Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway
Abstract
1. Introduction
2. Materials and Methods
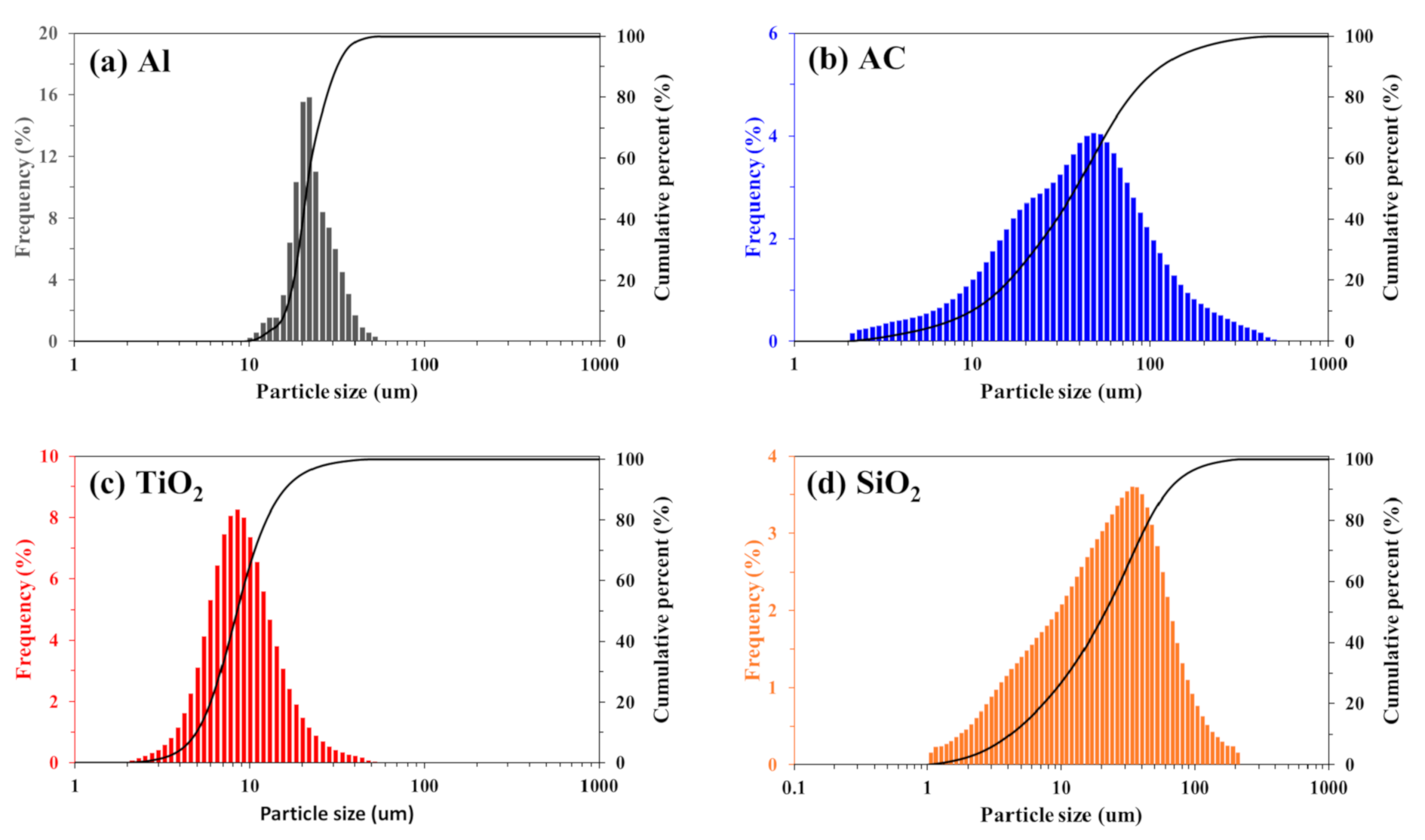
2.1. Materials
2.2. Recovery of Co2+ and Ni2+ from Sulfate and Chloride Solutions
2.2.1. Preparation of Co2+ and Ni2+ Solutions
2.2.2. Cementation Tests
2.2.3. Surface Analysis
3. Results and Discussion
3.1. Recovery of Co2+ and Ni2+
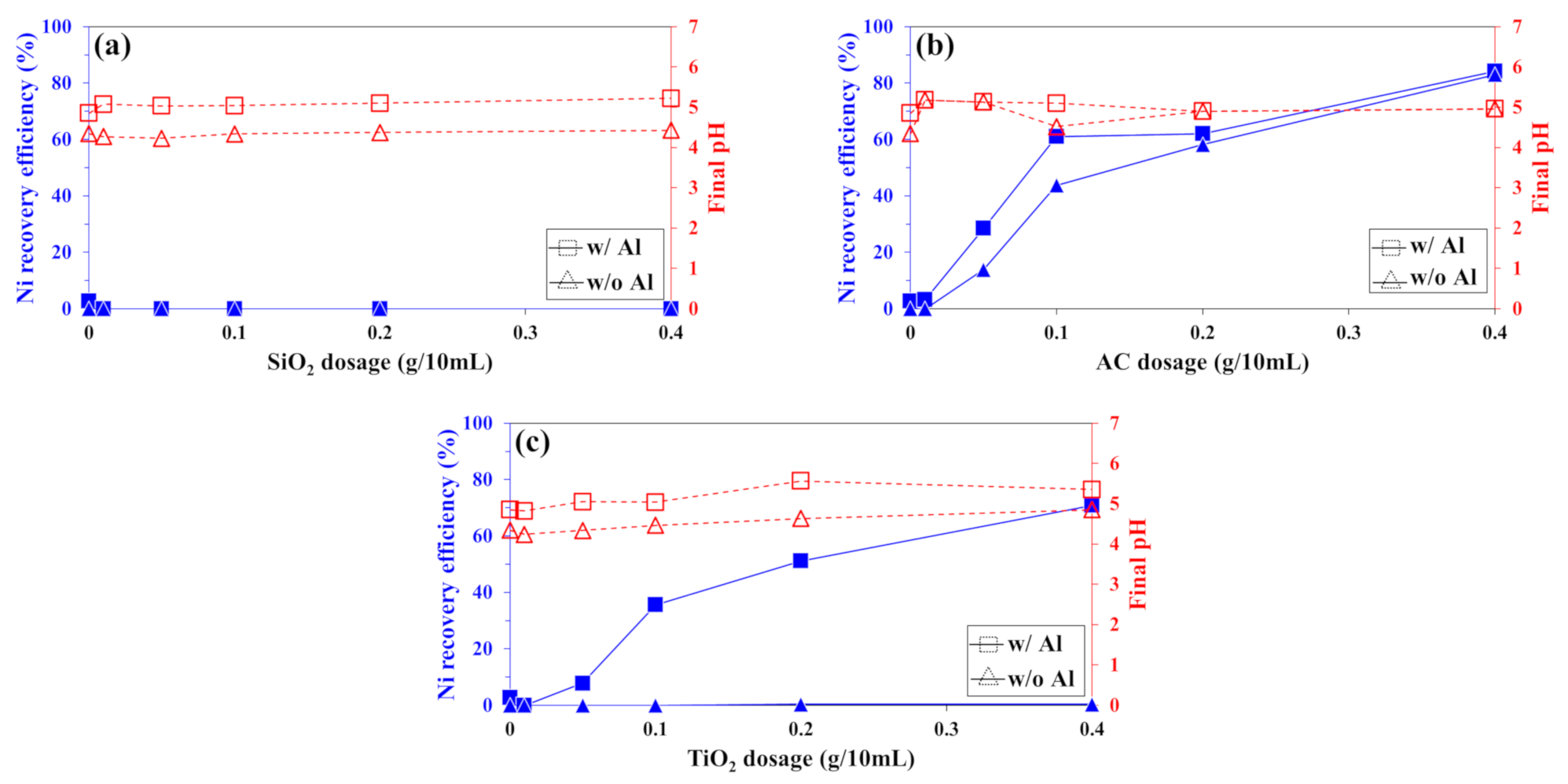
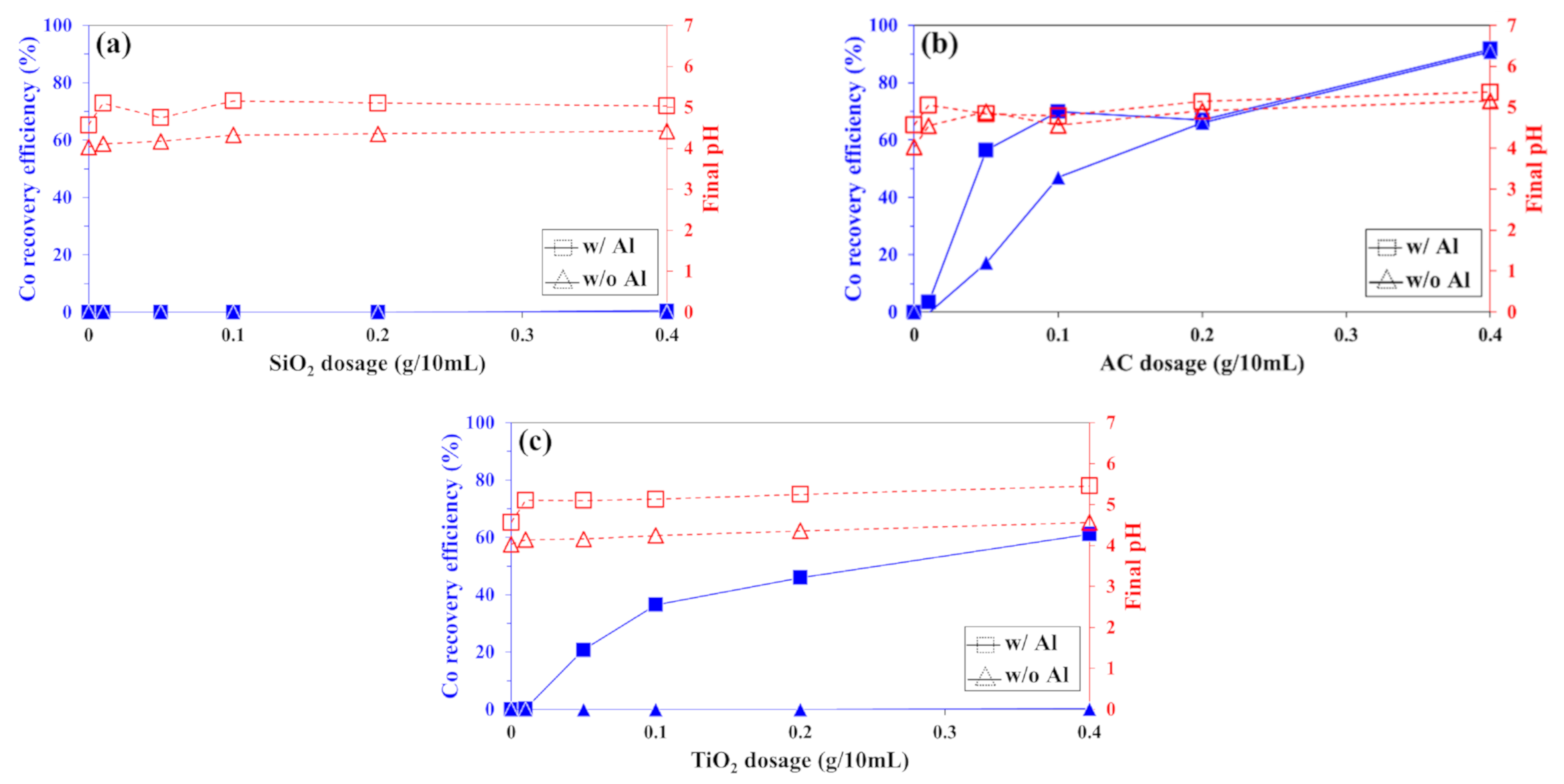
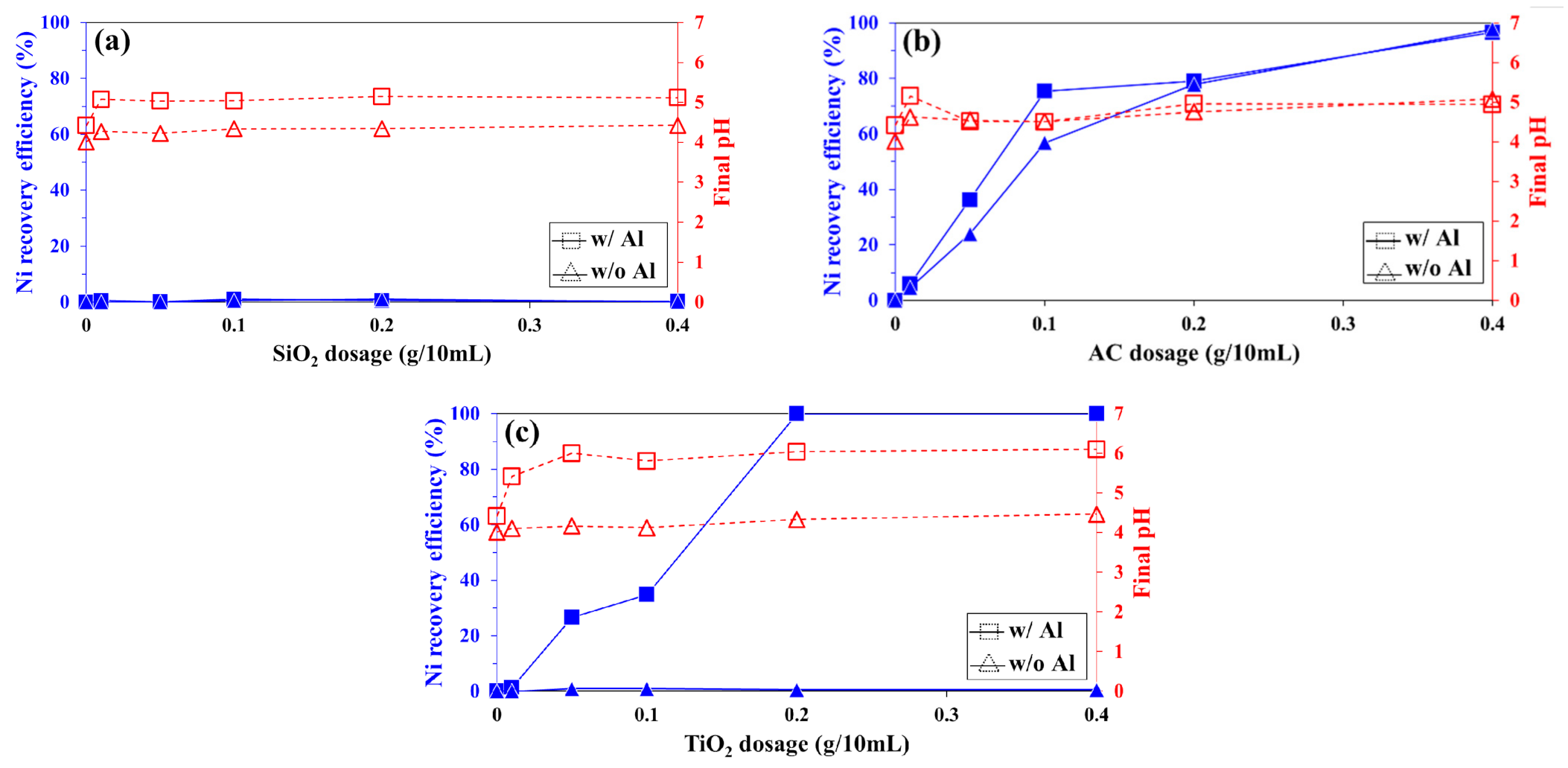
3.1.1. Recovery of Co2+ and Ni2+ from Sulfate Solution
3.1.2. Recovery of Co2+ and Ni2+ from Chloride Solution
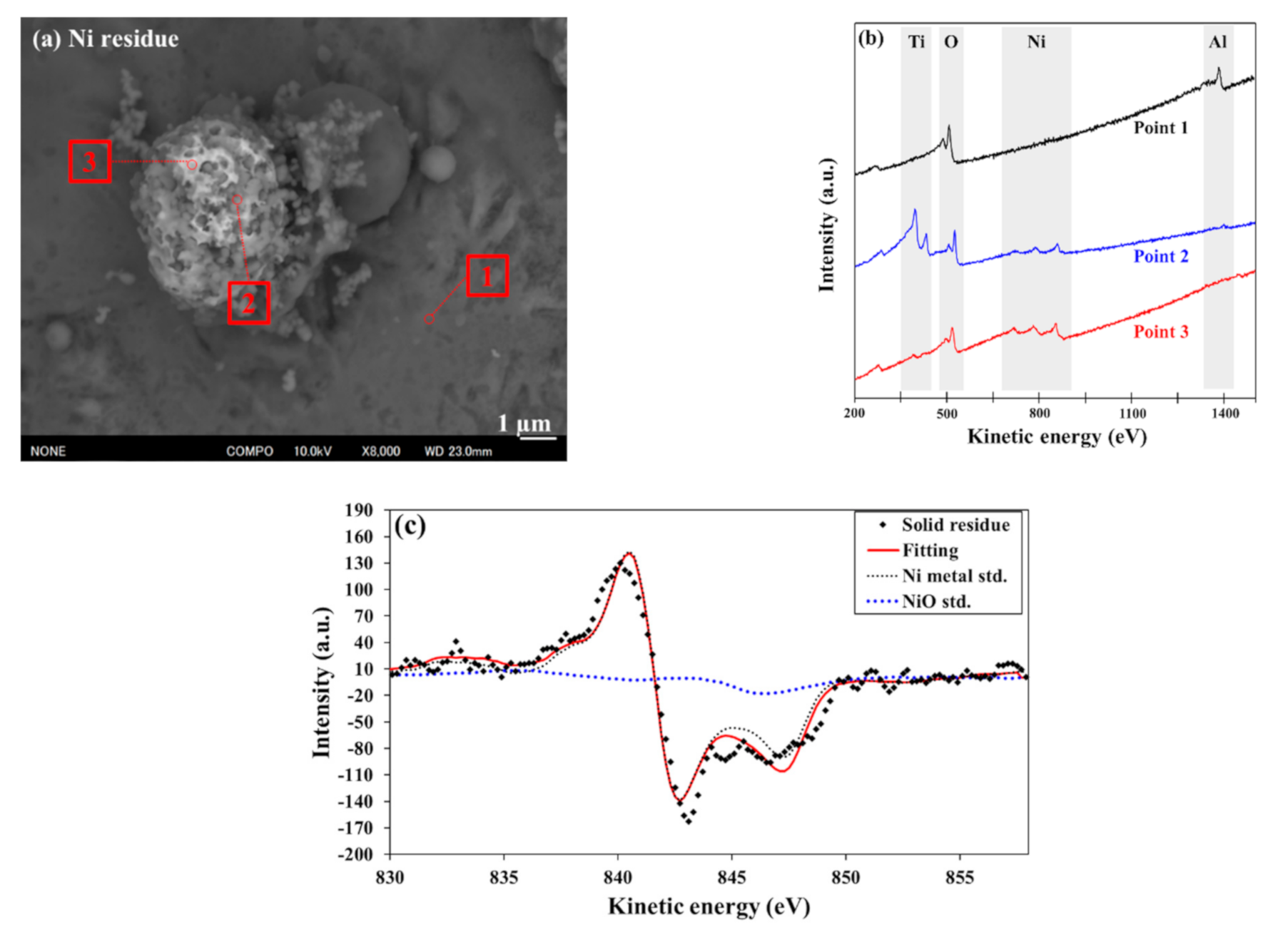
3.2. Surface Analysis of Deposited Co and Ni
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, A.; Wang, W.; Demopoulos, G.P.; Houlachi, G. Removal of cobalt from zinc electrolyte by cementation: A critical review. Miner. Process. Extr. Metall. Rev. 2000, 20, 325–356. [Google Scholar] [CrossRef]
- Demirkran, N.; Künkül, A. Recovering of copper with metallic aluminum. Trans. Nonferrous Met. Soc. China 2011, 21, 2778–2782. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B. Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation. Metals 2020, 10, 531. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, K.; Alorro, R.D.; Tabelin, C.B. Cementation of Co ion in leach solution using Zn powder followed by magnetic separation of cementation-precipitate for recovery of unreacted Zn powder. Miner. Eng. 2020, 145. [Google Scholar] [CrossRef]
- Farahmand, F.; Moradkhani, D.; Sadegh Safarzadeh, M.; Rashchi, F. Optimization and kinetics of the cementation of lead with aluminum powder. Hydrometallurgy 2009, 98, 81–85. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; El-Ashtoukhy, E.S.Z.; Bassyouni, M. Recovery of Copper from Effluents by Cementation on Aluminum in a Multirotating Cylinder-Agitated Vessel. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2016, 47, 657–665. [Google Scholar] [CrossRef]
- Abdollahi, P.; Yoozbashizadeh, H.; Moradkhani, D.; Behnian, D. A Study on Cementation Process of Lead from Brine Leaching Solution by Aluminum Powder. OALib 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Boisvert, L.; Turgeon, K.; Boulanger, J.; Bazin, C.; Houlachi, G. Recovery of Cobalt from the Residues of an Industrial Zinc Refinery. Metals 2020, 10, 1553. [Google Scholar] [CrossRef]
- Li, W.; Cochell, T.; Manthiram, A. Activation of aluminum as an effective reducing agent by pitting corrosion for wet-chemical synthesis. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Ito, M.; Hiroyoshi, N. Simultaneous suppression of acid mine drainage formation and arsenic release by Carrier-microencapsulation using aluminum-catecholate complexes. Chemosphere 2018, 205, 414–425. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I.; et al. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Annamalai, V.; Murr, L.E. Effects of the source of chloride ion and surface corrosion patterns on the kinetics of the copper-aluminum cementation system. Hydrometallurgy 1978, 3, 249–263. [Google Scholar] [CrossRef]
- Artamonov, V.V.; Moroz, D.R.; Bykov, A.O.; Artamonov, V.P. Experimental studies of cementation of tin in a dispersed form. Russ. J. Non-Ferrous Met. 2013, 54, 128–131. [Google Scholar] [CrossRef]
- Ekmekyapar, A.; Tanaydin, M.; Demirkiran, N. Investigation of copper cementation kinetics by rotating aluminum disc from the leach solutions containing copperions. Physicochem. Probl. Miner. Process. 2012, 48, 355–367. [Google Scholar] [CrossRef]
- Djokić, S.S. Cementation of Copper on Aluminum in Alkaline Solutions. J. Electrochem. Soc. 1996, 143, 1300–1305. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Park, I.; Nagata, Y.; Ito, M.; Hiroyoshi, N. Ammonium thiosulfate extraction of gold from printed circuit boards (PCBs) of end-of-life mobile phones and its recovery from pregnant leach solution by cementation. Hydrometallurgy 2020, 191, 105214. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Takahashi, H.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced cementation of gold via galvanic interactions using activated carbon and zero-valent aluminum: A novel approach to recover gold ions from ammonium thiosulfate medium. Hydrometallurgy 2020, 191, 105165. [Google Scholar] [CrossRef]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost adsorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: A review. Polish J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef]
- Negem, M.; Nady, H.; El-Rabiei, M.M. Nanocrystalline nickel–cobalt electrocatalysts to generate hydrogen using alkaline solutions as storage fuel for the renewable energy. Int. J. Hydrog. Energy 2019, 44, 11411–11420. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, B.; Li, B.; Lai, Y.; Zheng, W.; Wang, H.; Wang, W.; Wang, M. Study on the characteristics of a high capacity nickel manganese cobalt oxide (NMC) lithium-ion battery-an experimental investigation. Energies 2018, 11, 2275. [Google Scholar] [CrossRef]
- Lee, B.-R.; Noh, H.-J.; Myung, S.-T.; Amine, K.; Sun, Y.-K. High-Voltage Performance of Li[Ni[sub 0.55]Co[sub 0.15]Mn[sub 0.30]]O[sub 2] Positive Electrode Material for Rechargeable Li-Ion Batteries. J. Electrochem. Soc. 2011, 158, A180. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2020; OECD: Paris, France, 2020; ISBN 9789264616226. [Google Scholar]
- Zeng, X.; Li, J.; Singh, N. Recycling of spent lithium-ion battery: A critical review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Hext, P.M.; Tomenson, J.A.; Thompson, P. Titanium dioxide: Inhalation toxicology and epidemiology. Ann. Occup. Hyg. 2005, 49, 461–472. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Bancroft, G.M.; Davidson, R.; McIntyre, N.S.; Pratt, A.R. Minimum XPS core-level line widths of insulators, including silicate minerals. Am. Mineral. 2004, 89, 878–882. [Google Scholar] [CrossRef]
- Chen, H.J.; Lee, C. Effects of the Type of Chelating Agent and Deposit Morphology on the Kinetics of the Copper-Aluminum Cementation System. Langmuir 1994, 10, 3880–3886. [Google Scholar] [CrossRef]
- Gao, X.; Wu, L.; Xu, Q.; Tian, W.; Li, Z.; Kobayashi, N. Adsorption kinetics and mechanisms of copper ions on activated carbons derived from pinewood sawdust by fast H3PO4 activation. Environ. Sci. Pollut. Res. 2018, 25, 7907–7915. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Ghaedi, A.M.; Asfaram, A.; Goudarzi, A.; Hajati, S.; Soylak, M.; Agarwal, S.; Gupta, V.K. Modeling of quaternary dyes adsorption onto ZnO-NR-AC artificial neural network: Analysis by derivative spectrophotometry. J. Ind. Eng. Chem. 2016, 34, 186–197. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Inano, H.; Ito, M.; Hiroyoshi, N. Carrier-microencapsulation of arsenopyrite using Al-catecholate complex: Nature of oxidation products, effects on anodic and cathodic reactions, and coating stability under simulated weathering conditions. Heliyon 2020, 6, e03189. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Annamalai, V. An Electron Microscopic Study of Nucleation and Growth in Electrochemical Displacement Reactions: A Comparison of the Cu/Fe and Cu/AI Cementation Systems. Metall. Trans. B 1978, 9, 515–525. [Google Scholar] [CrossRef]
- Murr, L.E.; Annamalai, V. Characterization of copper nucleation and growth from aqueous solution on aluminum: A transmission electron microscopy study of copper cementation. Thin Solid Films 1978, 54, 189–195. [Google Scholar] [CrossRef]
- Reboul, M.C.; Warner, T.J.; Mayer, H.; Barouk, B. A Ten Step Mechanism for the Pitting Corrosion of Aluminium Alloys. Corros. Rev. 1997, 15, 471–496. [Google Scholar] [CrossRef]
- Andersson, M.; Kiselev, A.; Österlund, L.; Palmqvist, A.E.C. Microemulsion-mediated room-temperature synthesis of high-surface-area rutile and its photocatalytic performance. J. Phys. Chem. C 2007, 111, 6789–6797. [Google Scholar] [CrossRef]
- Inada, M.; Mizue, K.; Enomoto, N.; Hojo, J. Synthesis of rutile TiO2 with high specific surface area by self-hydrolysis of TiOCl2 in the presence of SDS. J. Ceram. Soc. Jpn. 2009, 117, 819–822. [Google Scholar] [CrossRef][Green Version]
- Scherer, J. Auger Electron Spectroscopy (AES): A Versatile Microanalysis Technique in the Analyst’s Toolbox. Microsc. Microanal. 2020, 26, 1564–1565. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Jeon, S.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway. Metals 2021, 11, 248. https://doi.org/10.3390/met11020248
Choi S, Jeon S, Park I, Ito M, Hiroyoshi N. Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway. Metals. 2021; 11(2):248. https://doi.org/10.3390/met11020248
Chicago/Turabian StyleChoi, Sanghyeon, Sanghee Jeon, Ilhwan Park, Mayumi Ito, and Naoki Hiroyoshi. 2021. "Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway" Metals 11, no. 2: 248. https://doi.org/10.3390/met11020248
APA StyleChoi, S., Jeon, S., Park, I., Ito, M., & Hiroyoshi, N. (2021). Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway. Metals, 11(2), 248. https://doi.org/10.3390/met11020248






