Abstract
In this study, in situ Al2O3-reinforced Ti3Al composite was fabricated after 8 h of milling and sintering at 850 °C. A mixture of TiO2 and Al powders were mechanically milled in a planetary mill, cold-compacted and sintered under a protected argon atmosphere. The microstructure and microhardness of the Al2O3 embedded in Ti3Al matrix at both room and elevated temperature has been reported. The obtained results showed that the Ti3Al/Al2O3 composite was successfully synthesized via the powder metallurgy method. Ti3Al phase and Al2O3 particles were formed after 8 h of milling and sintering at 850 °C. The microstructure formation of round and uniformly distributed Al2O3 particles in the Ti3Al matrix improved the microhardness of the composite. At normal temperature, the microhardness of the material measured about 11.5 GPa. Meanwhile, at elevated temperatures, from 600 to 800 °C, it decreased from 4.18 GPa to 3.15 GPa.
1. Introduction
Ti-Al composite nanomaterials have been receiving considerable interest due to their specific properties, such as relatively high melting point, low density, high specific strength, high elastic modulus, good oxidation resistance, good mechanical properties and high creep resistance at elevated temperatures [1,2,3,4]. However, Ti-Al alloys are brittle at room temperature and have low fracture toughness [3,4]. The microhardness of intermetallic alloy was improved using various methods. Skvortsov et al. reported that the hardness of low-alloy steel was increased via the cold metal transfer method under applied external magnetic fields [5]. Zuev et al. showed that the contact potential difference and electric potential were important to affect the microhardness of metals [6]. Taotao et al. reported that the hardness of TiAl intermetallic material increased via the random distribution of Al2O3 particles in the host matrix of Ti-Al compounds [7]. In situ Al2O3-reinforced Ti-Al composites not only maintain the excellent properties of the Ti-Al matrix but also improve other properties due to the hardening property of Al2O3.
There have been quite a few studies on the synthesis of Al2O3-reinforced Ti-Al composites. Binh et al. reported that the Ti3Al/Al2O3 and TiAl3/Al2O3 composites were well-fabricated by using the thermal reaction of oxide TiO2 with metallic Al by using ball mining and sintering at high temperature [8]. Welham et al. showed the possible fabricated TiAl3/Al2O3 composite via the reaction of oxide TiO2 with metallic Al under vacuum [9]. Ying et al. pointed out that the TiAl3 intermetallic phase was formed from the reaction of Al with TiO2 at temperatures above 820 °C, while the Al2O3 phase was difficult to form at temperatures below 800 °C and the α-Ti(Al,O) phase proceeded at temperatures above 1000 °C [10]. The phase form during the reaction of TiO2 with Al powder was also reported to be dependent on the milling time in the high-energy ball mill method where the formation of TiAl3/Al2O3 was first formed and changed to TiAl/Al2O3 [11]. The TiAl/Ti3Al-Al2O3 composite was synthesized from nanosized TiO2 and micron-sized Al powders by applying high pressure during thermal sintering [12]. Travitzky et al. reported that the dense interpenetrating phase Al2O3-TiAl-Ti3Al composite was fabricated by the pressure-assisted thermal explosion of a TiO2–Al powder blend [13]. The complex Al-Ti-Al2O3 composite was reported to be fabricated by using the spark plasma sintering technique [14]. The results indicated that the complex phase formed as a function of the chemical ratio source between oxide TiO2 and metallic Al powder and also depended on the method of fabrication processing. In addition, during the synthesis process, the reaction between Al and Ti could form intermetallic phases such as TiAl, TiAl3 and Ti3Al compounds. The formation of these intermetallic phases has a great effect on the properties of the composites. Al2O3-reinforced Ti-Al composites synthesized by the mechanical method have been shown to have significantly reduced sintering temperature. Studies also show that the mechanical activation of the milling process lowers the temperature of the synthesizing process of Al2O3 and TiAl3 phases from TiO2 and Al as raw materials, from 1000 °C to 650 °C after 5 h of milling [9].
In this study, in situ Al2O3-reinforced Ti3Al composite was fabricated from aluminum powder and titanium dioxide powder via the powder metallurgy method. The formation of the composite occurred after 8 h of milling and sintering. Microstructure and microhardness at room temperature and elevated temperature were studied and compared with other titanium-based materials.
2. Materials and Methods
Raw materials used in this study were commercial aluminum (Al) and titanium dioxide (TiO2) powder from Merck KGaA (Darmstadt, Germany) and Xilong Chemical Co. Ltd. (Shantou, Guangdong, China), respectively. According to the particle size and chemical composition information provided by the manufacturers, the aluminum powder has a particle size of less than 50 μm and contains less than 1% Fe impurity, and the titanium dioxide powder has a chemical composition of 99.7% TiO2 and its particle size is 2 μm. The mixture of aluminum and titanium dioxide powder was prepared in accordance with the stoichiometry of reaction between Al and TiO2 (Equation (1)):
5 Al + 3 TiO2 → Ti3Al + 2 Al2O3
The powder mixture was weighed, mixed into a ball mill in a closed chamber under argon atmosphere. Samples were then milled for 8 h. The mixture of milled powder and mill ball was fed into a stainless steel mill with the ball-to-powder ratio of 10:1. The mechanical mill used was a vertical planetary ball mill NQM-4 of Yangzhou Nuoya Machinery Co., Ltd. In order to prevent the powder mixture from oxidizing during the milling process, the ball-milling tank was filled with argon. The milling parameters were set as follows: the rotation speed was 300 rpm, the zirconia milling-balls diameter was 10 mm and the milling time was 8 h. The milled sample was compressed with pressure at 100 MPa. The pressed sample was sintered at 650, 750 and 850 °C in a resistance furnace (EF 11/8B, Lenton, Hope Valley, United Kingdom) under argon atmosphere for 30 min. The sample disk was polished using sandpaper, increasing in roughness from 80 to 2000 CC-Cw and finally, using Al2O3 powder. A sketch of sample fabrication processing is shown in Figure 1.
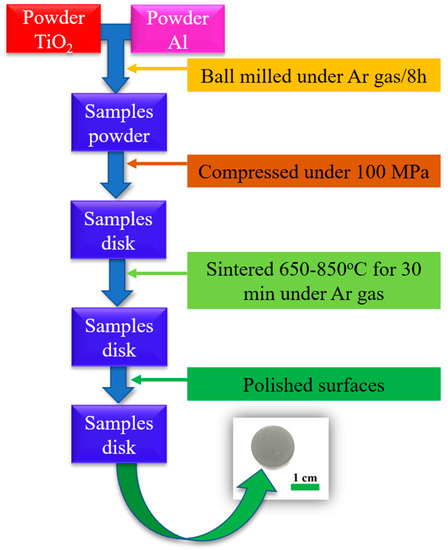
Figure 1.
Sketch of sample fabrication processing.
Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) was performed on milled powder using a sample weighing 10,852 mg, using a Setaram Labsys Evo S60 instrument, Lyon, France. The sample was heated to 1000 °C at a rate of 10 °C/min. All analyses were performed under high-purity argon atmosphere at a flow rate of 150 mL/min.
The phase composition and microstructure of the sintered products were characterized by employing X-Ray diffraction (XRD) (Smart Lab, Rigaku, Tokyo, Japan) and scanning electron microscopy (SEM) (JSM7001FD, JEOL, Kyoto, Japan). Microhardness was measured with the Vickers HMV-1 tester (Shimadzu Corporation, Kyoto, Japan), tested under 245.2 mN and 15 s. The microhardness of samples was measured in at least three positions.
3. Results and Discussion
3.1. Microstructure
Figure 2 shows the DTA curve of samples using a planetary ball mill after 8h-milled. It can be seen that, during the heating phase, a clear exothermic peak appeared at 580.1 °C, which is below the melting point of aluminum. This is the possible reaction temperature between TiO2 and Al powder; the energy then continued to climb as temperature increased up to 850 °C, beyond which no other energetic events were found. Experiments of Shi et al. indicated an exothermic peak at 891.3 °C when the mixture was not mechanically milled [15]. The results of this study show that the process of milling the sintered mixture is necessary to reduce the onset temperature to around the melting temperature of aluminum, which is also consistent with the study results of the previous reports [3,6].
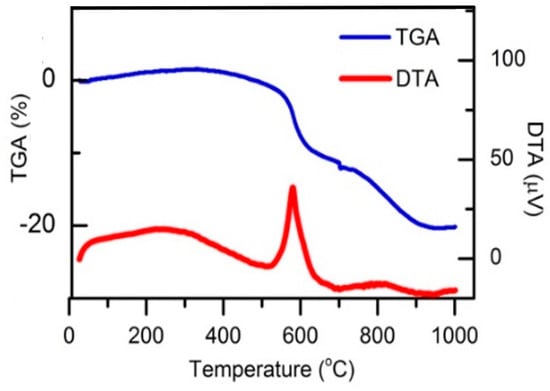
Figure 2.
Differential thermal analysis curve of samples using a planetary ball mill after 8h-milled.
Figure 3 exhibits the XRD patterns of the in situ composite samples sintered at 850 °C. As can be seen, titanium aluminide (Ti3Al) was formed in the reacted sample. The main peak of intermetallic phase Ti3Al was detected at 2θ angle of 39.92°; the remaining peaks mainly corresponded to Al2O3 detected in the reacted sample. On the basis of Figure 3, there is also a small amount of Ti2Al5 phase, but no peak indicates the presence of residual Ti, Al or TiO2. The above results suggest that the synthesis process occurred completely. The presence of these intermetallic phases in the mixed sample confirms the feasibility of the following in situ reactions:
4 Al + 3 TiO2 → 3 Ti + 2 Al2O3
Al + 3 Ti → Ti3Al
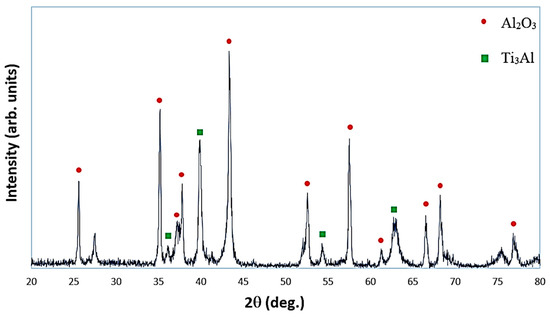
Figure 3.
X-ray diffraction patterns of reacted sample after sintering at 850 °C.
The microstructure of the obtained composite after 8 h of milling and sintering at 850 °C was studied. As shown in Figure 4, lighter particles are dispersed Al2O3 on the Ti3Al matrix as the darker region. Most Al2O3 (corundum) particles have a grain size of 0.2 ÷ 1.0 μm and a round and uniform shape in the composite, which increases the mechanical properties [16]. Figure 5a show the COMPO-SEM images of samples after being sintered at 850 °C. The contrasting dark and bright regions in COMPO-SEM images were related to the Al2O3 and Ti3Al matrix, respectively. Our results were consistent with recent reports on the microstructure of (γ + α2)-TiAl/Ti3Al/Al2O3 composites or TiAl3/Al2O3 composites [3,17]. The darker and lighter regions in contrast, in COMPO-SEM images, originated from the higher atomic number values of the Ti3Al phase than that of the Al2O3 phase [17]. The EDS mapping for Ti, Al and O elements for Ti3Al/Al2O3 samples are shown in Figure 5b–d, respectively. The results show that the Ti and Al elements were present everywhere in the samples but were inhomogeneously distributed in the samples as shown in Figure 5b,c, which is believed to relate to the contributions of Ti3Al or Al2O3 phases. The oxygen was found to contribute in all samples where the brighter image was related to Al2O3 regions and the darker image corresponded to Ti3Al regions [3,17]. The inhomogeneous distribution of Ti, Al and O in our samples further suggested that the Al2O3 phase was randomly distributed in the intermetallic Ti3Al matrix during the reaction of Al metal with oxide TiO2. This result agrees with the above XRD result that, when the milling time lasts up to 8 h, the reaction between oxide TiO2 and metallic Al occurred completely, which formed Ti3Al and Al2O3 phases under sintering processing.
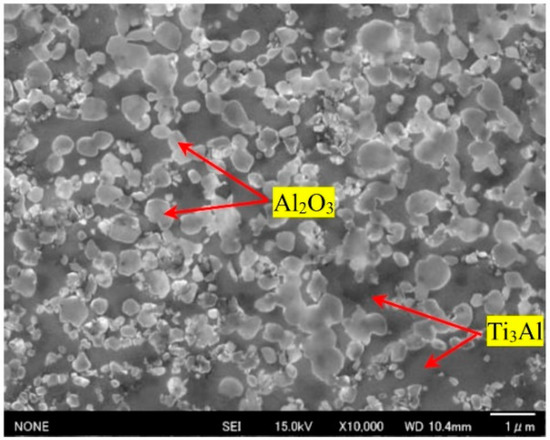
Figure 4.
SEM image of Al2O3/Ti3Al composite after 8 h of milling and sintering at 850 °C.
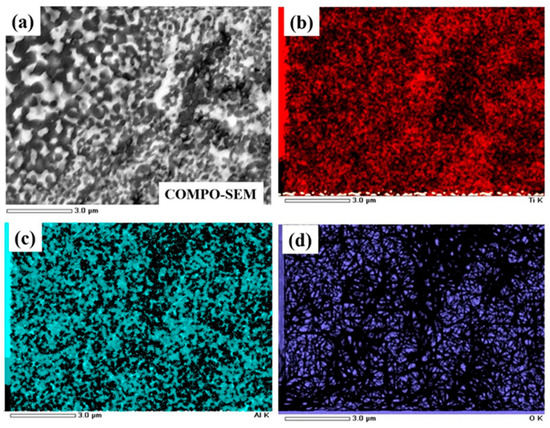
Figure 5.
(a) COMPO-SEM microphotograph of Al2O3/Ti3Al composite with EDS mapping photos of corresponding areas, (b) titanium (Ti), (c) aluminum (Al), (d) oxygen (O) of samples sintered at 850 °C.
3.2. Microhardness
Figure 6 shows that the microhardness of the sintered sample at 850 °C is considerably higher than that of the sample at 650 and 750 °C. This can result from the increased sintering temperature. Al2O3 particles formed by in situ synthesis have a finer grain size and are more uniformly dispersed into the Ti3Al matrix. In addition, a large number of dislocations were formed in the Ti3Al matrix, leading to the enhanced dispersion of the reinforcement particles, therefore increasing the microhardness of the material. The Vickers hardness value for the sample in this study reached 11.57 GPa at the condition of milling for 8 h. The microhardness of Ti3Al/Al2O3 composite samples at room temperature is compared with the results of TiAl3/Al2O3 composites in previously reported works [3,8].
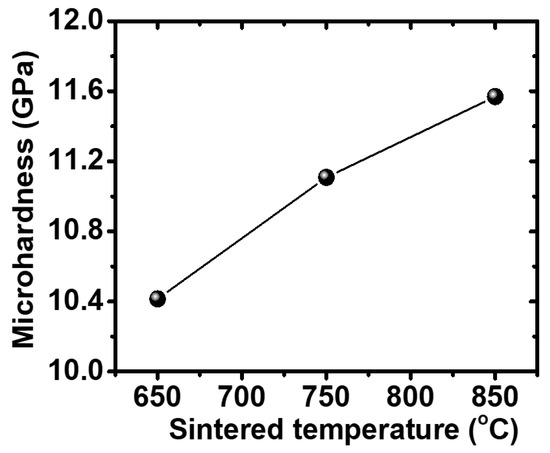
Figure 6.
Microhardness value of the Ti3Al/Al2O3 composite with selected sintering temperature.
Figure 7 shows that, as the temperature increased, the microhardness of the material decreased. This phenomenon occurs due to the fact that, at elevated temperatures, the Al-Ti intermetallic phase softened, leading to a reduction in interfacial adherence between the matrix and reinforcement particles. At the working temperature of the exhaust valve of a car engine (600–800 °C), the microhardness of the Ti3Al/Al2O3 material decreased from 4.18 to 3.15 GPa (around 35–45 in Rockwell C scale hardness (HRC) units). The observation of microhardness in our samples results at high temperature was comparable with the current used in martensitic heat-resistant steel (name JIS-SUH3, hardness of 30 HRC) and austenitic heat-resistant steel (name JIS-SUH35, hardness of 35 HRC) [18]. Therefore, we expected that our materials were valid replacements able to withstand more extreme conditions than the common materials being used. For a more general assessment of the microhardness of the Ti3Al/Al2O3 composite, the results of previous studies for the TiAl/Al2O3 composite, Ti metal and Ti-48Al alloy were compared [8]. The results indicate that, with the presence of the reinforcement particles and the intermetallic matrix Ti3Al, the hardness of the material is significantly improved even at elevated working temperatures. With the appearance of the reinforcement particles and titanium-rich intermetallic, the material produced in this study has the best mechanical properties. When the temperature is higher than 600 °C, we begin to see that the hardness of TiAl/Al2O3 composite decreases faster than that of Ti-48Al (a material that has been applied to the automobile engine exhaust valves of Mitsubishi cars) [19]. This illustrates that, at high temperature, the TiAl matrix has lower softening temperature than that of Ti3Al and Ti-48Al, causing interfacial adherence to decrease rapidly and mechanical properties at this time to depend greatly on the properties of the matrix material. Therefore, in this temperature range, the Ti-48Al alloy has better mechanical properties than the TiAl/Al2O3 composite. Meanwhile, the hardness of the TiAl3/Al2O3 in situ composite decreases as temperature increases.
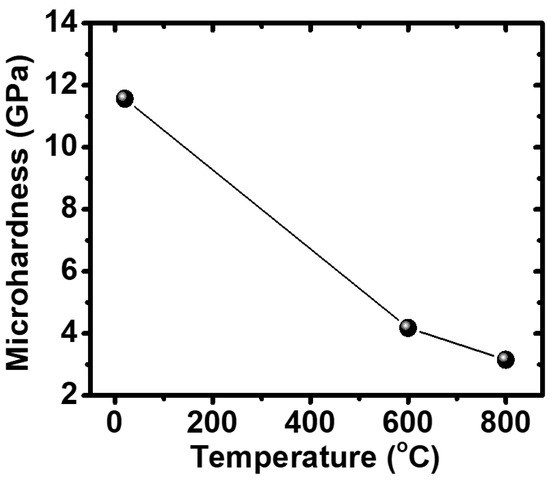
Figure 7.
Vickers hardness of the sample that was ball milled for 8 h and sintered at 850 °C at different temperatures.
4. Conclusions
In situ Al2O3-reinforced Ti3Al composite was successfully fabricated from aluminum and titanium dioxide powder after 8 h of grinding and sintering at 850 °C. The appearance of Al2O3 reinforcement particles increases the hardness of the Ti3Al matrix where the microhardness value is 4.20 GPa (430 Hv) at 600°C. Compared to other titanium-based materials, the Ti3Al/Al2O3 composite has the highest hardness. Under elevated working-temperature conditions, the hardness of the Ti3Al/Al2O3 composite material is superior. We expect that our observation of high microhardness at high temperatures of the Ti3Al/Al2O3 composite will be applied in car engine exhaust valves.
Author Contributions
Methodology, T.D.H.; Investigation, D.N.B.; Experimental verification, D.T.B. and T.V.T.; Data analysis, D.T.B.; Data curation, D.N.B. and H.M.; Visualization, H.M.; writing—original draft preparation, D.T.B.; writing—review and editing, T.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under Grant Number 103.02-2017.349.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are very grateful for support from the Vietnam National Foundation for Science and Technology Development (NAFOSTED), Hanoi University of Science and Technology, College of Mechanics and Metallurgy and Doshisha University from Japan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schuster, J.C.; Palm, M. Reassessment of the binary Aluminum-Titanium phase diagram. JPEDAV 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Milman, Y.V.; Miracle, D.B.; Chugunova, S.I.; Voskoboinik, I.V.; Korzhova, N.P.; Legkaya, T.N.; Poderezov, Y.N. Mechanical behaviour of Al3Ti intermetallic and L12 phases on its basis. Intermetallics 2001, 9, 839–845. [Google Scholar] [CrossRef]
- Huy, T.D.; Fujiwara, H.; Yoshida, R.; Binh, D.T.; Miyamoto, H. Microstructure and Mechanical Properties of TiAl3/Al2O3 in situ Composite by Combustion Process. Mater. Trans. 2014, 55, 1091–1093. [Google Scholar] [CrossRef]
- Cai, Z.H.; Zhang, D.L. Sintering behaviour and microstructures of Ti(Al,O)/Al2O3, Ti3Al(O)/Al2O3 and TiAl(O)/Al2O3 in situ composites. Mater. Sci. Eng. A 2006, 419, 310–317. [Google Scholar] [CrossRef]
- Skvortsov, A.A.; Pshonkin, D.E.; Lukyanov, M.N.; Rybakova, M.R. Influence of permanent magnetic fields on creep and microhardness of iron-containing aluminum alloy. J. Mater. Res. Technol. 2019, 8, 2481–2485. [Google Scholar] [CrossRef]
- Zuev, L.B.; Danilov, V.I.; Konovalov, S.V.; Filipev, R.A.; Gromov, V.E. Influence of contact potential difference and electric potential on the microhardness of metals. Phys. Solid State 2009, 51, 1137–1141. [Google Scholar] [CrossRef]
- Taotao, A. Microstructure and mechanical properties of in situ synthesized Al2O3/TiAl composites. Chin. J. Aeronaut. 2008, 21, 559–564. [Google Scholar] [CrossRef]
- Binh, D.T.; Huy, T.D.; Thuong, T.V.; Miyamoto, H. Microstructure and mechanical properties of in situ Al2O3 reinforced Ti-Al composite. J. Sci. Technol. Tech. Univ. 2016, 112, 76–79. [Google Scholar]
- Welham, N.J. Mechanical activation of the solid-state reaction between Al and TiO2. Mater. Sci. Eng. A 1998, 255, 81–89. [Google Scholar] [CrossRef]
- Ying, D.Y.; Zhang, D.L.; Newby, M. Solid-state reactions during heating mechanically milled Al/TiO2 composite powders. Metall. Mater. Trans. A 2004, 35, 2115–2125. [Google Scholar] [CrossRef]
- Alamolhoda, S.; Heshmati-Manesh, S. Role of intensive milling in mechano-thermal processing of TiAl/Al2O3 nano-composite. Adv. Powder Tech. 2012, 23, 343–348. [Google Scholar] [CrossRef]
- Horvitza, D.; Gotman, I.; Gutmanas, E.Y. In situ processing of dense Al2O3–TiAl interpenetrating phase composites. J. Eur. Ceram. Soc. 2002, 22, 947–954. [Google Scholar] [CrossRef]
- Travitzky, N.; Gotman, I.; Claussen, N. Alumina–Ti aluminide interpenetrating composites microstructure and mechanical properties. Mater. Lett. 2003, 57, 3422–3426. [Google Scholar] [CrossRef]
- Oke, S.R.; Falodun, O.E.; Motsa, B.G.; Ige, O.O.; Olubambi, P.A. Spark plasma sintering of Al-Ti-Al2O3 composite. Mater. Proc. 2019, 18, 3946–3951. [Google Scholar]
- Shi, S.; Sekino, T.; Cho, S.; Goto, T. Ti and TiC co-toughened Al2O3 composites by in situ synthesis from reaction of Ti and MWCNT. Mater. Sci. Eng. A 2020, 777, 139066. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, L.; Wang, Z.; Li, L.; Zhai, P.; Li, Q.; Wu, J. Effects of Nb doping on the mechanical properties and interfacial reactions of Ti/Al2O3 composites. Ceram. Int. 2018, 4, 14913–14919. [Google Scholar] [CrossRef]
- Li, H.Y.; Motamedi, P.; Hogan, J.D. Characterization and mechanical testing on novel (γ+α2)-TiAl/Ti3Al/Al2O3 cermet. Mater. Sci. Eng. A 2019, 750, 152–163. [Google Scholar] [CrossRef]
- Yamagata, H. (Ed.) The valve and valve seat. In The Science and Technology of Materials in Automotive Engines; Elsevier: Amsterdam, The Netherlands, 2005; pp. 132–151. [Google Scholar]
- Taguchi, K.; Ayada, M.; Ishihara, K.N.; Shingu, P.H. Densifing Process and Attempt to Produce Valve Spring Retainer of TiAl Intermetallic Compound by the Use of Pseudo HIP-SHS. J. Jpn. Soc. Powder Powder Metall. 1995, 42, 511–516. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).