Biofunctionalization of Porous Ti Substrates Coated with Ag Nanoparticles for Potential Antibacterial Behavior
Abstract
1. Introduction
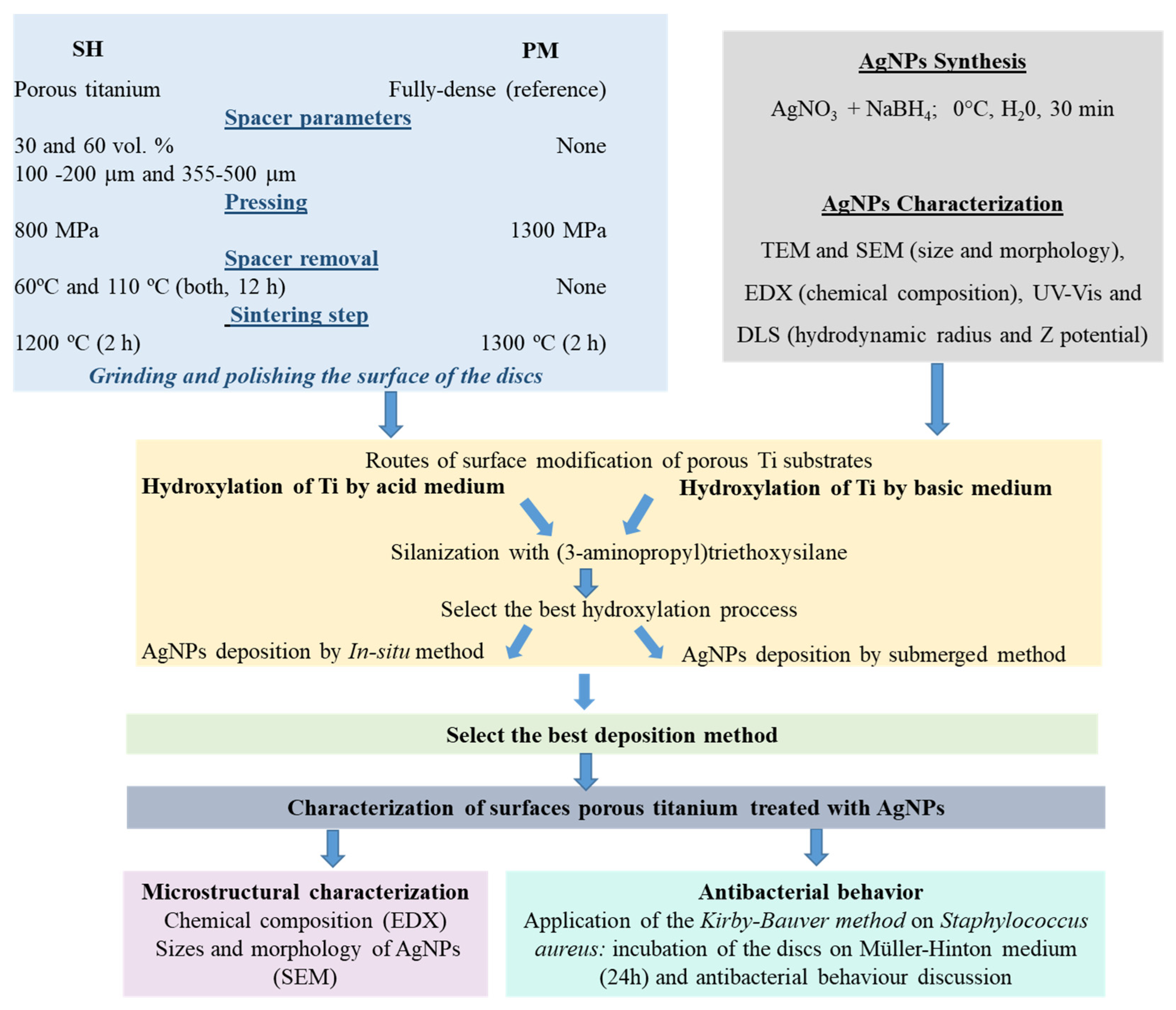
2. Materials and Methods
2.1. Fabrication and Characterization of the Ti Substrates
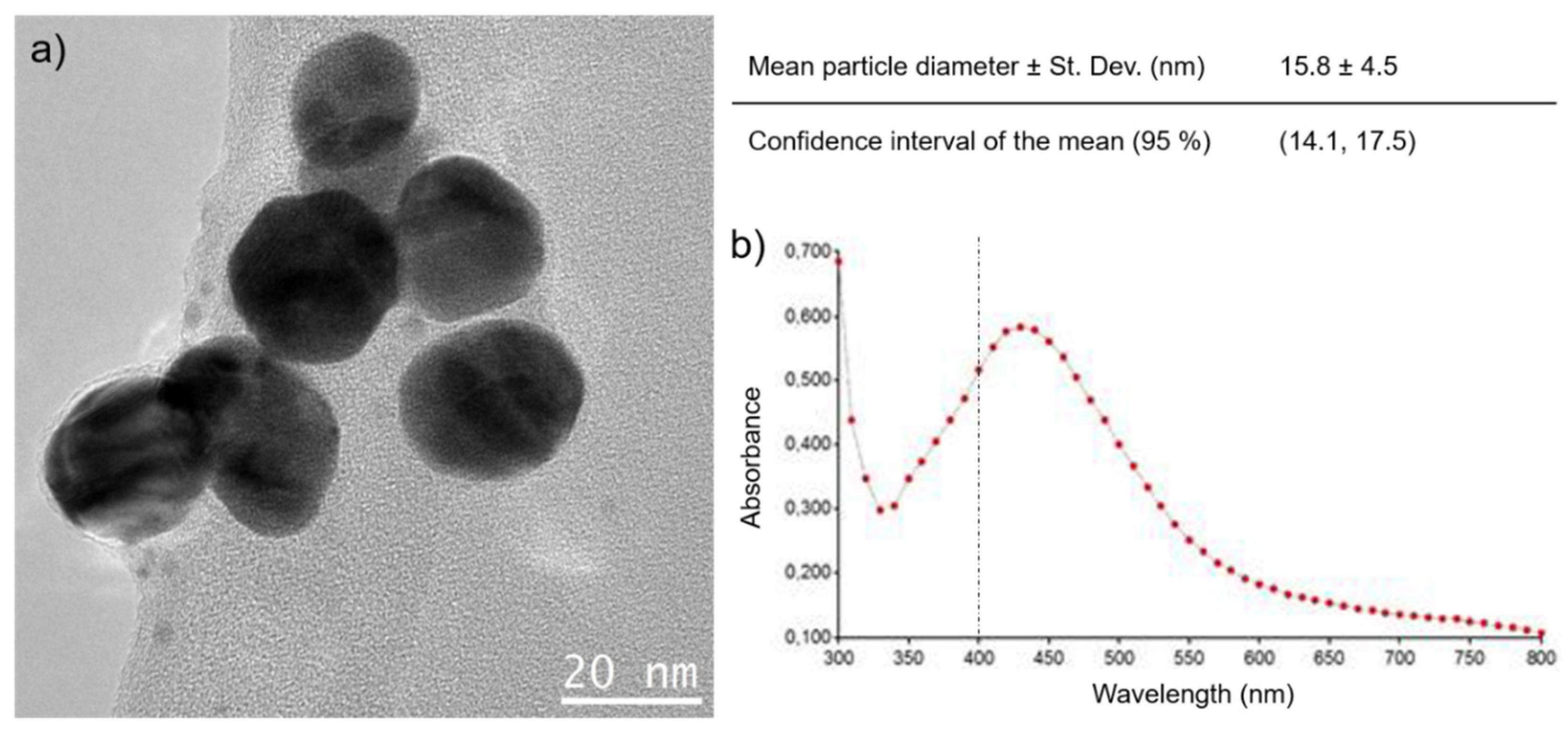
2.2. Synthesis and Characterization of the AgNPs
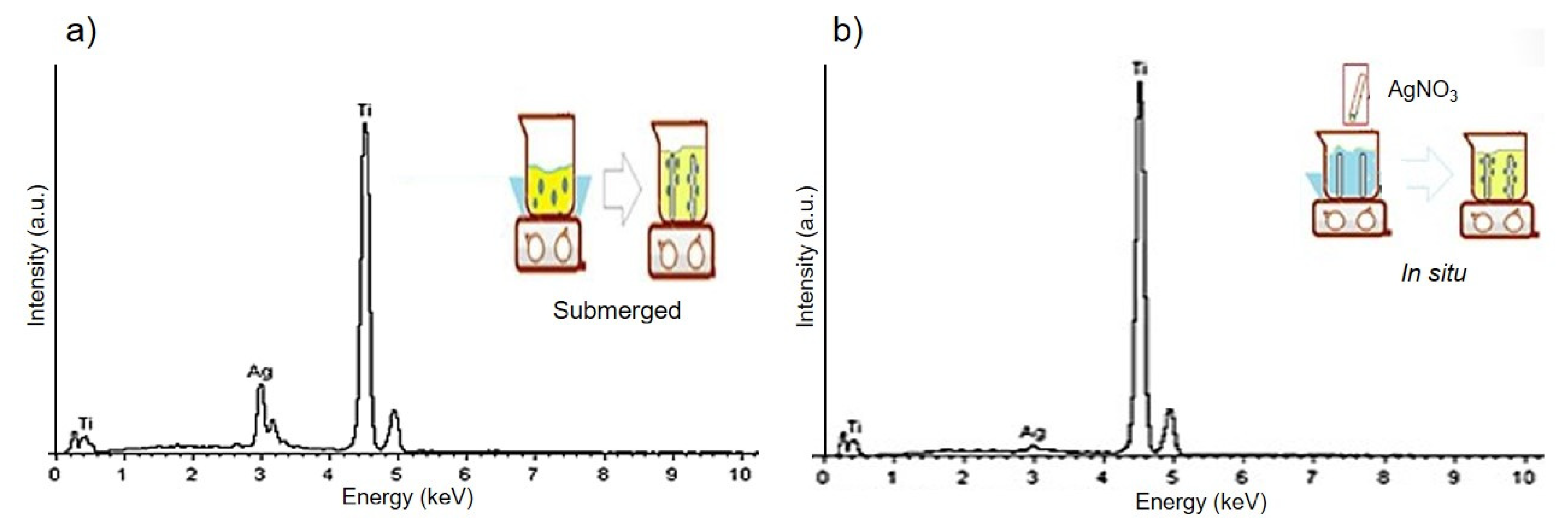
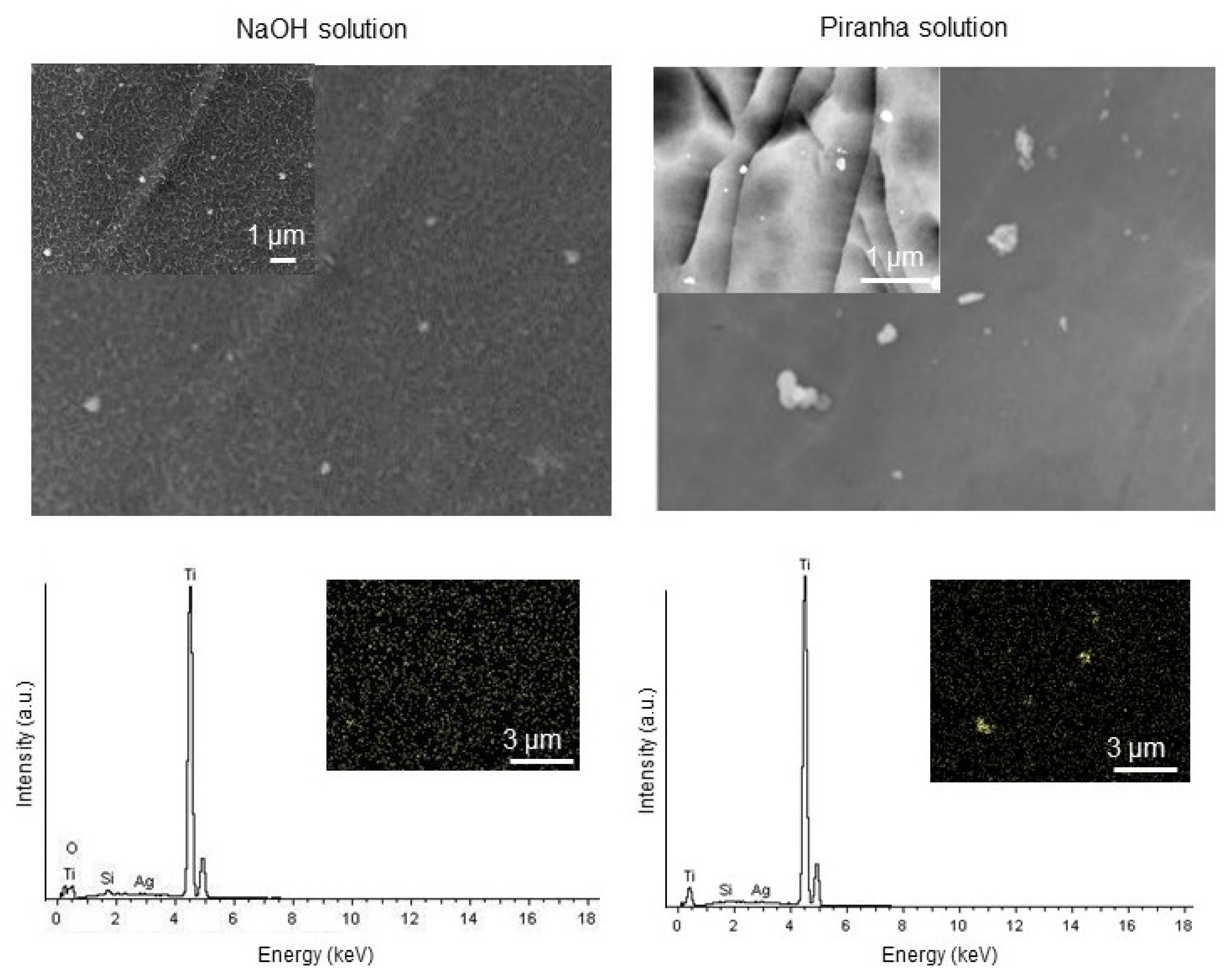
2.3. Modification and Characterization of the Surface of the Ti Substrates
3. Results and Discussion
3.1. Characterization of Surfaces of Ti Substrates with AgNPs
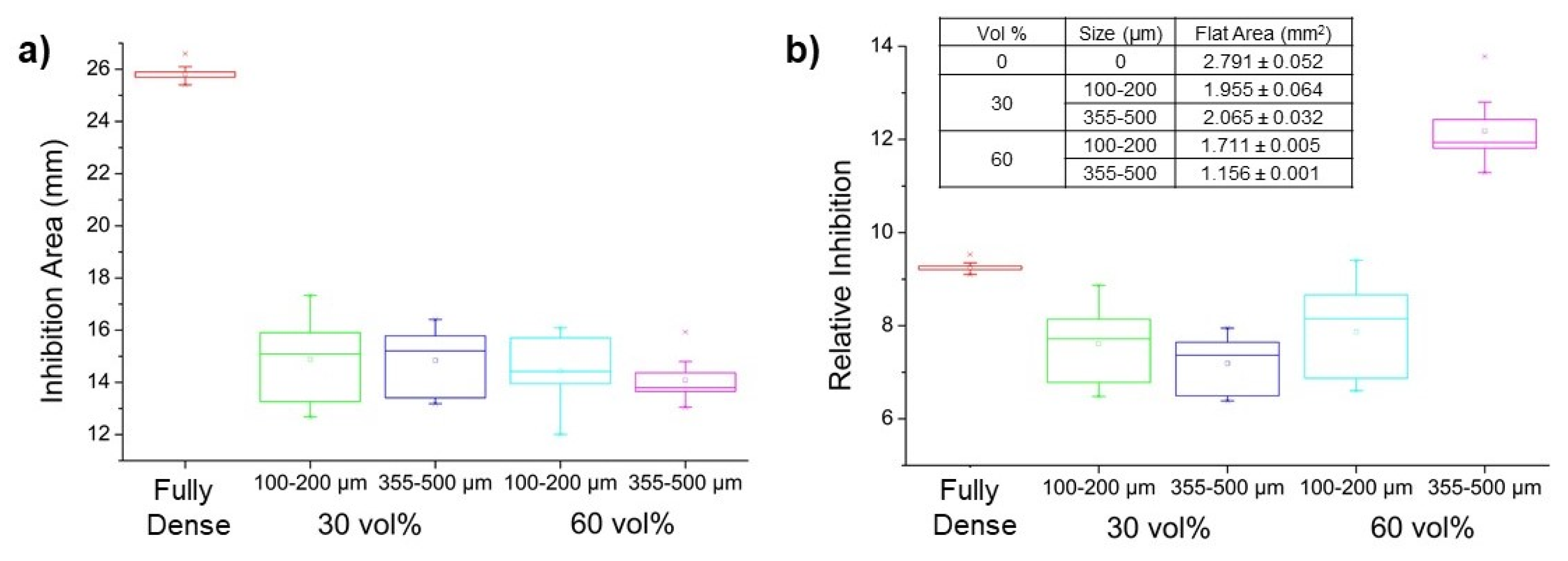
3.2. Characterization of the Antibacterial Behavior of Ti Substrates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couqueberg, Y.; Augoyard, R.; Augoyard, M.; Berry-Kromer, V.; Bouby, C.; Girod, L. A statistical study of metatarsal anatomy: Toward the design of wide-range prosthetic solutions. Foot Ankle Spec. 2017, 11, 277–287. [Google Scholar] [CrossRef]
- Chadwell, A.; Diment, L.; Micó-Amigo, M.; Morgado Ramírez, D.Z.; Dickinson, A.; Granat, M.; Kenney, L.; Kheng, S.; Sobuh, M.; Ssekitoleko, R.; et al. Technology for monitoring everyday prosthesis use: A systematic review. J. Neuroeng. Rehabil. 2020, 17. [Google Scholar] [CrossRef]
- Bartoníbek, J. Early history of operative treatment of fractures. Arch. Orthop. Trauma Surg. 2010, 130, 1385–1396. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Lascano, S.; Arévalo, C.; Montealegre-Melendez, I.; Muñoz, S.; Rodriguez-Ortiz, J.A.; Trueba, P.; Torres, Y. Porous titanium for biomedical applications: Evaluation of the conventional powder metallurgy frontier and space-holder technique. Appl. Sci. 2019, 9, 982. [Google Scholar] [CrossRef]
- Sidambe, A. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- González, J.E.; De Armas, G.; Negrin, J.; Beltrán, A.M.; Trueba, P.; Gotor, F.J.; Peón, E.; Torres, Y. Metals influence of successive chemical and thermochemical treatments on surface features of Ti6Al4V samples manufactured by SLM. Metals 2021, 11, 313. [Google Scholar] [CrossRef]
- Civantos, A.; Beltrán, A.M.; Domínguez-Trujillo, C.; Garvi, M.D.; Lebrato, J.; Rodríguez-Ortiz, J.A.; García-Moreno, F.; Cauich-Rodriguez, J.V.; Guzman, J.J.; Torres, Y. Balancing porosity and mechanical properties of titanium samples to favor cellular growth against bacteria. Metals 2019, 9, 1039. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Pałka, K.; Pokrowiecki, R. Porous titanium implants: A review. Adv. Eng. Mater. 2018, 20, 1–18. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Miao, X.; Sun, D. Graded/gradient porous biomaterials. Materials 2009, 3, 26–47. [Google Scholar] [CrossRef]
- Betts, C. Benefits of metal foams and developments in modelling techniques to assess their materials behaviour: A review. Mater. Sci. Technol. 2012, 28, 129–143. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Kobatake, R.; Doi, K.; Kubo, T.; Makihara, Y.; Oki, Y.; Yokoi, M.; Umehara, H.; Tsuga, K. Novel fabrication of porous titanium by a resin-impregnated titanium substitution technique for bone reconstruction. RSC Adv. 2019, 9, 1625–1631. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fang, G.; Leeflang, S.; Zadpoor, A.A.; Zhou, J. Topological design, permeability and mechanical behavior of additively manufactured functionally graded porous metallic biomaterials. Acta Biomater. 2019, 84, 437–452. [Google Scholar] [CrossRef]
- Bari, K.; Arjunan, A. Extra low interstitial titanium based fully porous morphological bone scaffolds manufactured using selective laser melting. J. Mech. Behav. Biomed. Mater. 2019, 95, 1–12. [Google Scholar] [CrossRef]
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Naebe, M.; Shirvanimoghaddam, K. Functionally graded materials: A review of fabrication and properties. Appl. Mater. Today 2016, 5, 223–245. [Google Scholar] [CrossRef]
- Chen, Y.J.; Feng, B.; Zhu, Y.P.; Weng, J.; Wang, J.X.; Lu, X. Fabrication of porous titanium implants with biomechanical compatibility. Mater. Lett. 2009, 63, 2659–2661. [Google Scholar] [CrossRef]
- Özbilen, S.; Liebert, D.; Beck, T.; Bram, M. Fatigue behavior of highly porous titanium produced by powder metallurgy with temporary space holders. Mater. Sci. Eng. C 2016, 60, 446–457. [Google Scholar] [CrossRef]
- de Vasconcellos, L.M.R.; de Oliveira, M.V.; de Alencastro Graça, M.L.; de Vasconcellos, L.G.O.; Carvalho, Y.R.; Cairo, C.A.A. Porous titanium scaffolds produced by powder metallurgy for biomedical applications. Mater. Res. 2008, 11, 275–280. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.H. A review on alloy design, biological response, and strengthening of β-titanium alloys as biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef]
- Palanivelu, R.; Kalainathan, S.; Ruban Kumar, A. CERAMICS Characterization studies on plasma sprayed (AT/HA) bi-layered nano ceramics coating on biomedical commercially pure titanium dental implant. Ceram. Int. 2014, 40, 7745–7751. [Google Scholar] [CrossRef]
- Yerokhin, A.; Parfenov, E.V.; Matthews, A. In situ impedance spectroscopy of the plasma electrolytic oxidation process for deposition of Ca- and P-containing coatings on Ti. Surf. Coat. Technol. 2016, 301, 54–62. [Google Scholar] [CrossRef]
- Zhang, X.; Aliasghari, S.; Němcová, A.; Burnett, T.L.; Kuběna, I.; Šmíd, M.; Thompson, G.E.; Skeldon, P.; Withers, P.J. X-ray computed tomographic investigation of the porosity and morphology of plasma electrolytic oxidation coatings. ACS Appl. Mater. Interfaces 2016, 8, 8801–8810. [Google Scholar] [CrossRef]
- Sobolev, A.; Zinigrad, M.; Borodianskiy, K. Ceramic coating on Ti-6Al-4V by plasma electrolytic oxidation in molten salt: Development and characterization. Surf. Coat. Technol. 2021, 408, 126847. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Begines, B.; Alcudia, A.; Rodríguez-Ortiz, J.A.; Torres, Y. Biofunctional and tribomechanical behavior of porous titanium substrates coated with a bioactive glass bilayer (45S5-1393). ACS Appl. Mater. Interfaces 2020, 12, 30170–30180. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, A.M.; Alcudia, A.; Begines, B.; Rodríguez-Ortiz, J.A.; Torres, Y. Porous titanium substrates coated with a bilayer of bioactive glasses. J. Non-Cryst. Solids 2020, 544, 120206. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Ternero, F.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; Montealegre-Meléndez, I.; Arévalo, C.; García-Moreno, F.; Torres, Y. Improvement of the balance between a reduced stress shielding and bone ingrowth by bioactive coatings onto porous titanium substrates. Surf. Coat. Technol. 2018, 338, 32–37. [Google Scholar] [CrossRef]
- D’almeida, M.; Amalric, J.; Brunon, C.; Grosgogeat, B.; Toury, B. Relevant insight of surface characterization techniques to study covalent grafting of a biopolymer to titanium implant and its acidic resistance. Appl. Surf. Sci. 2015, 327, 296–306. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Ma, P.; Sutrisno, L.; Luo, Z.; Hu, Y.; Yu, Y.; Tao, B.; Li, C.; Cai, K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials 2019, 212, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ballarre, J.; Aydemir, T.; Liverani, L.; Roether, J.A.; Goldmann, W.H.; Boccaccini, A.R. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: Improving early stage performance of titanium implants. Surf. Coat. Technol. 2020, 381, 125138. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface modification of titanium and titanium alloys: Technologies, developments, and future interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Saini, R.; Saini, S.; Sugandha, S. Nanotechnology: The future medicine. J. Cutan. Aesthet. Surg. 2010, 3, 32–33. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. An Introduction to Nanotechnology. In Interface Science and Technology; Elsevier, B.V., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 28, pp. 1–27. [Google Scholar]
- Zhou, W.; Ma, Y.; Yang, H.; Ding, Y.; Luo, X. A label-free biosensor based on silver nanoparticles array for clinical detection of serum p53 in head and neck squamous cell carcinoma. Int. J. Nanomed. 2011, 6, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, R.; Dhanasingh, S.; Kalimuthu, B.; Uthirappan, M.; Rose, C.; Baran Mandal, A. Phytosynthesis of silver nanoparticles using Coccinia grandis leaf extract and its application in the photocatalytic degradation. Colloids Surf. B Biointerfaces 2012, 94, 226–230. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. Engl. 2010, 49, 3280–3294. [Google Scholar] [CrossRef]
- Begines, B.; Alcudia, A.; Aguilera-Velazquez, R.; Martinez, G.; He, Y.; Wildman, R.; Sayagues, M.-J.; Jimenez-Ruiz, A.; Prado-Gotor, R. Design of highly stabilized nanocomposite inks based on biodegradable polymer-matrix and gold nanoparticles for Inkjet Printing. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, S.; Zhang, H.; Jiang, J.; Liu, X. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Reddy, G.R.K.; Hyder, M.; Kumar, P.S. Facile preparation of high-performance copper oxide sensors for electroanalysis of hydrogen peroxide. Mater. Today Proc. 2017, 4, 12457–12469. [Google Scholar] [CrossRef]
- Bhushan, M.; Kumar, Y.; Periyasamy, L.; Viswanath, A.K. Facile synthesis of Fe/Zn oxide nanocomposites and study of their structural, magnetic, thermal, antibacterial and cytotoxic properties. Mater. Chem. Phys. 2018, 209, 233–248. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Mathew, G.M.; Pancrecious, J.K.; Nair, J.B.; Rajan, T.P.D.; Maiti, K.K.; Pai, B.C. Biogenic Ag nanoparticles from neem extract: Their structural evaluation and antimicrobial effects against pseudomonas nitroreducens and aspergillus unguis (NII 08123). ACS Biomater. Sci. Eng. 2020, 6, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liang, G.; Zhang, W.; Jin, W.; Zhang, Y.; Chen, Q.; Cai, Y.; Zhang, W. Silver nanoparticles with low cytotoxicity: Controlled synthesis and surface modification with histidine. J. Mater. Sci. 2018, 53, 4768–4780. [Google Scholar] [CrossRef]
- Kongoli, F.; Marquis, F.; Chikhradze, N.; Prikhna, T. (Eds.) SIPS2019 Volume 11 New and Advanced Materials, Technologies, and Manufacturing; FLOGEN Stars Outreach: Mont-Royal, QC, Canada, 2019. [Google Scholar]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csík, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef]
- Juan, L.; Zhimin, Z.; Anchun, M.; Lei, L.; Jingchao, Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int. J. Nanomed. 2010, 5, 261–267. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Xu, G.; Liu, H.; Xiang, Y.; Cui, W. Accelerated fabrication of antibacterial and osteoinductive electrospun fibrous scaffolds via electrochemical deposition. RSC Adv. 2018, 8, 9546–9554. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, B.; Wang, Y.; Zhou, X.; Weng, J.; Qu, S.; Feng, B.; Watari, F.; Ding, Y.; Leng, Y. Nano-Ag-loaded hydroxyapatite coatings on titanium surfaces by electrochemical deposition. J. R. Soc. Interface 2011, 8, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Niño-Martínez, N.; Martínez-Castañón, G.A.; Aragón-Piña, A.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Characterization of silver nanoparticles synthesized on titanium dioxide fine particles. Nanotechnology 2008, 19, 65711. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, J.; Alcudia, A.; Begines, B.; Beltrán, A.M.; Villarraga, J.; Moriche, R.; Rodríguez-Ortiz, J.A.; Torres, Y. Synthesis and deposition of silver nanoparticles on porous titanium substrates for biomedical applications. Surf. Coat. Technol. 2021, 406, 126667. [Google Scholar] [CrossRef]
- ASTM F67-13(2017). Standard Specification for Unalloyed Titanium, for Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700); ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Torres, Y.; Trueba, P.; Pavón, J.; Montealegre, I.; Rodríguez-Ortiz, J.A. Designing, processing and characterisation of titanium cylinders with graded porosity: An alternative to stress-shielding solutions. Mater. Des. 2014, 63, 316–324. [Google Scholar] [CrossRef]
- ASTM C373-14(2014). Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products, Ceramic Tiles, and Glass Tiles; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Trueba, P.; Beltrán, A.M.; Bayo, J.M.J.M.; Rodríguez-Ortiz, J.A.; Larios, D.F.; Alonso, E.; Dunand, D.C.; Torres, Y.; Beltran, A.M.; Bayo, J.M.J.M.; et al. Porous titanium cylinders obtained by the freeze-casting technique: Influence of process parameters on porosity and mechanical behavior. Metals 2020, 10, 188. [Google Scholar] [CrossRef]
- Lee, P.C.; Melsel, D. Adsorption and surface-enhanced raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Vlčková, B.; Moskovits, M.; Pavel, I.; Šišková, K.; Sládková, M.; Šlouf, M. Single-molecule surface-enhanced Raman spectroscopy from a molecularly-bridged silver nanoparticle dimer. Chem. Phys. Lett. 2008, 455, 131–134. [Google Scholar] [CrossRef]
- Pavel, I.; McCarney, E.; Elkhaled, A.; Morrill, A.; Plaxco, K.; Moskovits, M. Label-free SERS detection of small proteins modified to act as bifunctional linkers. J. Phys. Chem. C Nanomater. Interfaces 2008, 112, 4880–4883. [Google Scholar] [CrossRef]
- Cuellar-Flores, M.; Acosta-Torres, L.S.; Martínez-Alvarez, O.; Sánchez-Trocino, B.; de la Fuente-Hernández, J.; Garcia-Garduño, R.; Garcia-Contreras, R. Effects of alkaline treatment for fibroblastic adhesion on titanium. Dent. Res. J. 2016, 13, 473–477. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.W.; Zhong, Y.; Wang, L. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.G.; de Oliveira, P.T.; Nanci, A.; Hawthorne, A.C.; Rosa, A.L.; Xavier, S.P. Treatment of a commercial, machined surface titanium implant with H2SO4/H2O2 enhances contact osteogenesis. Clin. Oral Implant. Res. 2007, 18, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Abe, Y.; Yoshida, Y.; Nakayama, Y.; Okazaki, M.; Akagawa, Y. Acid pretreatment of titanium implants. Biomaterials 2003, 24, 1821–1827. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Trueba, P.; Navarro, C.; Rodríguez-Ortiz, J.A.; Beltrán, A.M.; García-García, F.J.; Torres, Y. Fabrication and characterization of superficially modified porous dental implants. Surf. Coat. Technol. 2021, 408, 126796. [Google Scholar] [CrossRef]
- Wu, K.H.; Chang, Y.C.; Tsai, W.Y.; Huang, M.Y.; Yang, C.C. Effect of amine groups on the synthesis and antibacterial performance of Ag nanoparticles dispersed in aminosilanes-modified silicate. Polym. Degrad. Stab. 2010, 95, 2328–2333. [Google Scholar] [CrossRef]
- Frattini, A.; Pellegri, N.; Nicastro, D.; De Sanctis, O. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater. Chem. Phys. 2005, 94, 148–152. [Google Scholar] [CrossRef]
- Pris, M.; Krzysztof, T. Influence of Different Parameters on Wet Synthesis of Silver Nanoparticles. Bachelor’s Thesis, University of Twenty, Enschede, The Netherlands, 2008. [Google Scholar]
- Drew, W.L.; Barry, A.L.; O’Toole, R.; Sherris, J.C. Reliability of the Kirby-Bauer disc diffusion method for detecting methicillin-resistant strains of Staphylococcus aureus. Appl. Microbiol. 1972, 24, 240–247. [Google Scholar] [CrossRef] [PubMed]


| Microstructural Characterization | Estimated Mechanical Behavior | ||||||
|---|---|---|---|---|---|---|---|
| PT (%) | Pi (%) | Deq (μm) | Ff | Ed (GPa) | σy (MPa) | ||
| Fully dense | 2.4 ± 0.3 | 1.3 ± 0.3 | 5 ± 3 | 0.97 ± 0.1 | 103.1 ± 1.0 | 580 ± 3 | |
| 30 vol.% | 100–200 μm | 30.2 ± 0.2 | 18.1 ±0.1 | 192 ± 117 | 0.72 ± 0.2 | 56.8 ± 0.7 | 354 ± 6 |
| 355–500 μm | 30.1 ± 0.1 | 19.0 ± 0.1 | 435 ± 401 | 0.75 ± 0.1 | 57.0 ± 1.4 | 341 ± 7 | |
| 60 vol.% | 100–200 μm | 56.4 ± 0.5 | 51.8 ± 1.3 | 259 ± 287 | 0.74 ± 0.1 | 32.3 ± 0.6 | 88 ± 4 |
| 355–500 μm | 57.8 ± 0.5 | 53.0 ± 0.9 | 295 ± 331 | 0.77 ± 0.3 | 31.3 ± 1.5 | 80 ± 5 | |
| Fully Dense | Flat Area (%) | Macropores Area (%) | Micropores Area (%) | |
|---|---|---|---|---|
| 98.45 | 0 | 1.65 | ||
| 30 vol.% | 100–200 | 68.96 | 30.48 | 0.56 |
| 355–500 | 72.82 | 26.74 | 0.43 | |
| 60 vol.% | 100–200 | 60.36 | 39.46 | 0.17 |
| 355–500 | 40.77 | 59.00 | 0.22 | |
| Ti Hydroxylation | ZP (mV) | Mean Diameter (nm) | PdI | pH |
|---|---|---|---|---|
| acid medium | −29.6 | 116.2 | 0.332 | 7 |
| basic medium | −36.4 | 119.5 | 0.402 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaviria, J.; Alcudia, A.; Begines, B.; Beltrán, A.M.; Rodríguez-Ortiz, J.A.; Trueba, P.; Villarraga, J.; Torres, Y. Biofunctionalization of Porous Ti Substrates Coated with Ag Nanoparticles for Potential Antibacterial Behavior. Metals 2021, 11, 692. https://doi.org/10.3390/met11050692
Gaviria J, Alcudia A, Begines B, Beltrán AM, Rodríguez-Ortiz JA, Trueba P, Villarraga J, Torres Y. Biofunctionalization of Porous Ti Substrates Coated with Ag Nanoparticles for Potential Antibacterial Behavior. Metals. 2021; 11(5):692. https://doi.org/10.3390/met11050692
Chicago/Turabian StyleGaviria, Juliana, Ana Alcudia, Belén Begines, Ana María Beltrán, José Antonio Rodríguez-Ortiz, Paloma Trueba, Junes Villarraga, and Yadir Torres. 2021. "Biofunctionalization of Porous Ti Substrates Coated with Ag Nanoparticles for Potential Antibacterial Behavior" Metals 11, no. 5: 692. https://doi.org/10.3390/met11050692
APA StyleGaviria, J., Alcudia, A., Begines, B., Beltrán, A. M., Rodríguez-Ortiz, J. A., Trueba, P., Villarraga, J., & Torres, Y. (2021). Biofunctionalization of Porous Ti Substrates Coated with Ag Nanoparticles for Potential Antibacterial Behavior. Metals, 11(5), 692. https://doi.org/10.3390/met11050692









