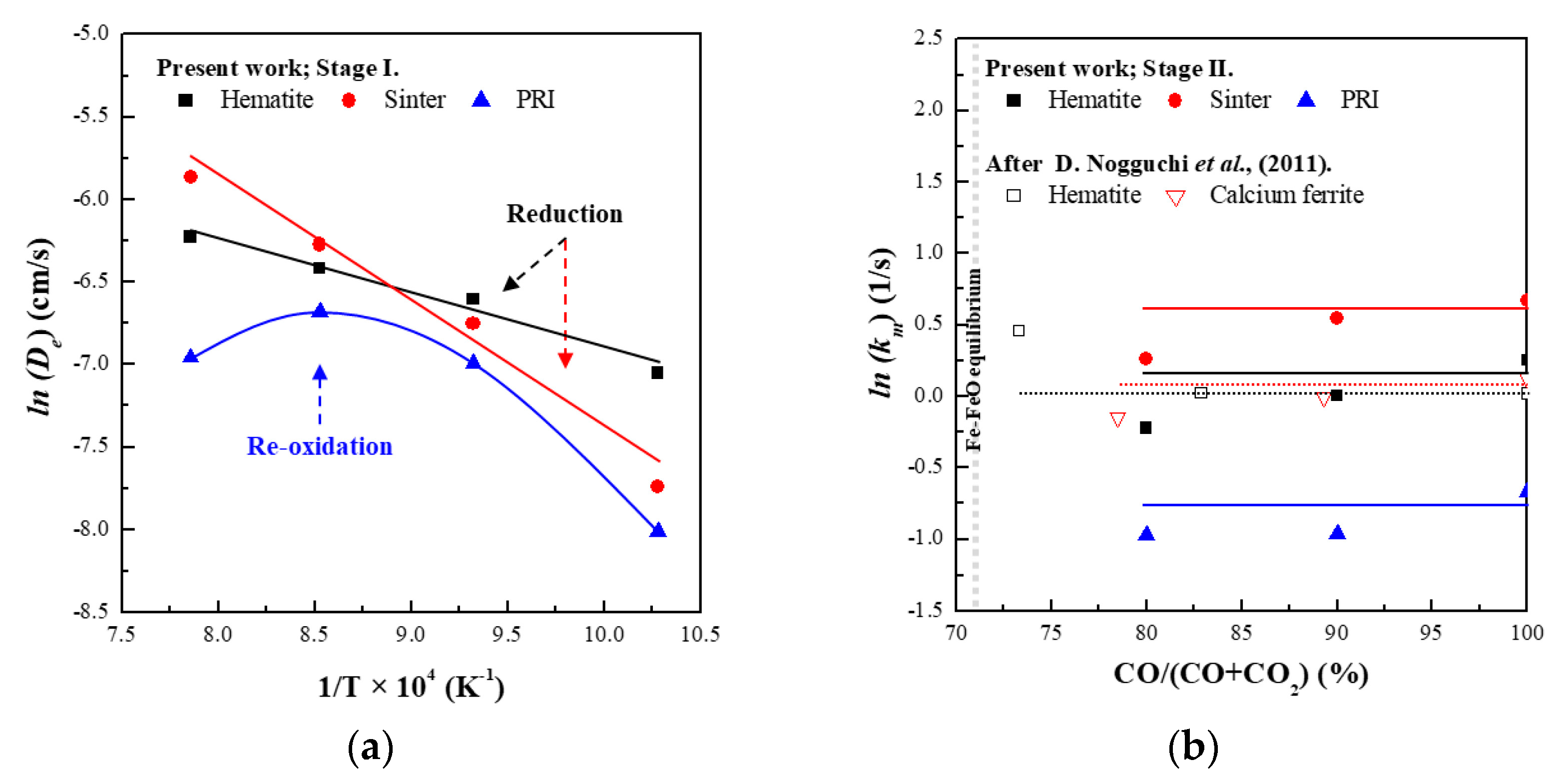
- Mixed controlled (interfacial chemical reaction + internal diffusion) reaction:where is the fractional degree of reduction at time t (), is the dimensionless time, is the shrinking core modulus (), and , and are the apparent rate constants (). The shrinking core modulus represents the ratio of the capacities of chemical reaction and diffusion based on the grain model. The relative resistance between the interfacial reaction and internal diffusion was evaluated according to Equations (8) and (9) to determine the rate-controlling step.
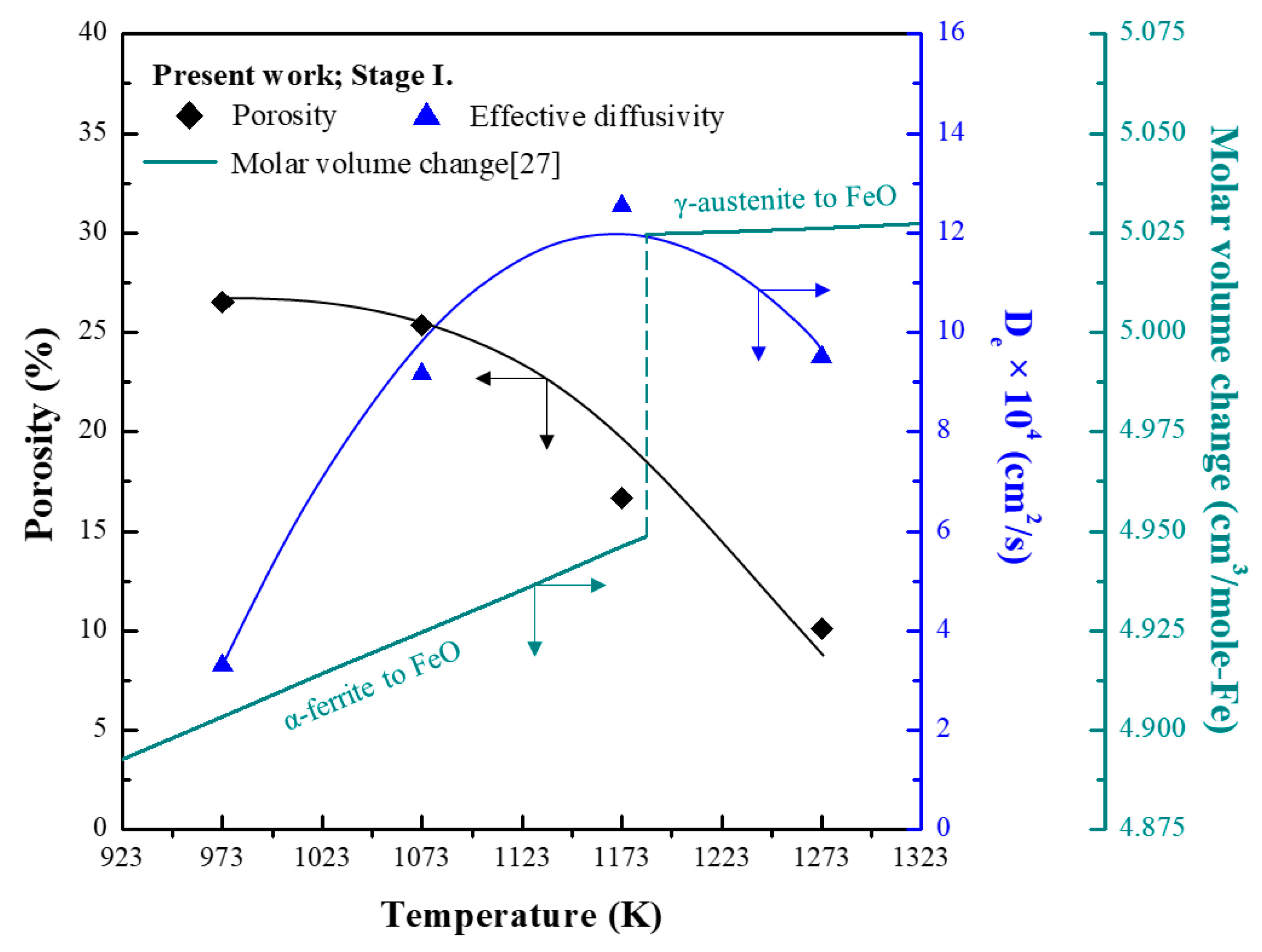
3.2. Analysis of Morphology and Porosity of PRI
3.3. Reaction Behaviour of PRI
4. Conclusions
- (1)
- PRI was reoxidised, whereas hematite and sinter were reduced in Stage I. The rate of reoxidation initially increased with increasing temperature and then decreased at 1273 K owing to the blockage of pores resulting from the significant increase in the molar volume changes during oxidation according to the phase transition of metallic iron.
- (2)
- The reduction rate of PRI in Stage II is retarded. It was confirmed that the reduction retardation of PRI was caused by a blockage of pores owing to the reoxidation of PRI that occurred in Stage I.
- (3)
- The reaction behaviour of the oxides was evaluated based on the effective diffusivity and mixed controlled rate constant. It was confirmed that the reaction behaviour of hematite in this study is consistent with the reaction behaviour of the ferrous burden in the theoretical blast furnace.
- (4)
- The degree of reduction of PRI at the final stage of Stage II is lower than that of hematite owing to the reduction retardation phenomenon of PRI. Consequently, the reduction retardation phenomenon of the PRI could deteriorate the stability of the blast furnace operation and negatively impact the heat balance and gas utilisation of the blast furnace.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ujisawa, Y.; Nakano, K.; Matsukura, Y.; Sunahara, K.; Komatsu, S.; Yamamoto, T. Subjects for achievement of blast furnace operation with low reducing agent rate. ISIJ Int. 2005, 45, 1379–1385. [Google Scholar] [CrossRef]
- Matsukura, Y.; Nakano, K.; Sunahara, K.; Ujisawa, Y.; Yamamoto, K. Effect of burden properties on permeability in blast furnace. Iron Steel 2001, 87, 350–356. [Google Scholar] [CrossRef][Green Version]
- Nomura, S.; Ayukawa, H.; Kitaguchi, H.; Tahara, T.; Matsuzaki, S.; Naito, M.; Koizumi, S.; Ogata, Y.; Nakayama, T.; Abe, T. Improvement in blast furnace reaction efficiency through the use of highly reactive calcium rich coke. ISIJ Int. 2005, 45, 316–324. [Google Scholar] [CrossRef]
- Moon, I.J.; Rhee, C.H.; Min, D.J. Reduction of hematite compacts by H2-CO gas mixtures. Steel Res. 1998, 69, 302–306. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Rajakumar, V. Gaseous reduction of wustite with H2, CO and H2-CO mixtures. Trans. Iron Steel Inst. Jpn. 1985, 25, 449–458. [Google Scholar] [CrossRef]
- Usui, T.; Kawabata, H.; Ono-Nakazato, H.; Kurosaka, A. Fundamental experiments on the H2 gas injection into the lower part of a blast furnace shaft. ISIJ Int. 2002, 42 (Suppl. S14), S14–S18. [Google Scholar] [CrossRef]
- Park, T.J.; Lee, J.H.; Kim, D.G.; Kim, H. Estimation of the H2 Gas Utilization Ratio Using a BF Shaft Inner Reaction Simulator. Metall. Mater. Trans. B 2020, 51, 417–421. [Google Scholar] [CrossRef]
- Kim, W.H.; Min, D.J. A mass and energy estimation for the hydrogen utilization in the iron-making process. Sci. China Technol. Sci. 2011, 54, 1655–1660. [Google Scholar] [CrossRef]
- Sundar Murti, N.S.; Seshadri, V. Kinetics of reduction of synthetic chromite with carbon. Trans. Iron Steel Inst. Jpn. 1982, 22, 925–933. [Google Scholar] [CrossRef]
- Rist, A.; Meysson, N. A dual graphic representation of the blast-furnace mass and heat balances. J. Mater. 1967, 19, 50–59. [Google Scholar] [CrossRef]
- Ono, Y. Rist operating diagram (I). Iron Steel 1993, 79, N618. [Google Scholar] [CrossRef][Green Version]
- Ono, Y. Rist operating diagram (II). Iron Steel 1993, 79, N711–N715. [Google Scholar] [CrossRef]
- Kaushik, P.; Fruehan, R. Behavior of direct reduced iron and hot briquetted iron in the upper blast furnace shaft: Part I. Fundamentals of kinetics and mechanism of oxidation. Metall. Mater. Trans. B 2006, 37, 715–725. [Google Scholar] [CrossRef]
- Kaushik, P.; Fruehan, R. Behavior of direct reduced iron and hot briquetted iron in the upper blast furnace shaft: Part II. A model of oxidation. Metall. Mater. Trans. B 2006, 37, 727–732. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; El-Kashif, F.O.; Nasr, M.I.; Omar, A.A. Kinetics and mechanisms of re-oxidation of freshly reduced iron compacts. ISIJ Int. 1994, 34, 541–547. [Google Scholar] [CrossRef][Green Version]
- Taniguchi, S.; Ohmi, M.; Nakaoka, S. Re-oxidation behaviour of a porous metallised iron pellet at relatively high temperatures. Trans. Jpn. Inst. Met. 1981, 22, 145–152. [Google Scholar] [CrossRef]
- Yang, L.; Belton, G. Iron redox equilibria in CaO-Al2O3-SiO2 and MgO-CaO-Al2O3-SiO2 slags. Metall. Mater. Trans. B 1998, 29, 837–845. [Google Scholar] [CrossRef]
- Park, Y.; Min, D.J. Effect of iron redox equilibrium on the foaming behavior of MgO-saturated slags. Metall. Mater. Trans. B 2018, 49, 1709–1718. [Google Scholar] [CrossRef]
- Biswas, A.K. Principles of Blast Furnace Ironmaking: Theory and Practice; Cootha Publishing House: Brisbane, Australia, 1981. [Google Scholar]
- Turkdogan, E. Blast furnace reactions. Metall. Trans. B 1978, 9, 163–179. [Google Scholar] [CrossRef]
- Szekely, J. Gas-Solid Reactions, 1st ed.; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Noguchi, D.; Ohno, K.I.; Maeda, T.; Nishioka, K.; Shimizu, M. Effect of CO gas concentration on reduction rate of major mineral phase in sintered iron ore. J. Iron Steel Inst. Jpn. 2011, 97, 548–553. [Google Scholar] [CrossRef][Green Version]
- Nasr, M.I.; Omar, A.A.; Hessien, M.M.; El-Geassy, A.A. Carbon monoxide reduction and accompanying swelling of iron oxide compacts. ISIJ Int. 1996, 36, 164–171. [Google Scholar] [CrossRef]
- Mousa, E.A. Effect of basicity on wüstite sinter reducibility under simulated blast furnace conditions. Ironmak. Steelmak. 2014, 41, 418–429. [Google Scholar] [CrossRef]
- Kim, W.-H.; Lee, Y.-S.; Suh, I.-K.; Min, D.-J. Influence of CaO and SiO2 on the reducibility of wüstite using H2 and CO gas. ISIJ Int. 2012, 52, 1463–1471. [Google Scholar] [CrossRef]
- Corbari, R.; Fruehan, R. Reduction of iron oxide fines to wustite with CO/CO2 gas of low reducing potential. Metall. Mater. Trans. B 2010, 41, 318–329. [Google Scholar] [CrossRef]
- McCammon, C.; Liu, L.-G. The effects of pressure and temperature on nonstoichiometric wüstite, FexO: The iron-rich phase boundary. Phys. Chem. Miner. 1984, 10, 106–113. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Suzuki, H.; Hayashi, S. Influence of mixing coal composite iron ore hot briquettes on blast furnace simulated reaction behavior in a packed mixed bed. ISIJ Int. 2011, 51, 1247–1254. [Google Scholar] [CrossRef]



| Raw Material | Chemical Composition (wt%) | Initial Degree of Reduciton (%) | |||||
|---|---|---|---|---|---|---|---|
| T.Fe | Fe2+ | M.Fe | CaO/MgO | SiO2 | Al2O3 | ||
| Hematite | 69.94 | – | 0.14 | – | – | – | 0.20 |
| Sinter | 58.01 | 3.91 | 0.24 | 10.26 | 5.47 | 1.99 | 2.66 |
| PRI | 75.07 | 46.83 | 23.78 | 0.12 | 6.11 | 3.41 | 52.47 |
| Stage | No. | Temperature (K) | Gas Composition (%) | |||
|---|---|---|---|---|---|---|
| N2 | CO | CO2 | CO/(CO + CO2) | |||
| Stage I (Fe/FeO equilibrium) | 1 | 973 | 55.00 | 27.23 | 17.77 | 60.50 |
| 2 | 1073 | 55.00 | 29.49 | 15.51 | 65.54 | |
| 3 | 1173 | 55.00 | 31.14 | 13.86 | 69.21 | |
| 4 | 1273 | 55.00 | 32.38 | 12.62 | 71.95 | |
| Stage II | 5 | 1273 | 55.00 | 36.00 | 9.00 | 80.00 |
| 6 | 1273 | 55.00 | 40.50 | 4.50 | 90.00 | |
| 7 | 1273 | 55.00 | 45.00 | 0.00 | 100.00 | |
| Sample | Stage | Temperature (K) | CO/(CO + CO2) (%) | Shrinking Core Modulus | Interfacial Reaction Resistance | Internal Diffusion Resistance | Rate Determining Step |
|---|---|---|---|---|---|---|---|
| Hematite | I. (Fe/FeO equilibrium) | 973 | 60.50 | 7.0511 | 0.1242 | 0.8758 | Internal Diffusion (D3) |
| 1073 | 65.54 | 7.2263 | 0.1216 | 0.8784 | |||
| 1173 | 69.21 | 7.1028 | 0.1234 | 0.8766 | |||
| 1273 | 71.95 | 7.1840 | 0.1222 | 0.8778 | |||
| II. | 1273 | 80.00 | 1.2925 | 0.4362 | 0.5638 | Mixed (R3 + D3) | |
| 1273 | 90.00 | 1.0941 | 0.4775 | 0.5225 | |||
| 1273 | 100.00 | 1.0464 | 0.4887 | 0.5113 | |||
| Sinter | I. (Fe/FeO equilibrium) | 973 | 60.50 | 7.8171 | 0.1134 | 0.8866 | Internal Diffusion (D3) |
| 1073 | 65.54 | 7.2175 | 0.1217 | 0.8783 | |||
| 1173 | 69.21 | 7.4243 | 0.1187 | 0.8813 | |||
| 1273 | 71.95 | 7.4144 | 0.1188 | 0.8812 | |||
| II. | 1273 | 80.00 | 1.3227 | 0.4305 | 0.5695 | Mixed (R3 + D3) | |
| 1273 | 90.00 | 1.2083 | 0.4528 | 0.5472 | |||
| 1273 | 100.00 | 1.2397 | 0.4465 | 0.5535 | |||
| PRI | I. (Fe/FeO equilibrium) | 973 | 60.50 | 2.2978 | 0.3032 | 0.6968 | Internal Diffusion (D3) |
| 1073 | 65.54 | 2.3341 | 0.2999 | 0.7001 | |||
| 1173 | 69.21 | 2.3483 | 0.2987 | 0.7013 | |||
| 1273 | 71.95 | 2.3255 | 0.3007 | 0.6993 | |||
| II. | 1273 | 80.00 | 1.4151 | 0.4141 | 0.5859 | Mixed (R3 + D3) | |
| 1273 | 90.00 | 1.1232 | 0.4170 | 0.5290 | |||
| 1273 | 100.00 | 0.9290 | 0.5184 | 0.4816 |
| Sample | Stage | Temperature (K) | Effective Diffusivity, De (cm/s) | Mixed Controlled Rate Constant, km (1/s) |
|---|---|---|---|---|
| Hematite | I. (Fe/FeO equilibrium) | 973 | 8.643 × 10−4 | – |
| 1073 | 1.350 × 10−3 | – | ||
| 1173 | 1.629 × 10−3 | – | ||
| 1273 | 1.977 × 10−3 | – | ||
| II. | 1273 | – | 1.180 | |
| Sinter | I. (Fe/FeO equilibrium) | 973 | 4.350 × 10−4 | – |
| 1073 | 1.172 × 10−3 | – | ||
| 1173 | 1.883 × 10−3 | – | ||
| 1273 | 2.828 × 10−3 | – | ||
| II. | 1273 | – | 1.854 | |
| PRI | I. (Fe/FeO equilibrium) | 973 | 3.314 × 10−4 | – |
| 1073 | 9.168 × 10−4 | – | ||
| 1173 | 1.254 × 10−3 | – | ||
| 1273 | 9.508 × 10−4 | – | ||
| II. | 1273 | – | 0.470 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, G.-H.; Choi, J.-S.; Min, D.-J. Investigation on the Reaction Behaviour of Partially Reduced Iron under Blast Furnace Conditions. Metals 2021, 11, 839. https://doi.org/10.3390/met11050839
La G-H, Choi J-S, Min D-J. Investigation on the Reaction Behaviour of Partially Reduced Iron under Blast Furnace Conditions. Metals. 2021; 11(5):839. https://doi.org/10.3390/met11050839
Chicago/Turabian StyleLa, Gi-Ho, Joon-Sung Choi, and Dong-Joon Min. 2021. "Investigation on the Reaction Behaviour of Partially Reduced Iron under Blast Furnace Conditions" Metals 11, no. 5: 839. https://doi.org/10.3390/met11050839
APA StyleLa, G.-H., Choi, J.-S., & Min, D.-J. (2021). Investigation on the Reaction Behaviour of Partially Reduced Iron under Blast Furnace Conditions. Metals, 11(5), 839. https://doi.org/10.3390/met11050839







