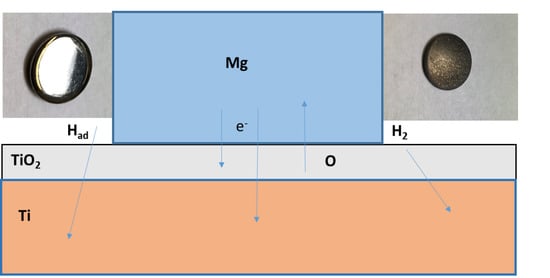
Degradation of Titanium Sintered with Magnesium: Effect of Hydrogen Uptake
Abstract
1. Introduction
2. Materials and Methods
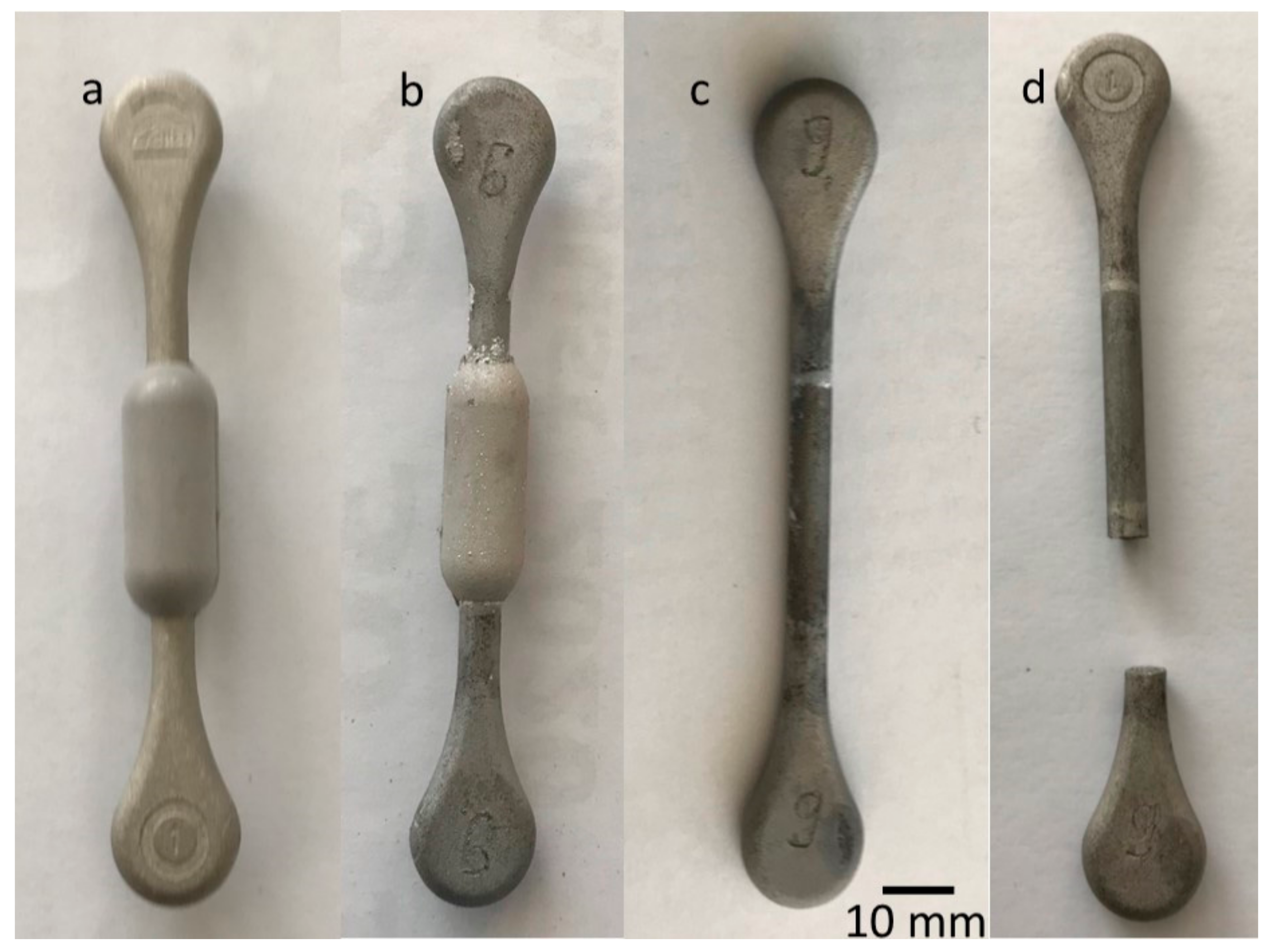
2.1. Preparation of Specimens
2.2. Characterisation
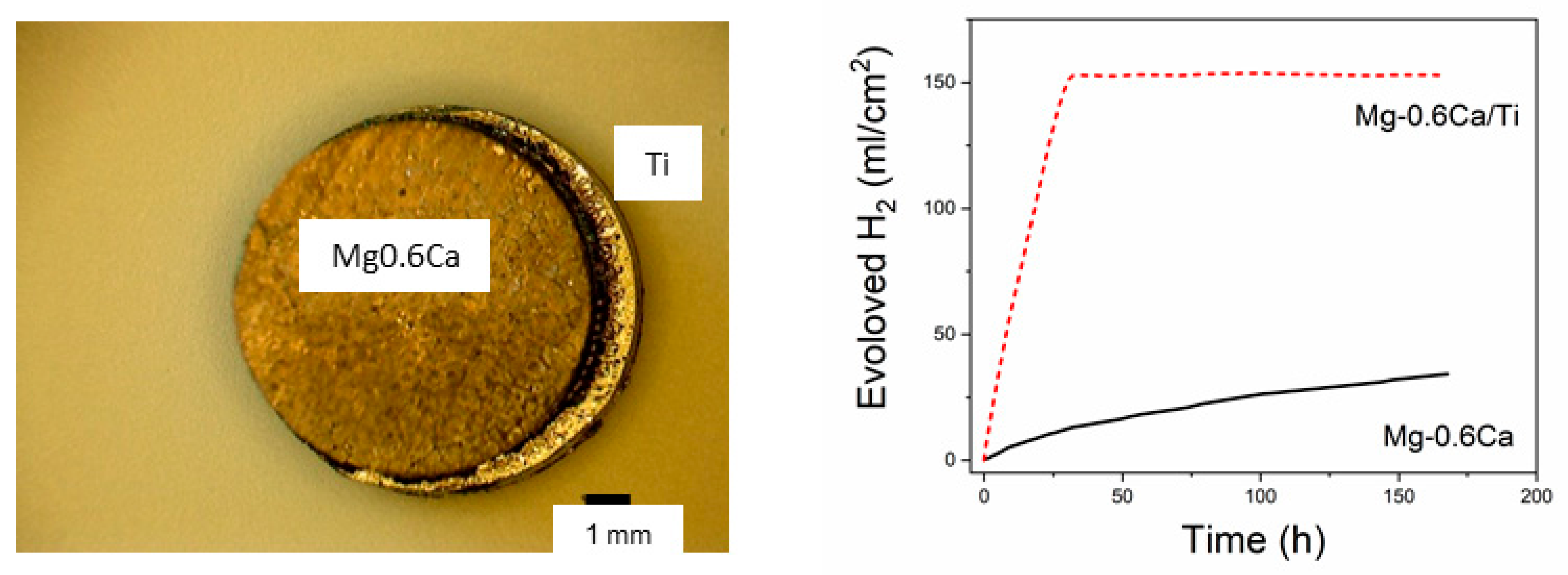
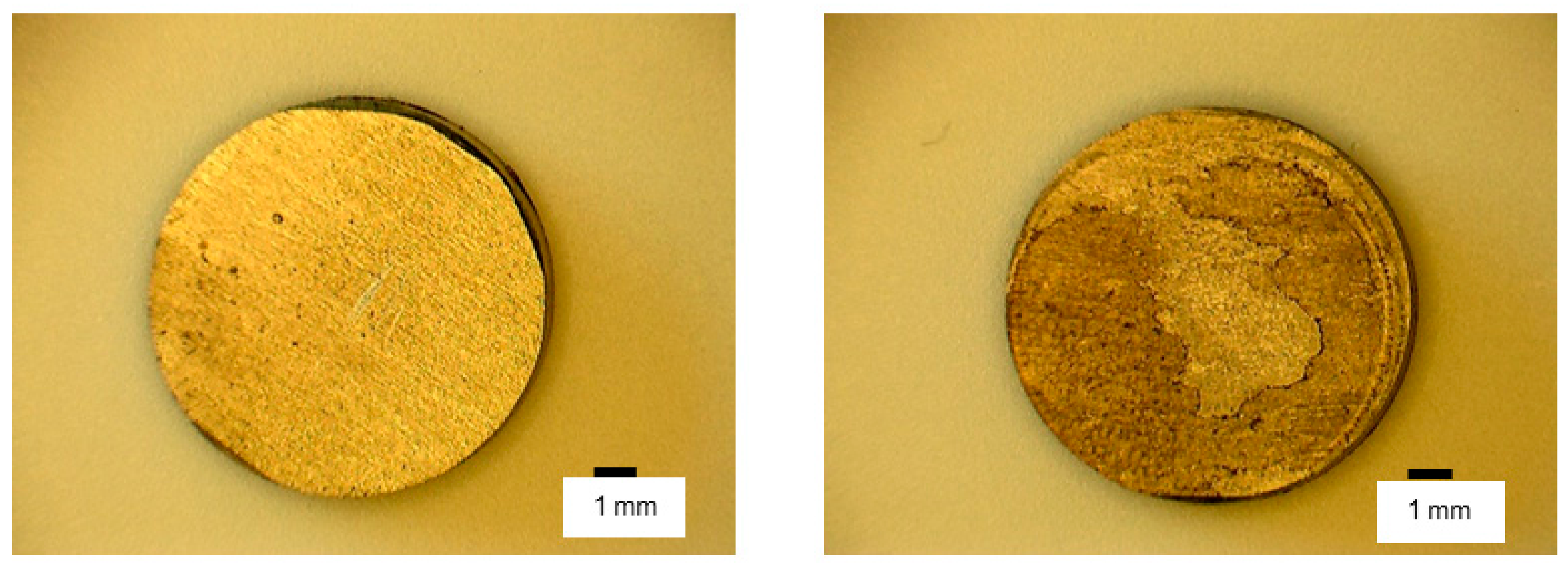
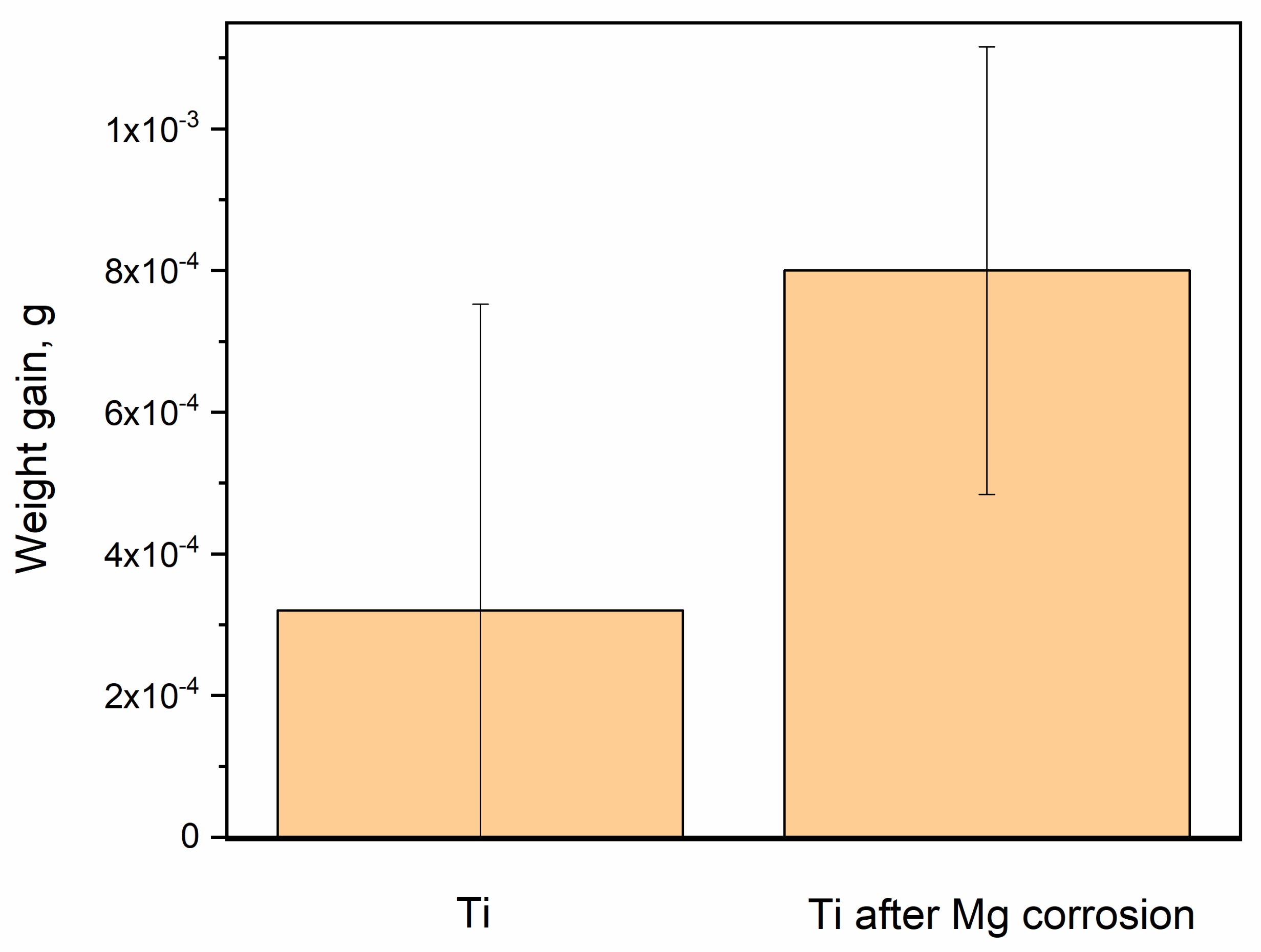
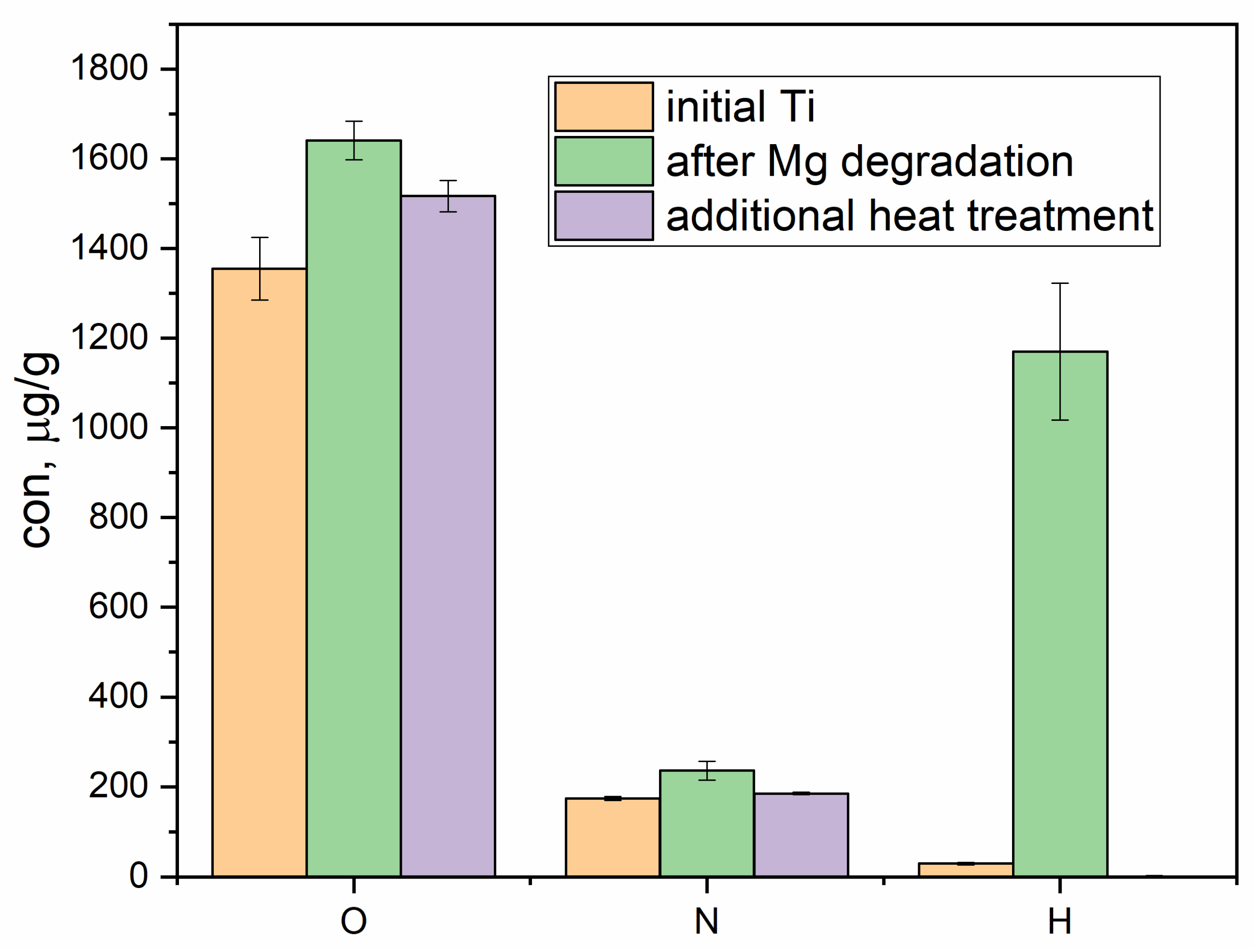
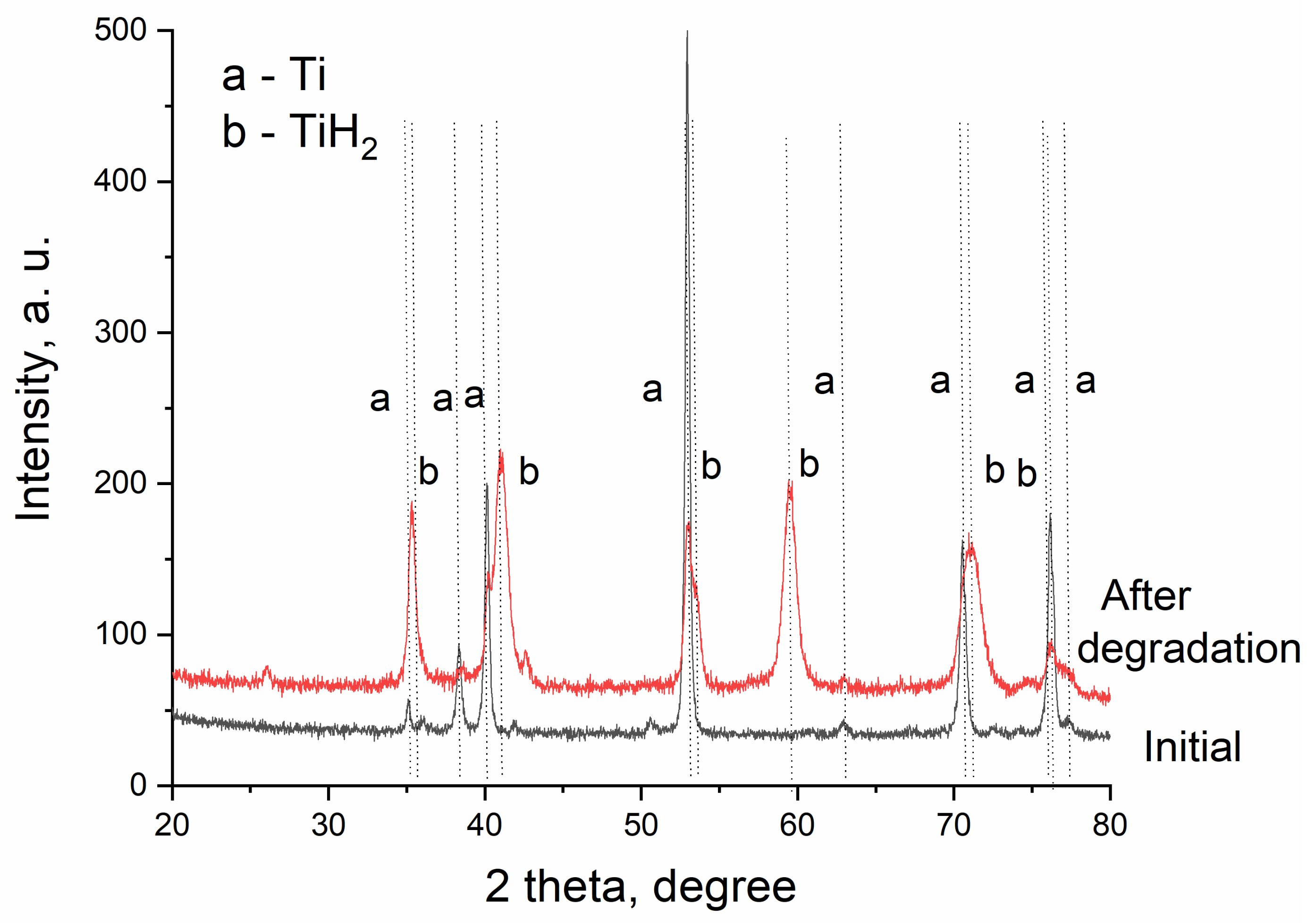
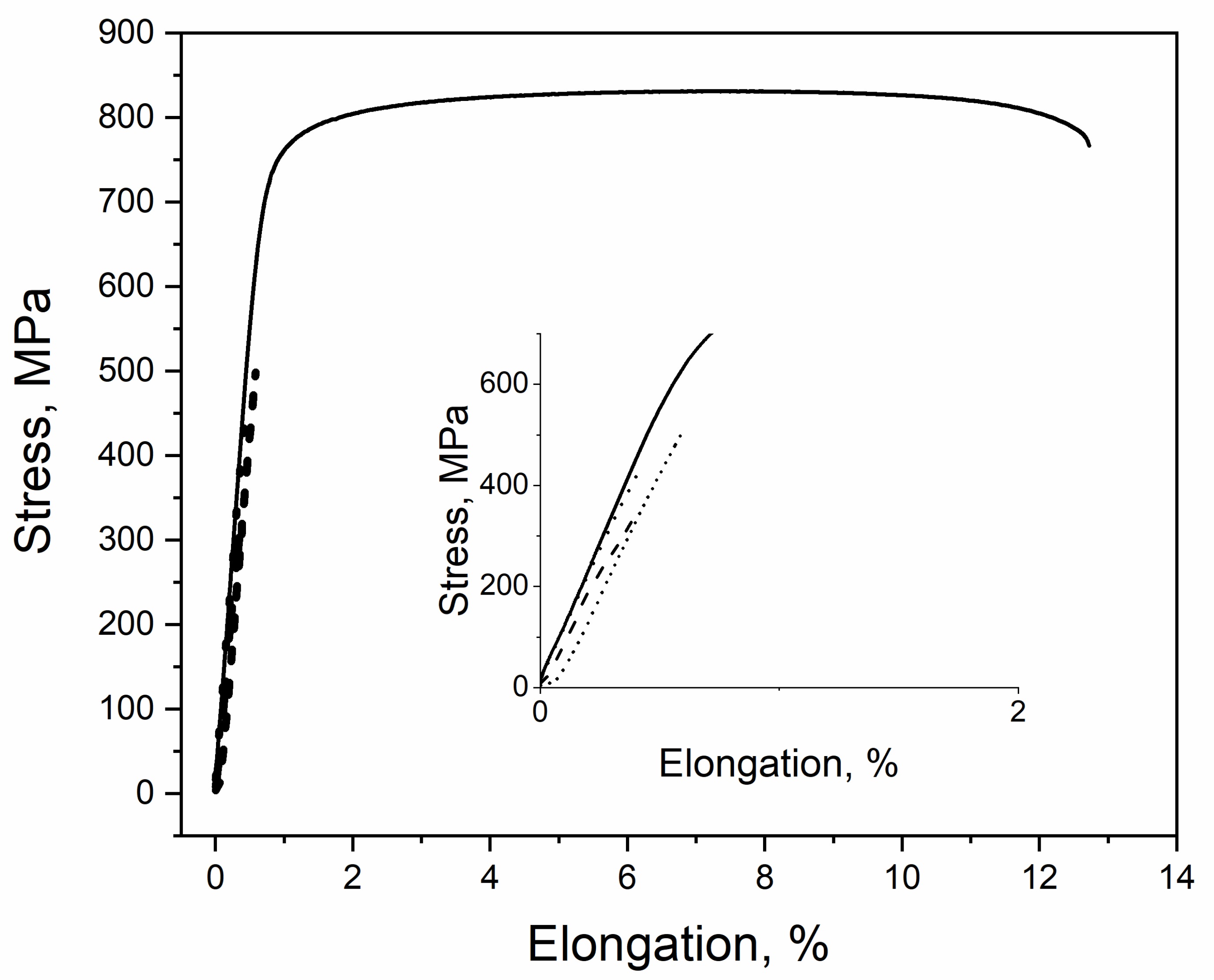
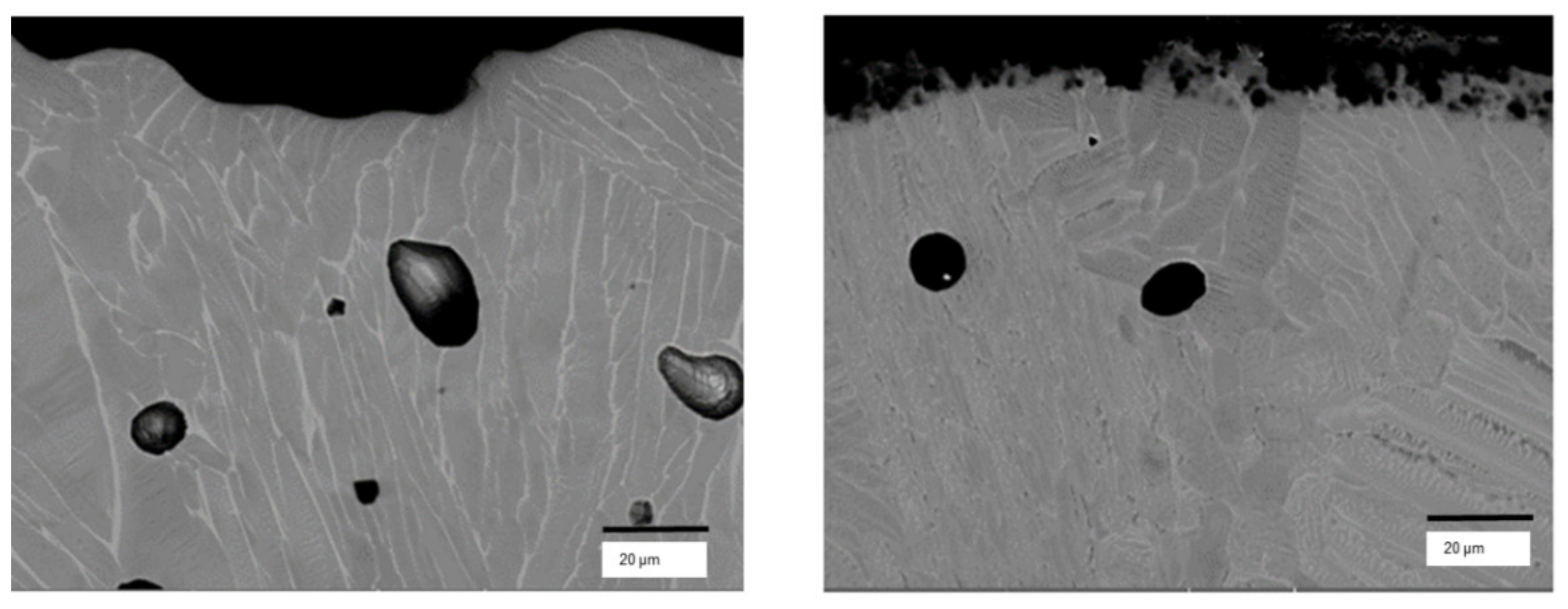
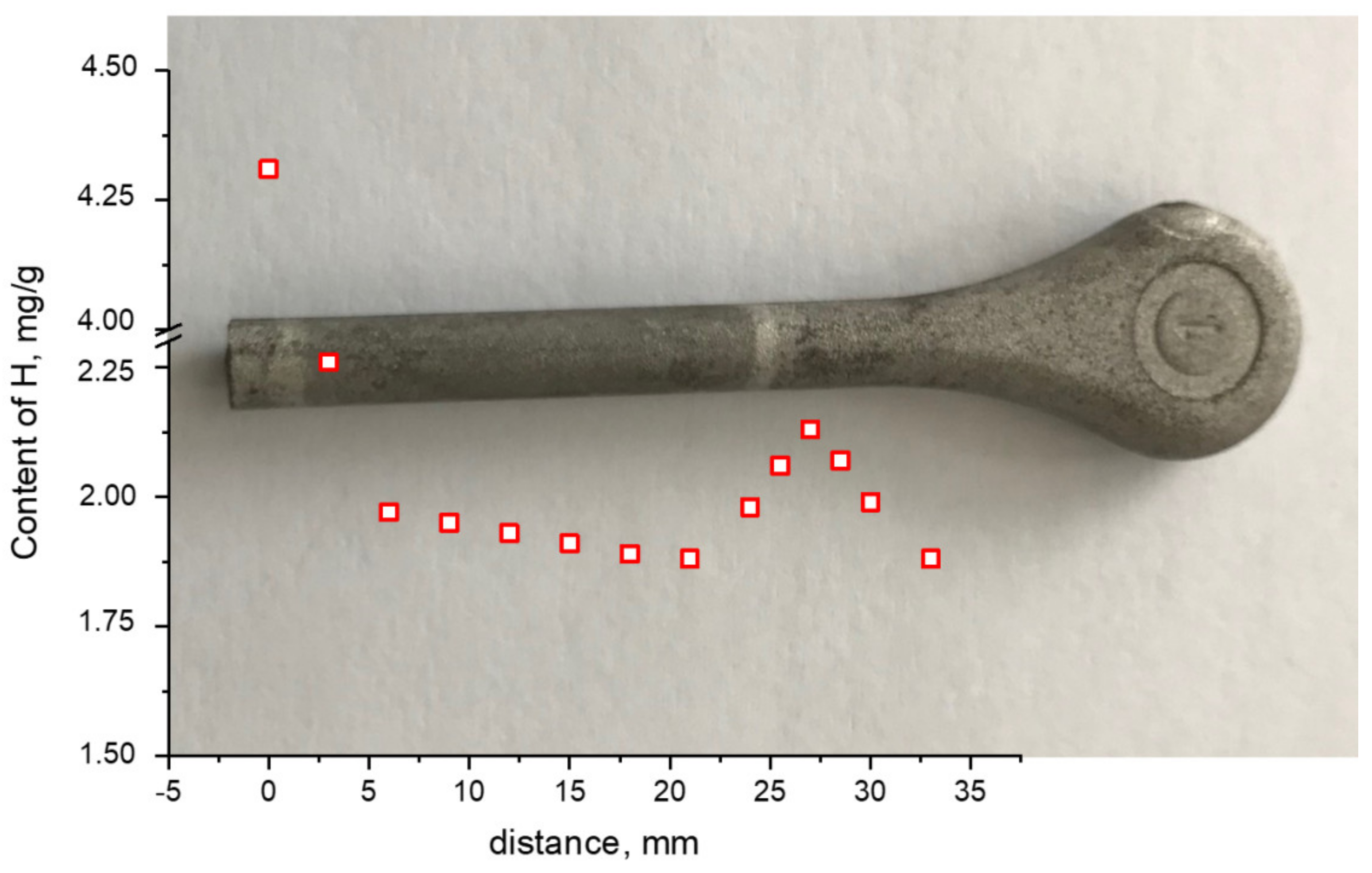
3. Results
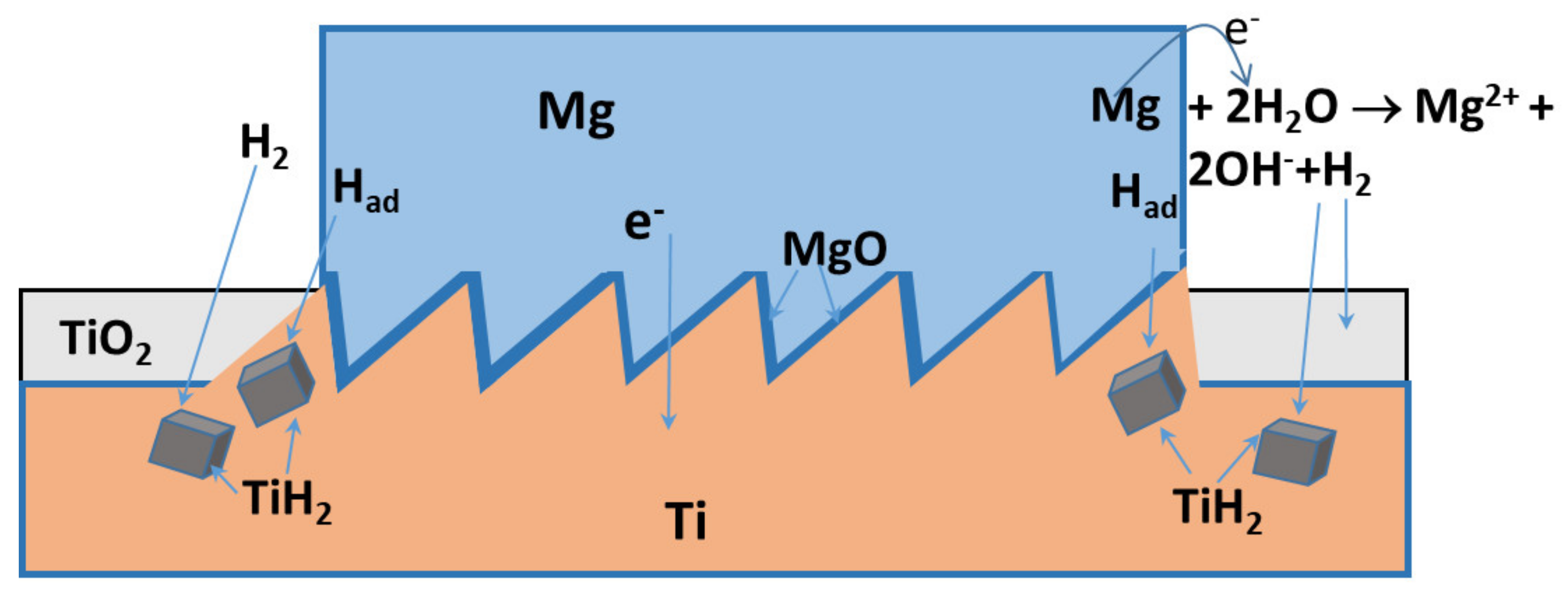
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Vandendolder, J.; Farber, E.; Spauwen, P.; Jansen, J. Bone tissue reconstruction using titanium fiber mesh combined with rat bone marrow stromal cells. Biomaterials 2003, 24, 1745–1750. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Sukotjo, C.; Lima-Neto, T.J.; Santiago Junior, J.F.; Faverani, L.P.; Miloro, M. Is There a Role for Absorbable Metals in Surgery? A Systematic Review and Meta-Analysis of Mg/Mg Alloy Based Implants. Materials 2020, 13, 3914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef]
- Zimmerli, W.; Moser, C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol. Med. Microbiol. 2012, 65, 158–168. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef]
- Gao, P.; Fan, B.; Yu, X.; Liu, W.; Wu, J.; Shi, L.; Yang, D.; Tan, L.; Wan, P.; Hao, Y.; et al. Biofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis in vitro and in vivo for orthopedic application. Bioact. Mater. 2020, 5, 680–693. [Google Scholar] [CrossRef]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Andersson, M.; Wennerberg, A.; Jimbo, R. Osteoconductive Potential of Mesoporous Titania Implant Surfaces Loaded with Magnesium: An Experimental Study in the Rabbit. Clin. Implant Dent. Relat. Res. 2015, 17, 1048–1059. [Google Scholar] [CrossRef]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Miyamoto, I.; Xue, Y.; Andersson, M.; Mustafa, K.; Wennerberg, A.; Jimbo, R. Local release of magnesium from mesoporous TiO2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014, 10, 5193–5201. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tang, N.; Ngai, T.; Wu, C.; Ruan, Y.; Huang, L.; Qin, L. Hybrid fracture fixation systems developed for orthopaedic applications: A general review. J. Orthop. Transl. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Sheng, Y.; Huang, L.; Chow, D.H.; Chau, W.H.; Tang, N.; Ngai, T.; Wu, C.; Lu, J.; Qin, L. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials 2018, 180, 173–183. [Google Scholar] [CrossRef]
- Murray, J.L. (Ed.) Mg-Ti (Magnesium-Titanium), 2nd ed.; ASM International: Novelty, OH, USA, 1990; Volume 3, pp. 2559–2560. [Google Scholar]
- Esen, Z.; Dikici, B.; Duygulu, O.; Dericioglu, A.F. Titanium–magnesium based composites: Mechanical properties and in-vitro corrosion response in Ringer’s solution. Mater. Sci. Eng. A 2013, 573, 119–126. [Google Scholar] [CrossRef]
- Hou, P.; Han, P.; Zhao, C.; Wu, H.; Ni, J.; Zhang, S.; Liu, J.; Zhang, Y.; Xu, H.; Cheng, P.; et al. Accelerating Corrosion of Pure Magnesium Co-implanted with Titanium in Vivo. Sci. Rep. 2017, 7, 41924. [Google Scholar] [CrossRef]
- Esen, Z.; Öcal, E.B.; Akkaya, A.; Gürçay, B.; Özcan, C.; Özgümüş, B.A.; Duygulu, Ö.; Dericioğlu, A.F. Corrosion behaviours of Ti6Al4V-Mg/Mg-Alloy composites. Corros. Sci. 2020, 166, 108470. [Google Scholar] [CrossRef]
- Sherif, E.M.; AlHazaa, A.N.; Abdo, H.S. Manufacturing of Mg-Ti Couples at Different Heat Treatment Temperatures and Their Corrosion Behavior in Chloride Solutions. Materials 2019, 12, 1300. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.F.; Tang, H.P.; Liu, C.T.; Liu, B.; Huang, B.Y. Design of powder metallurgy titanium alloys and composites. Mater. Sci. Eng. A 2006, 418, 25–35. [Google Scholar] [CrossRef]
- Ye, H.Z.; Liu, X.Y.; Hong, H.P. Fabrication of metal matrix composites by metal injection molding—A review. J. Mater. Process. Technol. 2008, 200, 12–24. [Google Scholar] [CrossRef]
- Wolff, M.; Ebel, T.; Dahms, M. Sintering of Magnesium. Adv. Eng. Mater. 2010, 12, 829–836. [Google Scholar] [CrossRef]
- Wolff, M.; Schaper, J.; Suckert, M.; Dahms, M.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. Metal Injection Molding (MIM) of Magnesium and Its Alloys. Metals 2016, 6, 118. [Google Scholar] [CrossRef]
- Haramus, V.; Ebel, T.; Ramakrishnegowda, N.; Bußacker, S.; Schaper, J. Method for Producing a Metallic Implant. EP3524280A1, 8 January 2020. Available online: https://patents.google.com/patent/EP3524280A1/en (accessed on 26 February 2021).
- Su, Y.; Wang, L.; Luo, L.; Liu, X.; Guo, J.; Fu, H. Investigation of melt hydrogenation on the microstructure and deformation behavior of Ti–6Al–4V alloy. Int. J. Hydrogen Energy 2011, 36, 1027–1036. [Google Scholar] [CrossRef]
- Shen, C.-C.; Wang, C.-M. Effects of hydrogen loading and type of titanium hydride on grain refinement and mechanical properties of Ti–6Al–4V. J. Alloys Compd. 2014, 601, 274–279. [Google Scholar] [CrossRef]
- Liang, C.H.; Jia, L.N.; Yuan, C.J.; Huang, N.B. Crevice Corrosion Behavior of CP Ti, Ti-6Al-4V Alloy and Ti-Ni Shape Memory Alloy in Artificial Body Fluids. Rare Met. Mater. Eng. 2015, 44, 781–785. [Google Scholar]
- Sun, P.; Fang, Z.Z.; Koopman, M.; Paramore, J.; Chandran, K.S.R.; Ren, Y.; Lu, J. An experimental study of the (Ti–6Al–4V)–xH phase diagram using in situ synchrotron XRD and TGA/DSC techniques. Acta Mater. 2015, 84, 29–41. [Google Scholar] [CrossRef]
- Lenning, G.A.; Craighead, C.M.; Jaffee, R.I. Constitution and mechanical properties of titanium-hydrogen alloys. JOM 1954, 6, 367–376. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Titanium and Titanium Alloy Bars and Billets; ASTM: West Consensehocken, PA, USA, 2009. [Google Scholar]
- Liu, H.J.; Zhou, L.; Liu, P.; Liu, Q.W. Microstructural evolution and hydride precipitation mechanism in hydrogenated Ti–6Al–4V alloy. Int. J. Hydrogen Energy 2009, 34, 9596–9602. [Google Scholar] [CrossRef]
- Luppo, M.I.; Politi, A.; Vigna, G. Hydrides in α-Ti: Characterization and effect of applied external stresses. Acta Mater. 2005, 53, 4987–4996. [Google Scholar] [CrossRef]
- Manchester, F.D.; San-Martinm, A. H–Ti (hydrogen–titanium). In Phase Diagrams of Binary Hydrogen Alloys; ASM International: Materials Park, OH, USA, 2000; p. 238. [Google Scholar]
- Fukai, Y. Phase-Diagrams of transition metal-hydrogen systems. J. Jpn. Inst. Met. 1991, 55, 17–21. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; He, H.; Tang, Y.; Ji, X.; Wang, H. Advanced Materials Prepared via Metallic Reduction Reactions for Electrochemical Energy Storage. Small Methods 2020, 4, 2000613. [Google Scholar] [CrossRef]
- Hoche, D.; Blawert, C.; Lamaka, S.V.; Scharnagl, N.; Mendis, C.; Zheludkevich, M.L. The effect of iron re-deposition on the corrosion of impurity-containing magnesium. Phys. Chem. Chem. Phys. 2016, 18, 1279–1291. [Google Scholar] [CrossRef]
- Wurger, T.; Feiler, C.; Vonbun-Feldbauer, G.B.; Zheludkevich, M.L.; Meissner, R.H. A first-principles analysis of the charge transfer in magnesium corrosion. Sci. Rep. 2020, 10, 15006. [Google Scholar] [CrossRef] [PubMed]
- Zu, D.; Xu, Z.; Zhang, A.; Wang, H.; Wei, H.; Ou, G.; Huang, K.; Zhang, R.; Li, L.; Hu, S.; et al. Room temperature Mg reduction of TiO2: Formation mechanism and application in photocatalysis. Chem. Commun. 2019, 55, 7675–7678. [Google Scholar] [CrossRef]
- Peng, P.-W.; Ou, K.-L.; Lin, H.-C.; Pan, Y.-N.; Wang, C.-H. Effect of electrical-discharging on formation of nanoporous biocompatible layer on titanium. J. Alloys Compd. 2010, 492, 625–630. [Google Scholar] [CrossRef]
- Ban, S.; Iwaya, Y.; Kono, H.; Sato, H. Surface modification of titanium by etching in concentrated sulfuric acid. Dent. Mater. 2006, 22, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Yu, X.; Tan, L.; Yang, K. Influence of Strontium phosphate Coating on the Degradation of Physical Vapor Deposition Sprayed Mg Coating on Ti6Al4V Substrate to Promote Bone Tissue Healing. Front. Mater. 2020, 7, 3240. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garamus, V.M.; Limberg, W.; Serdechnova, M.; Mei, D.; Lamaka, S.V.; Ebel, T.; Willumeit-Römer, R. Degradation of Titanium Sintered with Magnesium: Effect of Hydrogen Uptake. Metals 2021, 11, 527. https://doi.org/10.3390/met11040527
Garamus VM, Limberg W, Serdechnova M, Mei D, Lamaka SV, Ebel T, Willumeit-Römer R. Degradation of Titanium Sintered with Magnesium: Effect of Hydrogen Uptake. Metals. 2021; 11(4):527. https://doi.org/10.3390/met11040527
Chicago/Turabian StyleGaramus, Vasil M., Wolfgang Limberg, Maria Serdechnova, Di Mei, Sviatlana V. Lamaka, Thomas Ebel, and Regine Willumeit-Römer. 2021. "Degradation of Titanium Sintered with Magnesium: Effect of Hydrogen Uptake" Metals 11, no. 4: 527. https://doi.org/10.3390/met11040527
APA StyleGaramus, V. M., Limberg, W., Serdechnova, M., Mei, D., Lamaka, S. V., Ebel, T., & Willumeit-Römer, R. (2021). Degradation of Titanium Sintered with Magnesium: Effect of Hydrogen Uptake. Metals, 11(4), 527. https://doi.org/10.3390/met11040527