Numerical Simulation of Wire Rod Cooling in Eutectoid Steel under Forced-Convection
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Physical Model
3. Mathematical Model
- The problem is solved in a transitory state;
- The model considers a cartesian coordinate system in 3D;
- The thermophysical properties for steel are found as a function of temperature through polynomial functions for a eutectoid steel [20];
- The thermal condition at the boundary was determined from the thermal response on the specimen surface and the solution of the Inverse Heat Conduction Problem;
- The heat accumulation term was calculated from the transformation of austenite into pearlite through the JMAK model and the proposed UDF;
- Once the phase transformation starts, the cooling in the specimen followed a Newtonian behavior.
3.1. Thermal Model
3.2. Thermal Boundary Conditions
3.3. Microstructural Model
3.4. Solution Method Considerations
4. Results and Discussion
4.1. Thermal Boundary Condition
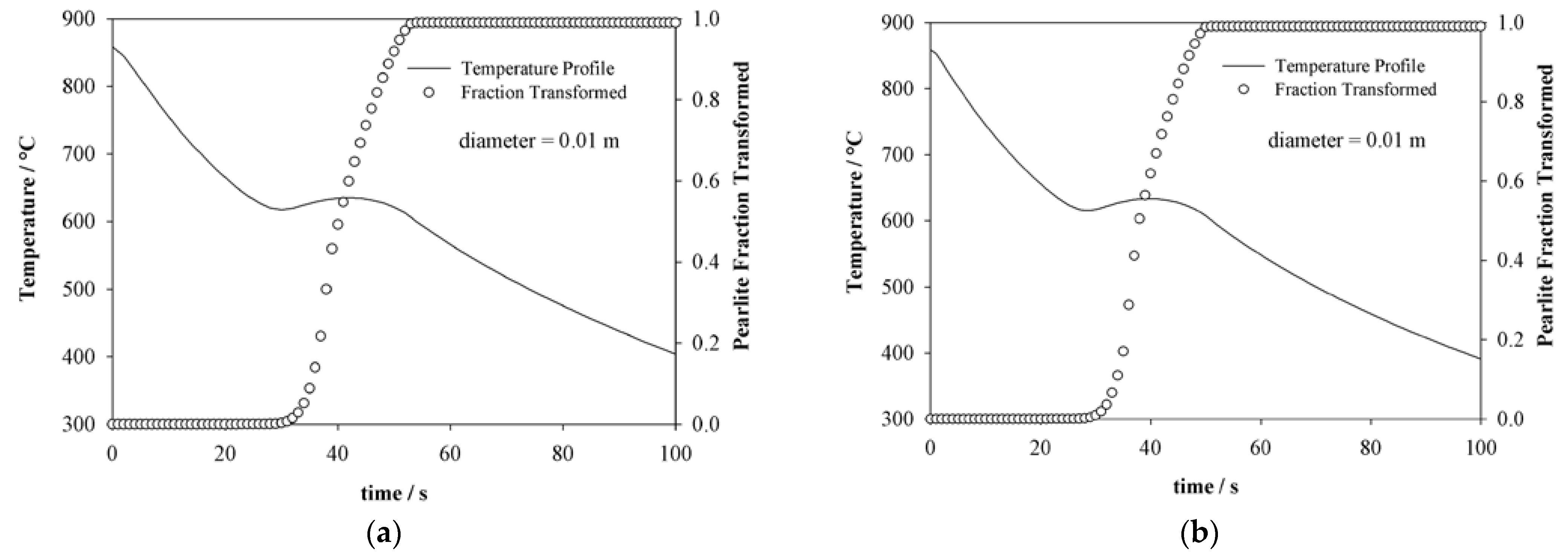
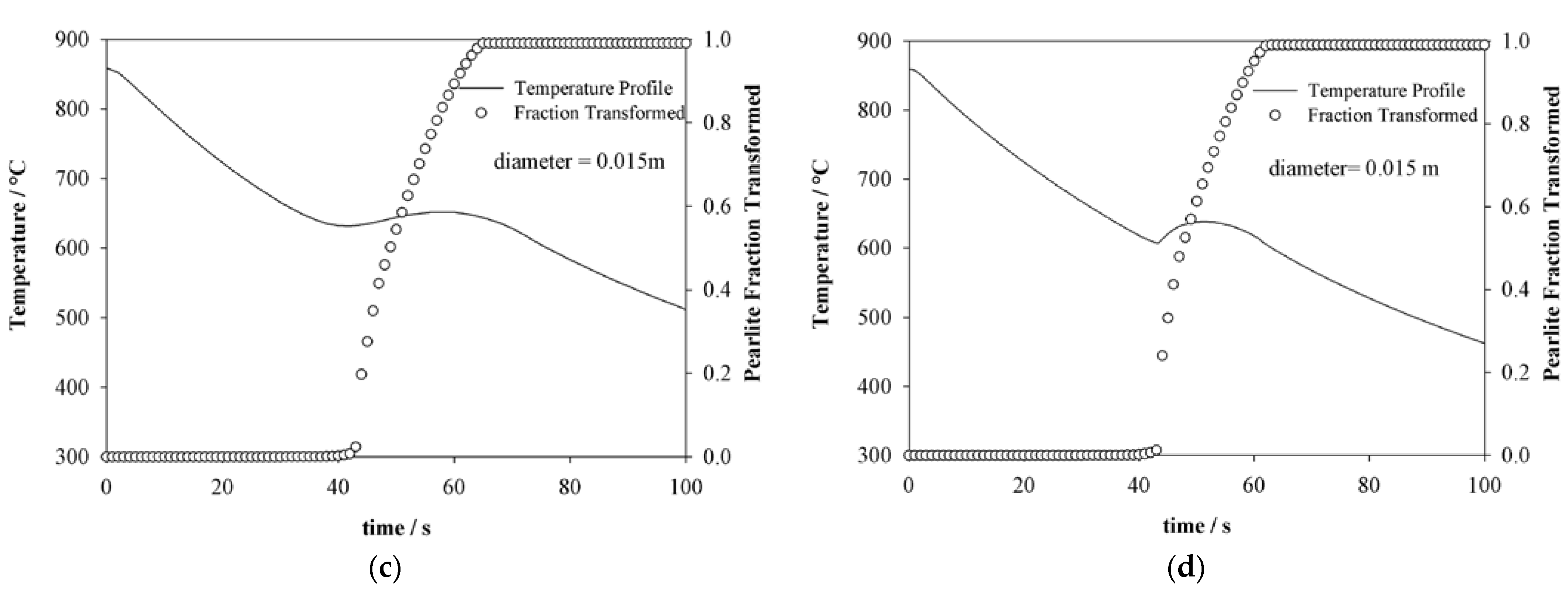
4.2. Thermal History Estimation
4.3. Pearlite Evolution Estimation
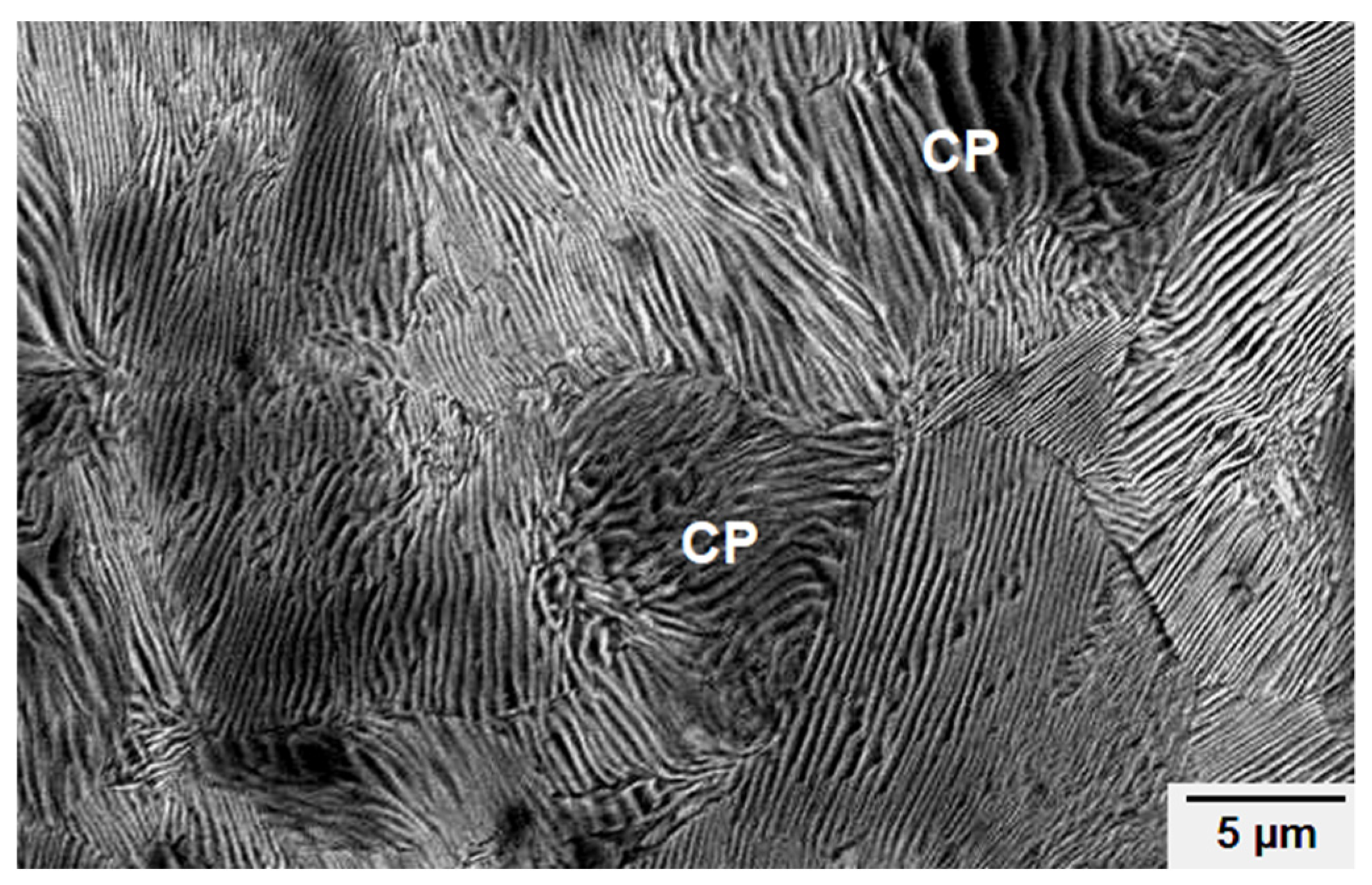
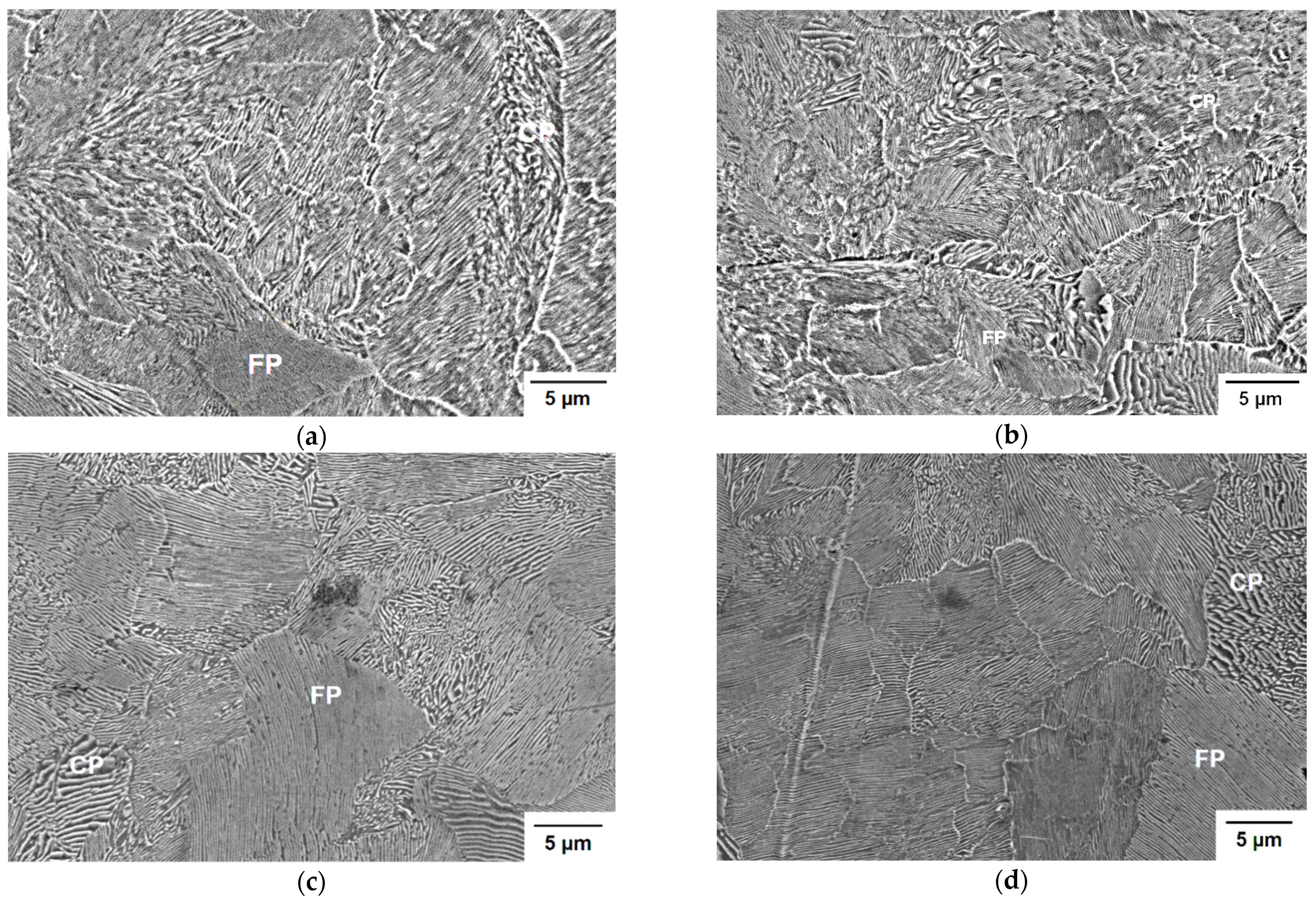
4.4. Material
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, I.; Lenka, S.; Ajmani, S.K.; Kundu, S. An approach to heat transfer analysis of wire loops over the stelmor conveyor to predict the microstructural and mechanical attributes of steel rods. J. Therm. Sci. Eng. Appl. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Goto, S.; Kirchheim, R.; Al-Kassab, T.; Borchers, C. Application of cold drawn lamellar microstructure for developing ultra-high strength wires. Trans. Nonferrous Met. Soc. China 2007, 17, 1129–1138. [Google Scholar] [CrossRef]
- Hyzak, J.M.; Bernstein, I.M. The role of microstructure on the strength and toughness of fully pearlitic steels. Metall. Mater. Trans. A 1976, 7, 1217–1224. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties; Butterworth-Heinemann: Oxford, UK, 1981; pp. 65–67. [Google Scholar]
- Rastegari, H.; Kermanpur, A.; Najafizadeh, A. Investigating the effects of short time austenitizing and cooling rate on pearlitic microstructure and mechanical properties of a hot rolled plain eutectoid carbon steel. Mater. Des. 2015, 67, 217–223. [Google Scholar] [CrossRef]
- Toribio, J.; González, B.; Matos, J.; Ayaso, F. Influence of microstructure on strength and ductility in fully pearlitic steels. Metals 2016, 6, 318. [Google Scholar] [CrossRef]
- Yu, W.H.; Chen, S.H.; Kuang, Y.H.; Cao, K.C. Development and application of online stelmor controlled cooling system. Appl. Therm. Eng. 2009, 29, 2949–2953. [Google Scholar]
- Kazeminezhad, M.; Karimi Taheri, A. The effect of controlled cooling after hot rolling on the mechanical properties of a commercial high carbon steel wire rod. Mater. Des. 2003, 24, 415–421. [Google Scholar] [CrossRef]
- Campbell, P.C.; Hawbolt, E.B.; Brimacombe, J.K. Microstructural engineering applied to the controlled cooling of steel wire rod: Part II. Microstructural evolution and mechanical properties correlations. Metall. Mater. Trans. A 1991, 22, 2779–2790. [Google Scholar] [CrossRef]
- Campbell, P.C.; Hawbolt, E.B.; Brimacombe, J.K. Microstructural engineering applied to the controlled cooling of steel wire rod: Part III. Mathematical model-formulation and predictions. Mettal. Mater. Trans. A 1991, 22, 2791–2805. [Google Scholar] [CrossRef][Green Version]
- Campbell, P.C.; Hawbolt, E.B.; Brimacombe, J.K. Microstructural engineering applied to the controlled cooling of steel wire rod: Part I. Experimental design and heat transfer. Metall. Mater. Trans. A 1991, 22, 2769–2778. [Google Scholar] [CrossRef]
- Lindemann, A.; Schmidt, J. ACMOD-2D—A heat transfer model for the simulation of the cooling of wire rod. J. Mater. Process. Technol. 2005, 169, 466–475. [Google Scholar] [CrossRef]
- Morales, R.D.; Lopéz, A.G.; Olivares, M. Mathematical simulation of stelmor process. Iron Steelmak. 1991, 18, 128–138. [Google Scholar]
- Agarwal, P.K.; Brimacombe, J.K. Mathematical model of heat flow and austenite-pearlite transformation in eutectoid carbon steel rods for wire. Metall. Mater. Trans. B 1981, 12, 121–133. [Google Scholar] [CrossRef]
- Pham, T.T.; Hawbolt, E.B.; Brimacombe, J.K. Predicting the onset of transformation under noncontinuous cooling conditions: Part II. Application to the austenite pearlite transformation. Metall. Mater. Trans. A 1995, 26, 1993–2000. [Google Scholar] [CrossRef]
- Hong, L.; Wang, B.; Feng, S.; Yang, Z.; Yu, Y.; Peng, W.; Zhang, J. A three-dimensional mathematical model to predict air-cooling flow and temperature distribution of wire loops in the stelmor air-cooling system. Appl. Therm. Eng. 2017, 116, 766–776. [Google Scholar] [CrossRef]
- Huang, J.; Wang, B.; Xue, F.; Liu, S.; Hong, L.; Yu, Y.; Zhang, J. Effect of controlled cold air distribution on temperature profile and phase transformation of wire loops in the stelmor air-cooling process. Appl. Therm. Eng. 2018, 143, 340–349. [Google Scholar] [CrossRef]
- Bringas, J. Handbook of Comparative World Steel Standards, 3rd ed.; ASTM International: West Conshohocken, PA, USA, 2004; p. 33. [Google Scholar]
- Quarmby, A.; Al-Farhri, A.A.M. Effect of finite length on forced convection heat transfer from cylinders. Int. J. Heat Mass Transf. 1980, 23, 463–469. [Google Scholar] [CrossRef]
- Fernandes, F.M.B.; Denis, S.; Simon, A. Mathematical model coupling phase transformation and temperature evolution during quenching of steels. Mater. Sci. Technol. 1985, 1, 838–844. [Google Scholar] [CrossRef]
- Pletcher, R.H.; Tannehill, J.C.; Anderson, D.A. Computational Fluid Mechanics and Heat Transfer, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 66–68. [Google Scholar]
- Kramer, J.J.; Pound, G.M.; Mehl, R.F. The free energy of formation and the interfacial enthalpy in pearlite. Acta Met. 1958, 6, 763–771. [Google Scholar] [CrossRef]
- Serajzadeh, S. Modelling of temperature history and phase transformations during cooling of steel. J. Mater. Process. Technol. 2004, 146, 311–317. [Google Scholar] [CrossRef]
- Carlone, P.; Palazzo, G.S.; Pasquino, R. Finite element analysis of the steel quenching process: Temperature field and solid-solid phase change. Comput. Math. Appl. 2010, 59, 585–594. [Google Scholar] [CrossRef]
- Puls, M.P.; Kirkaldy, J.S. The pearlite reaction. Metall. Mater. Trans. B. 1972, 7, 2777–2796. [Google Scholar] [CrossRef]
- Vázquez-Gómez, O.; Barrera-Godínez, J.; Hernández-Morales, B.; Vergara-Hernández, H.; López-Martínez, E. Mathematical model of thermal and microstructural evolution during austempering of ductile iron. Mater. Perform. Charact. 2012, 1, 1–14. [Google Scholar] [CrossRef]
- Li, M.V.; Niebuhr, D.V.; Meekisho, L.L.; Atteridge, D.G. A computational model for the prediction of steel hardenability. Metall. Mater. Trans. B. 1998, 29, 661–672. [Google Scholar] [CrossRef]
- Hawbolt, E.B.; Chau, B.; Brimacombe, J.K. Kinetics of austenite-pearlite transformation in eutectoid carbon steel. Metall. Mater. Trans. A 1983, 14, 1803–1815. [Google Scholar] [CrossRef]
- Umemoto, M.; Horoichi, K.; Tamura, I. Transformation kinetics of bainite during isothermal holding and continuous cooling. J. Iron Steel Inst. Jpn. 1982, 68, 461–470. [Google Scholar] [CrossRef]
- ASM International. Atlas of Isothermal Transformation and Cooling Transformation Diagrams; American Society for Metals: Cleveland, OH, USA, 1977; p. 28. [Google Scholar]
- Kirkaldy, J.S.; Baganis, E.A. Thermodynamic prediction of the Ae3 temperature of steels with additions of Mn,Si, Ni, Cr, Mo, Cu. Metall. Mater. Trans. A 1978, 9, 495–501. [Google Scholar] [CrossRef]
- Zener, C. Kinetics of the decomposition of austenite. Trans. Am. Inst. Min. Metall. Eng. 1946, 167, 550–595. [Google Scholar]
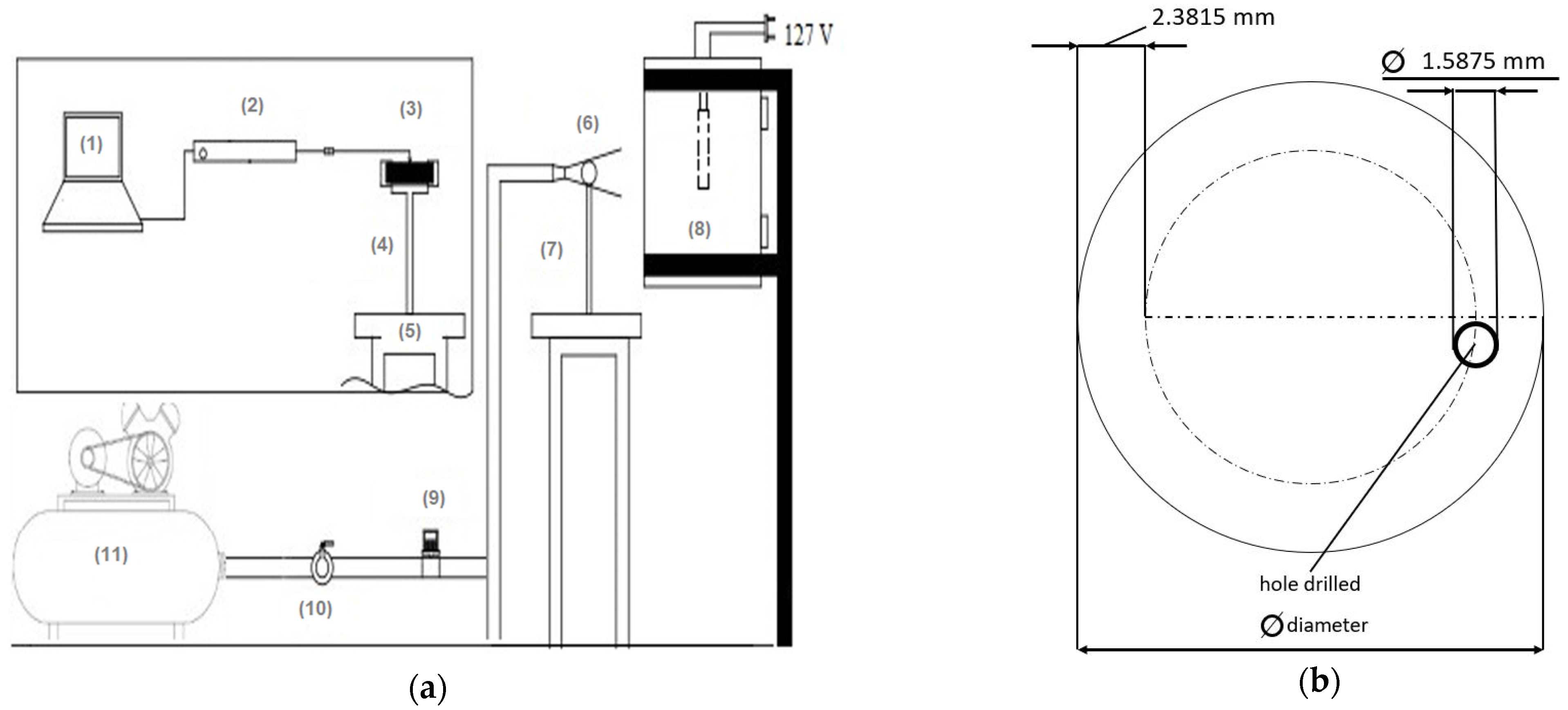
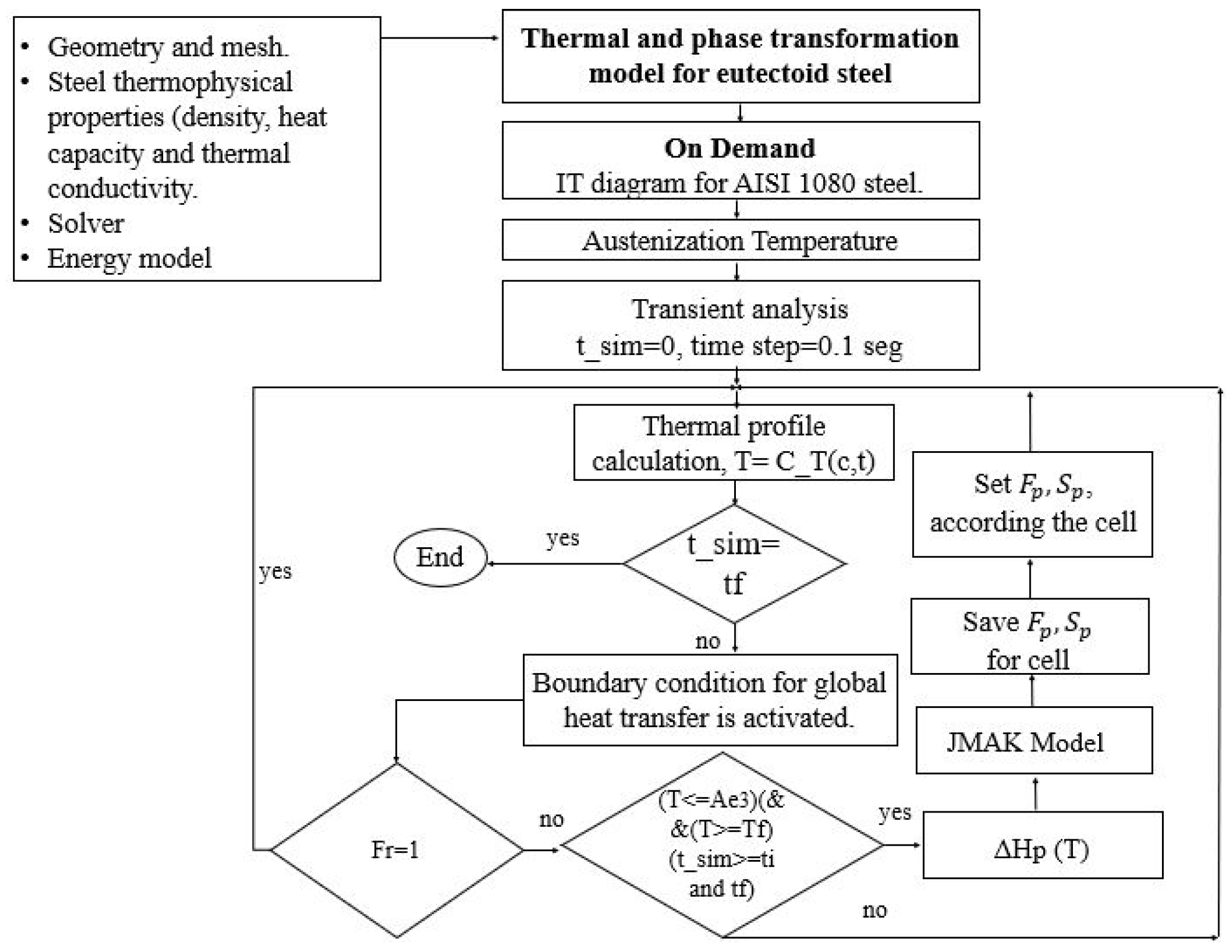
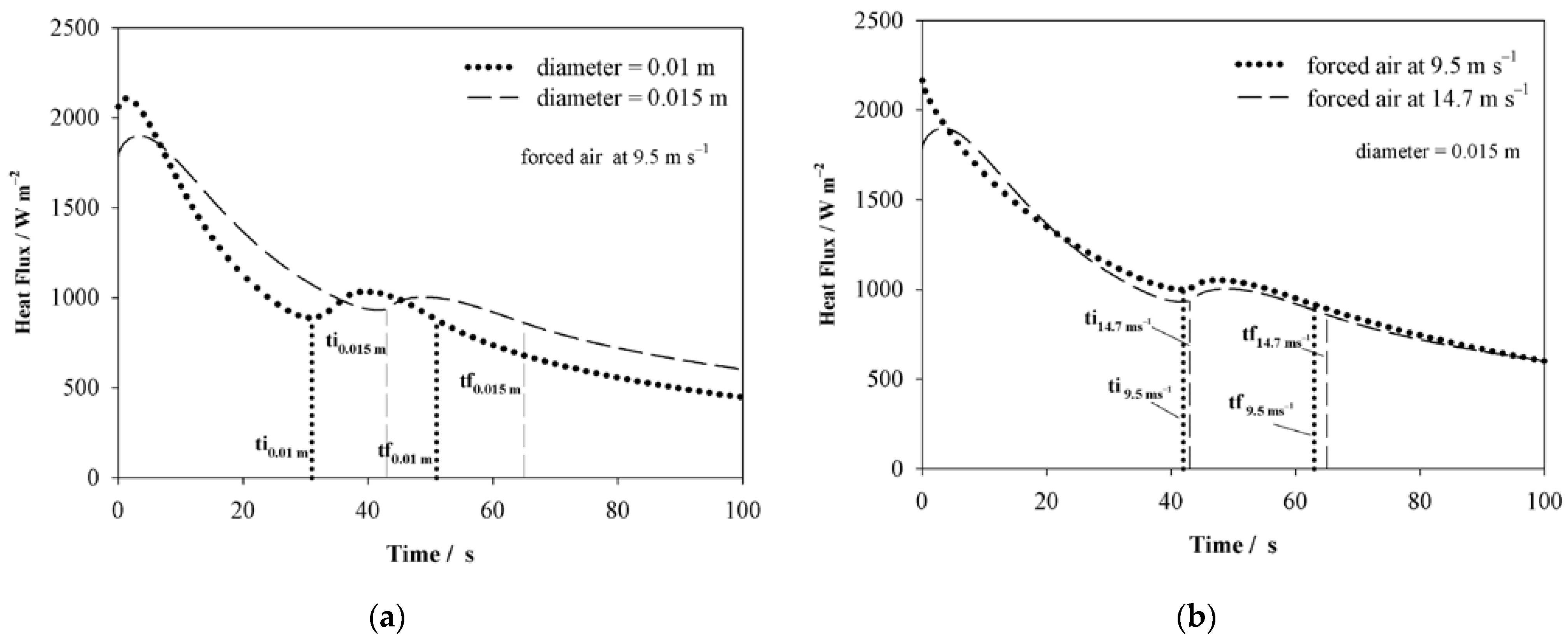
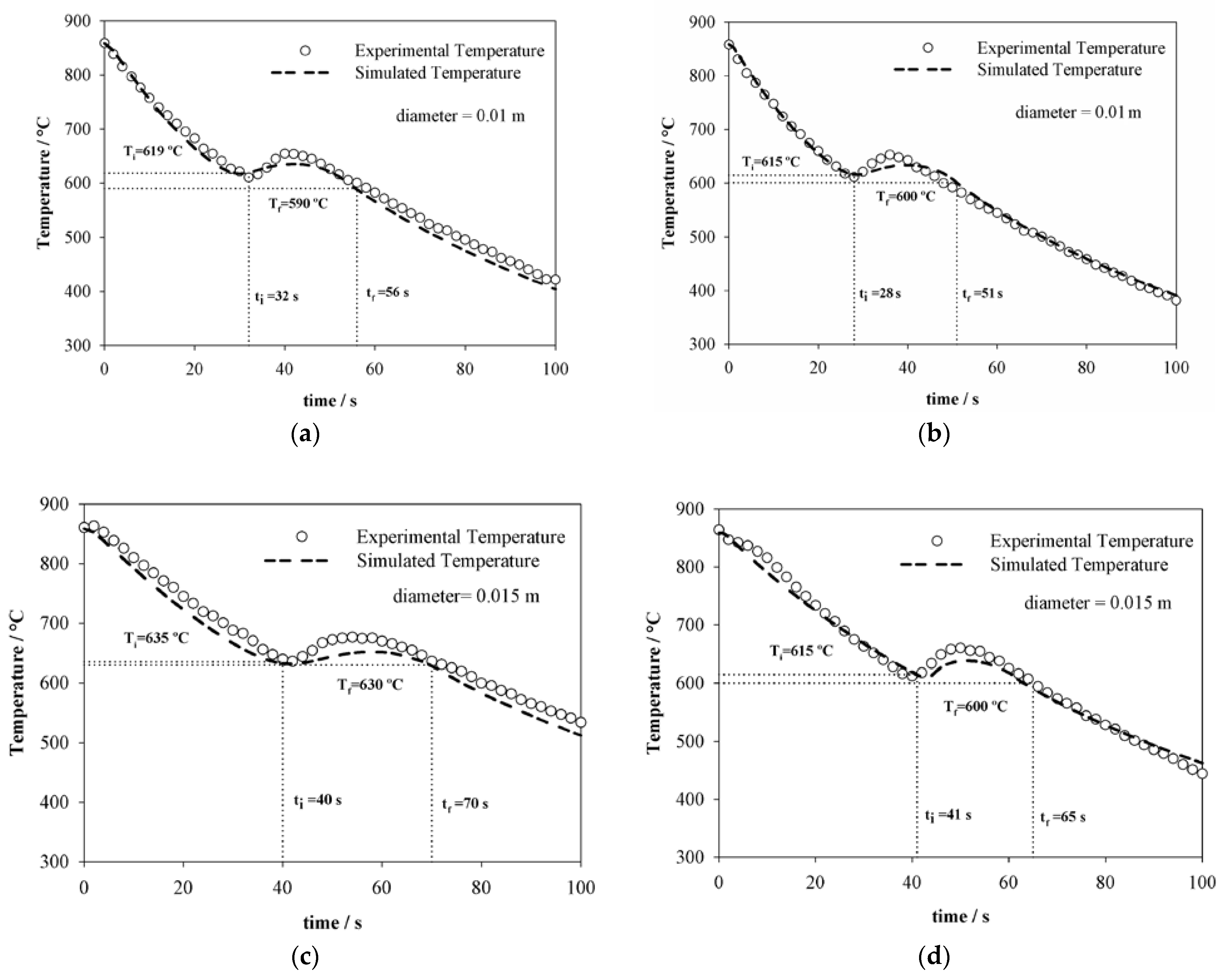
| C | Si | Mn | P | S | Cr | Al | Cu | Ni | Mo |
|---|---|---|---|---|---|---|---|---|---|
| 0.83 | 0.20 | 0.77 | 0.004 | 0.009 | 0.24 | 0.001 | 0.052 | 0.021 | 0.003 |
| Specimen | Diameter (m) | Average Cooling Rate (°C s−1) | Average Air Velocity (m s−1) |
|---|---|---|---|
| 1 | 0.010 | 10 | 7.8 |
| 2 | 0.010 | 12 | 9.5 |
| 3 | 0.015 | 7 | 9.5 |
| 4 | 0.015 | 10 | 14.7 |
| Specimen | D (m) | Va (m s−1) | Ar3-s (°C) | ti-s (s) | ΔT = Ae −Ar3-s | ΔtT (s) | Sp (nm) | HV0.1/15 |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.010 | 7.8 | 619 | 32 | 108 | 24 | 110 ± 15 | 337 ± 10 |
| 2 | 0.010 | 9.5 | 615 | 28 | 112 | 23 | 100 ± 10 | 343 ± 10 |
| 3 | 0.015 | 9.5 | 635 | 40 | 92 | 30 | 180 ± 17 | 313 ± 11 |
| 4 | 0.015 | 14.7 | 615 | 41 | 112 | 24 | 170 ± 16 | 343 ± 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cornejo, M.S.; Vergara-Hernández, H.J.; Arreola-Villa, S.A.; Vázquez-Gómez, O.; Herrejón-Escutia, M. Numerical Simulation of Wire Rod Cooling in Eutectoid Steel under Forced-Convection. Metals 2021, 11, 224. https://doi.org/10.3390/met11020224
López-Cornejo MS, Vergara-Hernández HJ, Arreola-Villa SA, Vázquez-Gómez O, Herrejón-Escutia M. Numerical Simulation of Wire Rod Cooling in Eutectoid Steel under Forced-Convection. Metals. 2021; 11(2):224. https://doi.org/10.3390/met11020224
Chicago/Turabian StyleLópez-Cornejo, Monserrat Sofía, Héctor Javier Vergara-Hernández, Sixtos Antonio Arreola-Villa, Octavio Vázquez-Gómez, and Martín Herrejón-Escutia. 2021. "Numerical Simulation of Wire Rod Cooling in Eutectoid Steel under Forced-Convection" Metals 11, no. 2: 224. https://doi.org/10.3390/met11020224
APA StyleLópez-Cornejo, M. S., Vergara-Hernández, H. J., Arreola-Villa, S. A., Vázquez-Gómez, O., & Herrejón-Escutia, M. (2021). Numerical Simulation of Wire Rod Cooling in Eutectoid Steel under Forced-Convection. Metals, 11(2), 224. https://doi.org/10.3390/met11020224









