Flotation Performance, Structure-Activity Relationship and Adsorption Mechanism of O-Isopropyl-N-Ethyl Thionocarbamate Collector for Elemental Sulfur in a High-Sulfur Residue
Abstract
1. Introduction
2. Experimental
2.1. Material and Reagents
2.2. Experimental Methods
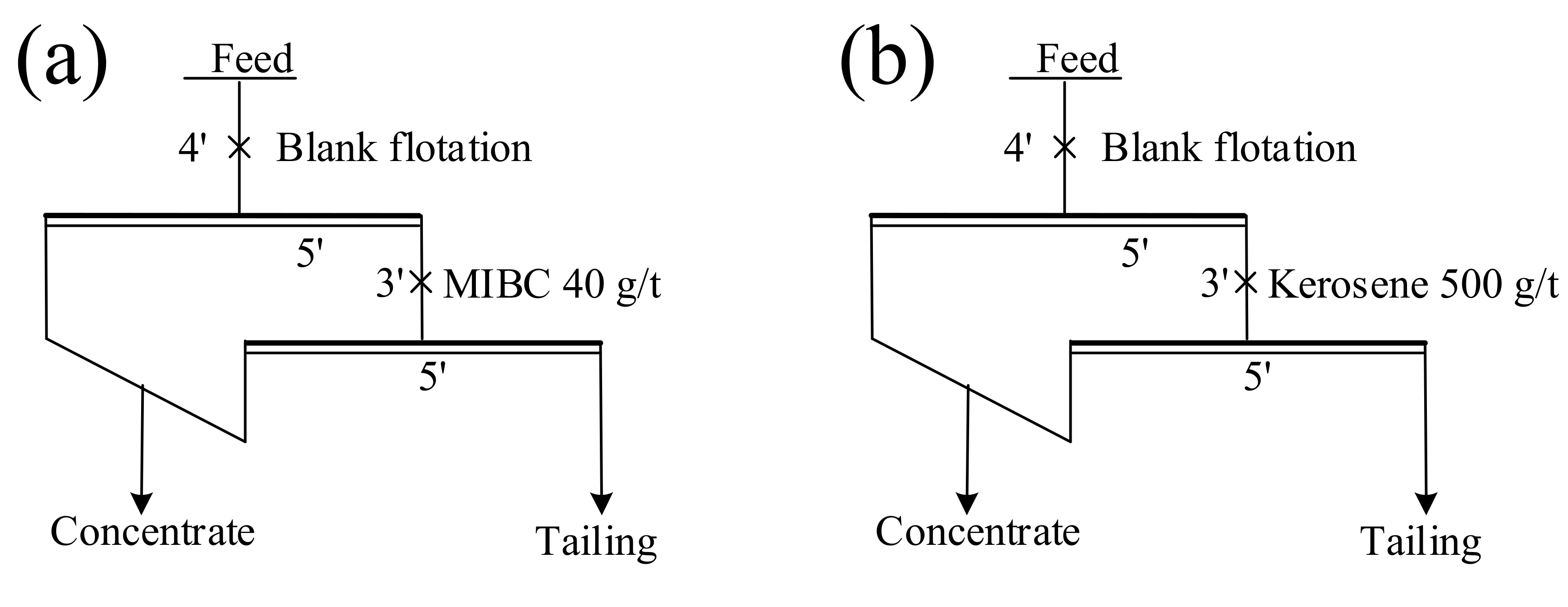
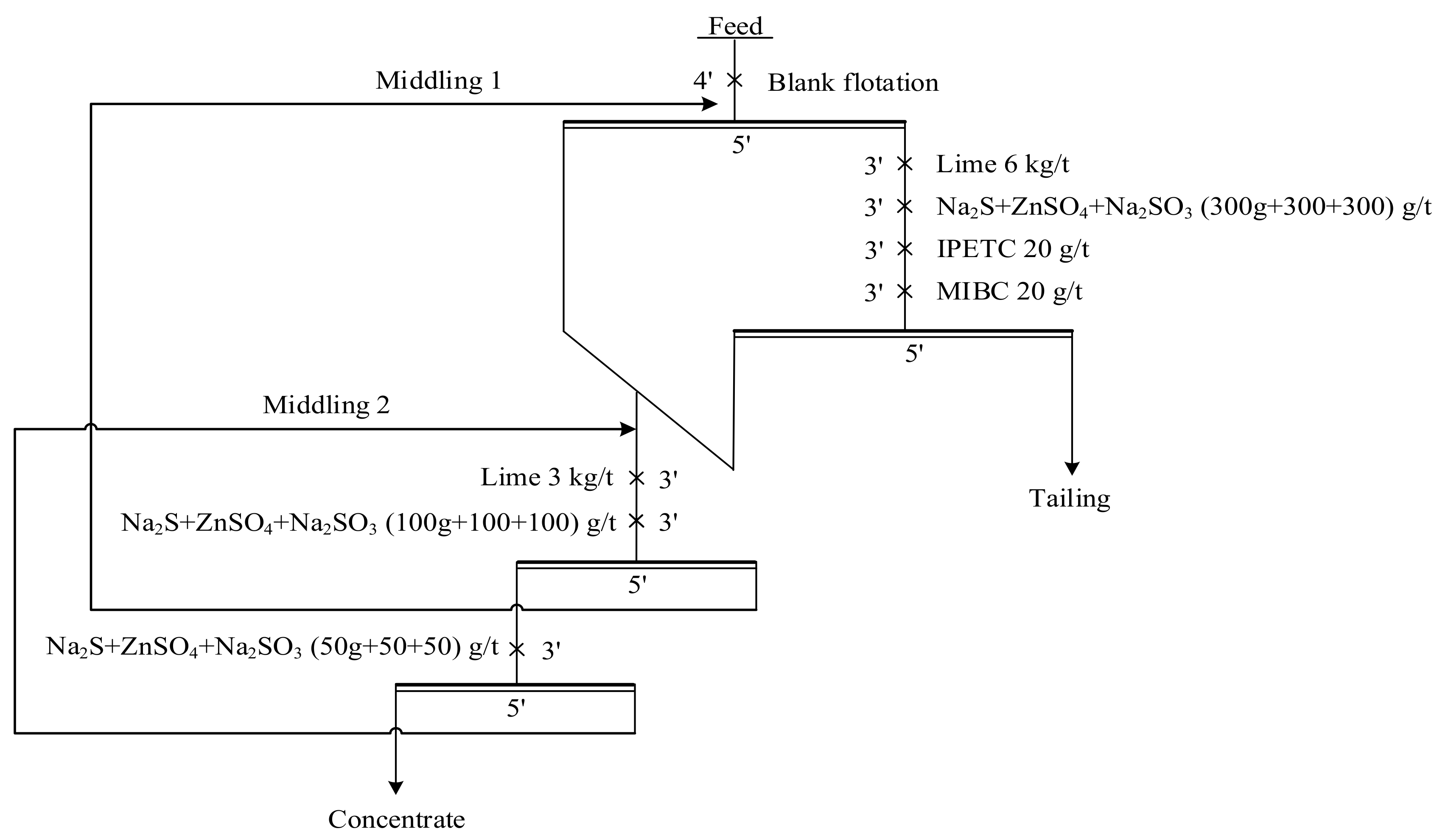
2.2.1. Flotation Experiment
Raw Ore Flotation
Pure Mineral Flotation
2.2.2. FTIR Spectrum
2.2.3. DFT Calculation
2.3. Analytical Methods
3. Results and Discussion
3.1. Flotation Performance of Collectors
3.2. Pure Mineral Flotation and Adsorption Experiments
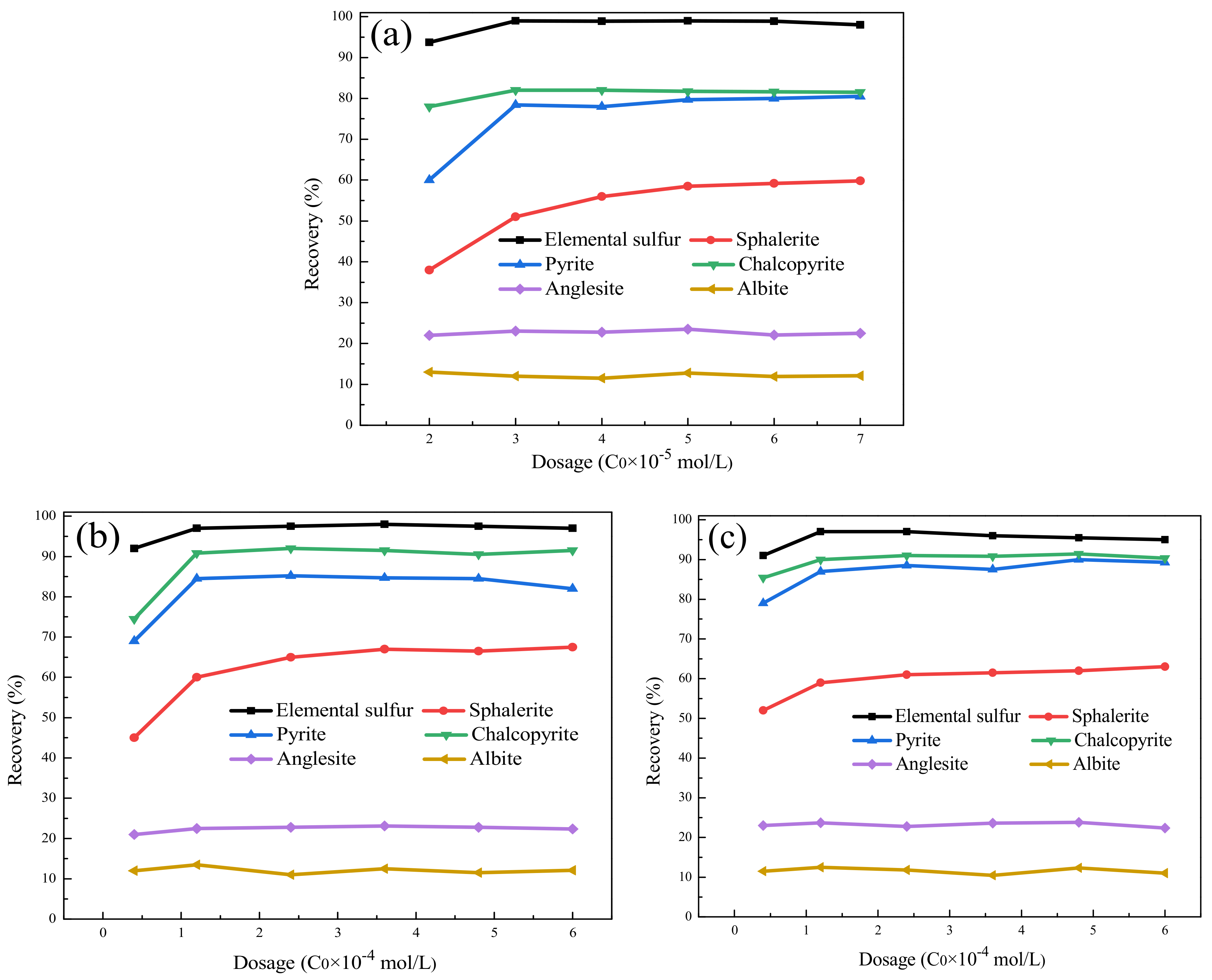
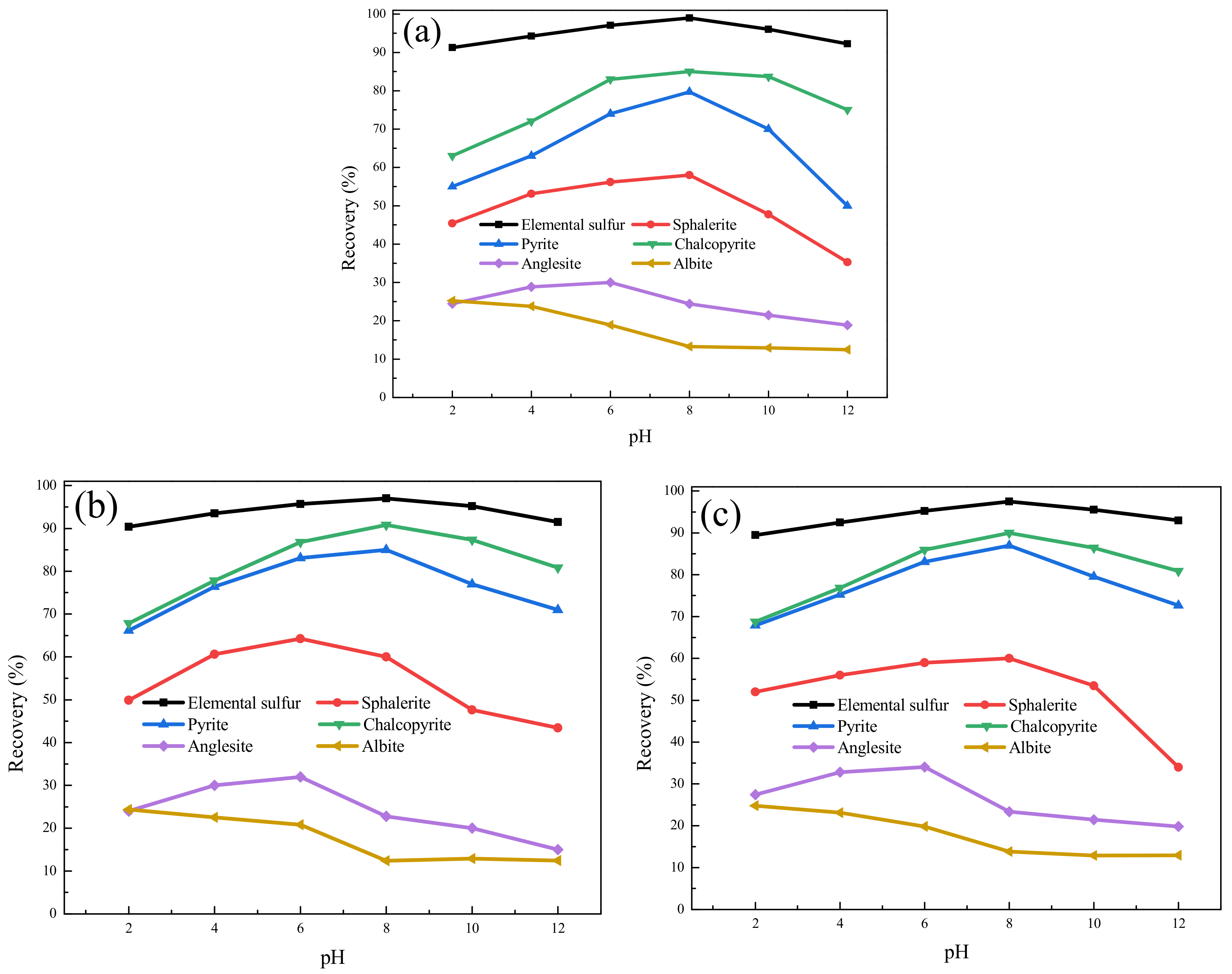
3.2.1. Pure Mineral Flotation Experiment
3.2.2. Pure Mineral Adsorption Experiment
3.3. Structure–Property Relationships of Collectors
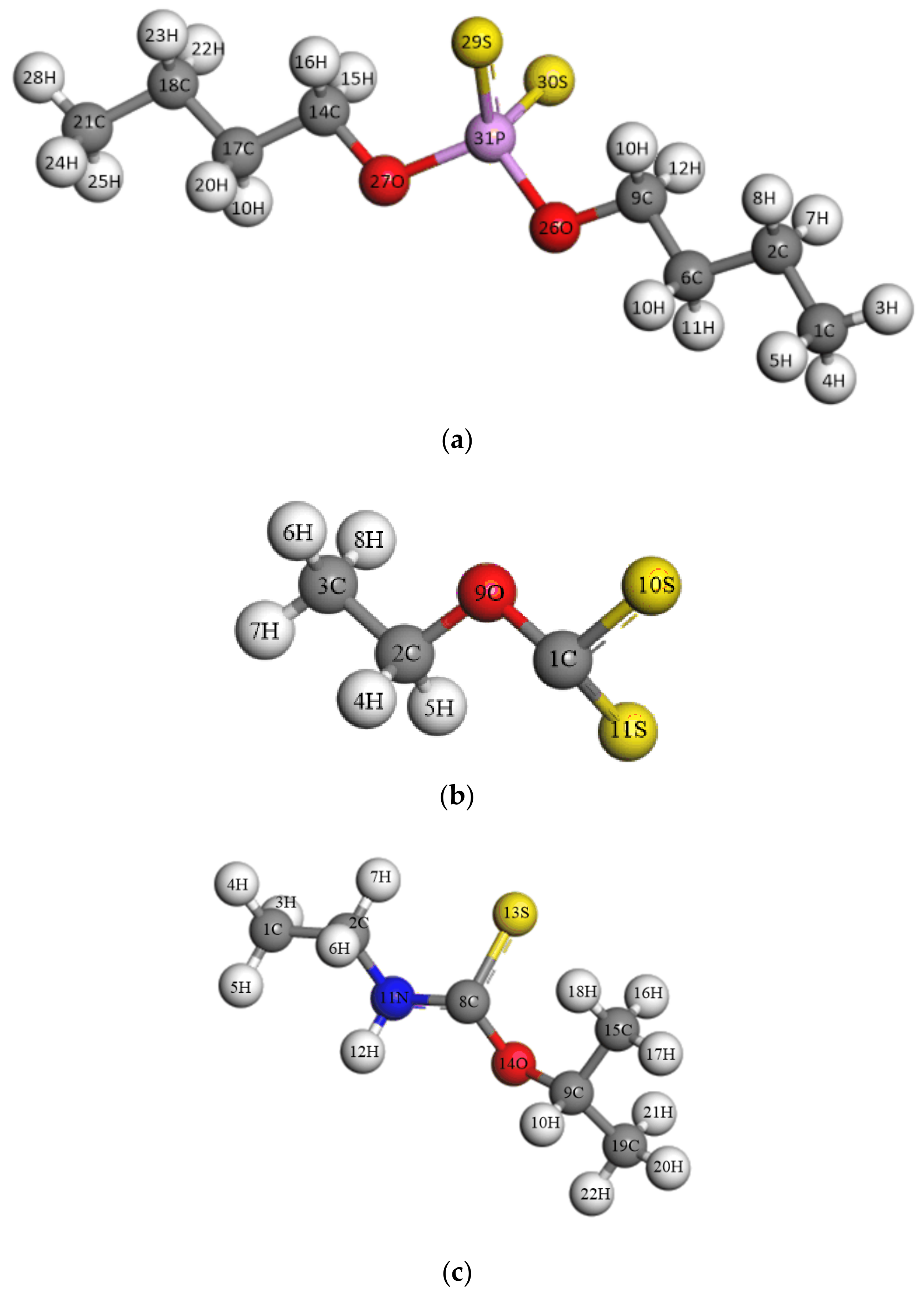
3.3.1. Geometry Configuration
3.3.2. Electronic Structure
Mulliken Population Analysis
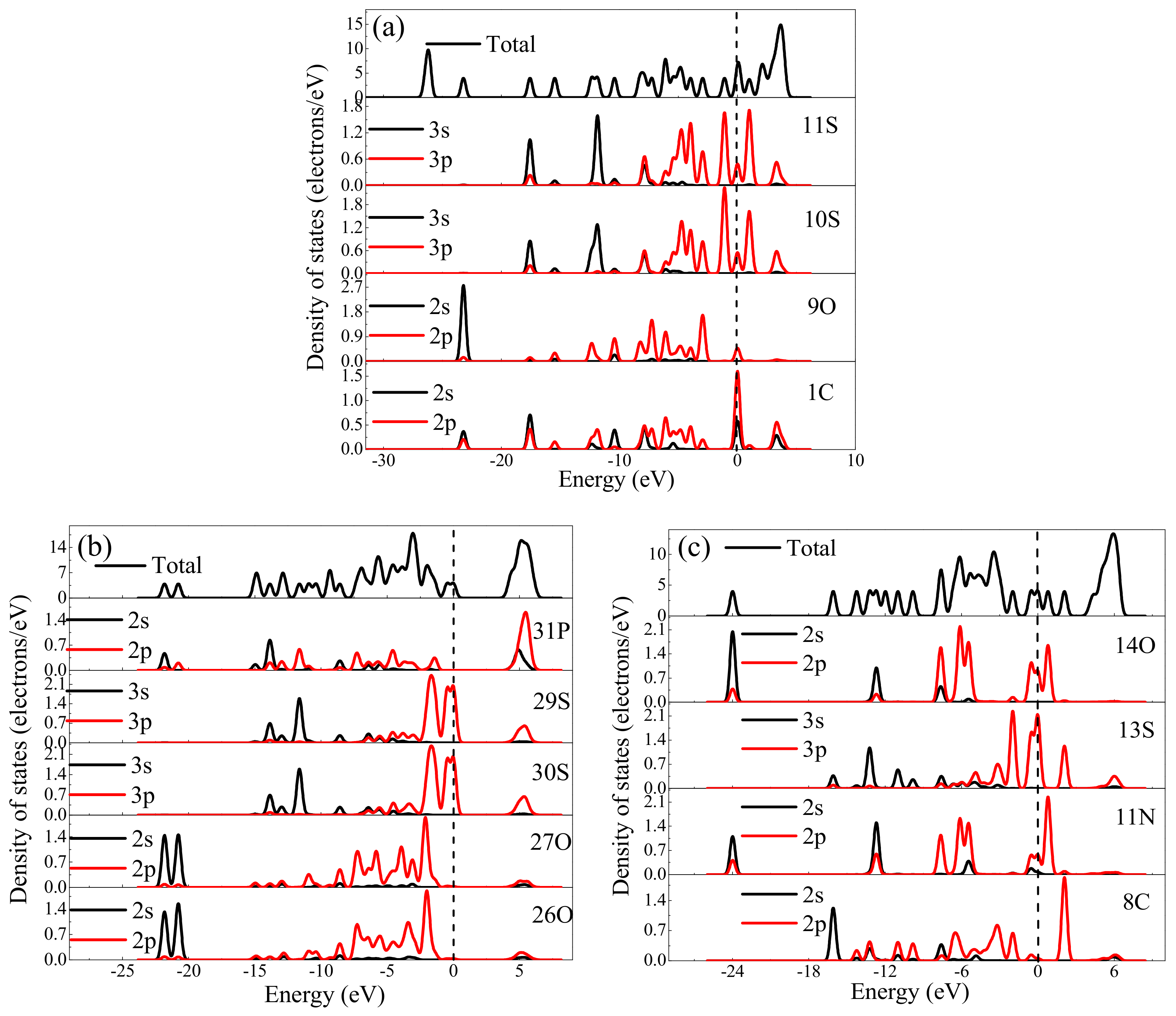
Density of States Analysis
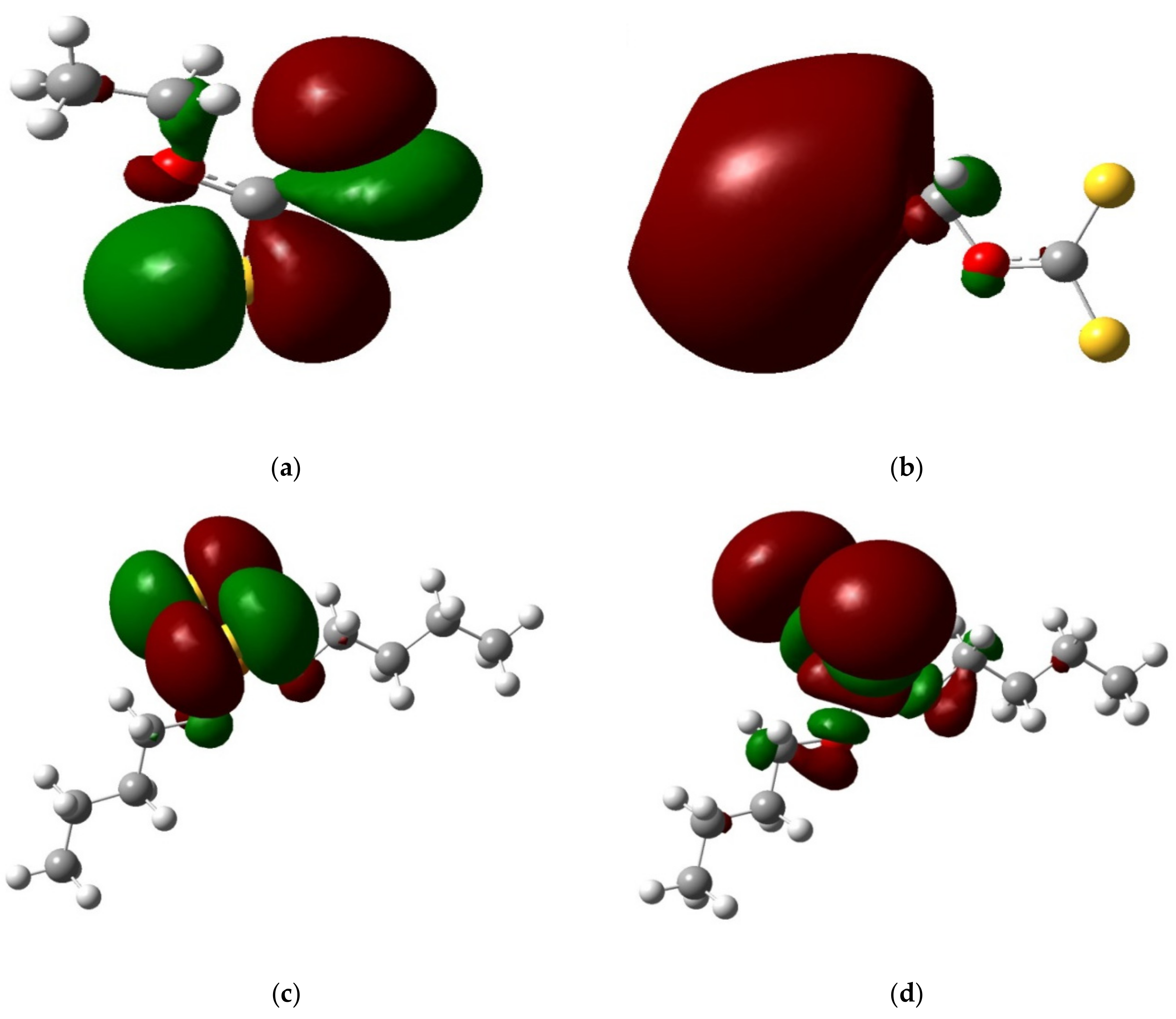
Frontier Orbital Analysis
3.4. Mechanism of IPETC Adsorption on Elemental Sulfur Surface
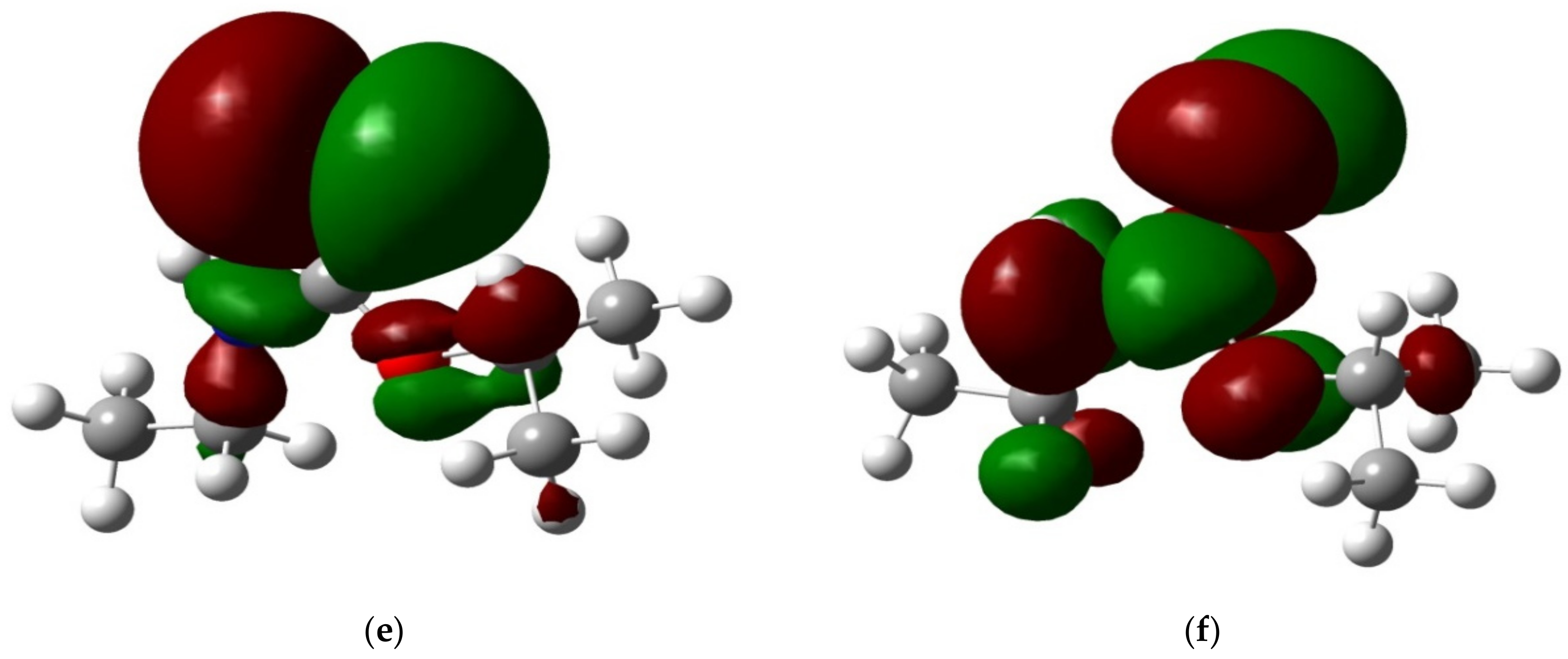
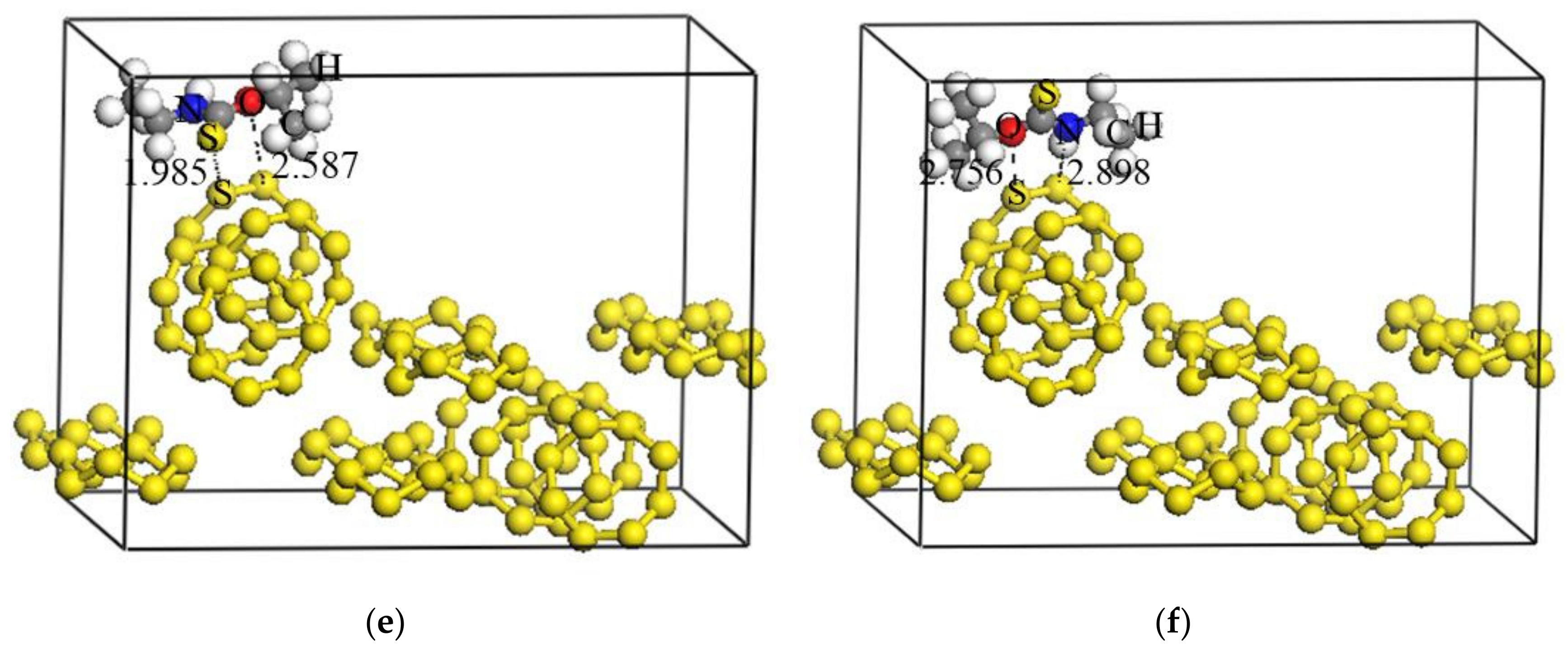
3.4.1. Adsorption Configuration and Adsorption Energy
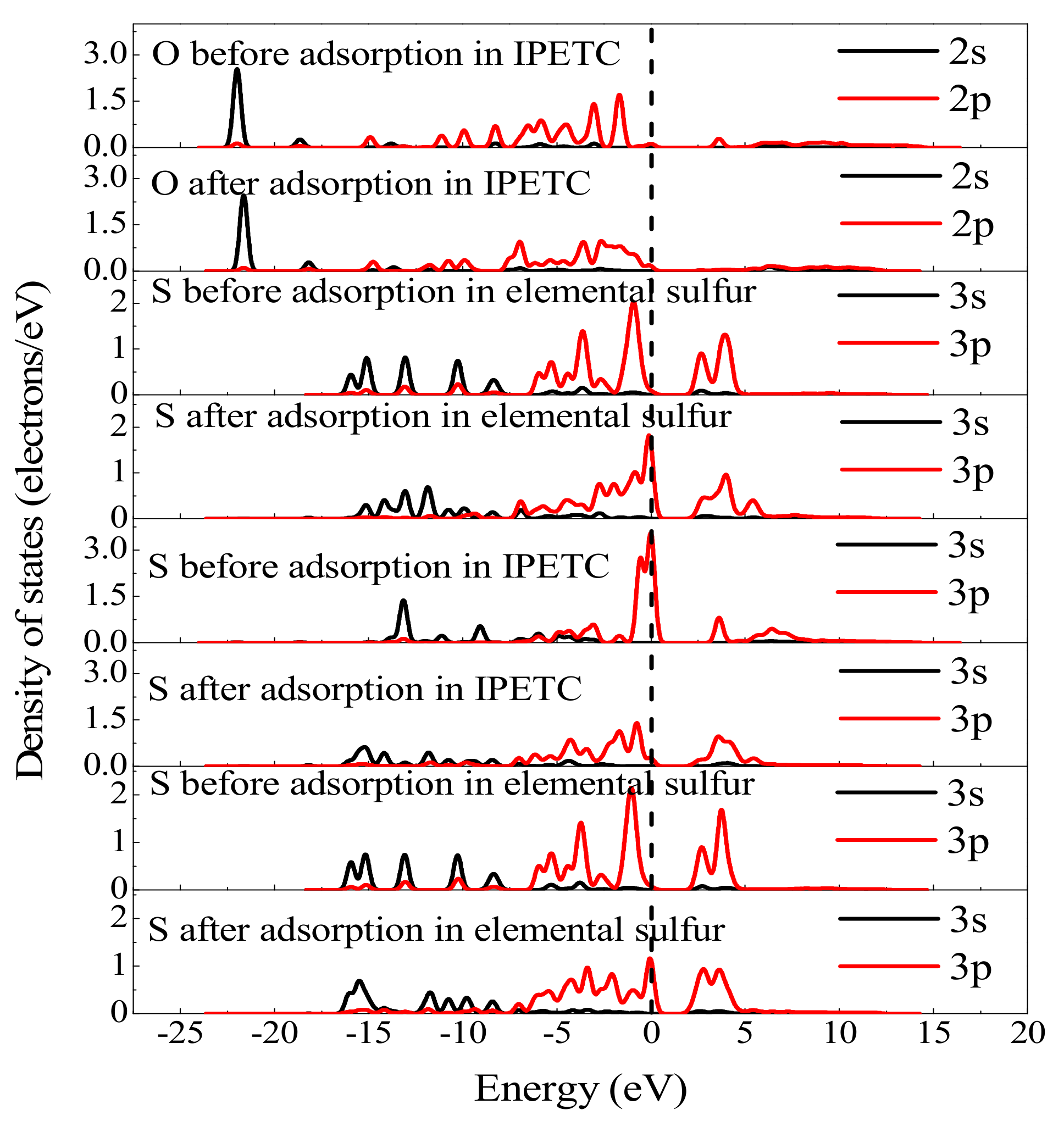
3.4.2. Mulliken Population and Density of States Analyses
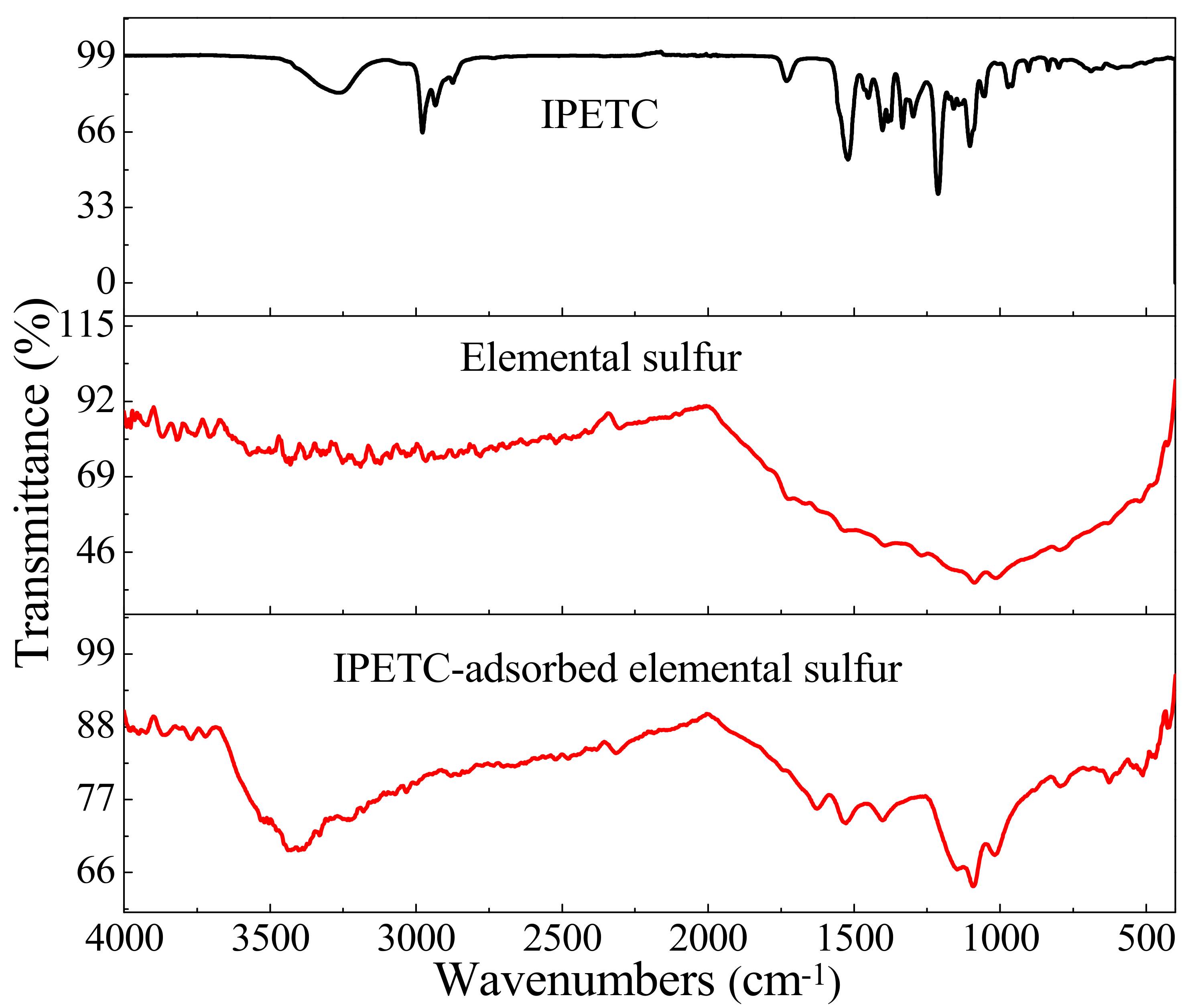
3.4.3. FTIR Spectrum Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbasi, A.; Nasef, M.M.; Yahya, W.Z.N. Sulfur based polymers by inverse vulcanization: A novel path to foster green chemistry. Green Mater. 2020, 8, 172–180. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Yang, Y.B.; Li, Q. Recovery of gold from pregnant thiosulfate solutions by the resin adsorption technique. Metals 2017, 7, 555. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Yang, Y.B.; Li, Q. An eco-friendly and efficient process of low potential thiosulfate leaching-resin adsorption recovery for extracting gold from a roasted gold concentrate. J. Clean. Prod. 2019, 229, 387–398. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Yang, J.K.; Chen, Y.Z.; Yang, Y.B.; Li, Q. Comprehensive recoveries of selenium, copper, gold, silver and lead from a copper anode slime with a clean and economical hydrometallurgical process. Chem. Eng. J. 2020, 393, 124762. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.H.; Li, Q.; Yang, Y.B.; Jiang, T. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Dong, Z.L.; Yang, Y.B.; Li, Q.; Liu, X.L.; Jiang, T. Eco-friendly and economical gold extraction by nickel catalyzed ammoniacal thiosulfate leaching-resin adsorption recovery. J. Clean. Prod. 2019, 233, 1475–1485. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Y.Z.; Dong, Z.L.; Jiang, T.; Zhang, B.S.; Liu, G.Q.; Yang, J.K.; Li, Q.; Yang, Y.B. Eco-friendly and efficient extraction of valuable elements from copper anode mud using an integrated pyro-hydrometallurgical process. Resour. Conserv. Recycl. 2021, 164, 105195. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, T.A.; Liu, Y.; Mu, W.Z.; Zhang, W.G.; Dou, Z.H.; Jiang, X.L. Pressure acid leaching of zinc sulfide concentrate. Trans. Nonferr. Met. Soc. China 2010, 20, s136–s140. [Google Scholar] [CrossRef]
- Padilla, R.; Vega, D.; Ruiz, M.C. Pressure leaching of sulfidized chalcopyrite in sulfuric acid–oxygen media. Hydrometallurgy 2010, 86, 80–88. [Google Scholar] [CrossRef]
- Jorjani, E.; Ghahreman, A. Challenges with elemental sulfur removal during the leaching of copper and zinc sulfides, and from the residues; a review. Hydrometallurgy 2017, 171, 333–343. [Google Scholar] [CrossRef]
- Forward, F.A.; Veltman, H. Direct leaching zinc-sulfide concentrates by Sherritt Gordon. JOM 1959, 11, 836–840. [Google Scholar] [CrossRef]
- Corriou, J.P.; Gély, R.; Viers, P. Thermodynamic and kinetic study of the pressure leaching of zinc sulfide in aqueous sulfuric acid. Hydrometallurgy 1988, 21, 85–102. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Kinetic model for direct leaching of zinc sulfide concentrates at high slurry and solute concentration. Hydrometallurgy 2015, 153, 160–169. [Google Scholar] [CrossRef]
- Mu, W.Z.; Zhang, T.A.; Liu, Y.; Gu, Y.; Dou, Z.H.; Lv, G.Z.; Bao, L.; Zhang, W.G. E-pH diagram of ZnS-H2O system during high pressure leaching of zinc sulfide. Hydrometallurgy 2010, 20, 2012–2019. [Google Scholar] [CrossRef]
- Liu, G.Q.; Jiang, K.X.; Zhang, B.S.; Dong, Z.L.; Zhang, F.; Wang, F.; Jiang, T.; Xu, B. Selective flotation of elemental sulfur from pressure acid leaching residue of zinc sulfide. Minerals 2021, 11, 89. [Google Scholar] [CrossRef]
- Qin, S.C.; Jiang, K.X.; Wang, H.B.; Zhang, B.S.; Wang, Y.F.; Zhang, X.D. Research on behavior of iron in the zinc sulfide pressure leaching process. Minerals 2020, 10, 224. [Google Scholar] [CrossRef]
- Rao, S.; Wang, D.X.; Liu, Z.Q.; Zhang, K.F.; Cao, H.Y.; Tao, J.Z. Selective extraction of zinc, gallium, and germanium from zinc refinery residue using two stage acid and alkaline leaching. Hydrometallurgy 2019, 183, 38–44. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cai, X.L.; Zhang, Z.B.; Zhang, L.B.; Wang, S.X.; Peng, J.H. Separation and enrichment of elemental sulfur and mercury from hydrometallurgical zinc residue using sodium sulfide. Trans. Nonferr. Met. Soc. China 2015, 25, 640–646. [Google Scholar] [CrossRef]
- Halfyard, J.E.; Hawboldt, K. Separation of elemental sulfur from hydrometallurgical residue: A review. Hydrometallurgy 2011, 109, 80–89. [Google Scholar] [CrossRef]
- Li, H.L.; Yao, X.L.; Wang, M.X.; Wu, S.K.; Ma, W.W.; Wei, W.W.; Li, L.Q. Recovery of elemental sulfur from zinc concentrate direct leaching residue using atmospheric distillation: A pilot-scale experimental study. J. Air Waste Manag. 2014, 64, 95–103. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, X.Y.; Wang, M.X.; Wang, J.; Wu, S.K.; Yao, X.L.; Li, L.Q. Separation of elemental sulfur from zinc concentrate direct leaching residue by vacuum distillation. Sep. Purif. Technol. 2014, 138, 41–46. [Google Scholar] [CrossRef]
- Chen, J.H.; Lan, L.H.; Chen, Y. Computational simulation of adsorption and thermodynamic study of xanthate, dithiophosphate and dithiocarbamate on galena and pyrite surfaces. Miner. Eng. 2013, 46–47, 136–143. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Zhong, H.; Wang, S.; Xia, L.Y.; Zou, W.B.; Liu, G.Y. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: Ethane-1,2-bis (dimethyl-dodecyl-ammonium bromide). Chem. Eng. J. 2014, 257, 218–228. [Google Scholar] [CrossRef]
- Ma, X.; Xia, L.Y.; Wang, S.; Zhong, H.; Jia, H. Structural modification of xanthate collectors to enhance the flotation selectivity of chalcopyrite. Ind. Eng. Chem. Res. 2017, 56, 6307–6316. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.H.; Wang, J.M.; Wang, L.; Xiao, J.H. A comparison study of adsorption of benzohydroxamic acid and amyl xanthate on smithsonite with dodecylamine as co-collector. Appl. Surf. Sci. 2017, 426, 1141–1147. [Google Scholar] [CrossRef]
- Liu, F.P.; Wang, J.L.; Peng, C.; Liu, Z.H.; Wilson, B.P.; Lundström, M. Recovery and separation of silver and mercury from hazardous zinc refinery residues produced by zinc oxygen pressure leaching. Hydrometallurgy 2019, 185, 38–45. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Liu, Y.; Niu, L.P.; Jing, T.L.; Zhang, T.A. Separation and purification of elemental sulfur from sphalerite concentrate direct leaching residue by liquid paraffin. Hydrometallurgy 2019, 186, 162–169. [Google Scholar] [CrossRef]
- Xing, P.; Ma, B.Z.; Wang, C.Y.; Wang, L.; Chen, Y.Q. A simple and effective process for recycling zinc-rich paint residue. Waste Manag. 2018, 76, 234–241. [Google Scholar] [CrossRef]
- Qiu, T.S.; He, Y.Q.; Qiu, X.H.; Yang, X.L. Density functional theory and experimental studies of Cu2+ activation on a cyanide-leached sphalerite surface. J. Ind. Eng. Chem. 2017, 45, 307–315. [Google Scholar] [CrossRef]
- Rashchi, F.; Dashti, A.; Arabpour-Yazdi, M.; Abdizadeh, H. Anglesite flotation: A study for lead recovery from zinc leach residue. Miner. Eng. 2005, 18, 205–212. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Li, Q.; Zhong, H.; Yang, Y.B. Selective flotation of galena using a novel collector S-benzyl-N-ethoxycarbonyl thiocarbamate: An experimental and theoretical investigation. J. Mol. Liq. 2021, 330, 115643. [Google Scholar] [CrossRef]
- Xu, B.; Wu, J.T.; Dong, Z.L.; Jiang, T.; Li, Q.; Yang, Y.B. Flotation performance, structure-activity relationship and adsorption mechanism of a newly-synthesized collector for copper sulfide minerals in Gacun polymetallic ore. Appl. Surf. Sci. 2021, 551, 149420. [Google Scholar] [CrossRef]
- Le, M.N.; Lee, M.S. Hydrometallurgical treatment of elemental sulfur in spent catalysts by aqueous and nonaqueous solutions at low temperature. Miner. Process. Extr. Metall. Rev. 2020, 41, 217–226. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, K.H.; Wang, S.; Cao, Z.F.; Zhong, H. The selective flotation behavior and adsorption mechanism of thiohexanamide to chalcopyrite. Miner. Eng. 2019, 137, 187–199. [Google Scholar] [CrossRef]



| Mineral | Cu | Pb | Zn | Fe | S | Purity |
|---|---|---|---|---|---|---|
| Elemental sulfur | ---- | ---- | ---- | 0.02 | 98.68 | 98.68 |
| Sphalerite | ---- | 0.04 | 66.20 | 0.52 | 32.9 | 98.65 |
| Pyrite | ---- | 0.21 | 0.07 | 45.25 | 53.26 | 97.21 |
| Chalcopyrite | 34.13 | 0.01 | 0.27 | 30.10 | 34.22 | 98.76 |
| Mineral | Na2O | Al2O3 | SiO2 | Purity |
|---|---|---|---|---|
| Albite | 11.36 | 19.19 | 67.86 | 96.27 |
| Mineral | PbO | SO3 | Fe2O3 | Purity |
|---|---|---|---|---|
| Anglesite | 69.32 | 28.23 | 0.052 | 96.27 |
| Raw Material | Recovery (%) | |||||
|---|---|---|---|---|---|---|
| Elemental Sulfur | Sphalerite | Pyrite | Chalcopyrite | Anglesite | Albite | |
| Pure minerals | 99.2 | 51.6 | 78.3 | 82.5 | 23.6 | 13.3 |
| Pure minerals after oxygen pressure acid leaching | 99.0 | 51.3 | 78.2 | 82.1 | 23.4 | 13.5 |
| Collector | Product | Yield (%) | Elemental Sulfur Grade (%) | Elemental Sulfur Recovery (%) |
|---|---|---|---|---|
| MIBC | Elemental sulfur concentrate | 57.31 | 71.17 | 88.83 |
| Tailing | 42.69 | 12.02 | 11.17 | |
| Feed residue | 100.00 | 45.92 | 100.00 | |
| Kerosene | Elemental sulfur concentrate | 56.70 | 71.24 | 88.00 |
| Tailing | 43.30 | 12.72 | 12.00 | |
| Feed residue | 100.00 | 45.90 | 100.00 |
| Collector | Product | Yield (%) | Grade (%) | Recovery (%) | ||
|---|---|---|---|---|---|---|
| Elemental Sulfur | Zinc | Elemental Sulfur | Zinc | |||
| SEX | Elemental sulfur concentrate | 43.55 | 81.53 | 1.33 | 93.67 | 13.33 |
| Tailing | 56.45 | 4.25 | 6.67 | 6.33 | 86.67 | |
| Feed residue | 100 | 37.91 | 4.34 | 100 | 100 | |
| ADDTP | Elemental sulfur concentrate | 45.47 | 82.25 | 1.15 | 97.83 | 12.08 |
| Tailing | 54.53 | 1.52 | 6.98 | 2.17 | 87.92 | |
| Feed residue | 100 | 38.23 | 4.33 | 100 | 100 | |
| IPETC | Elemental sulfur concentrate | 44.78 | 84.47 | 0.88 | 99.87 | 9.06 |
| Tailing | 55.22 | 0.09 | 7.16 | 0.13 | 90.94 | |
| Feed residue | 100 | 37.88 | 4.35 | 100 | 100 | |
| Collector | Bond Length (Å) | Dihedral Angle (°) | ||
|---|---|---|---|---|
| SEX | 1C-10S | 1C-11S | 9O-1C-10S-11S | |
| 1.707 | 1.699 | −179.992 | ||
| ADDTP | 29S-31P | 30S-31P | 14C-27O-31P-29S | 9C-26O-31P-30S |
| 2.006 | 2.006 | −59.008 | −59.003 | |
| IPETC | 8C-13S | 13S-8C-11N-2C | 11N-8C-14O-9C | |
| 1.679 | −178.654 | 177.847 | ||
| Collector | Bond Population | Atom Charge Population | ||||
|---|---|---|---|---|---|---|
| SEX | 10S-1C | 11S-1C | 1C | 9O | 10S | 11S |
| 0.41 | 0.31 | −0.099 | −0.174 | −0.379 | −0.405 | |
| ADDTP | 29S-31P | 30S-31P | 27O | 29S | 30S | 31P |
| 0.72 | 0.74 | −0.729 | −0.759 | −0.811 | 1.521 | |
| IPETC | 8C-13S | 8C | 11N | 13S | 14O | |
| 1.00 | 0.067 | 0.022 | −0.206 | −0.238 | ||
| Collector | Orbital Energy (eV) | ΔEa | Elemental Sulfur | Chalcopyrite | Pyrite | Sphalerite | Anglesite | Albite | |
|---|---|---|---|---|---|---|---|---|---|
| SEX | HOMO | −4.57 | ΔE1 | 0.39 | 0.64 | 0.78 | 1.54 | 1.69 | 1.77 |
| LUMO | −3.07 | ΔE2 | 2.55 | 2.60 | 2.21 | 2.38 | 2.44 | 2.67 | |
| ADDTP | HOMO | −5.56 | ΔE1 | 0.32 | 0.51 | 0.53 | 0.73 | 0.95 | 1.23 |
| LUMO | −4.67 | ΔE2 | 2.42 | 1.00 | 1.37 | 2.04 | 2.31 | 2.45 | |
| IPETC | HOMO | −5.18 | ΔE1 | 0.15 | 0.76 | 1.04 | 1.43 | 1.51 | 1.76 |
| LUMO | −3.76 | ΔE2 | 1.91 | 1.86 | 1.77 | 1.91 | 1.97 | 2.12 | |
| Adsorption Configuration | Adsorption Energy (kJ/mol) |
|---|---|
| Adsorption of carbonyl S | −19.79 |
| Adsorption of N | −10.23 |
| Adsorption of O | −6.16 |
| Simultaneous adsorption of carbonyl S together with N | −21.82 |
| Simultaneous adsorption of carbonyl S together with O | −26.98 |
| Simultaneous adsorption of N and O | −16.53 |
| Adsorption Configuration | Adsorption Energy (kJ/mol) | ||||
|---|---|---|---|---|---|
| Chalcopyrite (112) Plane | Pyrite (100) Plane | Sphalerite (110) Plane | Anglesite (001) Plane | Albite (001) Plane | |
| Adsorption of carbonyl S | −12.53 | −11.12 | −10.02 | −3.53 | 1.54 |
| Adsorption of N | −6.88 | −3.78 | −2.12 | −1.38 | 4.10 |
| Adsorption of O | −9.12 | −8.00 | −7.79 | −3.19 | 2.16 |
| Simultaneous adsorption of carbonyl S together with N | −8.01 | −4.56 | −3.45 | −2.41 | 2.33 |
| Simultaneous adsorption of carbonyl S together with O | −16.89 | −14.69 | −12.21 | −4.67 | 1.23 |
| Simultaneous adsorption of N and O | −5.13 | −6.69 | −5.63 | −0.43 | 3.49 |
| Crystal Plane | Adsorption Bond | Bond Length (Å) | Mulliken Population |
|---|---|---|---|
| Elemental sulfur (110) | S-S | 1.985 | 0.42 |
| O-S | 2.587 | 0.15 | |
| Chalcopyrite (112) | S-Cu | 2.461 | 0.35 |
| O-Cu | 2.667 | 0.12 | |
| Pyrite (100) | S-Fe | 2.561 | 0.33 |
| O-Fe | 2.773 | 0.08 | |
| Sphalerite (110) | S-Zn | 2.791 | 0.23 |
| O-Zn | 2.891 | 0.11 | |
| Anglesite (001) | S-Pb | 2.956 | 0.19 |
| O-Pb | 2.983 | 0.12 |
| Bonding Atom | Adsorption State | Electron Charge (e) | Charge Population | ||
|---|---|---|---|---|---|
| s | p | d | |||
| O (O-S) | Before adsorption | 1.77 | 4.66 | 0.00 | −0.44 |
| After adsorption | 1.77 | 4.71 | 0.00 | −0.46 | |
| S (O-S) | Before adsorption | 1.89 | 4.19 | 0.00 | −0.01 |
| After adsorption | 1.89 | 4.12 | 0.00 | −0.07 | |
| Carbonyl S (S-S) | Before adsorption | 1.82 | 4.41 | 0.00 | −0.23 |
| After adsorption | 1.82 | 4.10 | 0.00 | 0.05 | |
| S (S-S) | Before adsorption | 1.89 | 4.10 | 0.00 | 0.01 |
| After adsorption | 1.89 | 4.12 | 0.00 | −0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zhang, B.; Dong, Z.; Zhang, F.; Wang, F.; Jiang, T.; Xu, B. Flotation Performance, Structure-Activity Relationship and Adsorption Mechanism of O-Isopropyl-N-Ethyl Thionocarbamate Collector for Elemental Sulfur in a High-Sulfur Residue. Metals 2021, 11, 727. https://doi.org/10.3390/met11050727
Liu G, Zhang B, Dong Z, Zhang F, Wang F, Jiang T, Xu B. Flotation Performance, Structure-Activity Relationship and Adsorption Mechanism of O-Isopropyl-N-Ethyl Thionocarbamate Collector for Elemental Sulfur in a High-Sulfur Residue. Metals. 2021; 11(5):727. https://doi.org/10.3390/met11050727
Chicago/Turabian StyleLiu, Guiqing, Bangsheng Zhang, Zhonglin Dong, Fan Zhang, Fang Wang, Tao Jiang, and Bin Xu. 2021. "Flotation Performance, Structure-Activity Relationship and Adsorption Mechanism of O-Isopropyl-N-Ethyl Thionocarbamate Collector for Elemental Sulfur in a High-Sulfur Residue" Metals 11, no. 5: 727. https://doi.org/10.3390/met11050727
APA StyleLiu, G., Zhang, B., Dong, Z., Zhang, F., Wang, F., Jiang, T., & Xu, B. (2021). Flotation Performance, Structure-Activity Relationship and Adsorption Mechanism of O-Isopropyl-N-Ethyl Thionocarbamate Collector for Elemental Sulfur in a High-Sulfur Residue. Metals, 11(5), 727. https://doi.org/10.3390/met11050727





