Preparation of W-Plated Diamond and Improvement of Thermal Conductivity of Diamond-WC-Cu Composite
Abstract
1. Introduction
2. Experimental Procedures and Characterization
2.1. Raw Materials
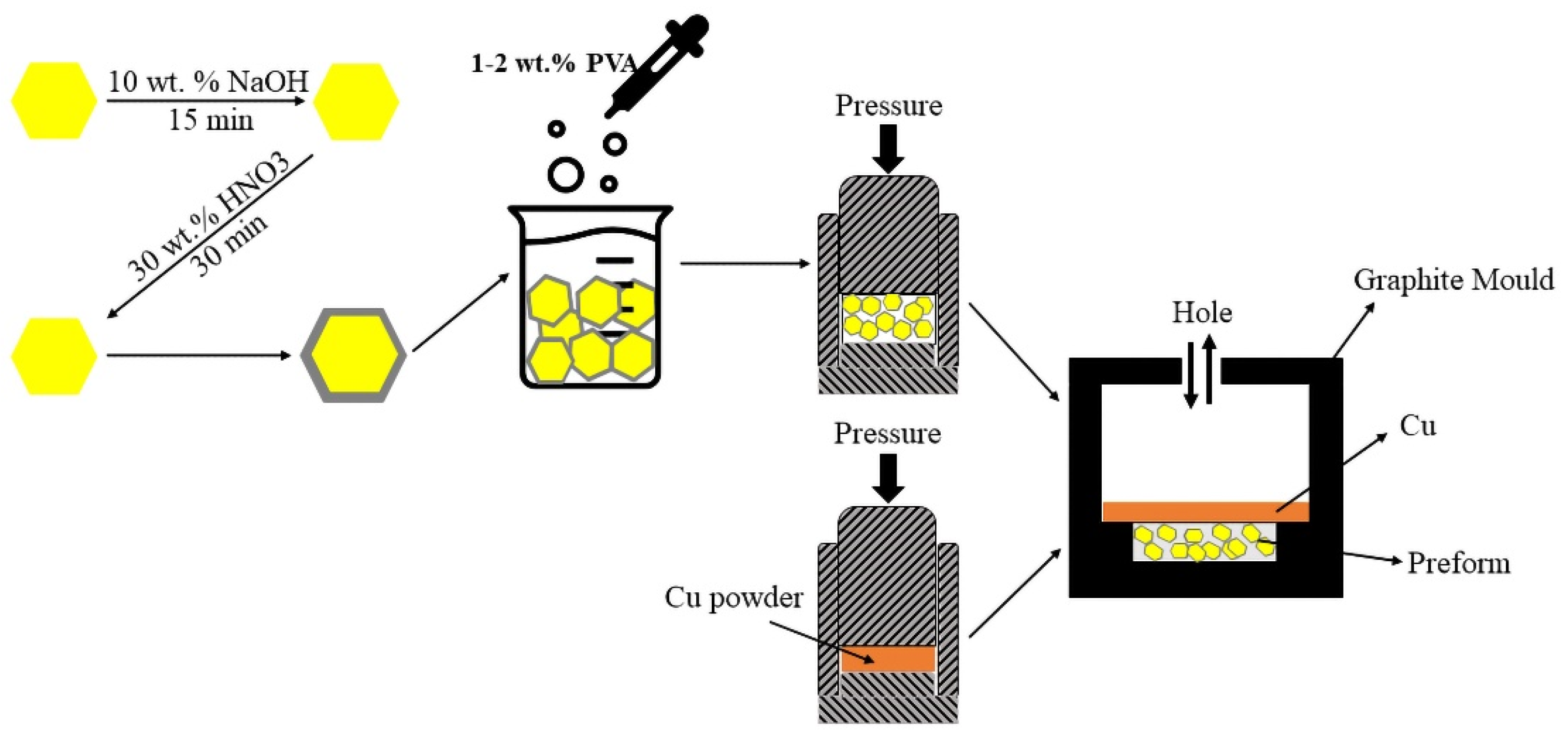
2.2. Composite Material Preparation
2.3. Characterization
3. Results and Discussion
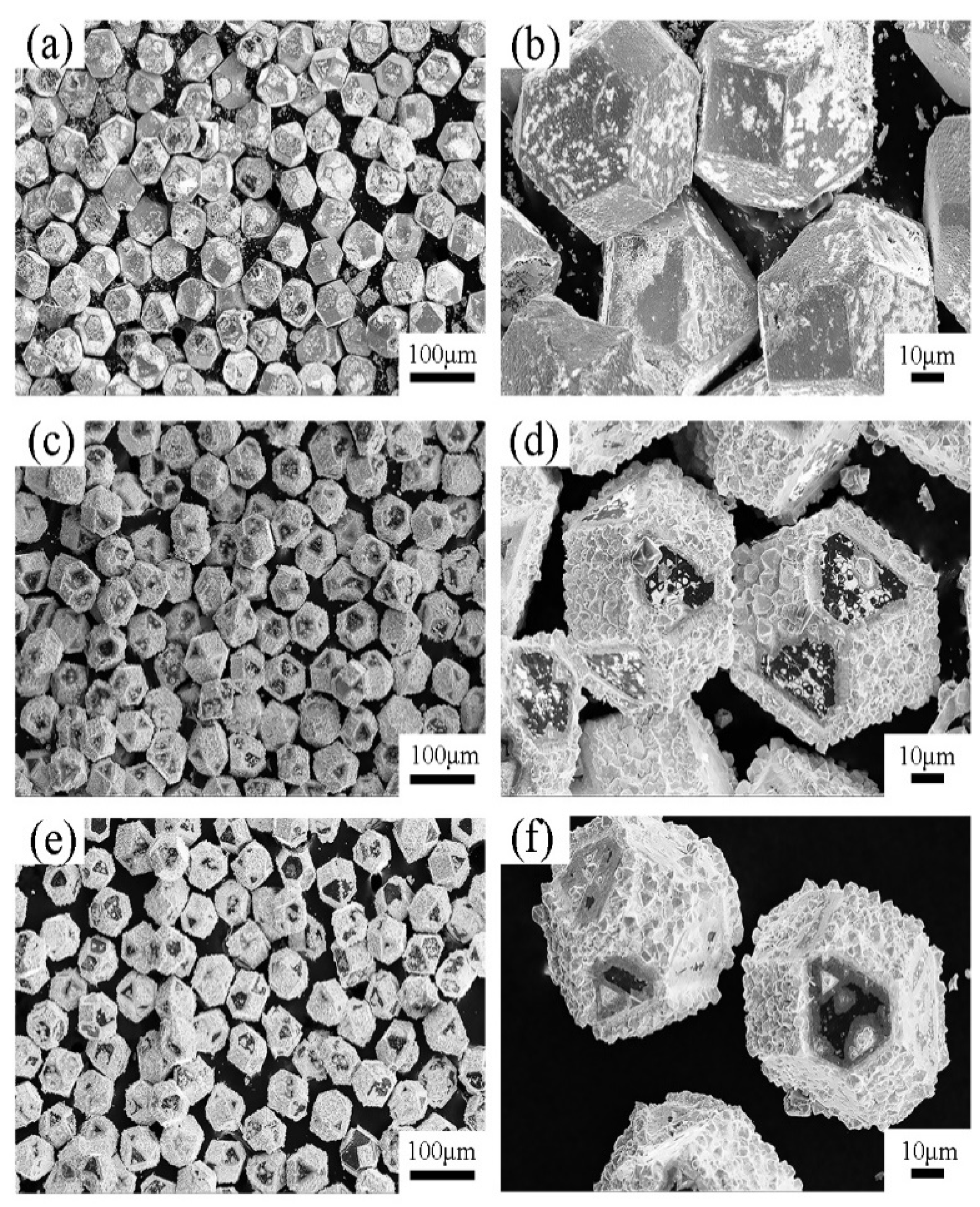
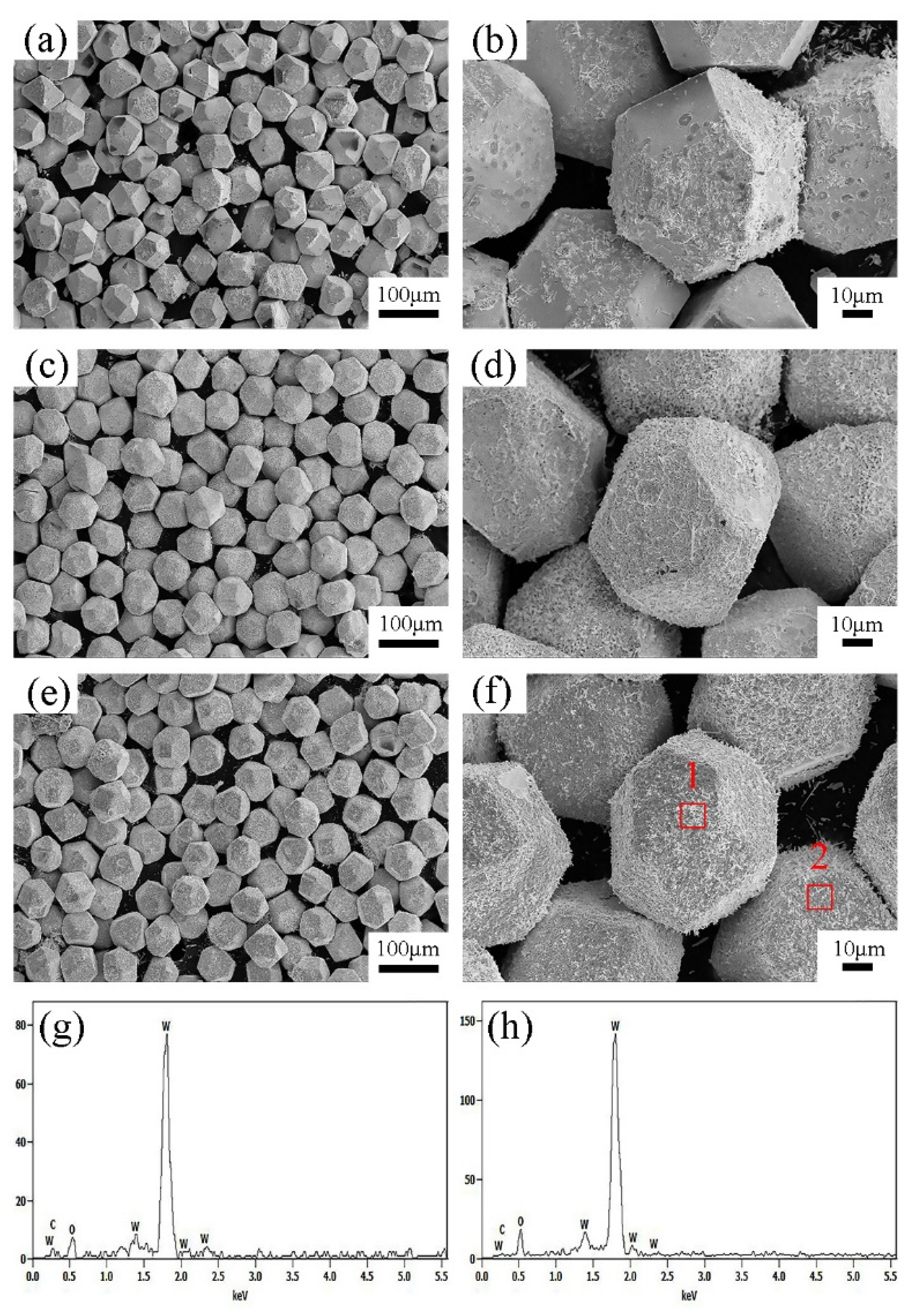
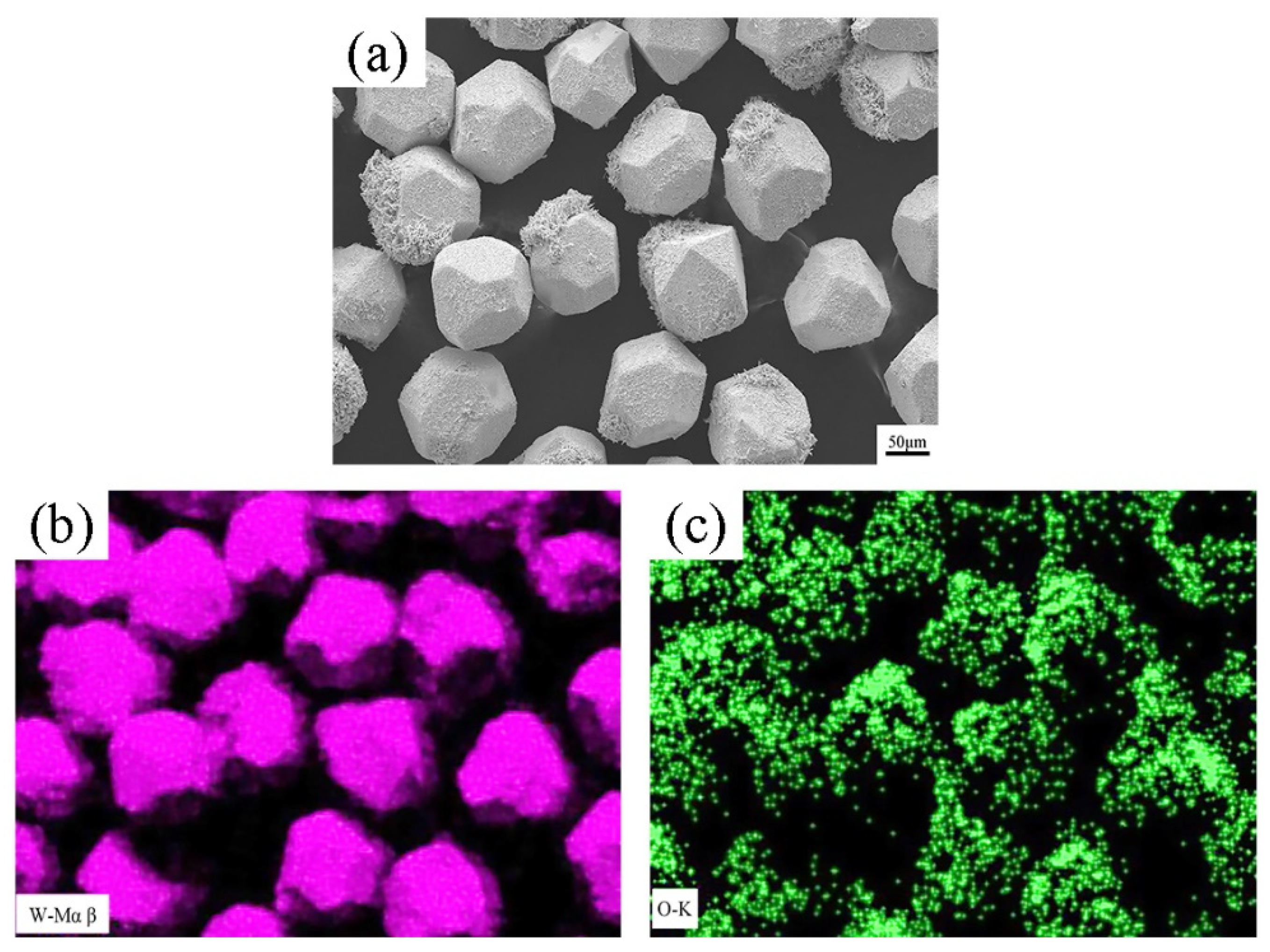
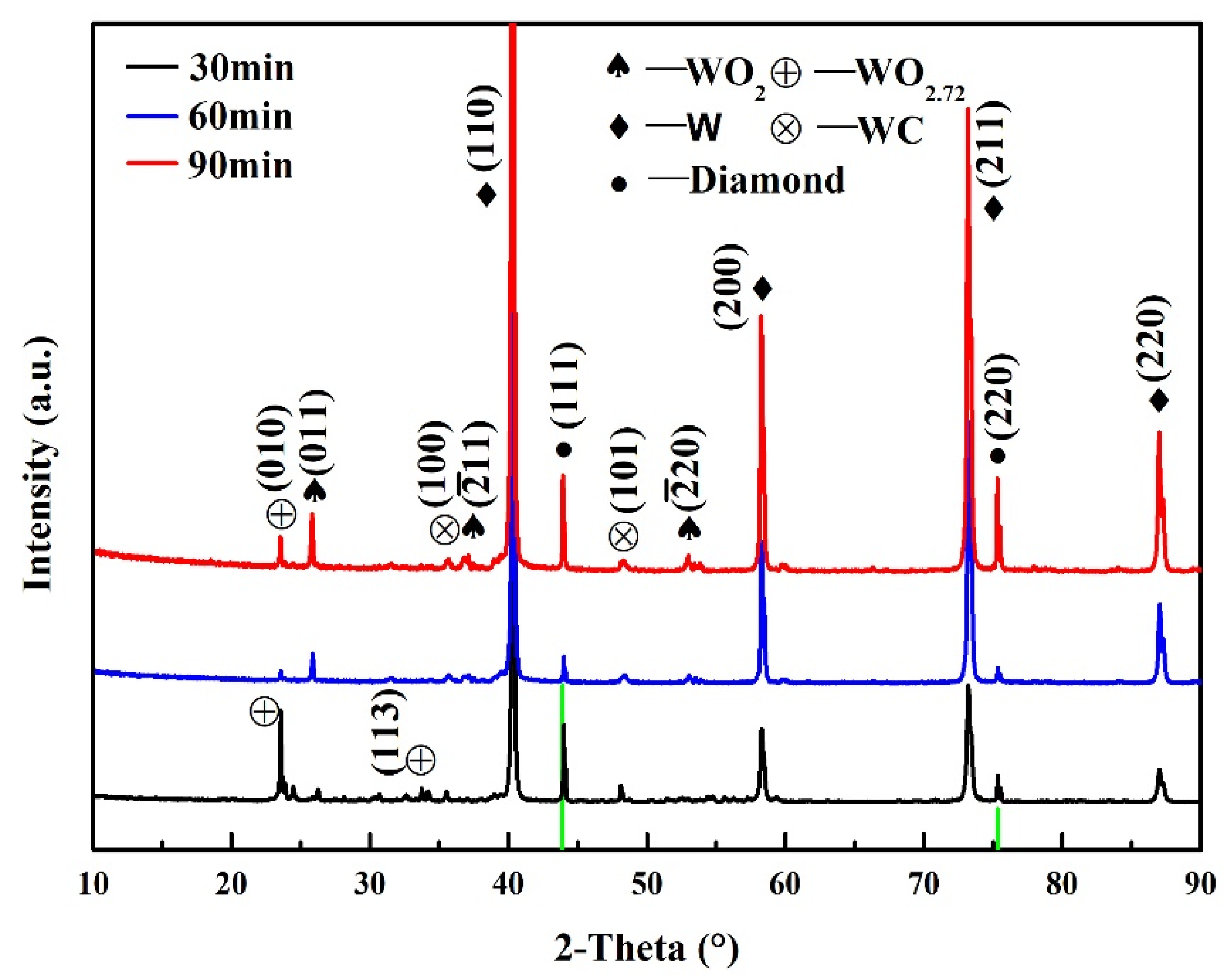
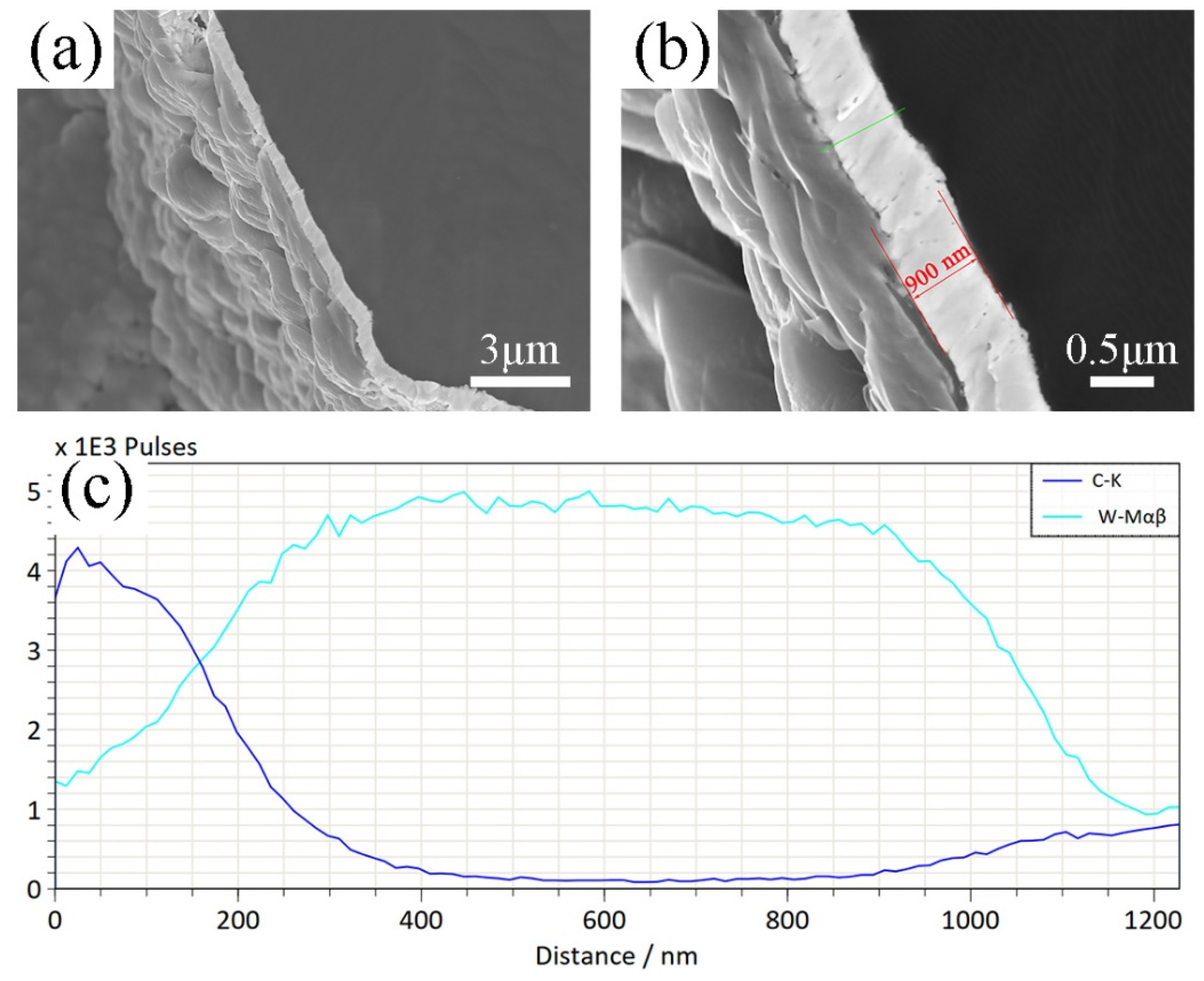
3.1. Microstructure and Composition of W-Plated Diamond Particles
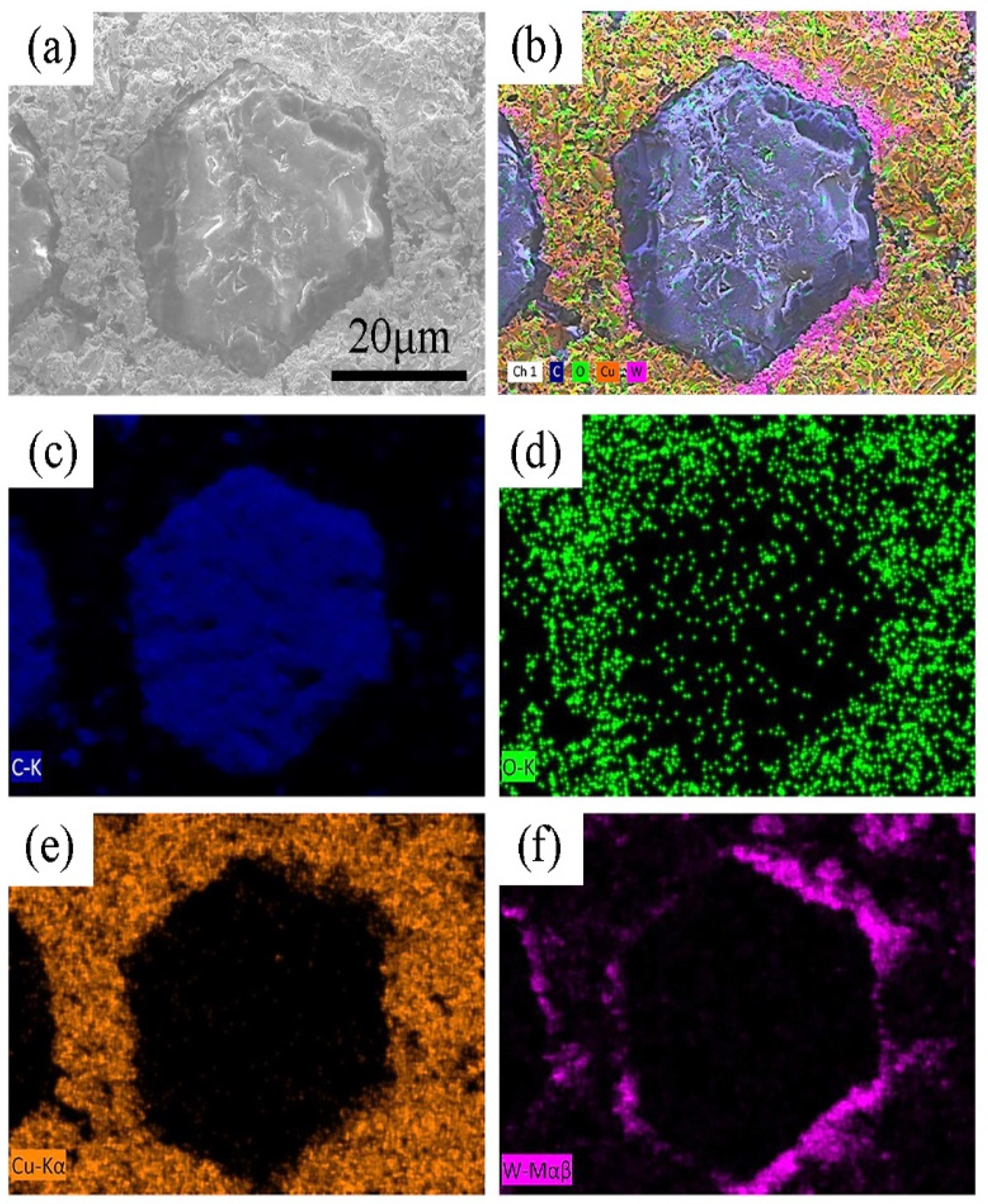
3.2. Micro-Characterization of Diamond-WC-Cu Composites
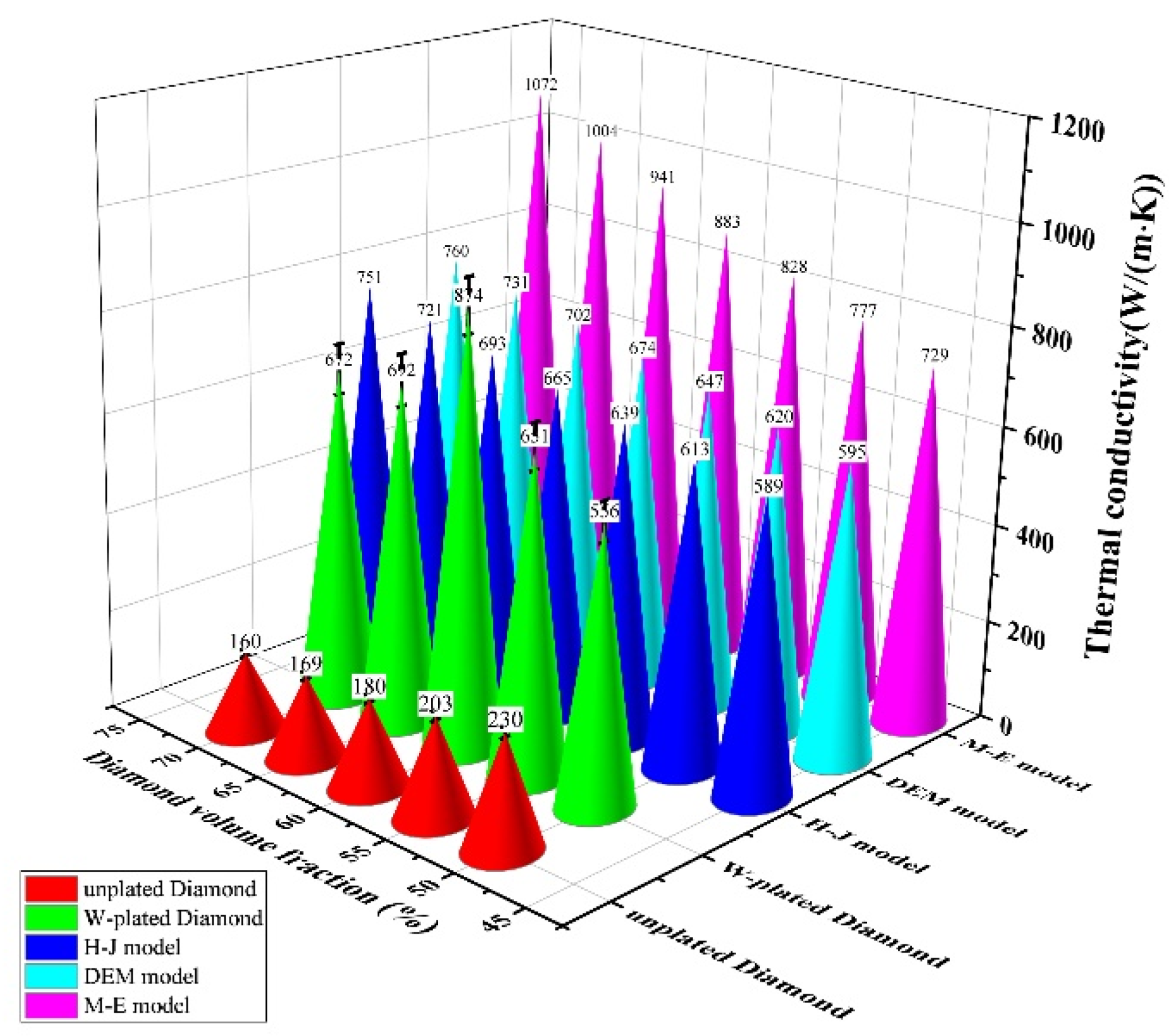
3.3. Thermal Conductivity of Diamond-WC-Cu Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Xue, Y.; Li, X.W.; Wen, Y.F.; Liu, J.W.; Xue, Z.G.; Shi, D.A.; Zhou, X.P.; Xie, X.L.; Mai, Y.W. High-performance epoxy/binary spherical alumina composite as underfill material for electronic packaging. Compos. Part A Appl. Sci. Manuf. 2019, 118, 67–74. [Google Scholar] [CrossRef]
- Gao, S.; Nan, Z.; Li, Y.; Zhao, N.; Liu, Q.; Xu, G.; Cheng, X.; Yang, J. Copper matrix thermal conductive composites with low thermal expansion for electronic packaging. Ceram. Int. 2020, 46, 18019–18025. [Google Scholar] [CrossRef]
- Khan, J.; Momin, S.A.; Mariatti, M. A review on advanced carbon-based thermal interface materials for electronic devices. Carbon 2020, 168, 65–112. [Google Scholar] [CrossRef]
- Wan, Y.J.; Li, G.; Yao, Y.M.; Zeng, X.L.; Zhu, P.L.; Sun, R. Recent advances in polymer-based electronic packaging materials. Compos. Commun. 2020, 19, 154–167. [Google Scholar] [CrossRef]
- Dong, L.L.; Ahangarkani, M.; Chen, W.G.; Zhang, Y.S. Recent progress in development of tungsten-copper composites: Fabrication, modification and applications. Int. J. Refract. Met. Hard Mater. 2018, 75, 30–42. [Google Scholar] [CrossRef]
- Niu, Y.; Lu, D.; Huang, L.; Zhao, J.; Zheng, X.; Chen, G. Comparison of W–Cu composite coatings fabricated by atmospheric and vacuum plasma spray processes. Vacuum 2015, 117, 98–103. [Google Scholar] [CrossRef]
- Qu, X.H.; Zhang, L.; Wu, M.; Ren, S.B. Review of metal matrix composites with high thermal conductivity for thermal management applications. Prog. Nat. Sci. Mater. Int. 2011, 21, 189–197. [Google Scholar] [CrossRef]
- Wu, D.; Yang, L.; Shi, C.D.; Wu, Y.C.; Tang, W.M. Effects of rolling and annealing on microstructures and properties of Cu/Invar electronic packaging composites prepared by powder metallurgy. Trans. Nonferrous Met. Soc. China 2015, 25, 1995–2002. [Google Scholar] [CrossRef]
- Lei, L.; Su, Y.; Bolzoni, L.; Yang, F. Evaluation on the interface characteristics, thermal conductivity, and annealing effect of a hot-forged Cu-Ti/diamond composite. J. Mater. Sci. Technol. 2020, 49, 7–14. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, H.; Zhang, X.; Huang, S. Enhancing thermal conductivity of Diamond/Cu composites by regulating distribution of bimodal diamond particles. Diam. Relat. Mater. 2019, 100, 107564. [Google Scholar] [CrossRef]
- Sui, R.; Lin, Q.; Wang, L.; Yang, F. Wetting of molten cerium on typical carbon materials (graphite, CVD-diamond and NWCNT) and molten Cu–Ce alloys on graphite at 950 °C. J. Rare Earths 2020, 331–339. [Google Scholar] [CrossRef]
- Yang, L.; Sun, L.; Bai, W.; Li, L. Thermal conductivity of Cu-Ti/diamond composites via spark plasma sintering. Diam. Relat. Mater. 2019, 94, 37–42. [Google Scholar] [CrossRef]
- Jia, S.Q.; Bolzoni, L.; Li, T.; Yang, F. Unveiling the interface characteristics and their influence on the heat transfer behavior of hot-forged Cu–Cr/Diamond composites. Carbon 2021, 172, 390–401. [Google Scholar] [CrossRef]
- Bai, G.; Wang, L.; Zhang, Y.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. Tailoring interface structure and enhancing thermal conductivity of Cu/diamond composites by alloying boron to the Cu matrix. Mater. Charact. 2019, 152, 265–275. [Google Scholar] [CrossRef]
- Bai, G.; Zhang, Y.; Dai, J.; Wang, L.; Wang, X.; Wang, J.; Kim, M.J.; Chen, X.; Zhang, H. Tunable coefficient of thermal expansion of Cu-B/diamond composites prepared by gas pressure infiltration. J. Alloys Compd. 2019, 794, 473–481. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Zhang, Y.; Zhao, Y.; Zhang, H. Thermal conductivity of Cu–Zr/diamond composites produced by high temperature–high pressure method. Compos. Part B Eng. 2015, 68, 22–26. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Bai, G.; Li, N.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Interfacial structure evolution and thermal conductivity of Cu-Zr/diamond composites prepared by gas pressure infiltration. J. Alloys Compd. 2019, 781, 800–809. [Google Scholar] [CrossRef]
- Lei, L.; Bolzoni, L.; Yang, F. High thermal conductivity and strong interface bonding of a hot-forged Cu/Ti-coated-diamond composite. Carbon 2020, 168, 553–563. [Google Scholar] [CrossRef]
- Sun, J.; Zang, J.; Li, H.; Feng, X.; Shen, Y. Influence of diamond content and milling duration on microstructure and thermal conductivity of Ti-coated diamond/copper composite coating on copper substrate. Mater. Chem. Phys. 2021, 259, 124017. [Google Scholar] [CrossRef]
- Chu, K.; Liu, Z.; Jia, C.; Chen, H.; Liang, X.; Gao, W.; Tian, W.; Guo, H. Thermal conductivity of SPS consolidated Cu/diamond composites with Cr-coated diamond particles. J. Alloys Compd. 2010, 490, 453–458. [Google Scholar] [CrossRef]
- Ma, S.D.; Zhao, N.Q.; Shi, C.S.; Liu, E.Z.; He, C.N.A.; He, F.; Ma, L.Y. Mo2C coating on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites. Appl. Surf. Sci. 2017, 402, 372–383. [Google Scholar] [CrossRef]
- Jia, J.; Bai, S.; Xiong, D.; Xiao, J.; Yan, T. Enhanced thermal conductivity in diamond/copper composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. Mater. Chem. Phys. 2020, 252, 123422. [Google Scholar] [CrossRef]
- Ukhina, A.V.; Dudina, D.V.; Esikov, M.A.; Samoshkin, D.A.; Stankus, S.V.; Skovorodin, I.N.; Galashov, E.N.; Bokhonov, B.B. The influence of morphology and composition of metal–carbide coatings deposited on the diamond surface on the properties of copper–diamond composites. Surf. Coat. Technol. 2020, 401, 126272. [Google Scholar] [CrossRef]
- Shiomi, T.H.H. Calculations for ledge energies on the diamond (111) surface. Surf. Sci. 1993, 295, 154–159. [Google Scholar]
- Zhang, J.M.; Li, H.Y.; Xu, K.W.; Ji, V. Calculation of surface energy and simulation of reconstruction for diamond cubic crystals (001) surface. Appl. Surf. Sci. 2008, 254, 4128–4133. [Google Scholar] [CrossRef]
- Butenko, Y.V.; Kuznetsov, V.L.; Chuvilin, A.L.; Kolomiichuk, V.N.; Stankus, S.V.; Khairulin, R.A.; Segall, B. Kinetics of the graphitization of dispersed diamonds at “low” temperatures. J. Appl. Phys. 2000, 88, 4380–4388. [Google Scholar] [CrossRef]
- Abyzov, A.M.; Kruszewski, M.J.; Ciupiński, Ł.; Mazurkiewicz, M.; Michalski, A.; Kurzydłowski, K.J. Diamond–tungsten based coating–copper composites with high thermal conductivity produced by Pulse Plasma Sintering. Mater. Des. 2015, 76, 97–109. [Google Scholar] [CrossRef]
- Kang, Q.; He, X.; Ren, S.; Zhang, L.; Wu, M.; Guo, C.; Cui, W.; Qu, X. Preparation of copper–diamond composites with chromium carbide coatings on diamond particles for heat sink applications. Appl. Therm. Eng. 2013, 60, 423–429. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Catalano, M.; Bai, G.; Li, N.; Dai, J.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Enhanced thermal conductivity in Cu/diamond composites by tailoring the thickness of interfacial TiC layer. Compos. Part A: Appl. Sci. Manuf. 2018, 113, 76–82. [Google Scholar] [CrossRef]
- Abyzov, A.M.; Kidalov, S.V.; Shakhov, F.M. High thermal conductivity composites consisting of diamond filler with tungsten coating and copper (silver) matrix. J. Mater. Sci. 2010, 46, 1424–1438. [Google Scholar] [CrossRef]
- He, J.; Zhang, H.; Zhang, Y.; Zhao, Y.; Wang, X. Effect of boron addition on interface microstructure and thermal conductivity of Cu/diamond composites produced by high temperature-high pressure method. Phys. Status Solidi A 2014, 211, 587–594. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, C.; Lang, J.; Ma, Y.; Ma, N. Si-Coated Diamond Particles Reinforced Copper Composites Fabricated by Spark Plasma Sintering Process. Mater. Manuf. Process. 2013, 28, 143–147. [Google Scholar] [CrossRef]
- Lee, H.G.; Kim, D.; Lee, S.J.; Park, J.Y.; Kim, W.J. Thermal conductivity analysis of SiC ceramics and fully ceramic microencapsulated fuel composites. Nucl. Eng. Des. 2017, 311, 9–15. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Cai, Z.; Peng, C.; Feng, Y.; Zhang, L. Effects of dual-layer coatings on microstructure and thermal conductivity of diamond/Cu composites prepared by vacuum hot pressing. Surf. Coat. Technol. 2015, 277, 299–307. [Google Scholar] [CrossRef]
- Every, A.G.; Tzou, Y.; Hasselman, D.P.H.; Raj, R. The effect of particle size on the thermal conductivity of ZnS/diamond composites. Acta Metall. Et Mater. 1992, 40, 123–129. [Google Scholar] [CrossRef]
- Pietrak, T.S.W.s.K. A review of models for effective thermal conductivity of composite materials. J. Power Technol. 2015, 95, 14–24. [Google Scholar]
- Zhai, S.; Zhang, P.; Xian, Y.; Zeng, J.; Shi, B. Effective thermal conductivity of polymer composites: Theoretical models and simulation models. Int. J. Heat Mass Transf. 2018, 117, 358–374. [Google Scholar] [CrossRef]
- Tavangar, R.; Molina, J.M.; Weber, L. Assessing predictive schemes for thermal conductivity against diamond-reinforced silver matrix composites at intermediate phase contrast. Scr. Mater. 2007, 56, 357–360. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Peng, C.; Tang, Y.; Cai, Z. Influence of titanium coating on the microstructure and thermal behavior of Dia./Cu composites. Diam. Relat. Mater. 2019, 97, 107449. [Google Scholar] [CrossRef]
- Pan, Y.; He, X.; Ren, S.; Wu, M.; Qu, X. Optimized thermal conductivity of diamond/Cu composite prepared with tungsten-copper-coated diamond particles by vacuum sintering technique. Vacuum 2018, 153, 74–81. [Google Scholar] [CrossRef]



| Interface Layer | Thickness (μm) | Diamond Content (vol%) | TC (W∙m−1∙K−1) | Ref |
|---|---|---|---|---|
| Mo2C | 0.50 | 60 | 657 | [21] |
| Cr7C3 | 1.00 | 65 | 562 | [28] |
| TiC | 0.22 | 60 | 811 | [29] |
| WC | 0.26 | 50 | 690 | [27] |
| WC | 0.11 | 63 | 900 | [30] |
| B4C | 2.11 | 90 | 731 | [31] |
| Si | 0.30 | 50 | 535 | [32] |
| WC | 0.90 | 60 | 874 | This study |
| Material | Density ρ (kg·m−3) | Specific Heat C (J·kg−1·K−1) | Shear Modulus G (GPa) | Phonon Velocity ν (m·s−1) | TC Λ (W·m−1·K−1) | Ref |
|---|---|---|---|---|---|---|
| Diamond | 3520 | 515 | 504 | 11,970 | 1500 | [39] |
| Cu | 8960 | 385 | 50 | 2362 | 400 | [39,40] |
| WC | 15,630 | 171 | 270 | 4156 | 120 | [34] |
| H-J Model/W·m−1·K−1 | DEM Model/W·m−1·K−1 | |||
|---|---|---|---|---|
| 50 | 613 | 0.93 | 620 | 0.95 |
| 55 | 639 | 0.98 | 647 | 0.99 |
| 60 | 665 | 1.02 | 674 | 1.03 |
| 65 | 693 | 1.06 | 702 | 1.07 |
| 70 | 721 | 1.10 | 731 | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; He, X.; Xu, Z.; Qu, X. Preparation of W-Plated Diamond and Improvement of Thermal Conductivity of Diamond-WC-Cu Composite. Metals 2021, 11, 437. https://doi.org/10.3390/met11030437
Wang X, He X, Xu Z, Qu X. Preparation of W-Plated Diamond and Improvement of Thermal Conductivity of Diamond-WC-Cu Composite. Metals. 2021; 11(3):437. https://doi.org/10.3390/met11030437
Chicago/Turabian StyleWang, Xulei, Xinbo He, Zhiyang Xu, and Xuanhui Qu. 2021. "Preparation of W-Plated Diamond and Improvement of Thermal Conductivity of Diamond-WC-Cu Composite" Metals 11, no. 3: 437. https://doi.org/10.3390/met11030437
APA StyleWang, X., He, X., Xu, Z., & Qu, X. (2021). Preparation of W-Plated Diamond and Improvement of Thermal Conductivity of Diamond-WC-Cu Composite. Metals, 11(3), 437. https://doi.org/10.3390/met11030437






