4. Discussion
To investigate the effect of cooling rate on the hardness of a Pd-Ag-based metal–ceramic alloy with or without ice-quenching, the hardness at each firing step was measured. The specimen in the as-cast state was homogenized by oxidation treatment, and the homogenized state was maintained at room temperature by ice-quenching (
Figure 3 and
Figure 6). On the other hand, by oxidation and then cooling at a relatively high (S0) or low (S3) cooling rate (OX-S0, OX-S3), hardening by precipitation occurred (
Figure 2 and
Figure 3). In particular, the rapidly cooled specimens (S0) showed an 8% higher hardness through the glaze step (
p < 0.05), indicating that the optimum cooling rate to maintain a high hardness has a relatively high value (S0). Although the lowest hardness was obtained with the ice-quenched specimens after oxidation (
p < 0.05), the hardness of the ice-quenched specimen was recovered in the very next firing step at both the relatively high and low cooling rates, with a greater increase in hardness observed at a higher cooling rate. The recovered hardness value did not exceed those of the non-quenched specimens at both cooling rates through the glaze step (
p < 0.05). From the above, it can be determined that oxidation followed by ice-quenching reduced the hardness temporarily without sacrificing the final hardness at both, the relatively high and low cooling rates (
p < 0.05). In previous studies that used a single cooling rate, such a recovery of hardness was observed in Pd−Au and Pd−Cu alloys, as well as in Pd-Ag alloy [
8,
9,
13]. In particular, in Pd−Cu alloys, the final hardness far exceeded that of the non-quenched alloy [
13].
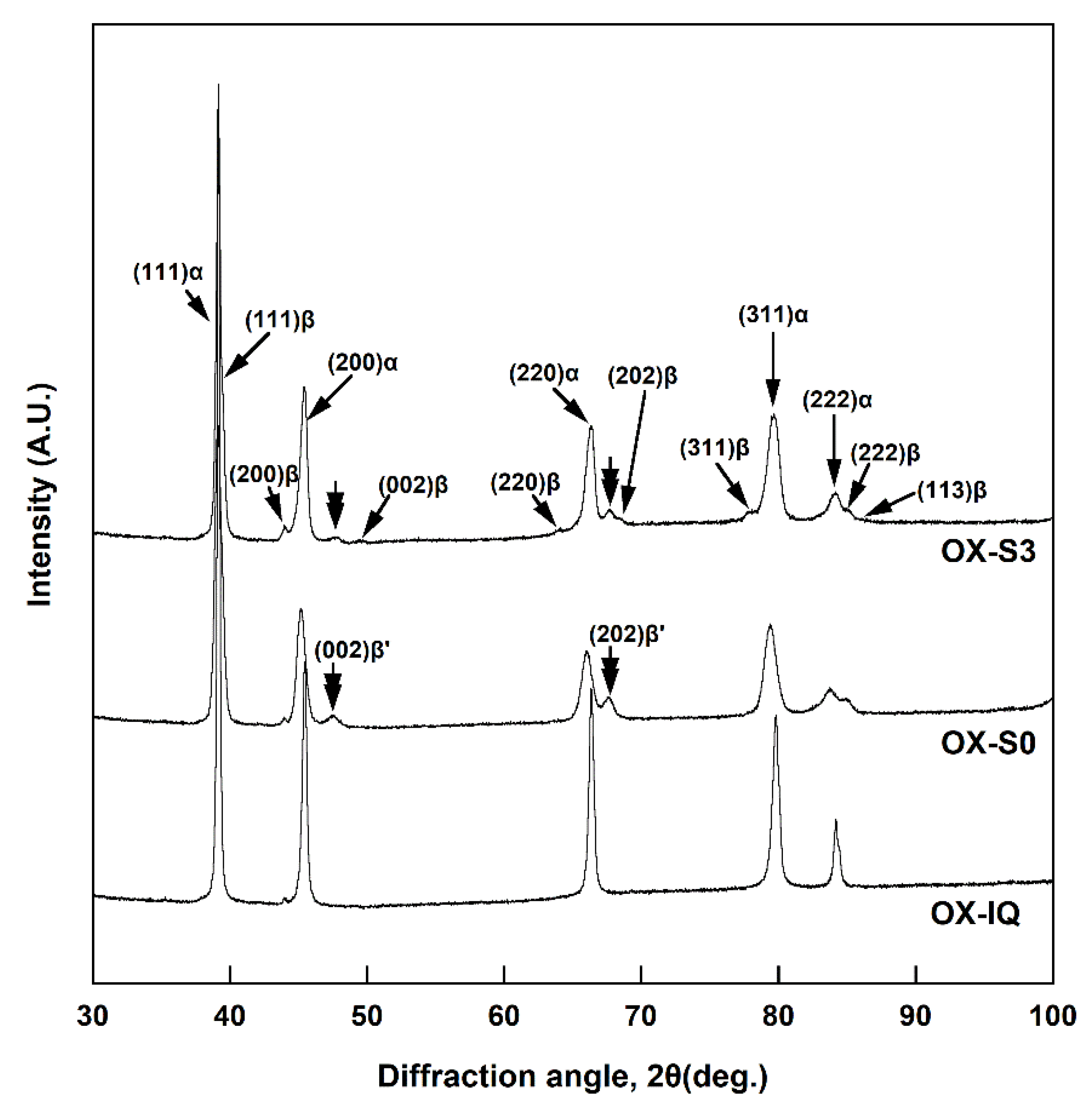
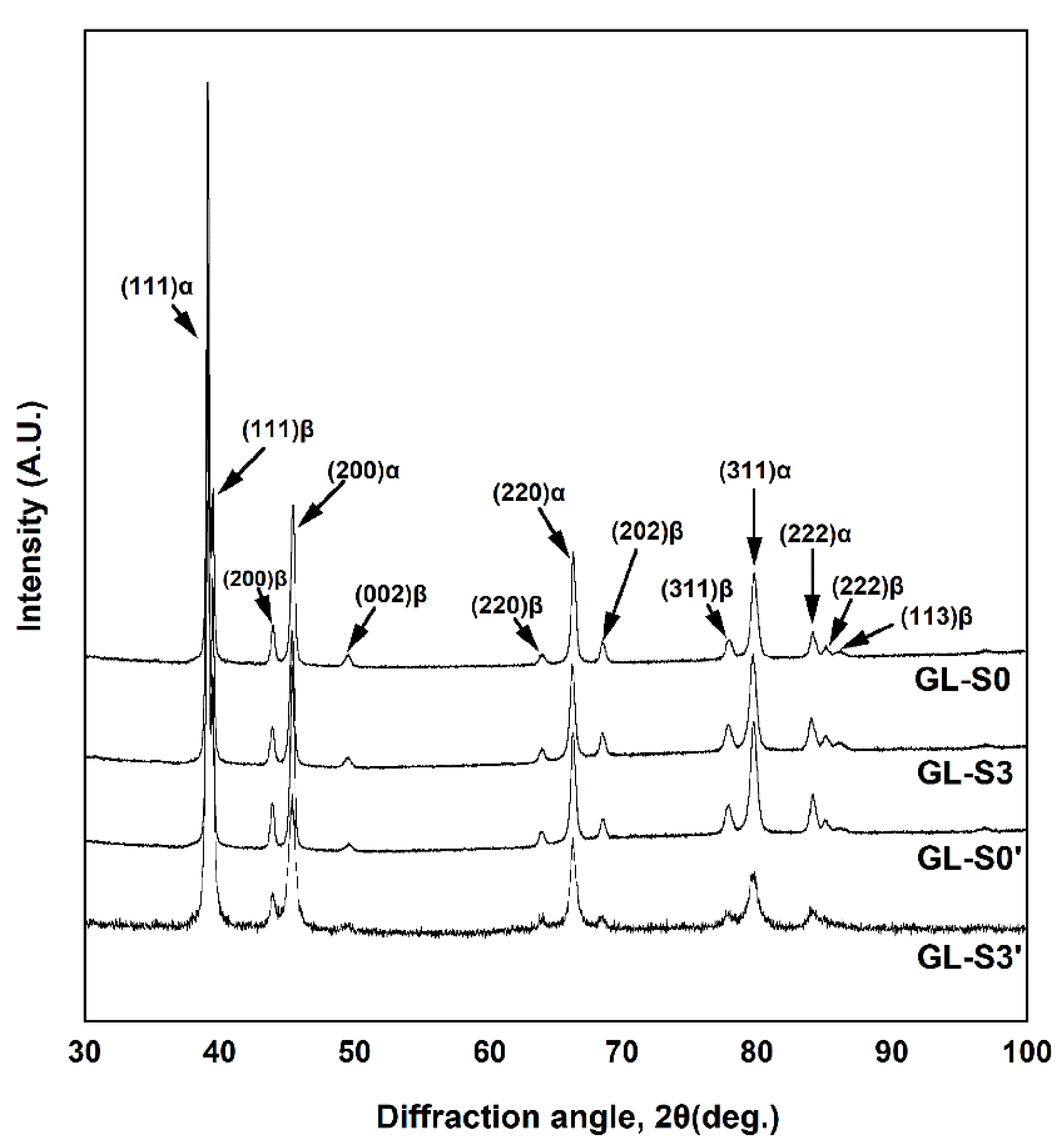
XRD was performed to investigate the phase transformation according to the multiple firing simulation of each specimen. All the specimens at the glaze step (GL-(S0′, S0, S3′, S3)) consisted of the Pd-rich matrix (α) and InPd
3-based precipitates (β) of tetragonal structure regardless of cooling rate and ice-quenching after oxidation (
Figure 7). However, XRD patterns at the oxidation step (OX-(S0, S3)) show a metastable InPd
3-based phase (β′) that has a
c/
a ratio larger than that of the stable InPd
3 phase (
Figure 6, double arrows). Particularly, in case of OX-S3, which was cooled at a lower cooling rate, the diffraction peaks for the metastable and stable InPd
3 phases coexisted, indicating that the precipitated phase was transforming from the metastable phase to the stable phase (
Figure 6). Such a precipitation of the metastable phase before the formation of a stable phase minimizes the gap in the lattice constant between the matrix and the precipitates with different crystal structures, and lowers the interphase boundary energy [
14].
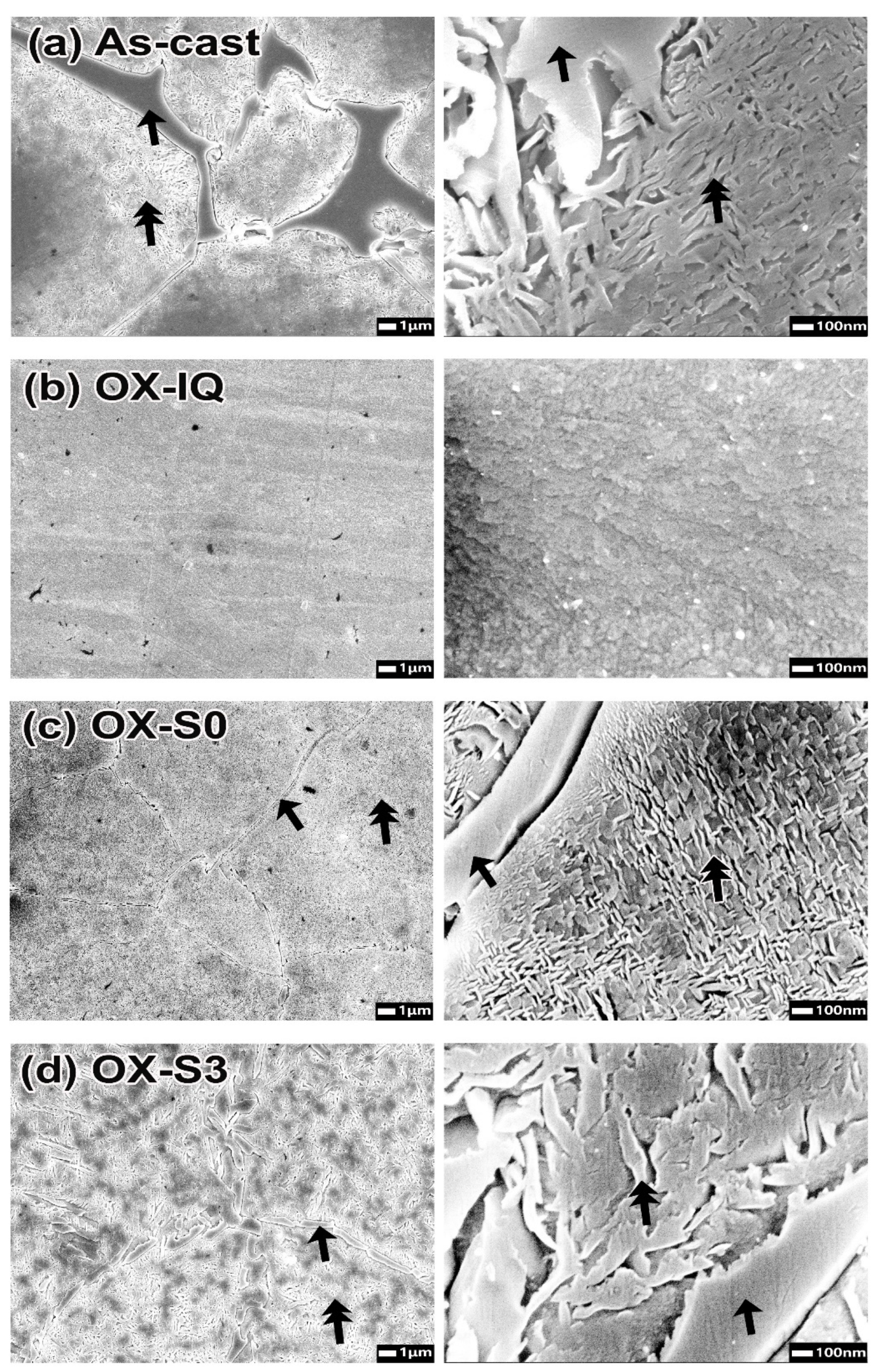
The oxidation-treated specimens that were then cooled at a relatively high or low cooling rate showed much higher hardness than the ice-quenched specimen after oxidation. In the OX-(S0, S3) specimens (
Figure 6), the full width at half maximum (FWHM) of the diffraction peaks for the α phase (matrix) increased compared to that in the homogenized specimen by ice-quenching after oxidation (OX-IQ). This indicated that a coherency strain field was generated at the interface between the matrix and the metastable precipitates owing to the slight gap between their lattice constants [
15], resulting in a significant increase in hardness (
p < 0.05). Such hardening through the formation of a metastable phase has been reported in several Pd-Ag-based metal–ceramic alloys [
10,
16], as well as in dental Au-based alloys that form a tetragonal AuCu I phase [
17,
18]. The relatively lower hardness of OX-S3 than that of OX-S0 must be resulted from the fact that the coherency strain field was released to some extent as the precipitates composed of the stable phase grew at the expense of the fine precipitates composed of the metastable phase [
19].
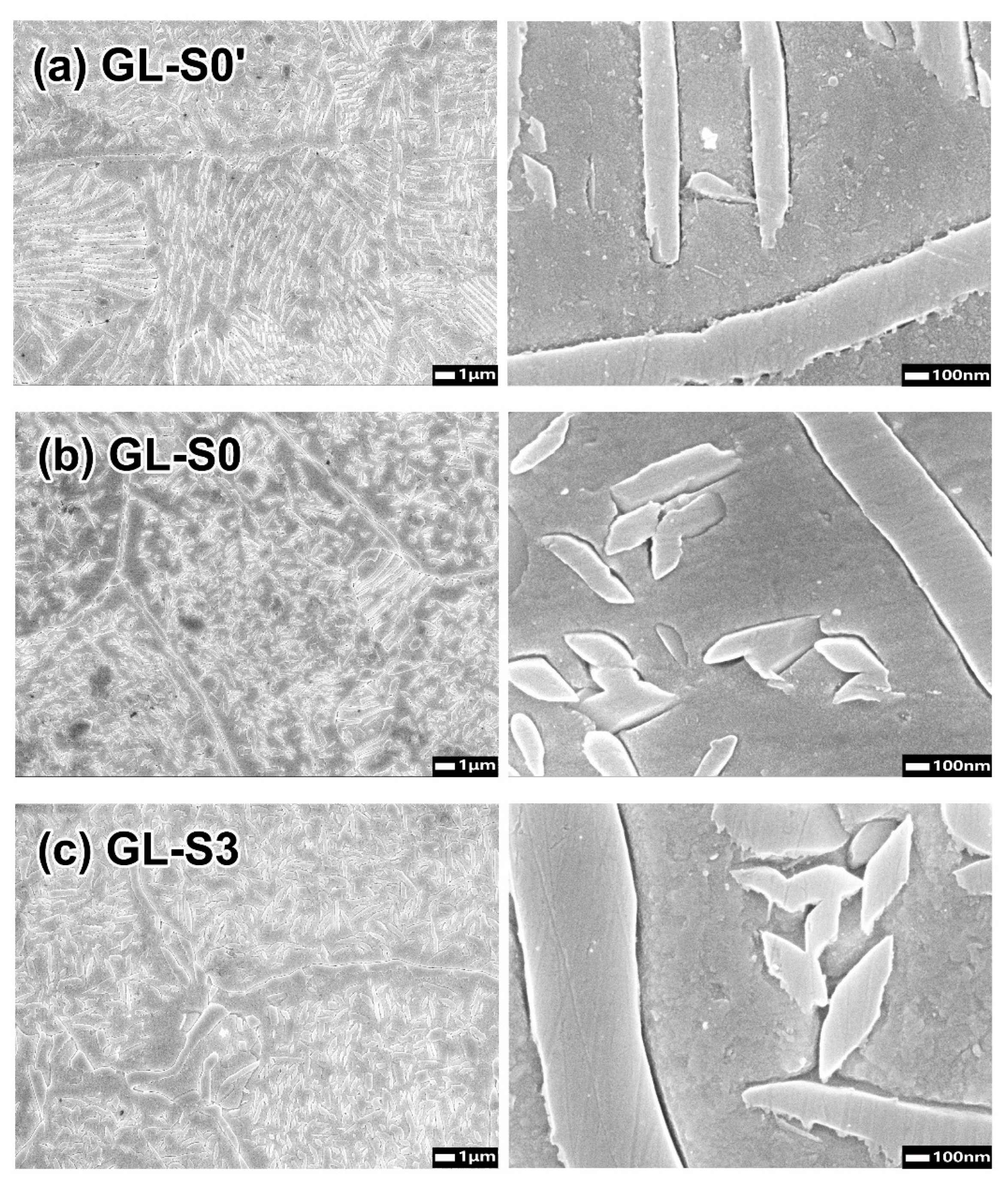
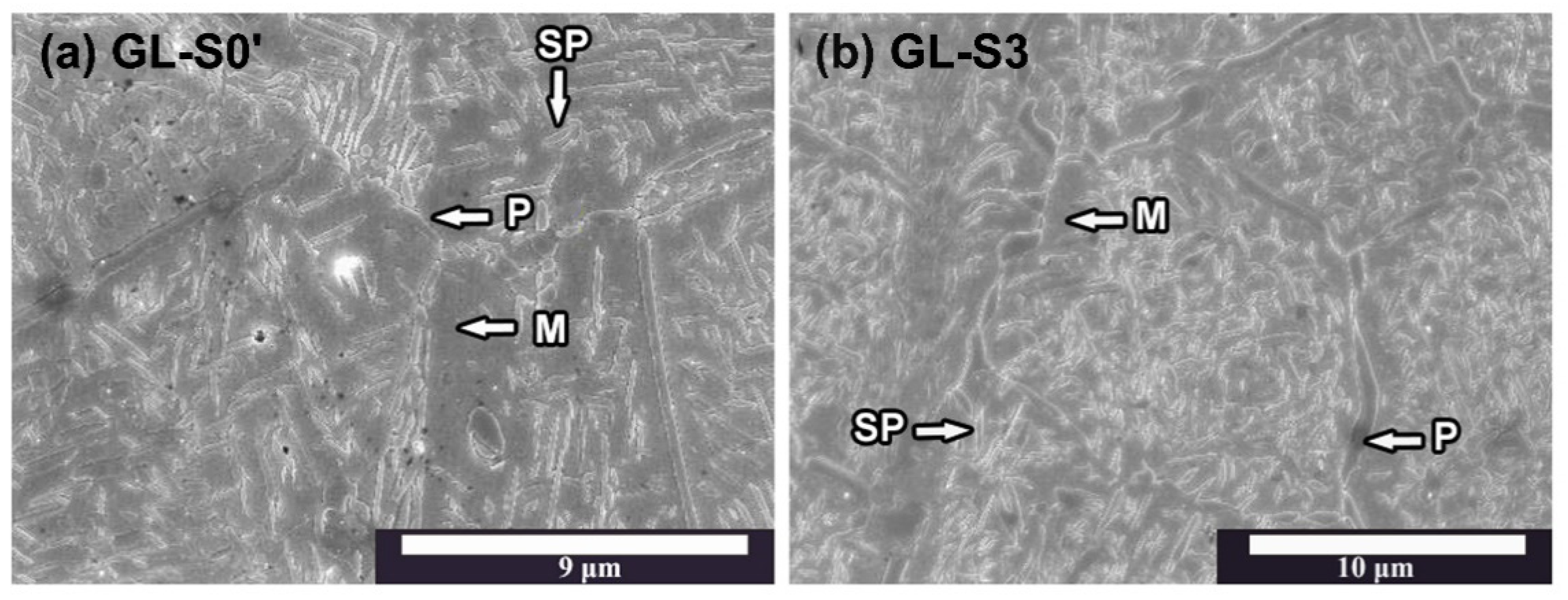
There was a tendency for the hardness to decrease as the firing progressed. In the microstructure of each specimen at the glaze step (
Figure 4), the precipitates were coarser than those in the oxidation step. Among the specimens at the glaze step, the GL-S3 specimen, which exhibited a lower hardness, had slightly coarser precipitates than those of the GL-(S0′, S0) specimens. The XRD patterns at glaze step revealed that these precipitates consist of only the stable phase. Therefore, the increased gap between the
c-axis lattice constant of the matrix and that of the precipitates composed of the stable phase led to a loss of coherency strain by introducing interface dislocations, which contributed to a decrease in hardness [
19,
20,
21]. In addition, the progress of coarsening of the precipitates reduced the interface between the matrix and precipitates, reducing lattice distortion and lowering the hardness [
20].
The general firing schedule for bonding porcelain starts at approximately 1000 °C and goes through several steps [
22,
23]. In order to prevent the deformation of the porcelain superstructure in the next firing step with a new porcelain layer, the firing temperature is gradually lowered for each step. The firing schedule implemented in this study was applied to porcelain powder with a coefficient of thermal expansion close to that of the used specimen, starting at 1040 °C for the oxidation step and ending at 880 °C in the final glaze step. In this study, the introduction of ice-quenching after glazing did not result in a significant decrease in hardness compared to that of the non-quenched specimens after glazing (
Figure 2; GL-(S0′, S0, S3)IQ). This is possibly attributed to the fact that the temperature for the glaze step was not high enough to homogenize the specimen alloy. To validate this, the GL-(S0′)IQ specimen was further treated at the glaze temperature (880 °C) for 80 min, but homogenization did not occur (
Figure 5). In previous study with 60.55 Pd-27.72 Ag-6.12 In-2.24 Sn-3.21 Ga-0.16 Ru (at%) alloy [
10], the homogenization proceeded during glazing at 930 °C, showing apparent hardening while cooling after glazing, unlike the results of this study. Considering that the specimen alloy in this study showed remarkable hardening while cooling at the oxidation temperature (1040 °C), it can be said that the influence of the cooling rate on the hardness became stronger when the specimen was in a homogenized state before cooling.
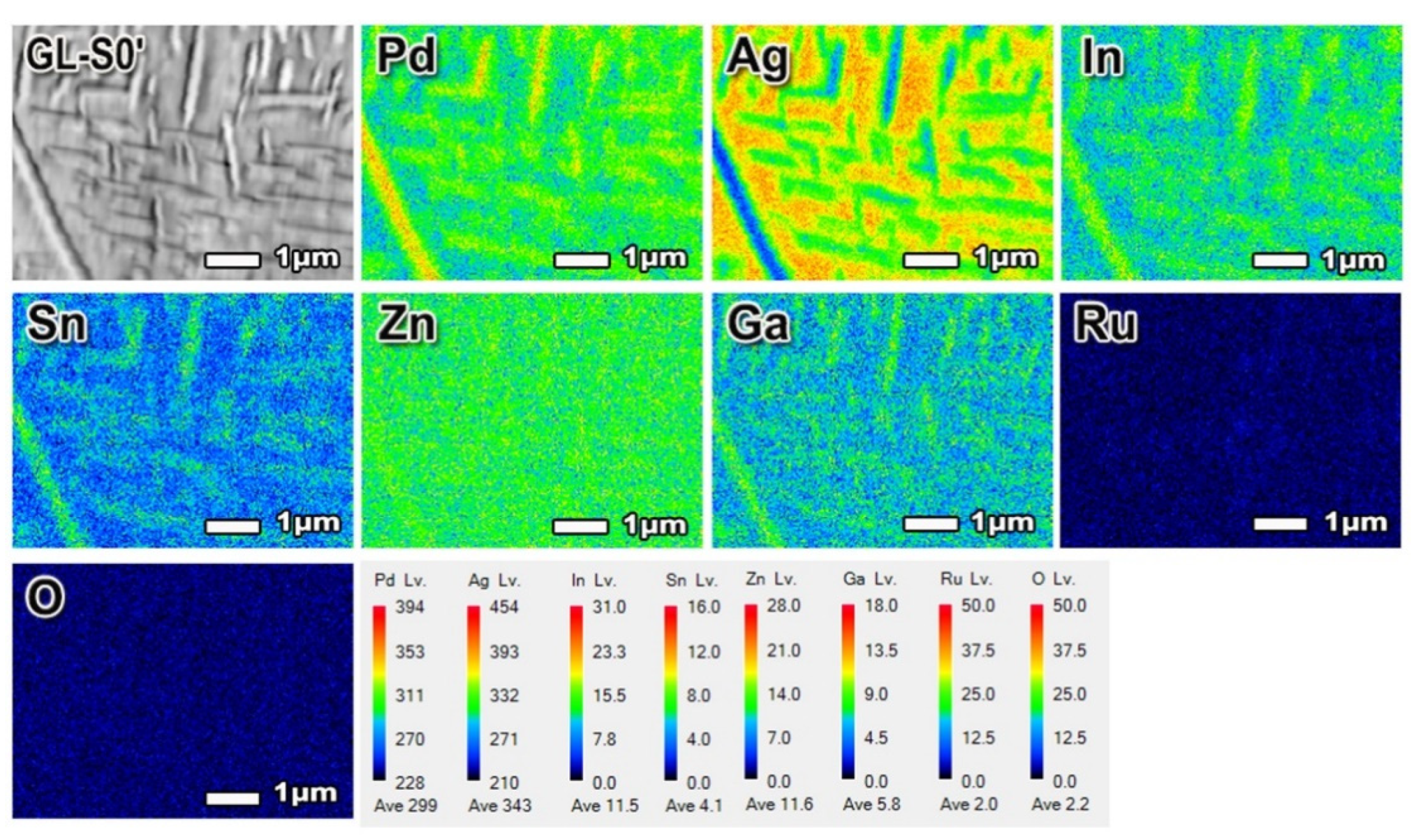
In this study, the homogenized specimen at an oxidation temperature was separated into the Pd-rich matrix and the InPd
3-based precipitates during cooling process, which corresponded to the precipitation reaction presented in the phase diagram of the In–Pd binary system. In the phase diagram, the Pd content must be approximately 85 at% or higher for the alloy to be homogenized at 1040 °C [
24]. Even though the Pd content was only 55.2 at% in the specimen alloy, a large amount of Ag contained in alloy made it possible by substituting Pd, as shown in the FE-EPMA results (
Table 7). This trend was also reported in several Pd-Ag-based alloys [
10,
25].
In this study, the oxide layer that formed on the alloy specimen was removed by polishing for the hardness test. Therefore, the effect of ice-quenching after oxidation on the degree of oxide film formation could not be detected. Nevertheless, the effect seems to be weak, as sandblasting is usually suggested by the manufacturer for Pd-Ag-based alloys to remove the over-produced oxide layer after oxidation.