A Preliminary Analysis of the Wear Pathways of Sliding Contacts on Temporomandibular Joint Total Joint Replacement Prostheses
Abstract
1. Introduction
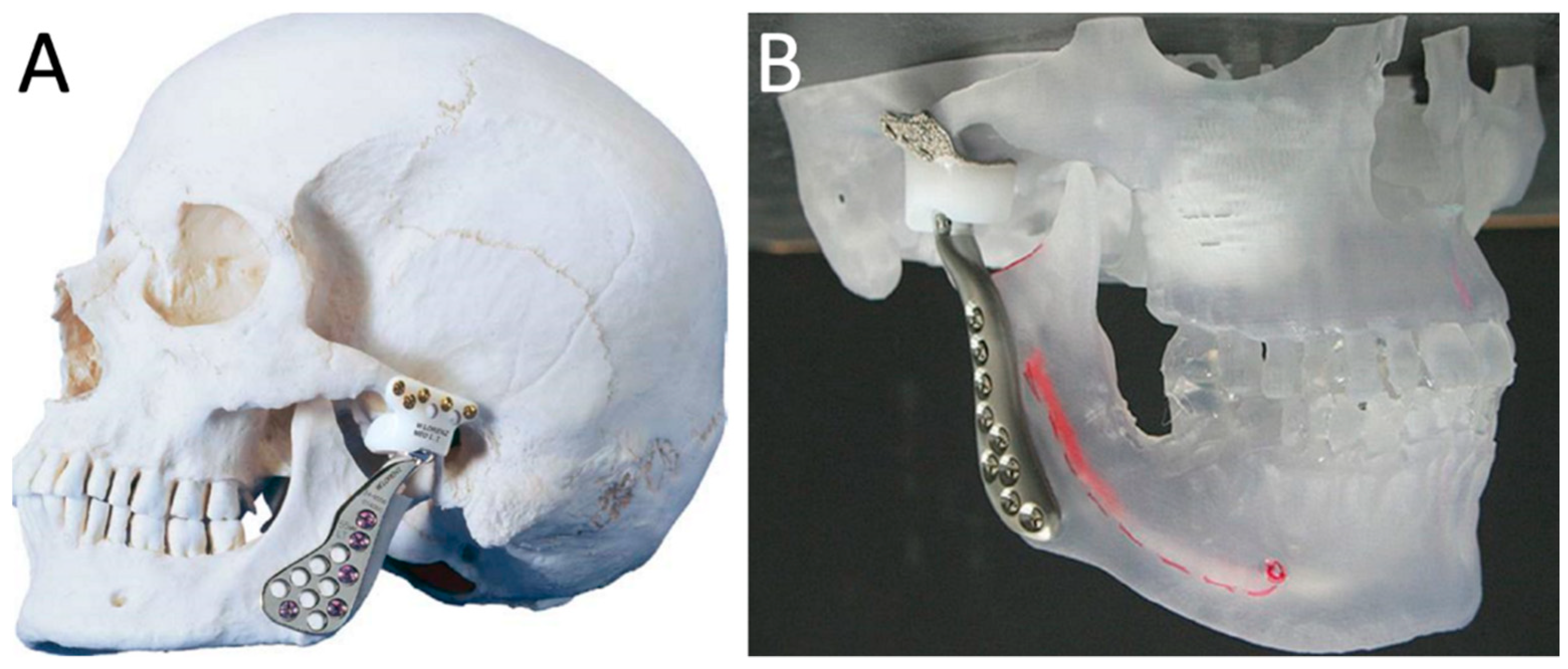
2. Standard and Custom-Made TMJ TJR Prostheses
3. Degradation of TMJ TJR Components
4. Adverse Biological Effects of Debris Released from TMJ TJR
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Duraccio, D.; Mussano, F.; Giulia, M. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Steinemann, S.G. Titanium—The material of choice? Periodontology 2000 1998, 17, 7–21. [Google Scholar] [CrossRef]
- Mercuri, L.G. Temporomandibular Joint Disorder Management in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2017, 75, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, S.K.; Klutcharch, K.; Rao, S.; Souza, J.C.M.M.; Mercuri, L.G.; Mathew, M.T. Advancements in temporomandibular joint total joint replacements (TMJR). Biomed. Eng. Lett. 2019, 9, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G. Total Joint Reconstruction-Autologous or Alloplastic. Oral Maxillofac. Surg. Clin. N. Am. 2006, 18, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Onoriobe, U.; Miloro, M.; Sukotjo, C.; Mercuri, L.G.; Lotesto, A.; Eke, R. How Many Temporomandibular Joint Total Joint Alloplastic Implants Will Be Placed in the United States in 2030? J. Oral Maxillofac. Surg. 2016, 74, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Kerwell, S.; Alfaro, M.; Pourzal, R.; Lundberg, H.J.; Liao, Y.; Sukotjo, C.; Mercuri, L.G.; Mathew, M.T. Examination of failed retrieved temporomandibular joint (TMJ) implants. Acta Biomater. 2016, 32, 324–335. [Google Scholar] [CrossRef]
- Gakhal, M.K.; Gupta, B.; Sidebottom, A.J. Analysis of outcomes after revision replacement of failed total temporomandibular joint prostheses. Br. J. Oral Maxillofac. Surg. 2020, 58, 220–224. [Google Scholar] [CrossRef]
- Rodrigues, Y.L.; Mathew, M.T.; Mercuri, L.G.; da Silva, J.S.P.; Henriques, B.; Souza, J.C.M. Biomechanical Simulation of Temporomandibular Joint Replacement (TMJR) Devices: A Scoping Review of the Finite Element Method; Churchill Livingstone: London, UK, 2018; Volume 47, pp. 1032–1042. [Google Scholar]
- De Meurechy, N.; Mommaerts, M.Y. Alloplastic temporomandibular joint replacement systems: A systematic review of their history. Int. J. Oral Maxillofac. Surg. 2018, 47, 743–754. [Google Scholar] [CrossRef]
- Wolford, L.; Movahed, R.; Teschke, M.; Fimmers, R.; Havard, D.; Schneiderman, E. Temporomandibular joint ankylosis can be successfully treated with TMJ concepts patient-fitted total joint prosthesis and autogenous fat grafts. J. Oral Maxillofac. Surg. 2016, 74, 1215–1227. [Google Scholar] [CrossRef]
- Sadoghi, P.; Liebensteiner, M.; Agreiter, M.; Leithner, A.; Böhler, N.; Labek, G. Revision surgery after total joint arthroplasty: A complication-based analysis using worldwide arthroplasty registers. J. Arthroplast. 2013, 28, 1329–1332. [Google Scholar] [CrossRef]
- Wolford, L.M.; Mercuri, L.G.; Schneiderman, E.D.; Movahed, R.; Allen, W. Twenty-year follow-up study on a patient-fitted temporomandibular joint prosthesis: The Techmedica/TMJ Concepts device. J. Oral Maxillofac. Surg. 2015, 73, 952–960. [Google Scholar] [CrossRef]
- Germain, M.A.; Hatton, A.; Williams, S.; Matthews, J.B.; Stone, M.H.; Fisher, J.; Ingham, E. Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro. Biomaterials 2003, 24, 469–479. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Brown, C.; Schins, R.; Singh, S.; Newson, R.; Davis, S.; Fisher, J.; Ingham, E.; Case, C.P. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials 2007, 28, 2946–2958. [Google Scholar] [CrossRef] [PubMed]
- De Moura Silva, A.; de Figueiredo, V.M.G.; do Prado, R.F.; de Fatima Santanta-Melo, G.; Del Valle El Abras Ankha, M.; de Vasconcellos, L.M.R.; da Silva Sobrinho, A.S.; Borges, A.L.S.; Nogueira, L., Jr. Diamond-like carbon films over reconstructive TMJ prosthetic materials: Effects in the cytotoxicity, chemical and mechanical properties. J. Oral Biol. Craniofacial Res. 2019, 9, 201–207. [Google Scholar] [CrossRef]
- Akbar, M.; Fraser, A.R.; Graham, G.J.; Brewer, J.M.; Grant, M.H. Acute inflammatory response to cobalt chromium orthopaedic wear debris in a rodent air-pouch model. J. R. Soc. Interface 2012, 9, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Drynda, A.; Ren, Q.; Buchhorn, G.H.; Lohmann, C.H. The induction of CXCR4 expression in human osteoblast-like cells (MG63) by CoCr particles is regulated by the PLC-DAG-PKC pathway. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Meng, H.; Freer, F.; Kwon, J.; Shelton, J.C.; Knight, M.M. Sub-toxic levels of Co2+ are anti-inflammatory and protect cartilage from degradation caused by IL-1β. Clin. Biomech. 2020. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Li, Z.; Wang, C.; Xiao, F.; Gao, Y.; Zhang, X.; Wang, P.; Peng, J.; Cai, G.; et al. The inhibition of RANKL expression in fibroblasts attenuate CoCr particles induced aseptic prosthesis loosening via the MyD88-independent TLR signaling pathway. Biochem. Biophys. Res. Commun. 2018, 503, 1115–1122. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ryu, D.-J.; Kim, H.-S.; Kim, H.-G.; Huh, J.-K. Alloplastic total temporomandibular joint replacement using stock prosthesis: A one-year follow-up report of two cases. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 297. [Google Scholar] [CrossRef]
- Abramowicz, S.; Barbick, M.; Rose, S.P.; Dolwick, M.F. Adaptability of stock TMJ prosthesis to joints that were previously treated with custom joint prosthesis. Int. J. Oral Maxillofac. Surg. 2012, 41, 518–520. [Google Scholar] [CrossRef]
- Mercuri, L.G. The role of custom-made prosthesis for temporomandibular joint replacement. Revista Española Cirugía Oral Maxilofacial 2013, 35, 1–10. [Google Scholar] [CrossRef]
- Dhanda, J.; Cooper, C.; Ellis, D.; Speculand, B. Technique of temporomandibular joint replacement using a patient-specific reconstruction system in the edentulous patient. Br. J. Oral Maxillofac. Surg. 2011, 49, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Cho, J.; Kim, H.M. Bilateral temporomandibular joint replacement using computer-assisted surgical simulation and three-dimensional printing. J. Craniofac. Surg. 2016, 27, e450–e452. [Google Scholar] [CrossRef] [PubMed]
- Kakuguchi, W.; Yamaguchi, H.O.; Inoue, N.; Totsuka, Y. Postoperative management of arthroplasty by using unique splints in almost edentulous patient. Br. J. Oral Maxillofac. Surg. 2012, 50, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Haq, J.; Patel, N.; Weimer, K.; Matthews, N.S. Single stage treatment of ankylosis of the temporomandibular joint using patient-specific total joint replacement and virtual surgical planning. Br. J. Oral Maxillofac. Surg. 2014, 52, 350–355. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Manfredini, D.; Ferronato, G. Temporomandibular joint total replacement prosthesis: Current knowledge and considerations for the future. Int. J. Oral Maxillofac. Surg. 2008, 37, 103–110. [Google Scholar] [CrossRef]
- Van Loon, J.P.; Verkerke, G.J.; De Vries, M.P.; De Bont, L.G.M. Design and wear testing of a temporomandibular joint prosthesis articulation. J. Dent. Res. 2000, 79, 715–721. [Google Scholar] [CrossRef]
- Mercuri, L.G.; Miloro, M.; Skipor, A.K.; Bijukumar, D.; Sukotjo, C.; Mathew, M.T. Serum Metal Levels in Maxillofacial Reconstructive Surgery Patients: A Pilot Study. J. Oral Maxillofac. Surg. 2018, 76, 2074–2080. [Google Scholar] [CrossRef]
- Mercuri, L.G.; Mathew, M.T.; Kerwell, S.; Lundberg, H.; Sukotjo, C. Temporomandibular Joint Replacement Device Research Wear and Corrosion Technology Transfer from Orthopedics. J. Bio Tribo Corros. 2015, 1. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Perez, L.M.; Gonzalez-Perez-Somarriba, B.; Centeno, G.; Vallellano, C.; Montes-Carmona, J.F.; Torres-Carranza, E.; Ambrosiani-Fernandez, J.; Infante-Cossio, P. Prospective study of five-year outcomes and postoperative complications after total temporomandibular joint replacement with two stock prosthetic systems. Br. J. Oral Maxillofac. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- De Meurechy, N.K.G.; Zaror, C.E.; Mommaerts, M.Y. Total Temporomandibular Joint Replacement: Stick to Stock or Optimization by Customization? Craniomaxillofac. Trauma Reconstr. 2020, 13, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Altaf, H.; Revell, P.A. Evidence for active antigen presentation by monocyte/macrophages in response to stimulation with particles: The expression of NFκB transcription factors and costimulatory molecules. Inflammopharmacology 2013, 21, 279–290. [Google Scholar] [CrossRef] [PubMed]
- De Meurechy, N.; Braem, A.; Mommaerts, M.Y. Biomaterials in temporomandibular joint replacement: Current status and future perspectives—a narrative review. Int. J. Oral Maxillofac. Surg. 2018, 47, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Driemel, O.; Braun, S.; Müller-Richter, U.D.A.; Behr, M.; Reichert, T.E.; Kunkel, M.; Reich, R. Historical development of alloplastic temporomandibular joint replacement after 1945 and state of the art. Int. J. Oral Maxillofac. Surg. 2009, 38, 909–920. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, L.; He, D.; Yang, C.; Zhao, J.; Ellis, E. Clinical and Radiologic Follow-Up of Zimmer Biomet Stock Total Temporomandibular Joint Replacement After Surgical Modifications. J. Oral Maxillofac. Surg. 2018, 76, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Mesnard, M. Christensen vs Biomet Microfixation alloplastic TMJ implant: Are there improvements? A numerical study. J. Cranio-Maxillofac. Surg. 2015, 43, 1398–1403. [Google Scholar] [CrossRef]
- Gerbino, G.; Zavattero, E.; Bosco, G.; Berrone, S.; Ramieri, G. Temporomandibular joint reconstruction with stock and custom-made devices: Indications and results of a 14-year experience. J. Cranio-Maxillofac. Surg. 2017, 45, 1710–1715. [Google Scholar] [CrossRef]
- Giannakopoulos, H.E.; Sinn, D.P.; Quinn, P.D. Biomet microfixation temporomandibular joint replacement system: A 3-year follow-up study of patients treated during 1995 to 2005. J. Oral Maxillofac. Surg. 2012, 70, 787–794. [Google Scholar] [CrossRef]
- Kanatsios, S.; Breik, O.; Dimitroulis, G. Biomet stock temporomandibular joint prosthesis: Long-term outcomes of the use of titanium condyles secured with four or five condylar fixation screws. J. Cranio-Maxillofac. Surg. 2018, 46, 1697–1702. [Google Scholar] [CrossRef]
- Baena, J.; Wu, J.; Peng, Z. Wear Performance of UHMWPE and Reinforced UHMWPE Composites in Arthroplasty Applications: A Review. Lubricants 2015, 3, 413–436. [Google Scholar] [CrossRef]
- Elledge, R.; Mercuri, L.G.; Attard, A.; Green, J.; Speculand, B. Review of emerging temporomandibular joint total joint replacement systems. Br. J. Oral Maxillofac. Surg. 2019, 57, 722–728. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Cunali, R.S.; Bonotto, D.; de Farias, A.C.; Cunali, P.A. Total temporomandibular joint alloplastic reconstruction. Revista Dor 2014, 15. [Google Scholar] [CrossRef]
- Mathew, M.T.; Kerwell, S.; Lundberg, H.J.; Sukotjo, C.; Mercuri, L.G. Tribocorrosion and oral and maxillofacial surgical devices. Br. J. Oral Maxillofac. Surg. 2014, 52, 396–400. [Google Scholar] [CrossRef]
- Johnson, N.R.; Roberts, M.J.; Doi, S.A.; Batstone, M.D. Total temporomandibular joint replacement prostheses: A systematic review and bias-adjusted meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Yang, C.; He, D.; Zhang, X.; Abdelrehem, A. Application of fossa bone graft to stabilize stock total joint prosthesis in temporomandibular joint surgery. J. Cranio-Maxillofac. Surg. 2015, 43, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; He, D.; Yang, C.; Chen, M.; Zhang, S.; Li, H.; Ellis, E., III. Simultaneous treatment of temporomandibular joint ankylosis with severe mandibular deficiency by standard TMJ prosthesis. Sci. Rep. 2017, 7, 45271. [Google Scholar] [CrossRef]
- Rhee, S.-H.; Baek, S.-H.; Park, S.-H.; Kim, J.-C.; Jeong, C.-G.; Choi, J.-Y. Total joint reconstruction using computer-assisted surgery with stock prostheses for a patient with bilateral TMJ ankylosis. Maxillofac. Plast. Reconstr. Surg. 2019, 41. [Google Scholar] [CrossRef]
- Moreira, C.V.A.; Serra, A.V.P.; Silva, L.O.R.; Fernandes, A.C.F.; de Azevedo, R.A. Total bilateral TMJ reconstruction for pain and dysfunction: Case report. Int. J. Surg. Case Rep. 2018, 42, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Westermark, A.; Leiggener, C.; Aagaard, E.; Lindskog, S. Histological findings in soft tissues around temporomandibular joint prostheses after up to eight years of function. Int. J. Oral Maxillofac. Surg. 2011, 40, 18–25. [Google Scholar] [CrossRef]
- Siegmund, B.J.; Winter, K.; Meyer-Marcotty, P.; Rustemeyer, J. Reconstruction of the temporomandibular joint: A comparison between prefabricated and customized alloplastic prosthetic total joint systems. Int. J. Oral Maxillofac. Surg. 2019, 48, 1066–1071. [Google Scholar] [CrossRef]
- Brown, Z.L.; Sarrami, S.; Perez, D.E. Will they fit? Determinants of the adaptability of stock TMJ prostheses where custom TMJ prostheses were utilized. Int. J. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Elledge, R.; Mercuri, L.G.; Speculand, B. Extended total temporomandibular joint replacements: A classification system. Br. J. Oral Maxillofac. Surg. 2018, 56, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G. Alloplastic temporomandibular joint replacement: Rationale for the use of custom devices. Int. J. Oral Maxillofac. Surg. 2012, 41, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, K.S.; Arce, K.; Fillmore, W.J.; Van Ess, J.M.; Yetzer, J.G.; Viozzi, C.F. Does the Amount of Screw Fixation Utilized for the Condylar Component of the TMJ Concepts Total Temporomandibular Joint Reconstruction Predispose to Hardware Loss or Postoperative Complications? J. Oral Maxillofac. Surg. 2016, 74, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Farzad, P. Reconstruction of nongrowing hemifacial microsomia patient with custom-made unilateral temporomandibular joint total joint prosthesis and orthognathic surgery. J. Oral Biol. Craniofac. Res. 2017, 7, 62–66. [Google Scholar] [CrossRef][Green Version]
- Gruber, E.A.; McCullough, J.; Sidebottom, A.J. Medium-term outcomes and complications after total replacement of the temporomandibular joint. Prospective outcome analysis after 3 and 5 years. Br. J. Oral Maxillofac. Surg. 2015, 53, 412–415. [Google Scholar] [CrossRef]
- Park, J.-H.; Jo, E.; Cho, H.; Kim, H.J. Temporomandibular joint reconstruction with alloplastic prosthesis: The outcomes of four cases. Maxillofac. Plast. Reconstr. Surg. 2017, 39. [Google Scholar] [CrossRef]
- Van Loon, J.P.; de Bont, L.G.M.; Boering, G. Evaluation of temporomandibular joint prostheses. Review of the literature from 1946 to 1994 and implications for future prosthesis designs. J. Oral Maxillofac. Surg. 1995, 53, 984–996. [Google Scholar] [CrossRef]
- Ramos, A.; Mesnard, M. A new condyle implant design concept for an alloplastic temporomandibular joint in bone resorption cases. J. Cranio-Maxillofac. Surg. 2016, 44, 1670–1677. [Google Scholar] [CrossRef]
- Mesnard, M.; Ramos, A. Experimental and numerical predictions of Biomet® alloplastic implant in a cadaveric mandibular ramus. J. Cranio-Maxillofac. Surg. 2016, 44, 608–615. [Google Scholar] [CrossRef]
- Ramos, A.; Mesnard, M.; Relvas, C.; Completo, A.; Simões, J.A. Theoretical assessment of an intramedullary condylar component versus screw fixation for the condylar component of a hemiarthroplasty alloplastic TMJ replacement system. J. Cranio-Maxillofac. Surg. 2014, 42, 169–174. [Google Scholar] [CrossRef]
- Ramos, A.; Mesnard, M. Comparison of load transfers in TMJ replacement using a standard and a custom-made temporal component. J. Cranio-Maxillofac. Surg. 2014, 42, 1766–1772. [Google Scholar] [CrossRef]
- Ramos, A.; Completo, A.; Relvas, C.; Mesnard, M.; Simões, J.A. Straight, semi-anatomic and anatomic TMJ implants: The influence of condylar geometry and bone fixation screws. J. Cranio-Maxillofac. Surg. 2011, 39, 343–350. [Google Scholar] [CrossRef]
- Sinno, H.; Tahiri, Y.; Gilardino, M.; Bobyn, D. Engineering alloplastic temporomandibular joint replacements. McGill J. Med. 2011, 13, 63. [Google Scholar]
- Souza, J.C.M.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.-P.; Rocha, L.A. Wear and Corrosion Interactions on Titanium in Oral Environment: Literature Review. J. Bio- Tribo-Corros. 2015, 1, 13. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Bagheri, A.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Tribocorrosion behavior of hot pressed CoCrMo alloys in artificial saliva. Tribol. Int. 2016. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Barbosa, S.L.; Ariza, E.; Celis, J.P.; Rocha, L.A. Simultaneous degradation by corrosion and wear of titanium in artificial saliva containing fluorides. Wear 2012, 292–293, 82–88. [Google Scholar] [CrossRef]
- Alves, S.A.; Beline, T.; Barão, V.A.R.; Sukotjo, C.; Mathew, M.T.; Rocha, L.A.; Celis, J.-P.; Souza, J.C.M. Chapter 3—Degradation of titanium-based implants. In Advanced Nanomaterials; Souza, J.C.M., Hotza, D., Henriques, B., Boccaccini, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–62. ISBN 978-0-12-814621-7. [Google Scholar] [CrossRef]
- Landolt, D. Corrosion and Surface Chemistry of Metals, 1st ed.; EPFL Press: Lausanne, Switzerland, 2007; ISBN 978-2-940222-11-7. [Google Scholar]
- Barril, S.; Debaud, N.; Mischler, S.; Landolt, D. A tribo-electrochemical apparatus for in vitro investigation of fretting-corrosion of metallic implant materials. Wear 2002, 252, 744–754. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.-P.; Rocha, L.A. Biofilms inducing ultra-low friction on titanium. J. Dent. Res. 2010, 89, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Barbosa, S.L.; Ariza, E.A.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.P.; Rocha, L.A. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater. Sci. Eng. C 2015, 47, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.-P.; Rocha, L.A. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling 2010, 26, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Doni, Z.; Alves, A.C.; Toptan, F.; Gomes, J.R.; Ramalho, A.; Buciumeanu, M.; Palaghian, L.; Silva, F.S. Dry sliding and tribocorrosion behaviour of hot pressed CoCrMo biomedical alloy as compared with the cast CoCrMo and Ti6Al4V alloys. Mater. Des. 2013, 52, 47–57. [Google Scholar] [CrossRef]
- Alves, A.C.; Oliveira, F.; Wenger, F.; Ponthiaux, P.; Celis, J.-P.; Rocha, L.A. Tribocorrosion behaviour of anodic treated titanium surfaces intended for dental implants. J. Phys. D Appl. Phys. 2013, 46, 404001. [Google Scholar] [CrossRef]
- Teeter, M.G.; MacLean, C.J.; Somerville, L.E.; Howard, J.L.; McCalden, R.W.; Lanting, B.A.; Vasarhelyi, E.M. Wear performance of cobalt chromium, ceramic, and oxidized zirconium on highly crosslinked polyethylene at mid-term follow-up. J. Orthop. 2018, 15, 620–623. [Google Scholar] [CrossRef]
- Patil, N.A.; Njuguna, J.; Kandasubramanian, B. UHMWPE for biomedical applications: Performance and functionalization. Eur. Polym. J. 2020, 125, 109529. [Google Scholar] [CrossRef]
- Lei, P.; Dai, Z.; Zhang, Y.S.; Liu, H.; Niu, W.; Li, K.; Wang, L.; Hu, Y.; Xie, J. Macrophage inhibits the osteogenesis of fibroblasts in ultrahigh molecular weight polyethylene (UHMWPE) wear particle-induced osteolysis. J. Orthop. Surg. Res. 2019, 14, 80. [Google Scholar] [CrossRef]
- McCalden, R.W.; MacDonald, S.J.; Rorabeck, C.H.; Bourne, R.B.; Chess, D.G.; Charron, K.D. Wear rate of highly cross-linked polyethylene in total hip arthroplasty. A randomized controlled trial. J. Bone Jt. Surg. Am. 2009, 91, 773–782. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Butt, A.; Patel, S.; Royhman, D.; Takoudis, C.; Sukotjo, C.; Yuan, J.; Jursich, G.; Mathew, M.T.; Hendrickson, W.; et al. Fabrication of Anti-Aging TiO2 Nanotubes on Biomedical Ti Alloys. PLoS ONE 2014, 9, e96213. [Google Scholar] [CrossRef]
- Mercuri, L.G.; Urban, R.M.; Hall, D.J.; Mathew, M.T. Adverse Local Tissue Responses to Failed Temporomandibular Joint Implants. J. Oral Maxillofac. Surg. 2017, 75, 2076–2084. [Google Scholar] [CrossRef]
- Noronha Oliveira, M.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Reddy, A.; Caicedo, M.S.; Samelko, L.; Jacobs, J.J.; Hallab, N.J. Implant debris particle size affects serum protein adsorption which may contribute to particle size-based bioreactivity differences. J. Long-Term Eff. Med. Implants 2014, 24, 77–88. [Google Scholar] [CrossRef]
- Bijukumar, D.R.; Segu, A.; Souza, J.C.M.; Li, X.J.; Barba, M.; Mercuri, L.G.; Jacobs, J.; Mathew, M.T. Systemic and local toxicity of metal debris released from hip prostheses: A review of experimental approaches. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Apaza-Bedoya, K.; Tarce, M.; Benfatti, C.A.M.; Henriques, B.; Mathew, M.T.; Teughels, W.; Souza, J.C.M. Synergistic interactions between corrosion and wear at titanium-based dental implant connections: A scoping review. J. Periodontal Res. 2017, 52, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, T.D.; Dolgova, N.V.; Lewis, J.S.; Hamaker, K.; Clare-Salzler, M.J.; Keselowsky, B.G. Macrophage integrins modulate response to ultra-high molecular weight polyethylene particles and direct particle-induced osteolysis. Biomaterials 2017, 115, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Richards, L.; Bladen, C.L.; Ingham, E.; Fisher, J.; Tipper, J.L. The biological response to nanometre-sized polymer particles. Acta Biomater. 2015, 23, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Gemini-Piperni, S.; Travassos, R.; Lemgruber, L.; Silva, R.C.; Rossi, A.L.; Farina, M.; Anselme, K.; Shokuhfar, T.; Shahbazian-Yassar, R.; et al. Trojan-Like Internalization of Anatase Titanium Dioxide Nanoparticles by Human Osteoblast Cells. Sci. Rep. 2016, 6, 23615. [Google Scholar] [CrossRef]
- Royhman, D.; Radhakrishnan, R.; Yuan, J.C.-C.; Mathew, M.T.; Mercuri, L.G.; Sukotjo, C. An electrochemical investigation of TMJ implant metal alloys in an artificial joint fluid environment: The influence of pH variation. J. Craniomaxillofac. Surg. 2014, 42, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Pourzal, R.; Catelas, I.; Theissmann, R.; Kaddick, C.; Fischer, A. Characterization of wear particles generated from CoCrMo alloy under sliding wear conditions. Wear 2011, 271, 1658–1666. [Google Scholar] [CrossRef]
- Messous, R.; Henriques, B.; Bousbaa, H.; Silva, F.S.; Teughels, W.; Souza, J.C.M. Cytotoxic effects of submicron- and nano-scale titanium debris released from dental implants: An integrative review. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef]
- Catelas, I.; Petit, A.; Vali, H.; Fragiskatos, C.; Meilleur, R.; Zukor, D.J.; Antoniou, J.; Huk, O.L. Quantitative analysis of macrophage apoptosis vs. necrosis induced by cobalt and chromium ions in vitro. Biomaterials 2005, 26, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’h, M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Jt. Surg. 2000, 82, 457. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, S.S.; Danscher, G.; Stoltenberg, M.; Larsen, A.; Bruun, J.M.; Mygind, T.; Kemp, K.; Soballe, K. Cobalt-chromium-molybdenum alloy causes metal accumulation and metallothionein up-regulation in rat liver and kidney. Basic Clin. Pharmacol. Toxicol. 2007, 101, 441–446. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto-Borges, H.; Carvalho, O.; Henriques, B.; Silva, F.; Ramos, A.; Souza, J.C.M. A Preliminary Analysis of the Wear Pathways of Sliding Contacts on Temporomandibular Joint Total Joint Replacement Prostheses. Metals 2021, 11, 685. https://doi.org/10.3390/met11050685
Pinto-Borges H, Carvalho O, Henriques B, Silva F, Ramos A, Souza JCM. A Preliminary Analysis of the Wear Pathways of Sliding Contacts on Temporomandibular Joint Total Joint Replacement Prostheses. Metals. 2021; 11(5):685. https://doi.org/10.3390/met11050685
Chicago/Turabian StylePinto-Borges, Henrique, Oscar Carvalho, Bruno Henriques, Filipe Silva, António Ramos, and Júlio C. M. Souza. 2021. "A Preliminary Analysis of the Wear Pathways of Sliding Contacts on Temporomandibular Joint Total Joint Replacement Prostheses" Metals 11, no. 5: 685. https://doi.org/10.3390/met11050685
APA StylePinto-Borges, H., Carvalho, O., Henriques, B., Silva, F., Ramos, A., & Souza, J. C. M. (2021). A Preliminary Analysis of the Wear Pathways of Sliding Contacts on Temporomandibular Joint Total Joint Replacement Prostheses. Metals, 11(5), 685. https://doi.org/10.3390/met11050685







