Review on the Use of Alternative Carbon Sources in EAF Steelmaking
Abstract
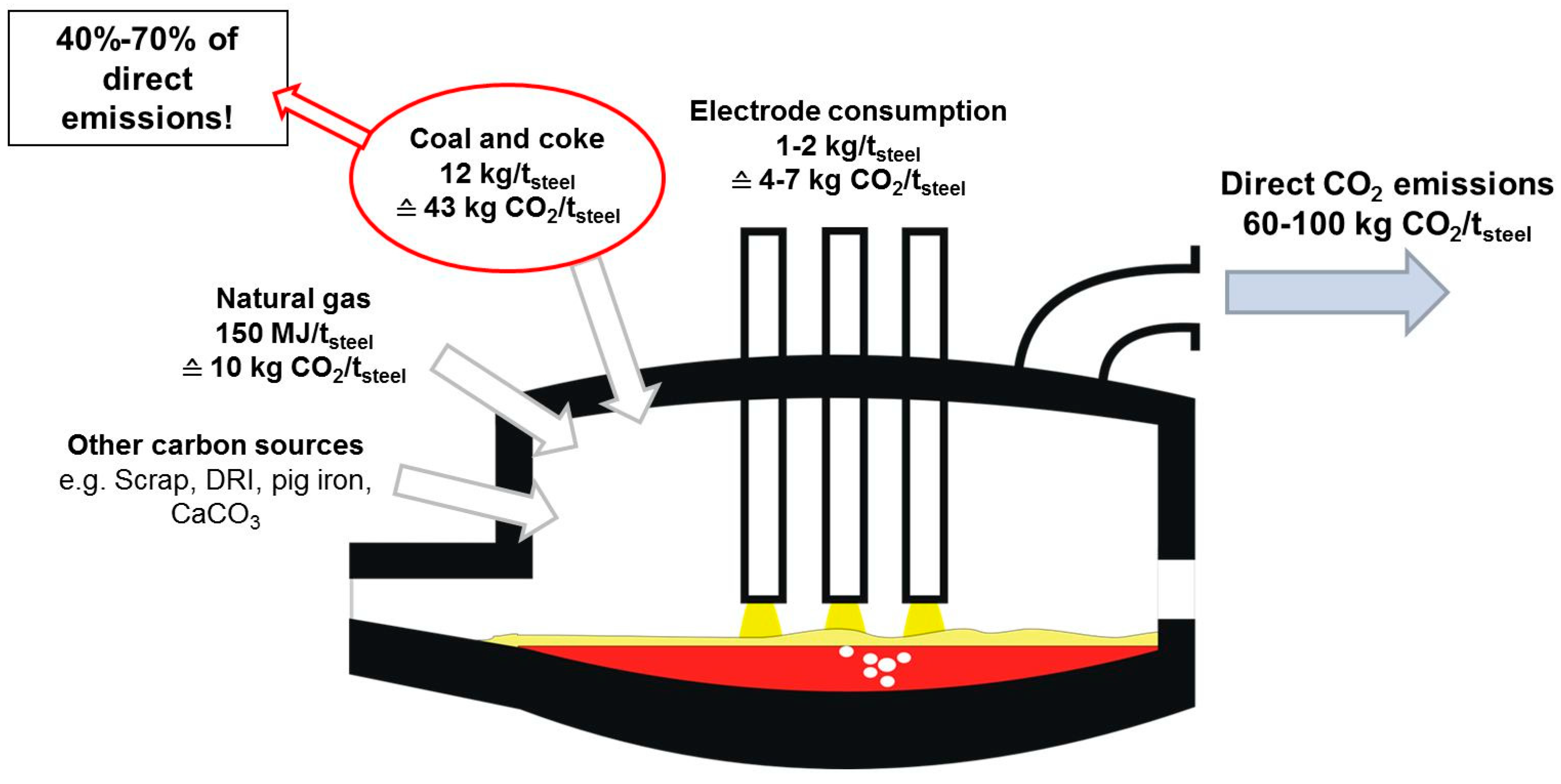
1. Introduction
2. Carbon Use in EAF Steelmaking
2.1. Charge Carbon
2.2. Injection Carbon
3. Alternative Carbon Sources
3.1. Biomass Based Alternatives
3.2. Rubber and Plastics Based Alternatives
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardo, N.; Moya, J.A.; Vatopoulos, K. Prospective Scenarios on Energy Efficiency and CO2 Emissions in the EU Iron & Steel Industry; Publications Office: Luxembourg, 2015; ISBN 978-92-79-54191-9. [Google Scholar]
- International Energy Agency. Coal Information 2015; IEA Publications: Paris, France, 2015; ISBN 978-92-64-23891-6. [Google Scholar]
- International Energy Agency. Energy Statistics of Non-OECD Countries 2015; IEA Publications: Paris, France, 2015; ISBN 978-92-64-23897-8. [Google Scholar]
- International Energy Agency. Renewables Information 2015; IEA Publications: Paris, France, 2015; ISBN 978-92-64-23887-9. [Google Scholar]
- Wörtler, M.; Schuler, F.; Voigt, N.; Schmidt, T.; Dahlmann, P.; Lüngen, H.B.; Genda, J.-T. Steel’s Contribution to a Low-Carbon Europe 2050. 2013. Available online: https://www.stahl-online.de/wp-content/uploads/2013/09/Schlussbericht-Studie-Low-carbon-Europe-2050_-Mai-20131.pdf (accessed on 22 December 2020).
- European Commission. The European Green Deal COM/2019/640 Final. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=COM:2019:640:FIN (accessed on 22 December 2020).
- World Steel Association. Steel Statistical Yearbook 2019; Concise Version; World Steel Association: Brussels, Belgium, 2019; Available online: https://www.worldsteel.org/en/dam/jcr:7aa2a95d-448d-4c56-b62b-b2457f067cd9/SSY19%2520concise%2520version.pdf (accessed on 22 December 2020).
- Gielen, D.J.; Moriguchi, Y. Technological potentials for CO2 emission reduction in the global iron and steel industry. Int. J. Energ. Tech. Pol. 2003, 1, 229–249. [Google Scholar] [CrossRef]
- Ariyama, T.; Murai, R.; Ishii, J.; Sato, M. Reduction of CO2 Emissions from Integrated Steel Works and Its Subjects for a Future Study. ISIJ Int. 2005, 45, 1371–1378. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D. Biomass use in the steel industry: Back to the future? Stahl und Eisen 2013, 133, 57–67. [Google Scholar]
- Jahanshahi, S.; Deev, A.; Haque, N.; Lu, L.; Mathieson, J.; Norgate, T.; Ridgeway, P.; Rogers, H.; Somerville, M.; Zughbi, H.; et al. Recent progress in R&D on assessment of the use of biomass/designer chars for steel production. In Proceedings of the Innovation of Ironmaking Technologies and Future International Collaboration-to Overcome Energy & Resource Restrictions in Accordance with Environments, Tokyo, Japan, 10–11 November 2014; 54th Committee on Ironmaking of Japan Society for Promotion of Science, Ed.; pp. 123–138. [Google Scholar]
- Jahanshahi, S.; Mathieson, J.G.; Somerville, M.A.; Haque, N.; Norgate, T.E.; Deev, A.; Pan, Y.; Xie, D.; Ridgeway, P.; Zulli, P. Development of Low-Emission Integrated Steelmaking Process. J. Sustain. Metall. 2015, 1, 94–114. [Google Scholar] [CrossRef]
- Mathieson, J.G.; Somerville, M.A.; Deev, A.; Jahanshahi, S. Utilization of biomass as an alternative fuel in ironmaking. In Iron Ore; Lu, L., Ed.; Elsevier: Waltham, MA, USA, 2015; pp. 581–613. [Google Scholar] [CrossRef]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sust. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of biomass in integrated steelmaking–Status quo, future needs and comparison to other low-CO2 steel production technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef]
- Bianco, L.; Baracchini, G.; Cirilli, F.; Di Sante, L.; Moriconi, A.; Moriconi, E.; Agorio, M.M.; Pfeifer, H.; Echterhof, T.; Demus, T.; et al. Sustainable Electric Arc Furnace Steel Production: GreenEAF. BHM Berg-und Hüttenmännische Monatshefte 2013, 158, 17–23. [Google Scholar] [CrossRef]
- Jellinghaus, M. Stahlerzeugung im Lichtbogenofen, 3rd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1994. [Google Scholar]
- International Iron and Steel Institute. EAF Technology. State of the Art & Future Trends; IISI: Brussels, Belgium, 2000. [Google Scholar]
- Neelis, M.; Worrell, E.; Mueller, N.; Angelini, T.; Cremer, C.; Schleich, J.; Eichhammer, W. Developing Benchmarking Criteria for CO2 Emissions Ecofys Project Number: PECSGB073248. 2009. Available online: https://ec.europa.eu/clima/sites/clima/files/ets/allowances/docs/benchm_co2emiss_en.pdf (accessed on 22 December 2020).
- Ecofys and Fraunhofer Institute for Systems and Innovation Research and Öko-Institut. Methodology for the free allocation of emission allowances in the EU ETS post 2012. Sector Report for the Iron and Steel Industry Ecofys Project Number: PECSNL082164. 2009. Available online: https://ec.europa.eu/clima/sites/clima/files/ets/allowances/docs/bm_study-iron_and_steel_en.pdf (accessed on 22 December 2020).
- Demus, T.; Echterhof, T.; Pfeifer, H.; Schulten, M.; Noel, Y.; Quicker, P. Investigations on the use of biogenic residues as a substitute for fossil coal in the EAF steelmaking process. In Proceedings of the 10th European Electric Steelmaking Conference (EEC), Graz, Austria, 25–28 September 2012. [Google Scholar]
- Zulhan, Z. Der Einfluss unterschiedlicher Kohlenstoffträger auf die Schaumschlackenbildung im Elektrolichtbogenofen; Shaker Verlag: Aachen, Germany, 2006. [Google Scholar]
- Bhoi, B.; Jouhari, A.K.; Ray, H.S.; Misra, V.N. Smelting reduction reactions by solid carbon using induction furnace: Foaming behaviour and kinetics of FeO reduction in CaO-SiO2-FeO slag. Ironmak. Steelmak. 2006, 33, 245–252. [Google Scholar] [CrossRef]
- Hayes, P.C. The Kinetics of Formation of H2O and CO2 During Iron Oxide Reduction. Metall. Mater. Trans. B 1979, 10B, 211–217. [Google Scholar] [CrossRef]
- Xie, D.; Belton, G.R. Kinetics of Reduction of Ferric Iron in Fe2O3-CaO-SiO2-Al2O3 Slags under Argon, CO-CO2, or H2-H2O. Metall. Mater. Trans. B 2003, 34B, 225–234. [Google Scholar] [CrossRef]
- King, M.P. The Effect of Hydrogen on Slag Foaming in Electric arc Furnace Slags. Presentation at Graduate Seminar McMaster University, Materials Science and Engineering, Hamilton, ON, Cananda, 24 October 2007. [Google Scholar]
- Mathieson, J.G.; Rogers, H.; Somerville, M.; Ridgeway, P.; Jahanshahi, S. Use of Biomass in the Iron and Steel Industry-An Australian Perspective. In Proceedings of the EECRsteel 2011, 1st International Conference on Energy Efficiency and CO2 Reduction in the Steel Industry, Düsseldorf, Germany, 27 June–1 July 2011. [Google Scholar]
- Norgate, T.; Langberg, D. Environmental and Economic Aspects of Charcoal Use in Steelmaking. ISIJ Int. 2009, 49, 587–595. [Google Scholar] [CrossRef]
- Norgate, T.; Haque, N.; Somerville, M.; Jahanshahi, S. Biomass as a Source of Renewable Carbon for Iron and Steelmaking. ISIJ Int. 2012, 52, 1472–1481. [Google Scholar] [CrossRef]
- Demus, T.; Reichel, T.; Echterhof, T.; Pfeifer, H. Biochar Usage in EAF-Steelmaking Potential and Feasibility. In Proceedings of the 1st European Steel Technology & Application Days (ESTAD) & 31st Journées Sidérurgiques Internationales (JSI), Paris, France, 7–8 April 2014. [Google Scholar]
- Sampaio, R.S.; Chevrand, L.J.d.S.; Filho, F.B.; Martins, T.B.; Fonseca, M.A.P.; de Oliveira, S.P. The Use of Self-Sustainable Pig Iron in EAF, a Clean and Economic Alternative Virgin Iron Source. A Brazilian Experience; Technical Report; Acesita: Belo Horizonte, Brazil, 1993. [Google Scholar]
- Sampaio, R.S.; Martins, T.B.; Nicacio, P.; Quadro, A.L.; Saab, M.W.; de Sales Chevrand, L.J.; Villanova, E.; Rezende, M.E.A. The Brazilian Experience in Utilizing Large Proportions of Cold Pig Iron in Electric Arc Furnace. In Proceedings of the 5th European Electric Steel Congress, European Electric Steel Congress (EEC), Paris, France, 19–23 June 1995; Fédération Française de l’Acier, Ed.; pp. 62–71. [Google Scholar]
- Sampaio, R.S.; Jones, J.; Vieira, J.B. Hot Metal Strategies for the EAF Industry. In Proceedings of the AISTech 2008 Proceedings. AISTech 2008, Iron and Steel Technology Conference, Pittsburgh, PA, USA, 5–8 May 2008; Association for Iron and Steel Technology: Warrendale, PA, USA, 2008; pp. 1–9. [Google Scholar]
- Sahajwalla, V.; Rahman, M.; Hong, L.; Saha-Chaudhury, N.; Spencer, D. Influence of Carbonaceous Materials on Slag Foaming Behavior during EAF steelmaking. In Proceedings of the AISTech 2005 Proceedings. AISTech 2005, Iron and Steel Technology Conference, Charlotte, NC, USA, 9–12 May 2005; Association for Iron and Steel Technology: Warrendale, PA, USA, 2005; pp. 639–650. [Google Scholar]
- Yunos, N.F.M.; Ahmad, K.R.; Zaharia, M.; Sahajwala, V. Combustion of Agricultural Waste and Coke Blends during High Temperature Processes: Effect of Physical, Chemical and Surface Properties. J. Jpn. Soc. Exp. Mech. 2011, 11, s261–s266. [Google Scholar] [CrossRef]
- Yunos, F.N.M.; Zaharia, M.; Idris, M.A.; Nath, D.; Khanna, R.; Sahajwalla, V. Recycling Agricultural Waste from Palm Shells during Electric Arc Furnace Steelmaking. Energ Fuel 2012, 26, 278–286. [Google Scholar] [CrossRef]
- Fidalgo, B.; Berrueco, C.; Millan, M. Chars from agricultural wastes as greener fuels for electric arc furnaces. J. Anal. Appl. Pyrol. 2015, 113, 274–280. [Google Scholar] [CrossRef]
- Kalde, A.; Demus, T.; Echterhof, T.; Pfeifer, H. Determining the Reactivity of Biochar-Agglomerates to Replace Fossil Coal in Electric Arc Furnace Steelmaking. In Proceedings of the EUBCE 2015 Online Conference Proceedings. 23rd European Biomass Conference and Exhibition, Vienna, Austria, 1–4 June 2015; pp. 497–507. [Google Scholar] [CrossRef]
- Kalde, A.; Willms, T.; Demus, T.; Echterhof, T.; Pfeifer, H. Determining the Potential of Biogenic Calcium- and Carbon-Rich Residues as Substitutes in the Electric Steelmaking. In Proceedings of the EUBCE 2016 Online Conference Proceedings. 24th European Biomass Conference and Exhibition, Amsterdam, The Netherlands, 6–9 June 2016; pp. 1642–1649. [Google Scholar] [CrossRef]
- Huang, X.-A.; Ng, K.W.; Giroux, L.; Duchesne, M. Carbonaceous Material Properties and Their Interactions with Slag During Electric Arc Furnace Steelmaking. Metall. Mater. Trans. B 2019, 50, 1387–1398. [Google Scholar] [CrossRef]
- Mansuri, I.; Farzana, R.; Rajarao, R.; Sahajwalla, V. Carbon Dissolution Using Waste Biomass—A Sustainable Approach for Iron-Carbon Alloy Production. Metals 2018, 8, 290. [Google Scholar] [CrossRef]
- Kongkarat, S. Recarburization of Liquid Steel Using Rubber Tree Bark: Implication for Utilizing Agricultural Waste in EAF Steelmaking. Iron Steel Technol. 2019, 16, 64–69. [Google Scholar]
- Bianco, L.; Baracchini, G.; Cirilli, F.; Di Sante, L.; Moriconi, A.; Moriconi, E.; Agorio, M.M.; Pfeifer, H.; Echterhof, T.; Demus, T.; et al. Sustainable Electric Arc Furnace Steel Production: GreenEAF. In Proceedings of the 10th European Electric Steelmaking Conference (EEC), Graz, Austria, 25–28 September 2012. [Google Scholar]
- Bianco, L.; Baracchini, G.; Cirilli, F.; Moriconi, A.; Moriconi, E.; Marcos, M.; Demus, T.; Echterhof, T.; Pfeifer, H.; Beiler, C.; et al. Sustainable EAF steel production (GREENEAF); Publications Office of the European Union: Luxembourg, 2013. [Google Scholar] [CrossRef]
- Demus, T.; Echterhof, T.; Pfeifer, H. Replacement of fossil carbon with biogenic residues in the electric steelmaking process. In Proceedings of the International Workshop EAF Perspectives on Automation, Materials, Energy & Environment, Milano, Italy, 29–30 March 2012. [Google Scholar]
- Demus, T.; Reichel, T.; Schulten, M.; Echterhof, T.; Pfeifer, H. Increasing the sustainability of steel production in the electric arc furnace by substituting fossil coal with biochar agglomerates. Ironmak. Steelmak. 2016, 43, 564–570. [Google Scholar] [CrossRef]
- Funke, A.; Demus, T.; Willms, T.; Schenke, L.; Echterhof, T.; Niebel, A.; Pfeifer, H.; Dahmen, N. Application of fast pyrolysis char in an electric arc furnace. Fuel Process Technol. 2018, 174, 61–68. [Google Scholar] [CrossRef]
- Baracchini, G.; Bianco, L.; Cirilli, F.; Echterhof, T.; Griessacher, T.; Marcos, M.; Mirabile, D.; Reichel, T.; Rekersdrees, T.; Sommerauer, H. Biochar for a Sustainable EAF Steel Production (GREENEAF2); Publications Office of the European Union: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Cirilli, F.; Baracchini, G.; Bianco, L. EAF long term industrial trials of utilization of char from biomass as fossil coal substitute. La Metallurgia Italiana 2017, 109, 13–17. [Google Scholar]
- Echterhof, T.; Demus, T.; Pfeifer, H.; Schlinge, L.; Schliephake, H. Investigation of palm kernel shells as a substitute for fossil carbons in a 140 t DC Electric Arc Furnace. In Proceedings of the 11th European Electric Steelmaking Conference & Expo. 11th European Electric Steelmaking Conference & Expo, Venice, Italy, 25–27 May 2016; pp. 1–10. [Google Scholar]
- Meier, T.; Hay, T.; Echterhof, T.; Pfeifer, H.; Rekersdrees, T.; Schlinge, L.; Elsabagh, S.; Schliephake, H. Process Modeling and Simulation of Biochar Usage in an Electric Arc Furnace as a Substitute for Fossil Coal. Steel Res. Int. 2017, 88, 1600458. [Google Scholar] [CrossRef]
- Robinson, R.; Brabie, L.; Pettersson, M.; Amovic, M.; Ljunggren, R. An Empirical Comparative Study of Renewable Biochar and Fossil Carbon as Carburizer in Steelmaking. ISIJ Int. 2020. [Google Scholar] [CrossRef]
- Zaharia, M.; Sahajwalla, V.; Khanna, R.; Koshy, P. Carbon/Slag Interactions between Coke/Rubber Blends and EAF Slag at 1 550 °C. ISIJ Int. 2009, 49, 1513–1521. [Google Scholar] [CrossRef]
- Zaharia, M.; Sahajwalla, V.; Saha-Chaudhury, N.; O’Kane, P.; Fontana, A.; Skidmore, C.; Knights, D. Recycling of Rubber Tyres in Electric Arc Furnace Steelmaking: Carbon/Slag Reactions of Coke/Rubber Blends. High Temp. Mater. Proc. 2012, 31, 593–602. [Google Scholar] [CrossRef]
- Zaharia, M.; Sahajwalla, V.; Kim, B.-C.; Khanna, R.; Saha-Chaudhury, N.; O’Kane, P.; Dicker, J.; Skidmore, C.; Knights, D. Recycling of Rubber Tires in Electric Arc Furnace Steelmaking: Simultaneous Combustion of Metallurgical Coke and Rubber Tyres Blends. Energy Fuel 2009, 23, 2467–2474. [Google Scholar] [CrossRef]
- Dankwah, J.R.; Koshy, P.; O’Kane, P.; Sahajwalla, V. Reduction of FeO in EAF Steelmaking Slag by Blends of Metallurgical Coke and End-of-Life Tyre. Steel Res. Int. 2012, 83, 766–774. [Google Scholar] [CrossRef]
- Sahajwala, V.; Khanna, R.; Rahman, M.; Huang, Z.; Tanaka, E.; Saha-Chaudhury, N.; Knights, D.; O’Kane, P. Recycling of Waste Plastics for Slag Foaming in EAF Steelmaking. In Proceedings of the AISTech 2006 Proceedings. AISTech 2006, Iron and Steel Technology Conference, Cleveland, OH, USA, 1–4 May 2006; Association for Iron and Steel Technology: Warrendale, PA, USA, 2006; pp. 547–553. [Google Scholar]
- Sahajwala, V.; Lee, J.; Rahman, M.; Zaharia, M.; Koshy, P.; Khanna, R.; Saha-Chaudhury, N.; O’Kane, P.; Dicker, J.; Skidmore, C.; et al. Gas Phase Reactions of Coke Blends with Plastics for Chemical Energy Input into EAF Steelmaking. In Proceedings of the AISTech 2009 Proceedings. AISTech 2009, Iron and Steel Technology Conference, St. Louis, MO, USA, 4–7 May 2009; Association for Iron and Steel Technology: Warrendale, PA, USA, 2009; pp. 627–636. [Google Scholar]
- Dankwah, J.R.; Sahajwala, V.; Koshy, P.; Saha-Chaudhury, N.M.; O’Kane, P.; Skidmore, C.; Knights, D. Kinetics of Reduction of FeO in EAF Steelmaking Slag by Metallurgical Coke and Waste Plastics Blends. In Proceedings of the AISTech 2010 Proceedings. AISTech 2010, Iron and Steel Technology Conference, Pittsburgh, PA, USA, 3–6 May 2010; Association for Iron and Steel Technology: Warrendale, PA, USA, 2010; pp. 895–903. [Google Scholar]
- Sahajwalla, V.; Kongkarat, S.; Koshy, P.; Khanna, R.; Zaharia, M.; Saha-Chaudhury, N.; O’Kane, P.; Skidmore, C.; Knights, D. Utilization of Waste Plastics in EAF Steelmaking: High-temperature Interactions between Slag and Carbonaceous Materials. In Proceedings of the AISTech 2010 Proceedings. AISTech 2010, Iron and Steel Technology Conference, Pittsburgh, PA, USA, 3–6 May 2010; Association for Iron and Steel Technology: Warrendale, PA, USA, 2010; pp. 127–136. [Google Scholar]
- Sahajwalla, V.; Zaharia, M.; Kongkarat, S.; Khanna, R.; Rahman, M.; Saha-Chaudhury, N.; O’Kane, P.; Dicker, J.; Skidmore, C.; Knights, D. Recycling End-of-Life Polymers in an Electric Arc Furnace Steelmaking Process: Fundamentals of Polymer Reactions with Slag and Metal. Energy Fuel 2012, 26, 58–66. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Zaharia, M.; Kongkarat, S.; Khanna, R.; Saha-Chaudhury, N.; O’Kane, P. Recycling Plastics as a Resource for Electric Arc Furnace (EAF) Steelmaking: Combustion and Structural Transformations of Metallurgical Coke and Plastic Blends. Energy Fuel 2010, 24, 379–391. [Google Scholar] [CrossRef]
- Dankwah, J.R.; Koshy, P. Reduction of FeO in EAF Steelmaking Slag by Blends of Metallurgical Coke and Waste Polypropylene. High Temp. Mater. Proc. 2014, 33, 107–114. [Google Scholar] [CrossRef]
- Dankwah, J.R.; Koshy, P.; Sahajwalla, V. Reduction of FeO in EAF steelmaking slag by blends of metallurgical coke and end-of-life polyethylene terephthalate. Ironmak. Steelmak. 2014, 41, 401–409. [Google Scholar] [CrossRef]
- Kongkarat, S.; Khanna, R.; Koshy, P.; O’kane, P.; Sahajwalla, V. Recycling Waste Polymers in EAF Steelmaking: Influence of Polymer Composition on Carbon/Slag Interactions. ISIJ Int. 2012, 52, 385–393. [Google Scholar] [CrossRef]
- Kongkarat, S.; Cherdhirunkorn, B.; Thongreang, R. Utilization of Waste HDPE for Sustainable EAF Steelmaking: Carbon Dissolution into Liquid Steel. Steel Res. Int. 2017, 88, 1600168. [Google Scholar] [CrossRef]
- Mansuri, I.; Khanna, R.; Sahajwalla, V. Recycling Carbonaceous Industrial/Commercial Waste as a Carbon Resource in Iron and Steelmaking. Steel Res. Int. 2017, 88, 1600333. [Google Scholar] [CrossRef]
- Gorez, J.-P.; Gros, B.; Birat, J.-P.; Grisvard, C.; Huber, J.-C.; Le Coq, X. Charging tires in the EAF as a substitute to carbon. Rev. Metall. 2003, 100, 17–23. [Google Scholar] [CrossRef]
- Ayed, P.; Clauzade, C.; Gros, B.; Huber, J.-C.; Lebrun, C.; Vassart, N. Charging used tyres in the EAF as a substitute for carbon: A success story for LME and Industeel Belgium. Rev. Metall. 2007, 104, 128–135. [Google Scholar] [CrossRef]
- Sahajwala, V.; Rahman, M.; Khanna, R.; Dicker, J.; Knights, D.; O’Kane, P.; Skidmore, C. Novel Industrial Trials Demonstrating The Use Of Plastics For EAF Slag Foaming. In Proceedings of the AISTech 2007 Proceedings. AISTech 2007, Iron and Steel Technology Conference, Indianapolis, IN, USA, 7–10 May 2007; Association for Iron and Steel Technology: Warrendale, PA, USA, 2007; pp. 1–12. [Google Scholar]
- Sahajwala, V.; Khanna, R.; Zaharia, M.; Kongkarat, S.; Rahman, M.; Kim, B.C.; Saha-Chaudhury, N.; O’Kane, P.; Dicker, J.; Skidmore, C.; et al. Environmentally Sustainable EAF Steelmaking Through Introduction of Recycled Plastics and Tires: Laboratory and Plant Studies. Iron Steel Technol. 2009, 6, 43–50. [Google Scholar]
- Sahajwalla, V.; Zaharia, M.; Rahman, M.; Khanna, R.; Saha-Chaudhury, N.; O’Kane, P.; Dicker, J.; Skidmore, C.; Knights, D. Recycling Rubber Tyres and Waste Plastics in EAF Steelmaking. Steel Res. Int. 2011, 82, 566–572. [Google Scholar] [CrossRef]
- Joulazadeh, M.H. Using Scrap Tires in EAFs as a Substitute for Carbon. Int. J. Iron Steel Soc. Iran 2008, 5, 36–40. [Google Scholar]
- Clauzade, C.; Osset, P.; Hugrel, C.; Chappert, A.; Durande, M.; Palluau, M. Life cycle assessment of nine recovery methods for end-of-life tyres. Int. J. Life Cycle Ass. 2010, 15, 883–892. [Google Scholar] [CrossRef]
- O’Kane, P.; Fontana, A.; Skidmore, C.; Jin, Z. Sustainable EAF Steelmaking Through The Use of Polymer Technology. In Proceedings of the AISTech 2016 Proceedings. AISTech 2016, Iron and Steel Technology Conference and Exposition, Pittsburgh, PA, USA, 16–19 May 2016; Association for Iron and Steel Technology: Warrendale, PA, USA, 2016; pp. 1–12, ISBN 9781935117551. [Google Scholar]
- Fontana, A.; Alvarez, A.C.A.; Iacuzzi, M.; O’Kane, P.; Jin, Z. Injection of Recycled Rubber Tires in the EAF as Foaming Slag Agent at CELSA Group. In Proceedings of the EEC 2016-11th European Electric Steelmaking Conference & Expo, Venice, Italy, 25–26 May 2016; Associazione Italiana di Metallurgia, Ed.; AIM: Milano, Italy, 2016; pp. 1–10. [Google Scholar]
- Cirilli, F.; Di Sante, L.; Faraci, E.; Fusato, M.; Foglio, G.; Frittella, P.; Filippini, E.; Tolettini, E. ASR utilization as carbon substitute into the EAF. In Proceedings of the Clean Tech 4, The 4th European Conference on Clean Technologies in the Steel Industry, Bergamo, Italy, 28–29 November 2018; Associazione Italiana di Metallurgia: Milano, Italy, 2018; pp. 1–10, ISBN 978-88-98990-18-4. [Google Scholar]
- Pei, M.; Petäjäniemi, M.; Regnell, A.; Wijk, O. Toward a Fossil Free Future with HYBRIT: Development of Iron and Steelmaking Technology in Sweden and Finland. Metals 2020, 10, 972. [Google Scholar] [CrossRef]
- Salzgitter, A.G. SALCOS-Climate Initiative for Low CO2 Steel Production. Available online: https://salcos.salzgitter-ag.com/en/ (accessed on 29 December 2020).
- Verbund Solutions GmbH. H2FUTURE Project. Available online: https://www.h2future-project.eu (accessed on 29 December 2020).
- European Commission. Technology readiness levels (TRL); Extract from Part 19-Commission Decision C(2014)4995. 2014. Available online: https://ec.europa.eu/research/participants/data/ref/h2020/wp/2014_2015/annexes/h2020-wp1415-annex-g-trl_en.pdf (accessed on 29 December 2020).
| Carbon Source | Use | Laboratory | Industrial Tests | SOP 1 | References |
|---|---|---|---|---|---|
| Biomass based | |||||
| Charcoal from various materials | Injection carbon | 3 | 3 | - | [35,37,38,39,40,41,42,44,45,49] |
| Virgin biomasses | Injection carbon | 3 | 3 | - | [36,39,40,43,49] |
| Charcoal from various materials | Charge carbon | 4 | 5 | - | [44,45,46,47,48,49,50,53] |
| Virgin biomasses | Charge carbon | 4 | 6 | - | [49,51,52] |
| Rubber and plastics based | |||||
| Rubber tire/coke blends | Injection carbon | 4 | 9 | SOP | [54,55,56,57,72,73,76,77] |
| Polymer/coke blends | Injection carbon | 4 | 7 | - | [58,59,60,61,62,63,64,65,66,67,71,72,73] |
| Pyrolyzed CFRP | Charge carbon | 3 | - | [68] | |
| Rubber tires | Charge carbon | 2 | 9 | SOP | [69,70,74] |
| Polymer/cokebriquettes | Charge carbon | 4 | 5 | - | [76] |
| ASR briquettes | Charge carbon | 2 | 5 | - | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. https://doi.org/10.3390/met11020222
Echterhof T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals. 2021; 11(2):222. https://doi.org/10.3390/met11020222
Chicago/Turabian StyleEchterhof, Thomas. 2021. "Review on the Use of Alternative Carbon Sources in EAF Steelmaking" Metals 11, no. 2: 222. https://doi.org/10.3390/met11020222
APA StyleEchterhof, T. (2021). Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals, 11(2), 222. https://doi.org/10.3390/met11020222






