The Evolution of Intermetallic Compounds in High-Entropy Alloys: From the Secondary Phase to the Main Phase
Abstract
:1. Introduction
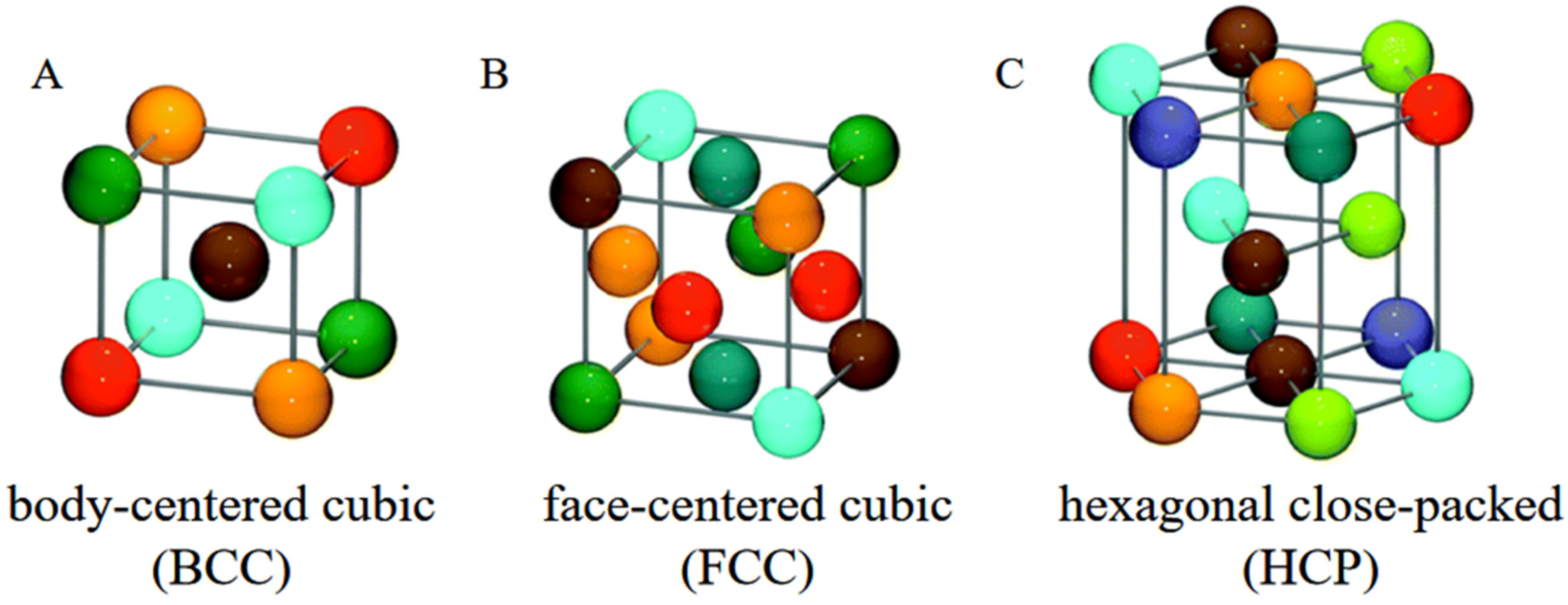
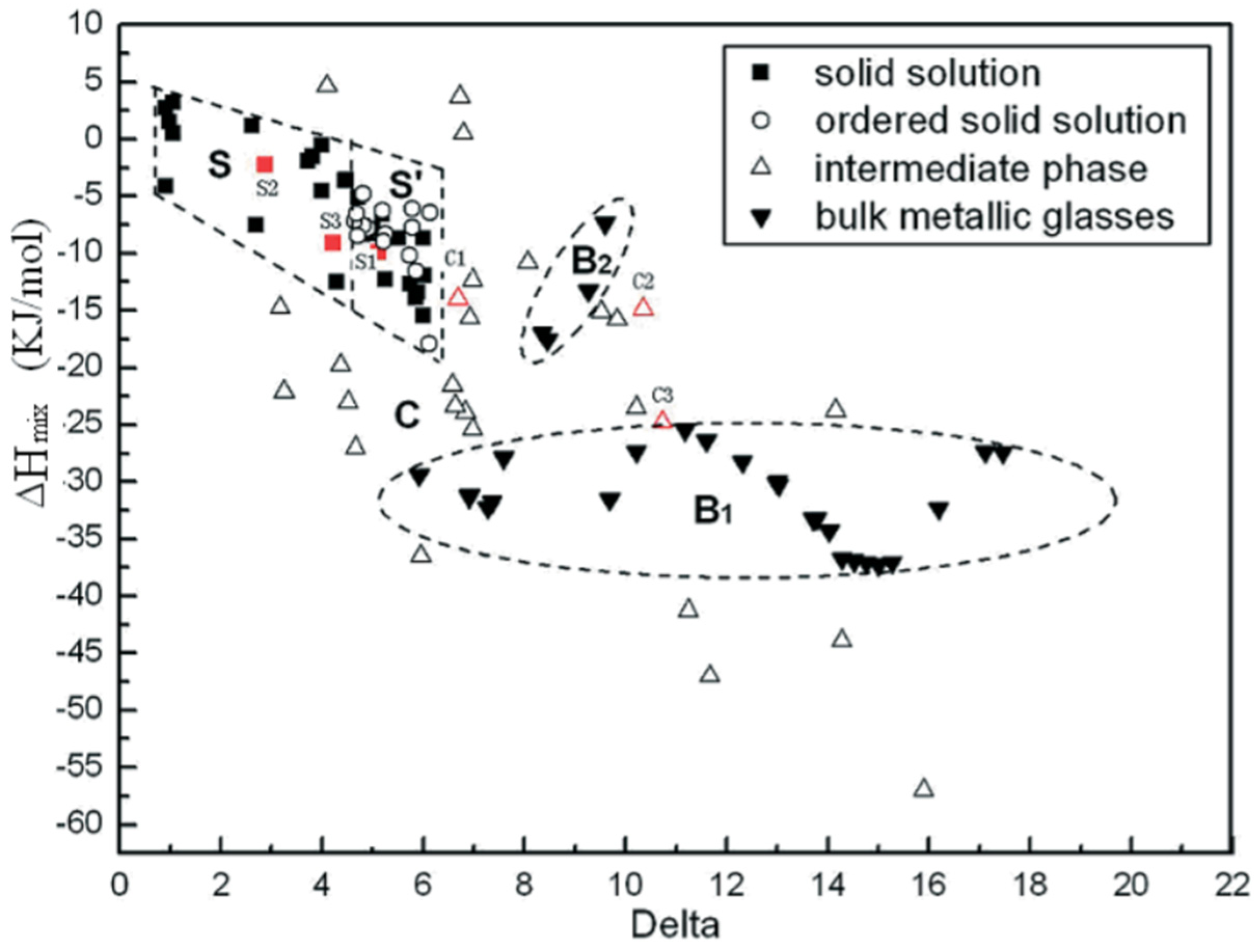
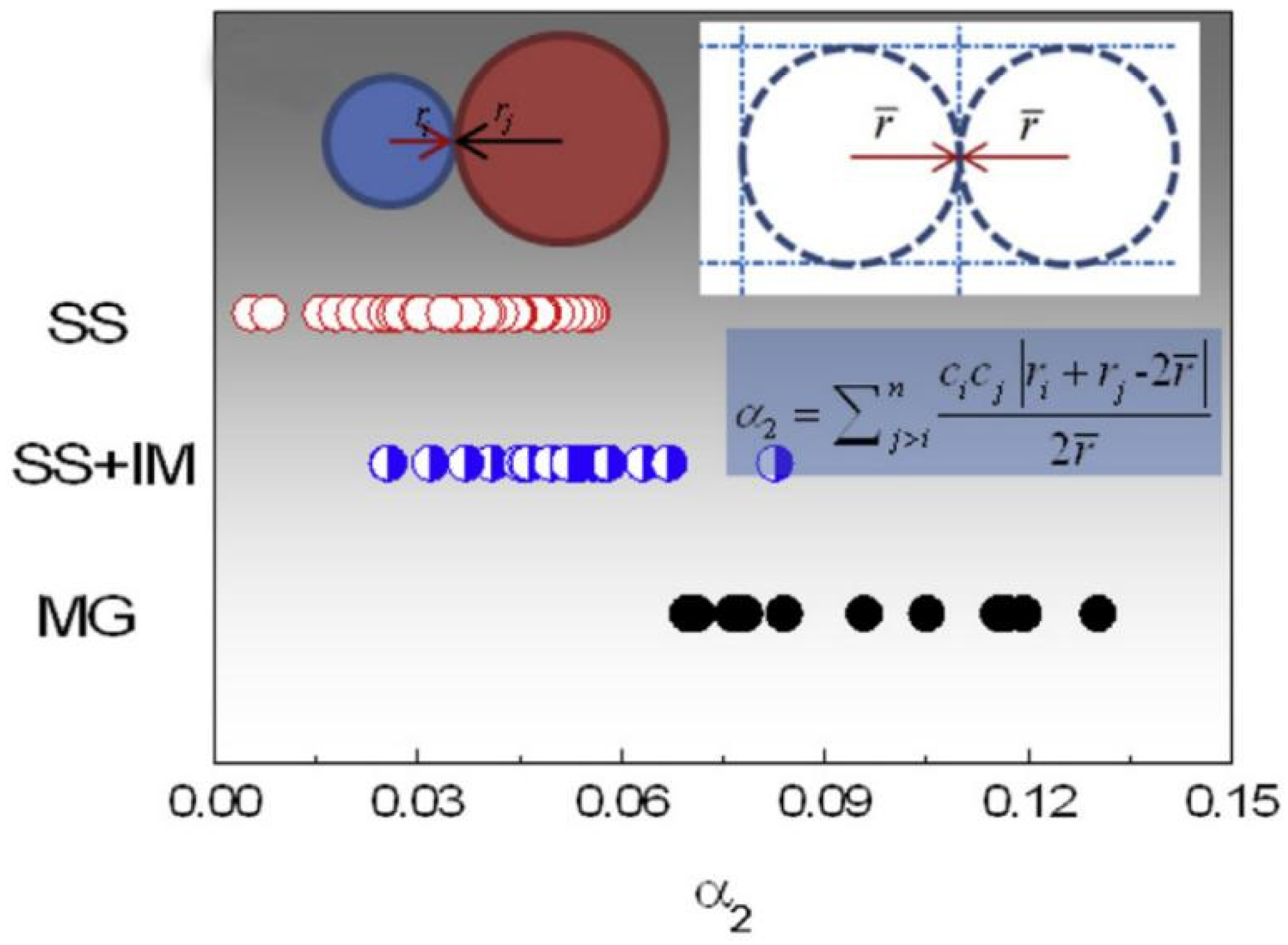
2. Intermetallic Compounds in High-Entropy Alloys
3. Intermetallic Compound-Strengthened High-Entropy Alloy
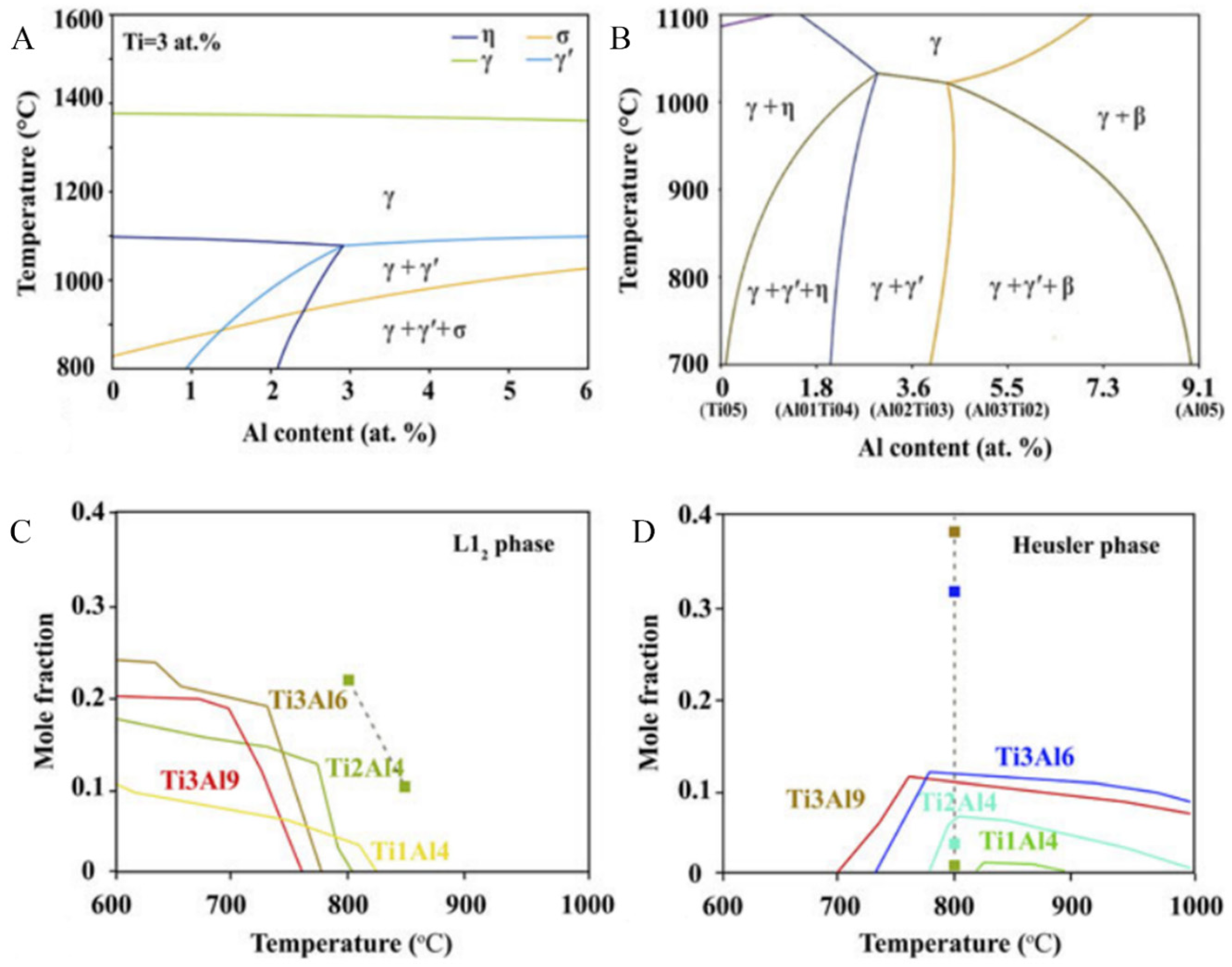
3.1. Composition Design Based on CALPHAD
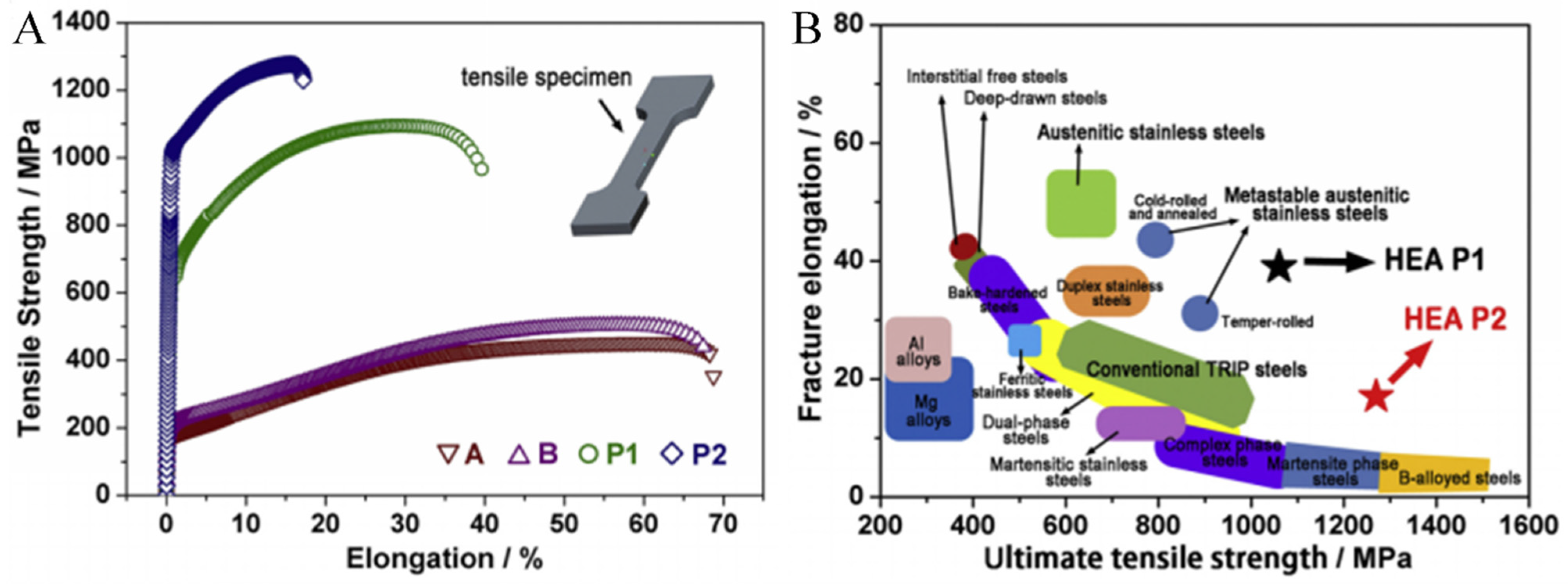
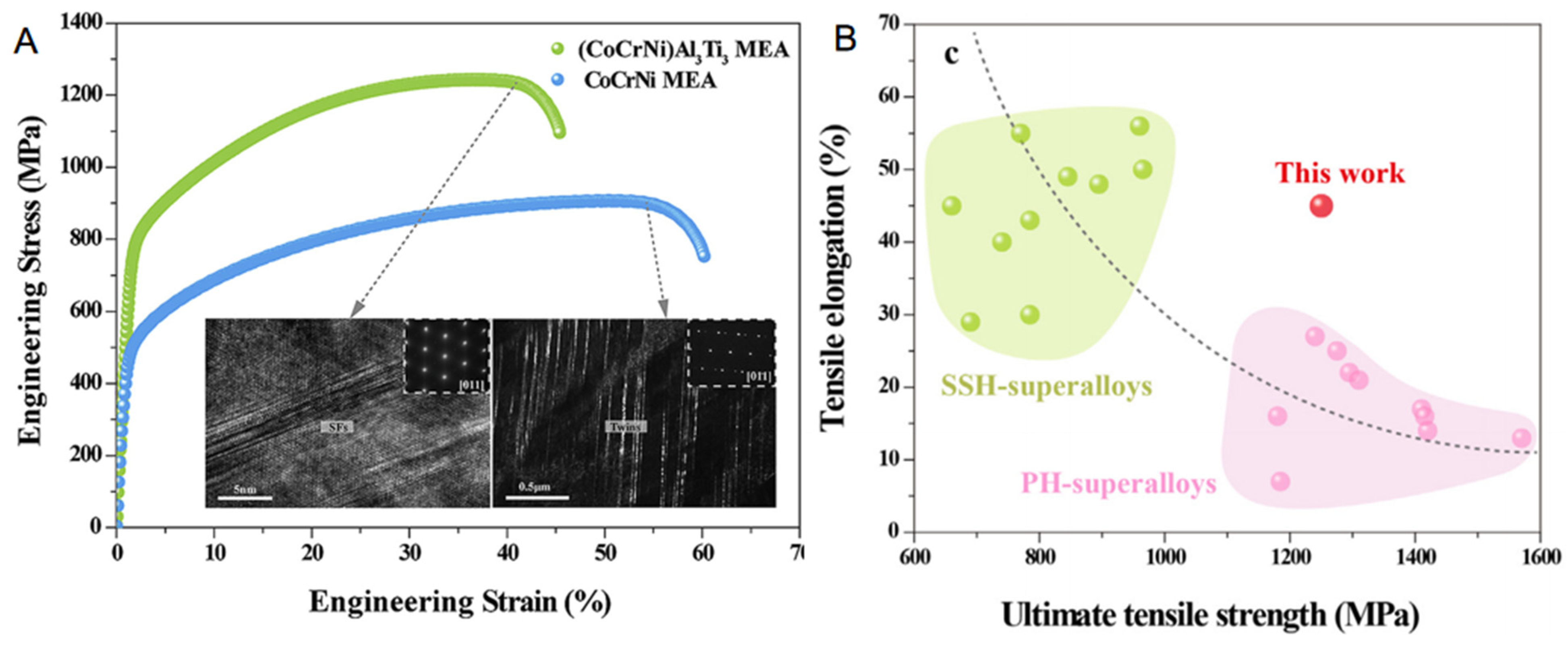
3.2. Unique Structure and Excellent Tensile Properties at Ambient Temperature
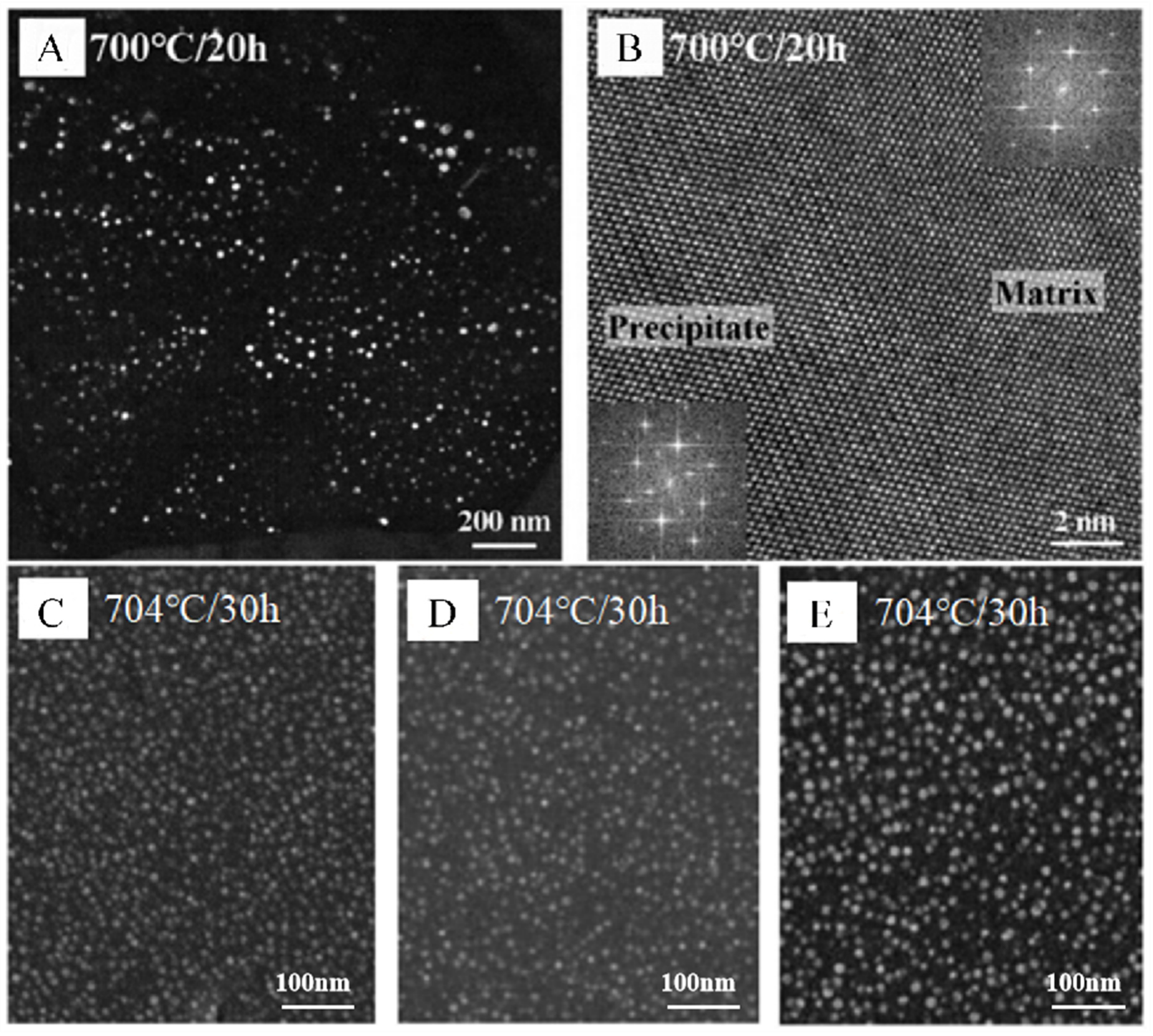
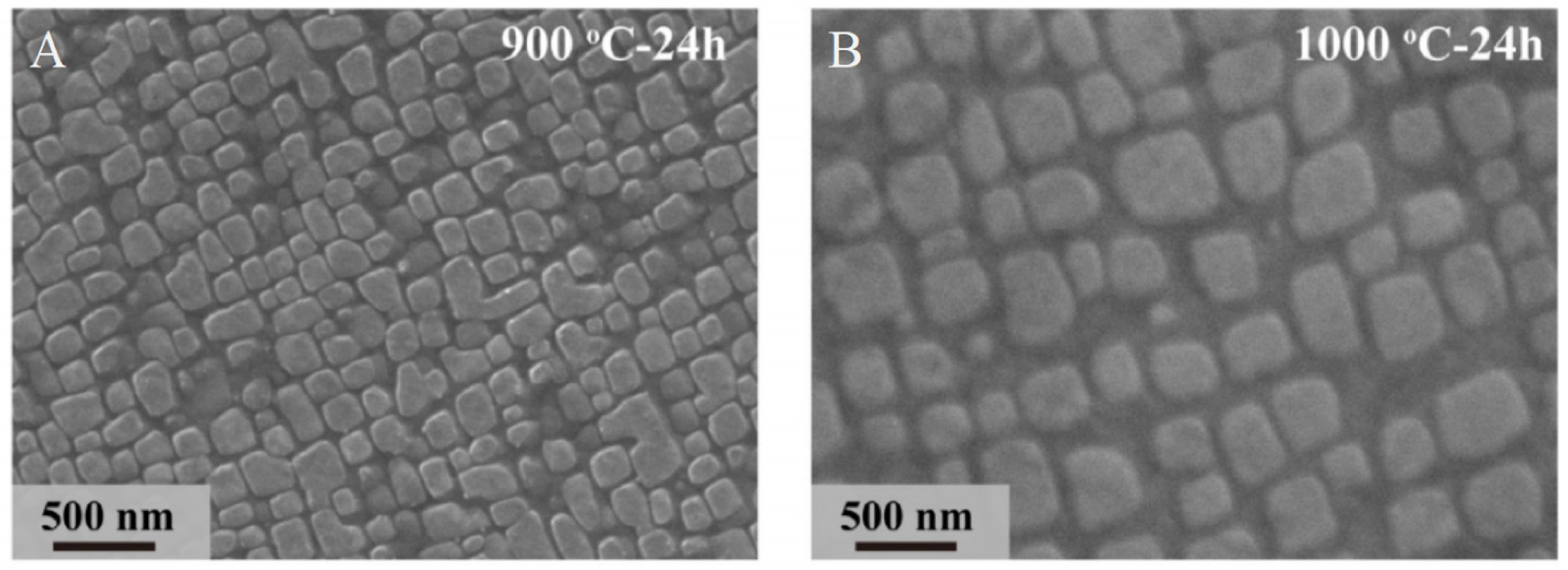
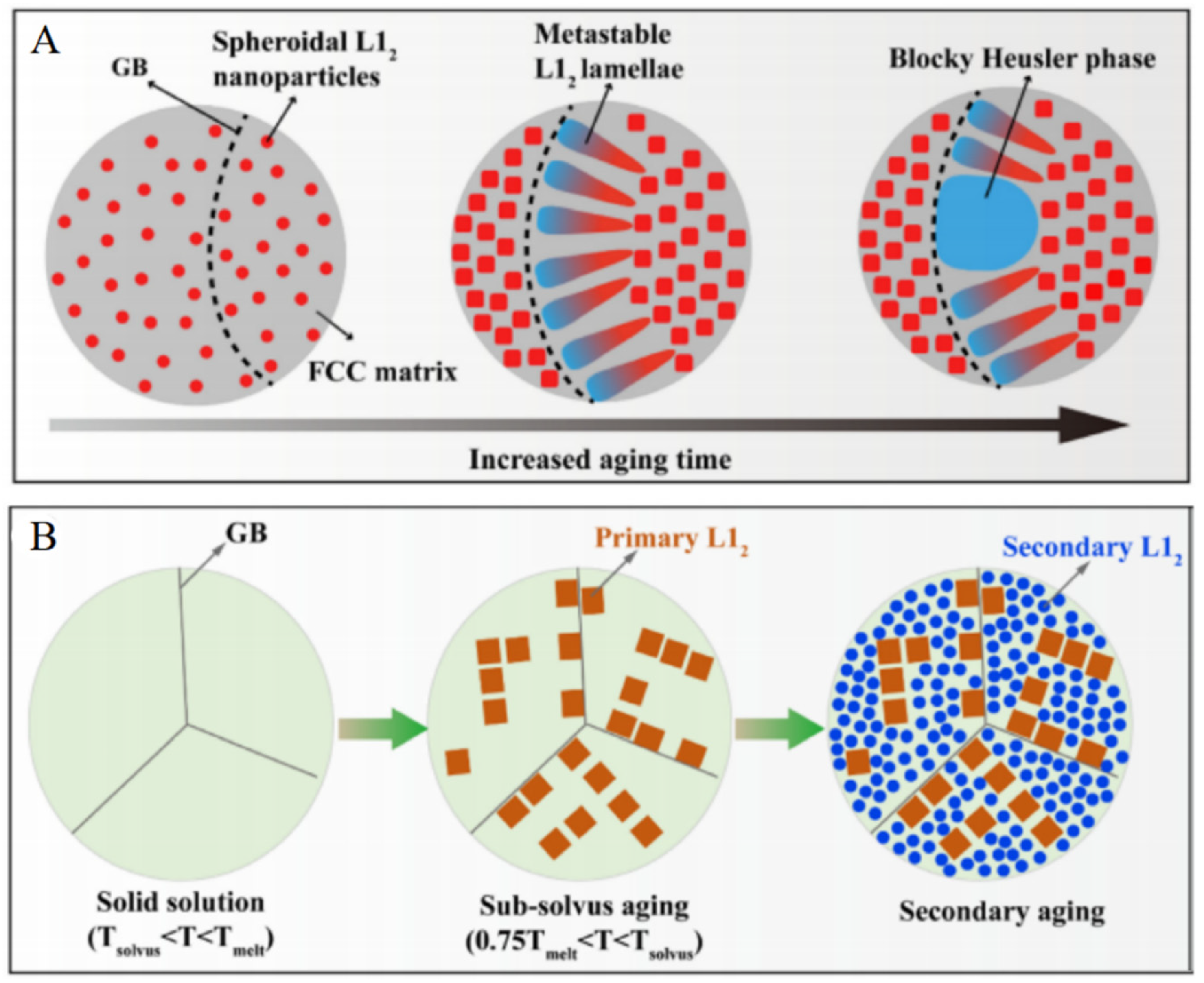
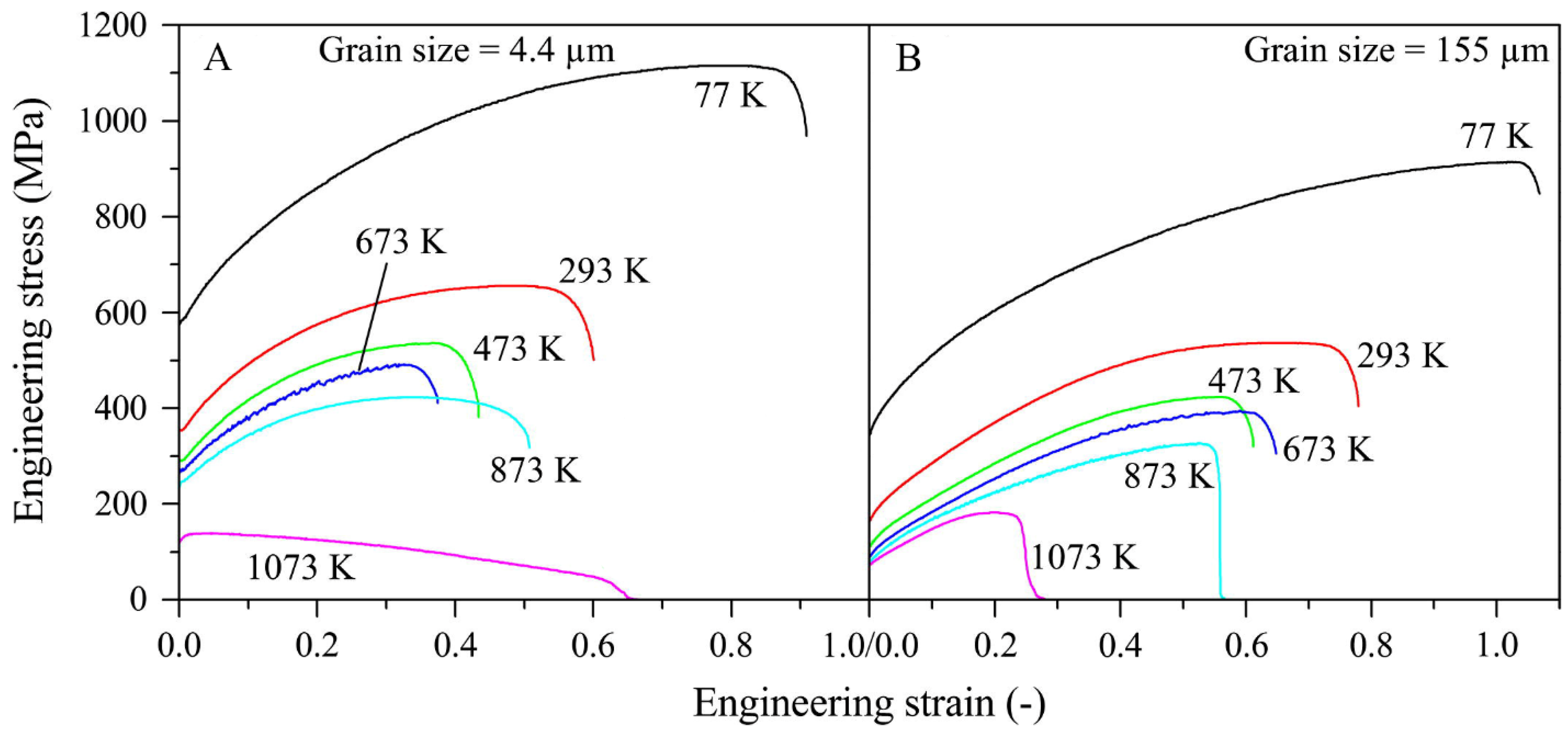
3.3. Outstanding Thermal Stability at High Temperatures
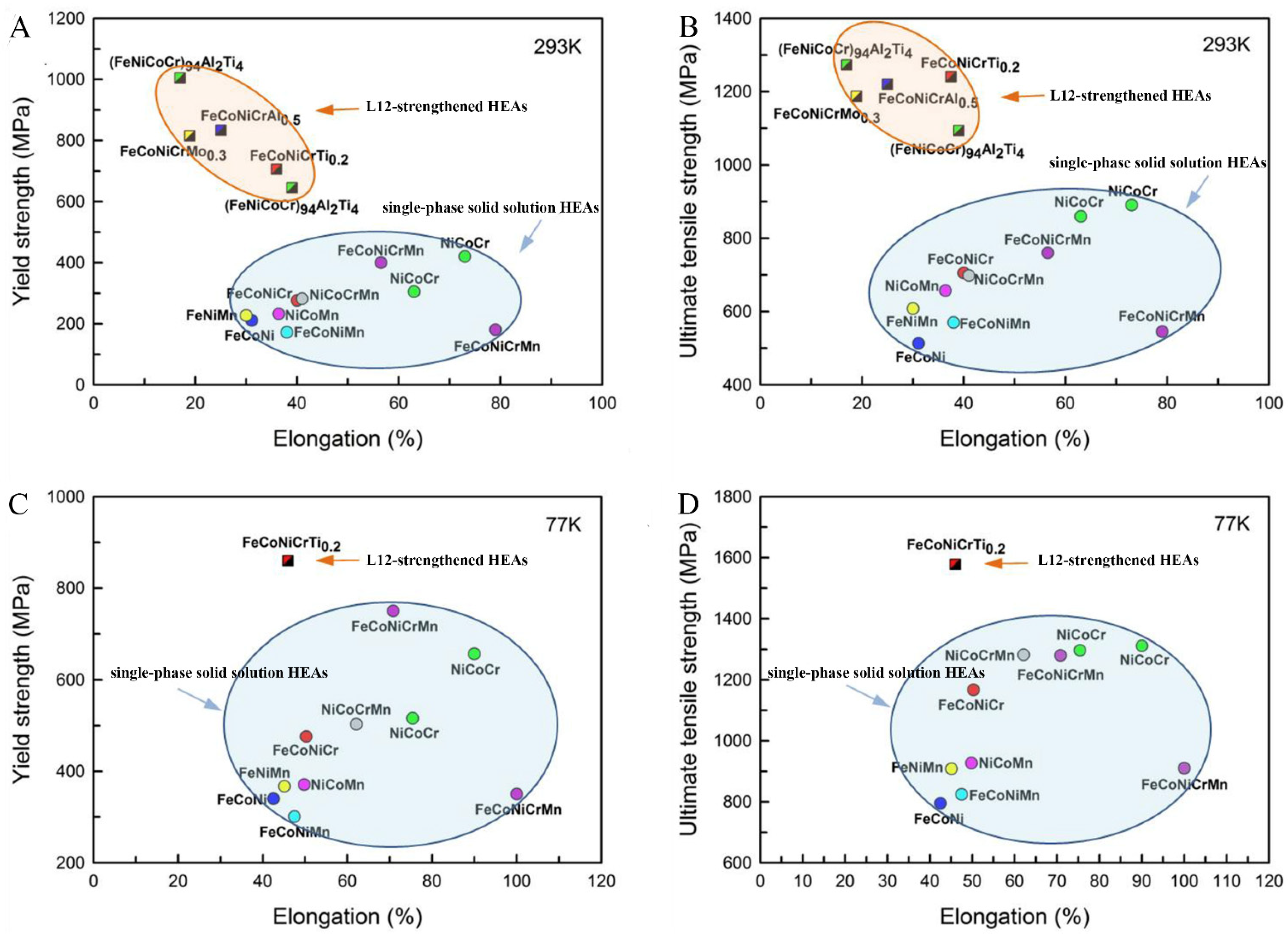
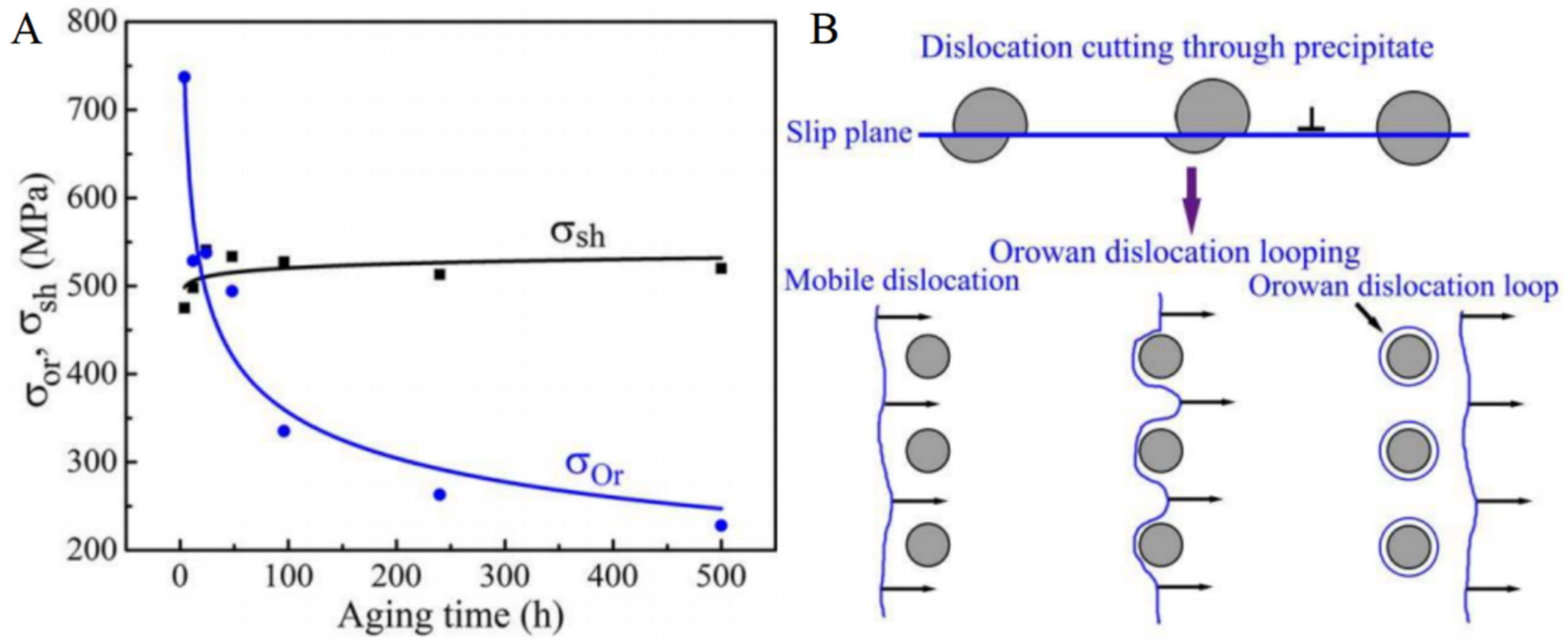
3.4. The Effect of Different Strengthening Mechanisms on the Properties of Alloys
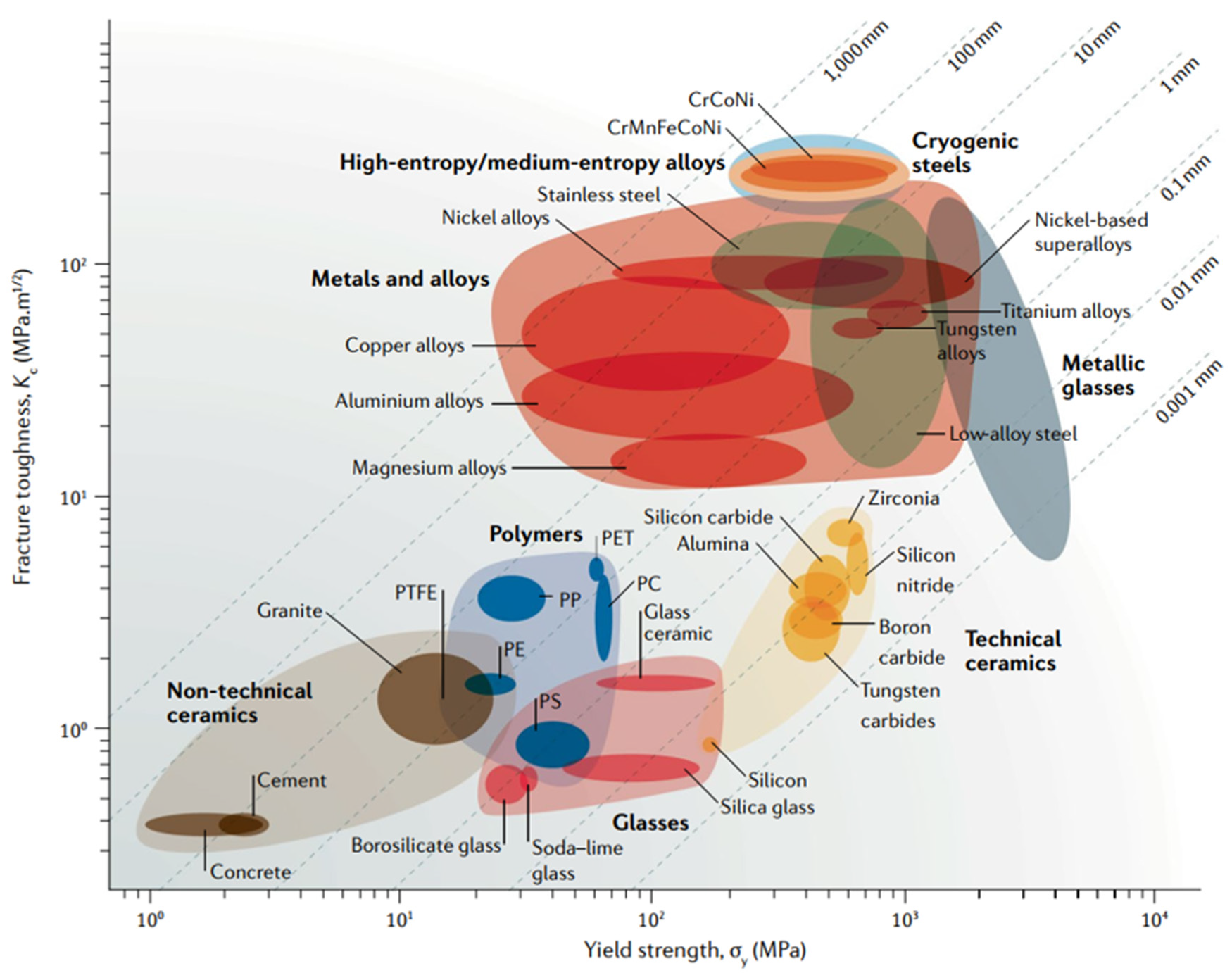
4. High-Entropy Intermetallics (HEIs)
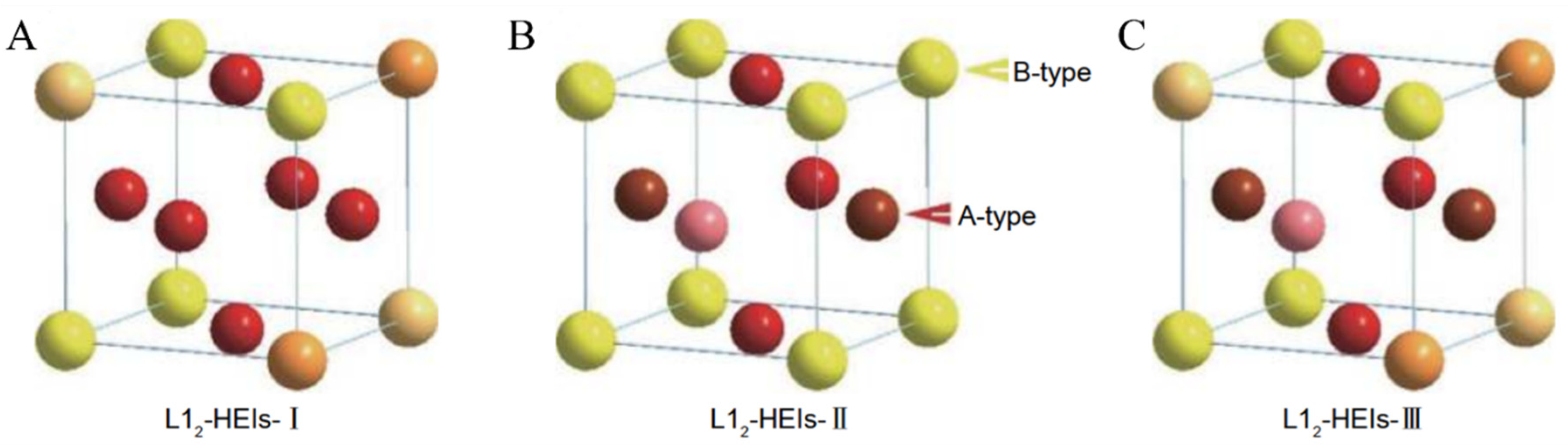
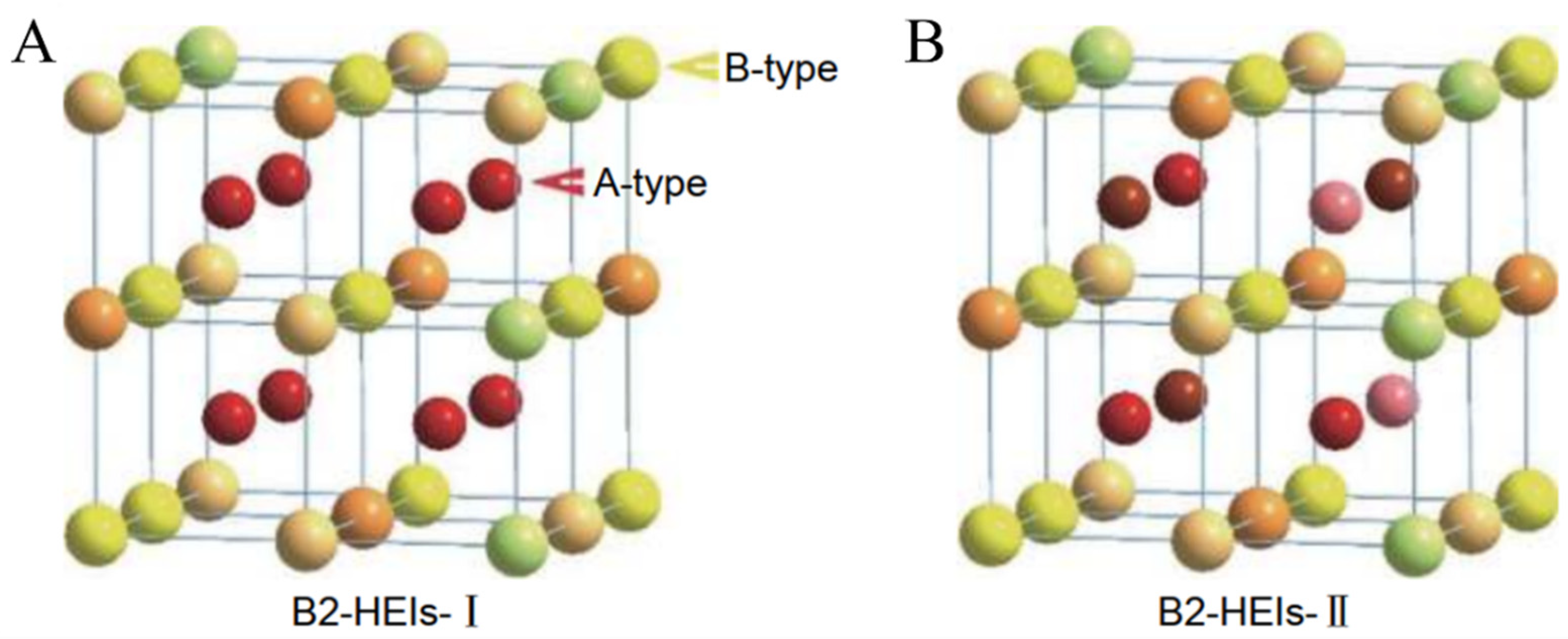
4.1. Experiments Combined with Calculations to Accelerate the Study of Atomic Occupancy
4.2. Unique Atomic Structure Provides Remarkable Comprehensive Mechanical Performance
4.3. Ingenious Structure Improves Grain Boundary Properties of Alloy
4.4. Various Plasticizing Mechanisms Effectively Improve Alloy Plasticity
4.5. Distinctive Electronic Structure Produces Attractive Catalytic Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high-entropy alloys. Eur. J. Control. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. Jom-Us 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Microstructure and compressive properties of multicomponent Alx(TiVCrMnFeCoNiCu)100-x high-entropy alloys. Mater. Sci. Eng. A 2007, 454, 260–265. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, C.; Dong, J.; Zhang, M. The effect of Mo and Ti elements on long-term microstructure stability of 617B nickel-base superalloy. J. Alloys Compd. 2020, 821, 153217. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.B.; Zhang, C.; Li, J.C.; Jiang, Q. Microstructure and the properties of FeCoCuNiSnx high entropy alloys. Mater. Sci. Eng. A. 2012, 548, 64–68. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.Z.; Zhou, W.; Fu, M.L.; Wang, F.Z.; Luo, C.R. Phase stability of non-equiatomic CoCrFeMnNi high entropy alloys. Acta Mater. 2015, 98, 288–296. [Google Scholar]
- Gali, A.; George, E.P. Tensile properties of high- and medium-entropy alloys. Intermetallics 2013, 39, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Liu, W.H.; Wu, Y.; He, J.Y.; Nieh, T.G.; Lu, Z.P. Grain growth and the Hall–Petch relationship in a high-entropy FeCrNiCoMn alloy. Scripta Mater. 2013, 68, 526–529. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Pharr, G.M.; George, E.P. Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures. Acta Mater. 2014, 81, 428–441. [Google Scholar] [CrossRef]
- He, J.Y.; Zhu, C.; Zhou, D.Q.; Liu, W.H.; Nieh, T.G.; Lu, Z.P. Steady state flow of the FeCoNiCrMn high entropy alloy at elevated temperatures. Intermetallics 2014, 55, 9–14. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Qiao, Y.; Chen, G.L. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Otto, F.; Hanold, N.L.; George, E.P. Microstructural evolution after thermomechanical processing in an equiatomic, single-phase CoCrFeMnNi high-entropy alloy with special focus on twin boundaries. Intermetallics 2014, 54, 39–48. [Google Scholar] [CrossRef]
- Haglund, A.; Koehler, M.; Catoor, D.; George, E.P.; Keppens, V. Polycrystalline elastic moduli of a high-entropy alloy at cryogenic temperatures. Intermetallics 2015, 58, 62–64. [Google Scholar] [CrossRef] [Green Version]
- Laplanche, G.; Gadaud, P.; Horst, O.; Otto, F.; Eggeler, G.; George, E.P. Temperature dependencies of the elastic moduli and thermal expansion coefficient of an equiatomic, single-phase CoCrFeMnNi high-entropy alloy. J. Alloys Compd. 2015, 623, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Laurent-Brocq, M.; Akhatova, A.; Perrière, L.; Chebini, S.; Sauvage, X.; Leroy, E.; Champion, Y. Insights into the phase diagram of the CrMnFeCoNi high entropy alloy. Acta Mater. 2015, 88, 355–365. [Google Scholar] [CrossRef]
- Laplanche, G.; Horst, O.; Otto, F.; Eggeler, G.; George, E.P. Microstructural evolution of a CoCrFeMnNi high-entropy alloy after swaging and annealing. J. Alloys Compd. 2015, 647, 548–557. [Google Scholar] [CrossRef]
- Laplanche, G.; Kostka, A.; Horst, O.M.; Eggeler, G.; George, E.P. Microstructure evolution and critical stress for twinning in the CrMnFeCoNi high-entropy alloy. Acta Mater. 2016, 118, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Bracq, G.; Laurent-Brocq, M.; Perrière, L.; Pirès, R.; Joubert, J.M.; Guillot, I. The fcc solid solution stability in the Co-Cr-Fe-Mn-Ni multi-component system. Acta Mater. 2018, 128, 327–336. [Google Scholar] [CrossRef]
- Laplanche, G.; Berglund, S.; Reinhart, C.; Kostka, A.; Fox, F.; George, E.P. Phase stability and kinetics of σ-phase precipitation in CrMnFeCoNi high-entropy alloys. Acta Mater. 2018, 161, 338–351. [Google Scholar] [CrossRef]
- Sebastian, H.; Mike, M.; Oleg, S.; Michael, F.; Jens, F.; Senol, G.; Rainer, V.L.; Uwe, G. Entropy determination of single-phase high-entropy alloys with different crystal structures over a wide temperature range. Entropy 2018, 20, 654. [Google Scholar]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.F. Microstructure and properties of a refractory NbCrMo0.5Ta0.5TiZr alloy. Mater. Sci. Eng. A 2011, 529, 311–320. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Miracle, D.B.; Woodward, C.F. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloys Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Meisenkothen, F.; Miracle, D.B.; Woodward, C.F. Microstructure and elevated temperature properties of a refractory TaNbHfZrTi alloy. J. Mater. Sci. 2012, 47, 4062–4074. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Senkov, O.N.; Semiatin, S.L. Microstructure and properties of a refractory high-entropy alloy after cold working. J. Alloys Compd. 2015, 649, 1110–1123. [Google Scholar] [CrossRef]
- Juan, C.C.; Tsai, M.H.; Tsai, C.W.; Lin, C.M.; Wang, W.R.; Yang, C.C.; Yeh, J.W. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 2015, 62, 76–83. [Google Scholar] [CrossRef]
- Couzinié, J.P.; Lilensten, L.; Champion, Y.; Dirras, G.; Perrière, L.; Guillot, I. On the room temperature deformation mechanisms of a TiZrHfNbTa refractory high-entropy alloy. Mater. Sci. Eng. A Struct. Mater. Prop. Misrostructure Process. 2015, 645, 255–263. [Google Scholar] [CrossRef]
- Sheikh, S.; Shafeie, S.; Hu, Q.; Ahlstr, M.J.; Persson, C.; Vesely, J.; Zyka, J.Í.; Klement, U.; Guo, S. Alloy design for intrinsically ductile refractory high-entropy alloys. J. Appl. Phys. 2016, 120, 164902. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Amiya, K.; Wada, T.; Yubuta, K.; Zhang, W. High-entropy alloys with a hexagonal close-packed structure designed by equi-atomic alloy strategy and binary phase diagrams. Jom-Us 2014, 66, 1984–1992. [Google Scholar] [CrossRef]
- Feuerbacher, B.M.; Heidelmann, M.; Thomas, A.C. Hexagonal high-entropy alloy. Mater. Res. Lett. 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Tong, X.; Zhang, H.; Wang, H.; Liu, X.J.; Ma, L.; Suo, H.L.; Lu, Z.P. Rare-earth high-entropy alloys with giant magnetocaloric effect. Acta Mater. 2017, 125, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.J.; Qiao, J.W.; Ma, S.G.; Gao, M.C.; Yang, H.J.; Chen, M.W.; Zhang, Y. A hexagonal close-packed high-entropy alloy: The effect of entropy. Mater. Des. 2016, 96, 10–15. [Google Scholar] [CrossRef]
- Soler, R.; Evirgen, A.; Yao, M.; Kirchlechner, C.; Stein, F.; Feuerbacher, M.; Raabe, D.; Dehm, G. Microstructural and mechanical characterization of an equiatomic YGdTbDyHo high-entropy alloy with hexagonal close-packed structure. Acta Mater. 2018, 156, 86–96. [Google Scholar] [CrossRef]
- Schuh, B.; Mendez-Martin, F.; Völker, B.; George, E.P.; Clemens, H.; Pippan, R.; Hohenwarter, A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater. 2015, 96, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Pickering, E.J.; Muñoz-Moreno, R.; Stone, H.J.; Jones, N.G. Precipitation in the equiatomic high-entropy alloy CrMnFeCoNi. Scr. Mater. 2016, 113, 106–109. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhy, A.; Pradeep, K.G.; Kuběnová, M.; Raabe, D.; Eggeler, G.; George, E.P. Decomposition of the single-phase high-entropy alloy CrMnFeCoNi after prolonged anneals at intermediate temperatures. Acta Mater. 2016, 112, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Schuh, B.V.; Lker, B.; Todt, J.; Schell, N.; Perriere, L.; Li, J.; Couzinié, J.P.; Hohenwarter, A. Thermodynamic instability of a nanocrystalline, single-phase TiZrNbHfTa alloy and its impact on the mechanical properties. Acta Mater. 2017, 142, 201–212. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Aging behavior of the HfNbTaTiZr high entropy alloy. Mater. Lett. 2017, 87–90. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.; Shun, T.T.; Chen, S.K. Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. Part A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 253. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhang, Y. Effect of Co addition on crystal structure and mechanical properties of Ti0.5CrFeNiAlCo high entropy alloy. Mater. Sci. Eng. A 2008, 496, 214–216. [Google Scholar] [CrossRef]
- Shun, T.T.; Hung, C.H.; Lee, C.F. Formation of ordered/disordered nanoparticles in fcc high entropy alloys. J. Alloys Compd. 2010, 493, 105–109. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Ma, Y.J.; Ma, Y.; Wang, Q.S.; Simon, S.; Miriam, B.; Fu, T.T.; Horst, H.; Torsten, B.; Ben, B. High-entropy energy materials: Challenges and new opportunities. Energ. Environ. Sci. 2021, 14, 2883–2905. [Google Scholar] [CrossRef]
- Chou, Y.L.; Yeh, R.W.; Shih, R.C. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys C1.5CrFeNi1.5Ti0.5MOx in aqueous environments. Corros. Sci. 2010, 52, 2571–2581. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Chen, H.W.; Lu, Z.P.; Nieh, T.G. Thermal stability and coarsening of coherent particles in a precipitation-hardened (NiCoFeCr)94Ti2Al4 high-entropy alloy. Acta Mater. 2018, 147, 184–194. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Wang, X.P.; Kong, F.T. Recent development in high-entropy alloys and other high-entropy materials. J. Aeronaut. Mater. 2019, 39, 1–19. [Google Scholar]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Yong, Z.A.; Ttz, A.; Zhi, T.B.; Mcgc, D.; Kad, E.; Pkl, B.; Zhao, P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar]
- Shi, P.; Ren, W.; Zheng, T.; Ren, Z.; Hou, X.; Peng, J.; Hu, P.; Gao, Y.; Zhong, Y.; Liaw, P.K. Enhanced strength–ductility synergy in ultrafine-grained eutectic high-entropy alloys by inheriting microstructural lamellae. Nat. Commun. 2019, 10, 489. [Google Scholar] [CrossRef]
- Cai, B.; Liu, B.; Kabra, S.; Wang, Y.; Yan, K.; Lee, P.D.; Liu, Y. Deformation mechanisms of Mo alloyed FeCoCrNi high entropy alloy: In situ neutron diffraction. Acta Mater. 2017, 127, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Chanho, L.; Song, G.; Gao, M.C.; Rui, F.; Chen, P.; Jamieson, B.; Yan, C.; Ke, A.; Wei, G.; Poplawsky, J.D. Lattice distortion in a strong and ductile refractory high-entropy alloy. Acta Mater. 2018, 160, S506314074. [Google Scholar]
- Zhang, Z.J.; Mao, M.M.; Wang, J.; Gludovatz, B.; Zhang, Z.; Mao, S.X.; George, E.P.; Qian, Y.; Ritchie, R.O. Nanoscale origins of the damage tolerance of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2015, 6, 10143. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural stability and mechanical behavior of FeNiMnCr high entropy alloy under ion irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef] [Green Version]
- Egami, T.; Guo, W.; Rack, P.D.; Nagase, T. Irradiation resistance of multicomponent alloys. Metall. Mater. Trans. A 2014, 45, 180–183. [Google Scholar] [CrossRef]
- Tang, Z.; Yuan, T.; Tsai, C.-W.; Yeh, J.-W.; Lundin, C.D.; Liaw, P.K. Fatigue behavior of a wrought Al0.5CoCrCuFeNi two-phase high-entropy alloy. Acta Mater. 2015, 2, 107–123. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Cheng, Y.Q.; Liaw, P.K. High-entropy alloys with high saturation magnetization, electrical resistivity, and malleability. Sci. Rep. UK 2013, 3, 1455. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Tsai, M.-H. Physical properties of high-entropy alloys. Entropy 2013, 15, 5338. [Google Scholar] [CrossRef] [Green Version]
- Chuang, M.; Tsai, M.; Wang, W.; Lin, S.; Yeh, J. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Lilensten, L.; Couzinié, J.; Bourgon, J.; Perrière, L.C.; Dirras, G.; Prima, F.; Guillot, I. Design and tensile properties of a bcc Ti-rich high-entropy alloy with transformation-induced plasticity. Mater. Res. Lett. 2017, 5, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Zhao, Y.; Liu, W.; Kai, J.; Liu, C. L12-strengthened high-entropy alloys for advanced structural applications. J. Mater. Res. 2018, 33, 2983–2997. [Google Scholar]
- Committe, A. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials. In ASM Metals Handbook; ASM International: Materials Park, OH, USA, 1990; Volume 2, p. 48. [Google Scholar]
- Jiao, Z.B.; Luan, J.H.; Liu, C.T. Strategies for improving ductility of ordered intermetallics. Prog. Nat. Sci. Mater. Int. 2016, 26, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Yu, Q.; Mark, A.; Ritchie, R.O. Tunable stacking fault energies by tailoring local chemical order in CrCoNi medium-entropy alloys. Proc. Natl. Acad. Sci. USA 2018, 115, 8919–8924. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Chung, Y.W.; Liu, C.T. Co-precipitation of nanoscale particles in steels with ultra-high strength for a new era. Mater. Today 2017, 20, 142–154. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Zhang, Z.W.; Miller, M.K.; Liu, C.T. High-strength steels hardened mainly by nanoscale NiAl precipitates. Scr. Mater. 2014, 87, 45–48. [Google Scholar] [CrossRef]
- Liu, W.H.; Yang, T.; Liu, C.T. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2017, 210, 2–11. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Age-hardening of the CoCrFeNiMo0.85 high-entropy alloy. Mater. Charact. 2013, 81, 92–96. [Google Scholar] [CrossRef]
- Shun, T.T.; Hung, C.H.; Lee, C.F. The effects of secondary elemental Mo or Ti addition in Al0.3CoCrFeNi high-entropy alloy on age hardening at 700 °C. J. Alloys Compd. 2010, 495, 55–58. [Google Scholar] [CrossRef]
- Ming, K.S.; Bi, X.F.; Wang, J. Precipitation strengthening of ductile Cr15Fe20Co35Ni20Mo10 alloys. Scripta Mater. 2017, 137, 88–93. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Wu, Y.; Liu, X.J.; Mao, H.H.; Nieh, T.G.; Lu, Z.P. Precipitation behavior and its effects on tensile properties of FeCoNiCr high-entropy alloys. Intermetallics 2016, 79, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.L.; Yang, T.; Tong, Y.; Wang, J.; Luan, J.H.; Jiao, Z.B.; Chen, D.; Yang, Y.; Hu, A.; Liu, C.T.; et al. Heterogeneous precipitation behavior and stacking-fault-mediated deformation in a CoCrNi-based medium-entropy alloy. Acta Mater. 2017, 138, 72–82. [Google Scholar] [CrossRef]
- Antonov, S.; Detrois, M.; Tin, S. Design of novel precipitate-strengthened Al-Co-Cr-Fe-Nb-Ni high-entropy superalloys. Metall. Mater. Trans. A 2018, 49, 305–320. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yeh, A.C. The formation of cellular precipitate and its effect on the tensile properties of a precipitation strengthened high entropy alloy. Mater. Chem. Phys. 2018, 210, 111–119. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.M.; Wanderka, N.; Glatzel, U. High-temperature tensile strength of Al10Co25Cr8Fe15Ni36Ti6 compositionally complex alloy (high-entropy alloy). Jom-Us 2015, 67, 2271–2277. [Google Scholar] [CrossRef]
- Gwalani, B.; Choudhuri, D.; Soni, V.; Ren, Y.; Styles, M.; Hwang, J.Y.; Nam, S.J.; Ryu, H.; Hong, S.H.; Banerjee, R. Cu assisted stabilization and nucleation of L12 precipitates in Al0.3CuFeCrNi2 fcc-based high entropy alloy. Acta Mater. 2017, 129, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, A.M.; Singh, S.; Daoud, H.M.; Popp, R.; Völkl, R.; Glatzel, U.; Wanderka, N. On the path to optimizing the Al-Co-Cr-Cu-Fe-Ni-Ti high entropy alloy family for high temperature applications. Entropy 2016, 18, 104. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.H.; Hao, Y.; Cheng, G.; Xu, W.; Tsai, K.Y.; Tsai, C.W.; Jian, W.W.; Juan, C.C.; Shen, W.J.; Chuang, M.H. Morphology, structure and composition of precipitates in Al0.3CoCrCu0.5FeNi high-entropy alloy. Intermetallics 2013, 32, 329–336. [Google Scholar] [CrossRef]
- Wang, G.Z.; Zhou, W.; Fu, M.L.; Wang, F.J.; Luo, C.R. Effect of coherent L12 nanoprecipitates on the tensile behavior of a fcc-based high-entropy alloy. Mater. Sci. Eng. A 2017, 696, 503–510. [Google Scholar] [CrossRef]
- Xu, X.D.; Liu, P.; Guo, S.; Hirata, A.; Chen, M.W. Nanoscale phase separation in a fcc-based CoCrCuFeNiAl0.5 high-entropy alloy. Acta Mater. 2015, 84, 145–152. [Google Scholar] [CrossRef]
- Guo, N.N.; Gao, X.J.; Wang, L.; Zhu, G.M. Phase formation rules and design methods of high-entropy alloys. Spec. Cast. Nonferrous Alloys 2019, 39, 1072–1076. [Google Scholar]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Room temperature mechanical properties of the AlxTiVCrMnFeCoNiCu high entropy alloy system with multi-principal elements. J. Univ. Sci. Technol. Beijing 2008, 30, 765–769. [Google Scholar]
- Zhang, Y.; Zhou, Y.; Lin, J.; Chen, G.; Liaw, P. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy design and properties optimization of high-entropy alloys. J. Miner. Met. Mater. Soc. 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Yang, Y.; Liu, C.T. Atomic-size and lattice-distortion effects in newly developed high-entropy alloys with multiple principal elements. Intermetallics 2015, 64, 63–69. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, N.; Dwivedi, A.; Subramaniam, A. A geometrical parameter for the formation of disordered solid solutions in multi-component alloys. Intermetallics 2014, 53, 112–119. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Liu, C.T. Precipitation mechanism and mechanical properties of an ultra-high strength steel hardened by nanoscale NiAl and Cu particles. Acta Mater. 2015, 97, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Luan, J.H.; Jiao, Z.B.; Heatherly, L.; George, E.P.; Chen, G.; Liu, C.T. Effects of boron on the fracture behavior and ductility of cast Ti–6Al–4V alloys. Scr. Mater. 2015, 100, 90–93. [Google Scholar] [CrossRef]
- Eggeler, Y.M.; Titus, M.S.; Suzuki, A.; Pollock, T.M. Creep deformation-induced antiphase boundaries in L12-containing single-crystal cobalt-base superalloys. Acta Mater. 2014, 77, 352–359. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Zhu, M.; Li, J.; Dang, Y.; Wang, J. The phase stability of Ni2CrFeMox multi-principal-component alloys with medium configurational entropy. Mater. Des. 2015, 85, 1–6. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yeh, A.C. The evolution of microstructures and high temperature properties of AlxCo1.5CrFeNi1.5Tiy high entropy alloys. J. Alloys Compd. 2015, 653, 379–385. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Tong, Y.; Jiao, Z.B.; Wei, J.; Cai, J.X.; Han, X.D.; Chen, D.; Hu, A.; Kai, J.J. Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys. Science 2018, 362, 933–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef] [Green Version]
- Lu, K. Stabilizing nanostructures in metals using grain and twin boundary architectures. Nat. Rev. Mater. 2016, 1, 16019. [Google Scholar] [CrossRef]
- Reed, R.C.; Rae, C. Physical metallurgy of the nickel-based superalloys. Phys. Metall. 2014, 22, 2215–2290. [Google Scholar]
- Kim, S.H.; Kim, H.; Kim, N.J. Brittle intermetallic compound makes ultrastrong low-density steel with large ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.H.; Lu, Z.P.; He, J.Y.; Luan, J.H.; Wang, Z.J.; Liu, B.; Liu, Y.; Chen, M.W.; Liu, C.T. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- He, B.B.; Hu, B.; Yen, H.W.; Cheng, G.J.; Wang, Z.K.; Luo, H.W.; Huang, M.X. High dislocation density–induced large ductility in deformed and partitioned steels. Science 2017, 357, 1029. [Google Scholar] [CrossRef] [Green Version]
- Raabe, D.; Ponge, D.; Dmitrieva, O.; Sander, B. Nanoprecipitate-hardened 1.5 GPa steels with unexpected high ductility. Scr. Mater. 2009, 60, 1141–1144. [Google Scholar] [CrossRef]
- Ty, A.; Ylza, C.; Bxc, B.; Jjkab, C.; Ctla, B. Towards superior mechanical properties of hetero-structured high-entropy alloys via engineering multicomponent intermetallic nanoparticles. Scr. Mater. 2020, 183, 39–44. [Google Scholar]
- Gwalani, B.; Soni, V.; Lee, M.; Mantri, S.A.; Ren, Y.; Banerjee, R. Optimizing the coupled effects of Hall-Petch and precipitation strengthening in a Al0.3CoCrFeNi high entropy alloy. Mater. Des. 2017, 121, 254–260. [Google Scholar] [CrossRef]
- Kuo, C.M.; Tsai, C.W. Effect of cellular structure on the mechanical property of Al0.2Co1.5CrFeNi1.5Ti0.3 high-entropy alloy. Mater. Chem. Phys. 2018, 210, 103–110. [Google Scholar] [CrossRef]
- Tsao, T.K.; Yeh, A.C.; Kuo, C.M.; Kakehi, K.; Murakami, H.; Yeh, J.W.; Jian, S.R. The high temperature tensile and creep behaviors of high-entropy superalloy. Sci. Rep. UK 2017, 7, 12658. [Google Scholar] [CrossRef]
- He, J.; Wang, H.; Wu, Y.; Liu, X.; Nieh, T.; Lu, Z. High-temperature plastic flow of a precipitation-hardened FeCoNiCr high entropy alloy. Mater. Sci. Eng. A Struct. Mater. Prop. Misrostructure Process. 2017, 686, 34–40. [Google Scholar] [CrossRef]
- Jones, N.G.; Frezza, A.; Stone, H.J. Phase equilibria of an Al0.5CrFeCoNiCu high-entropy alloy. Mater. Sci. Eng. A 2014, 615, 214–221. [Google Scholar] [CrossRef]
- Suzuki, A.; Inui, H.; Pollock, T.M. L12-strengthened cobalt-base superalloys. Annu. Rev. Mater. Res. 2015, 45, 345–368. [Google Scholar] [CrossRef]
- Reed, R.C. The superalloys (fundamentals and applications) || The physical metallurgy of nickel and its alloys. Open J. Geology 2006, 33–120. [Google Scholar] [CrossRef]
- Reed, R.C.; Rae, C. Physical Metallurgy of the Nickel-Based Superalloys. In Physical Metallurgy, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zhao, Y.L.; Yang, T.; Li, Y.R.; Fan, L.; Kai, J.J. Superior high-temperature properties and deformation-induced planar faults in a novel L12-strengthened high-entropy alloy. Acta Mater. 2020, 188, 517–527. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Yang, T.; Zhu, J.H.; Chen, D.; Yang, Y.; Hu, A.; Liu, C.T.; Kai, J.J. Development of high-strength Co-free high-entropy alloys hardened by nanosized precipitates. Scr. Mater. 2018, 148, 51–55. [Google Scholar] [CrossRef]
- Wang, W.Z.; Hong, H.U.; Kim, I.S.; Choi, B.G.; Jeong, H.W.; Kim, M.Y.; Jo, C.Y. Influence of γ′ and grain boundary carbide on tensile fracture behaviors of Nimonic 263. Mater. Sci. Eng. A 2009, 523, 242–245. [Google Scholar] [CrossRef]
- Pu, E.; Zheng, W.; Song, Z.; Feng, H.; Yang, F.; Dong, H. Effects of temperature and strain rate on tensile deformation behavior of superalloy UNS N10276. Mater. Sci. Eng. A Struct. Mater. Prop. Misrostructure Process. 2017, 699, 88–98. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Fan, L.; Wei, J.; Liu, C.T. Control of nanoscale precipitation and elimination of intermediate-temperature embrittlement in multicomponent high-entropy alloys. Acta Mater. 2020, 189, 47–59. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, D.; Han, B.; Wang, J.; Feng, R.; Yang, T.; Zhao, C.; Zhao, Y.L.; Guo, W.; Shimizu, Y.; et al. Outstanding tensile properties of a precipitation-strengthened FeCoNiCrTi0.2 high-entropy alloy at room and cryogenic temperatures. Acta Mater. 2019, 165, 228–240. [Google Scholar] [CrossRef]
- Kamikawa, N.; Sato, K.; Miyamoto, G.; Murayama, M.; Sekido, N.; Tsuzaki, K.; Furuhara, T. Stress–strain behavior of ferrite and bainite with nano-precipitation in low carbon steels. Acta Mater. 2015, 83, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Hu, X.; He, F.; Wu, Q.; Liu, C.T. Tailoring nanoprecipitates for ultra-strong high-entropy alloys via machine learning and prestrain aging. J. Mater. Sci. Technol. Shenyang 2021, 69, 156–167. [Google Scholar] [CrossRef]
- Peng, H.; Hu, L.; Li, L.; Zhang, W. Ripening of L12 nanoparticles and their effects on mechanical properties of Ni28Co28Fe21Cr15Al4Ti4 high-entropy alloys. Mater. Sci. Eng. A 2020, 772, 138803. [Google Scholar] [CrossRef]
- Ming, K.; Bi, X.; Jian, W. Realizing strength-ductility combination of coarse-grained Al0.2Co1.5CrFeNi1.5Ti0.3 alloy via nano-sized, coherent precipitates. Int. J. Plast. 2018, 100, 177–191. [Google Scholar] [CrossRef]
- Gladman, T. Precipitation hardening in metals. Met. Sci. J. 1999, 15, 30–36. [Google Scholar] [CrossRef]
- Vo, N.Q.; Liebscher, C.H.; Rawlings, M.J.; Asta, M.; Dunand, D.C. Creep properties and microstructure of a precipitation-strengthened ferritic Fe–Al–Ni–Cr alloy. Acta Mater. 2014, 71, 89–99. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhy, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.; Liu, Z.X.; Cai, B.; Wang, M.X.; Shi, L. Aging behavior of a CuCr2Fe2NiMn high-entropy alloy. Mater. Des. 2012, 33, 121–126. [Google Scholar] [CrossRef]
- Chen, S.T.; Tang, W.Y.; Kuo, Y.F.; Chen, S.Y.; Tsau, C.H.; Shun, T.T.; Yeh, J.W. Microstructure and properties of age-hardenable AlxCrFe1.5MnNi0.5 alloys. Mater. Sci. Eng. A 2010, 527, 5818–5825. [Google Scholar] [CrossRef]
- Zhao, Y. Study on Design, Microstructure and Properties of L12/B2-type High-Entropy Intermetallics. 2018. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201901&filename=1018892623.nh&uniplatform=NZKPT&v=FZahDJ--E2kQ2w0XosYUUFM9wdLRgbXVx3hR-rdla8OrLKy24PhQU2hp9EXCyIuy (accessed on 13 November 2021).
- Yang, B.F. Microstructure and Mechanical Properties of L12/B2-type High-Entropy Intermetallics. 2019. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD202001&filename=1019689431.nh&uniplatform=NZKPT&v=BPuvLStnvW3h492zZBHSDjOBxxtEb71ZeFLdvLLEKgjwBK-l9_2MQzMdKI7LOOij (accessed on 13 November 2021).
- Liu, J.Q. Composition Optimization and Microstructure and Properties of L12 Type High-Entropy Intermetallics. 2020. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD202101&filename=1020400608.nh&uniplatform=NZKPT&v=p_1PXehctP4c97hh-Bs_XxJRz8wPA4SI40RnjfDltWTi4K6VMl_Ujj1RFtFFhk84 (accessed on 13 November 2021).
- Zhang, J.; Ma, S.; Xiong, Y.; Xu, B.; Zhao, S. Elemental partitions and deformation mechanisms of L12-type multicomponent intermetallics. Acta Mater. 2021, 219, 117238. [Google Scholar] [CrossRef]
- Zhou, B.X. Three Dimensional Atom Probe—An instrument for microstructure investigation of materials with analyzing atoms one by one. Nat. Mag. 2005, 3, 125–129. [Google Scholar]
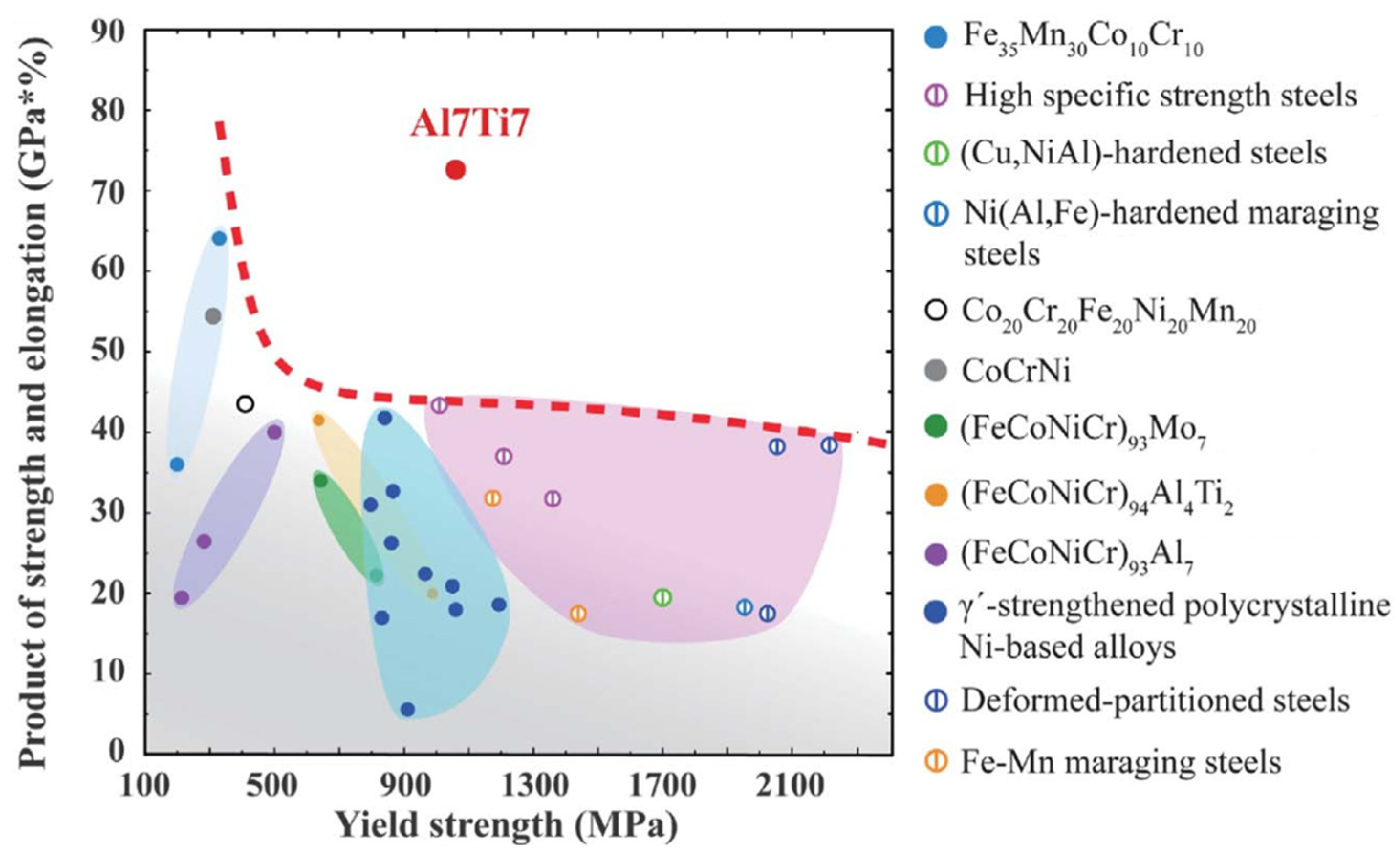
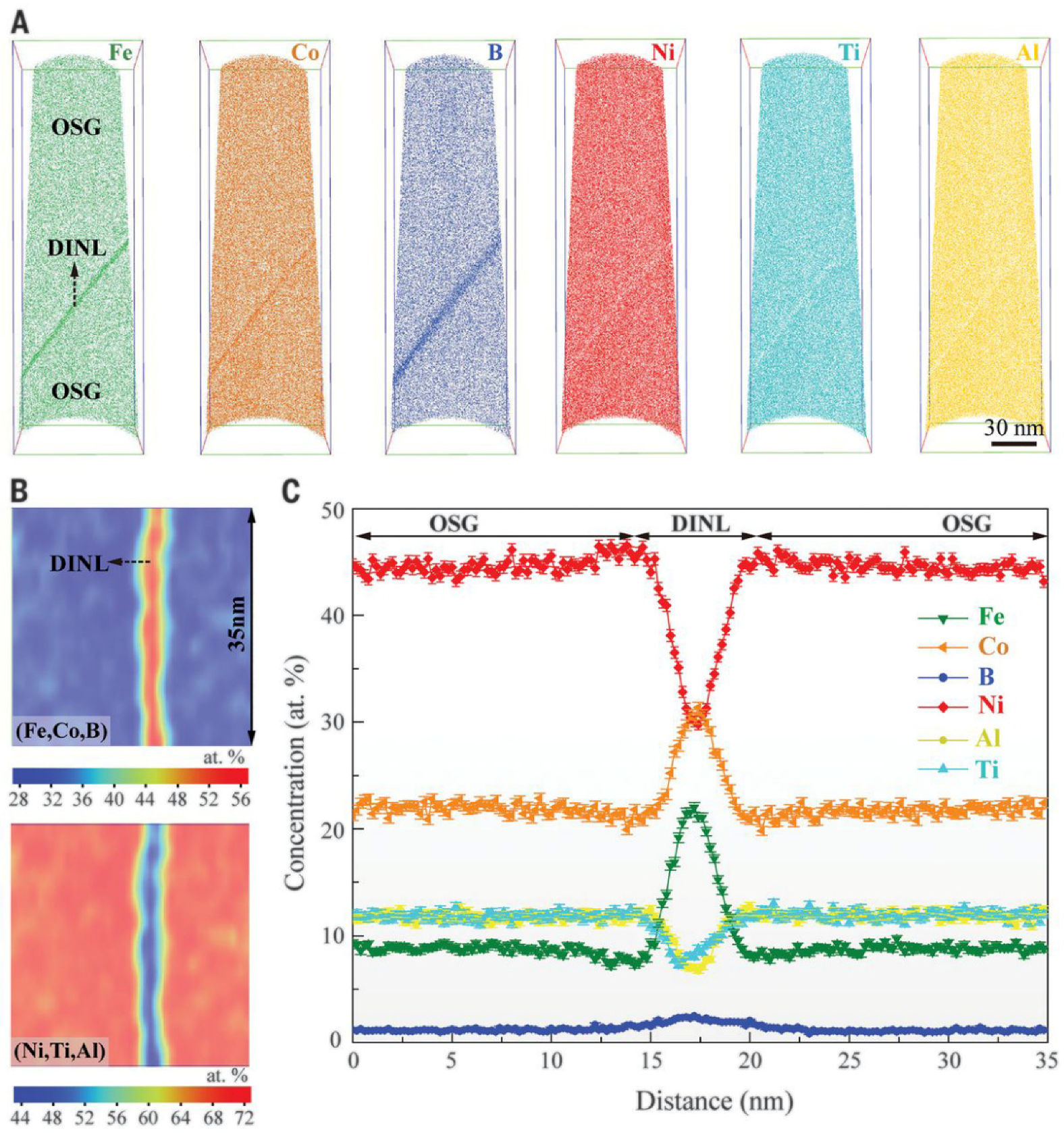
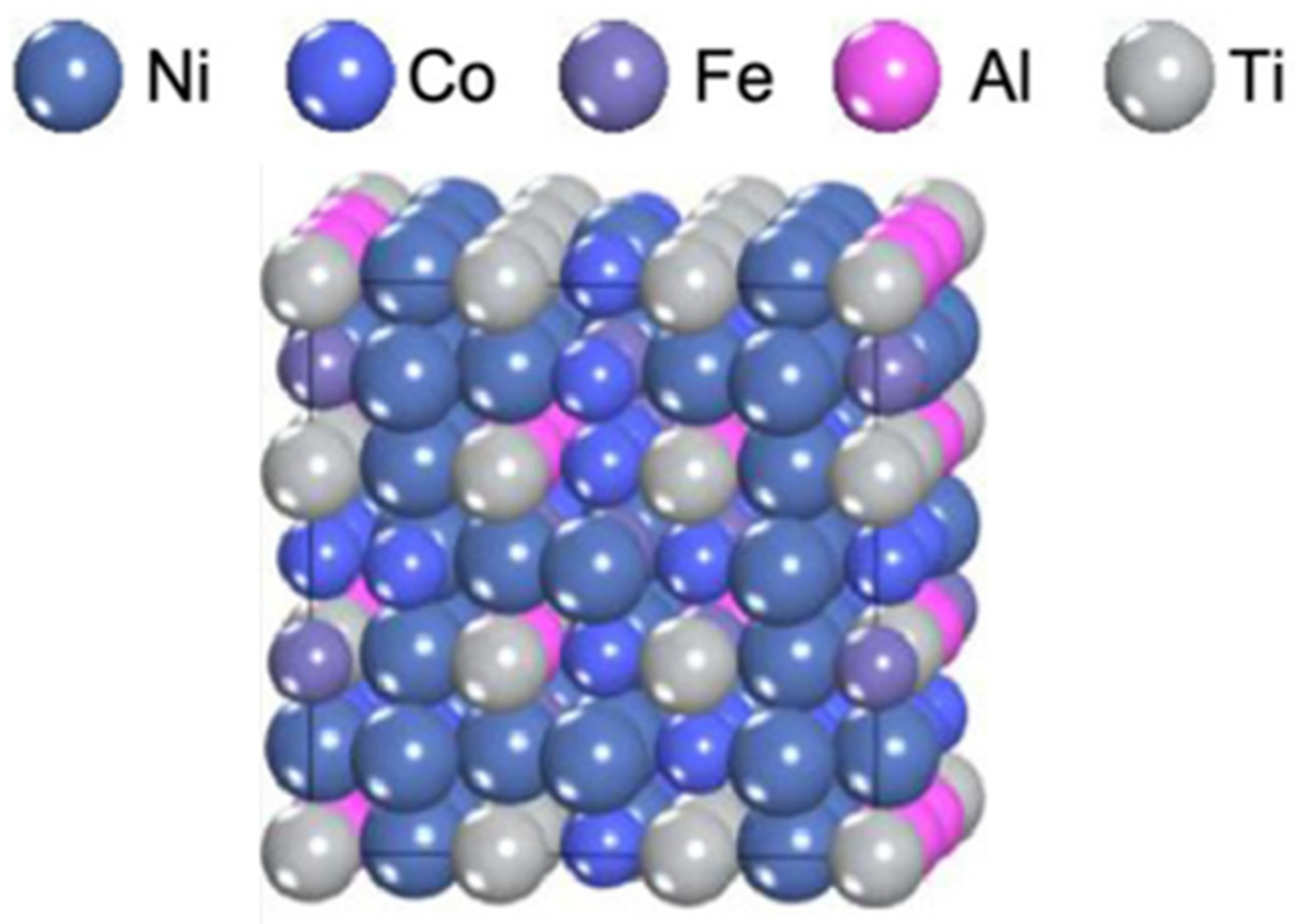
- Yang, T.; Zhao, Y.L.; Li, W.P.; Yu, C.Y.; Luan, J.H.; Lin, D.Y.; Fan, L.; Jiao, J.B.; Liu, W.H.; Liu, X.J.; et al. Ultrahigh-strength and ductile superlattice alloys with nanoscale disordered interfaces. Science 2020, 369, 427–432. [Google Scholar] [CrossRef]
- Russell, A.M.; Lee, K.L. Structure–Property Relations in Nonferrous Metals. Intermet. Compd. J. 2005, 44, 418. [Google Scholar]
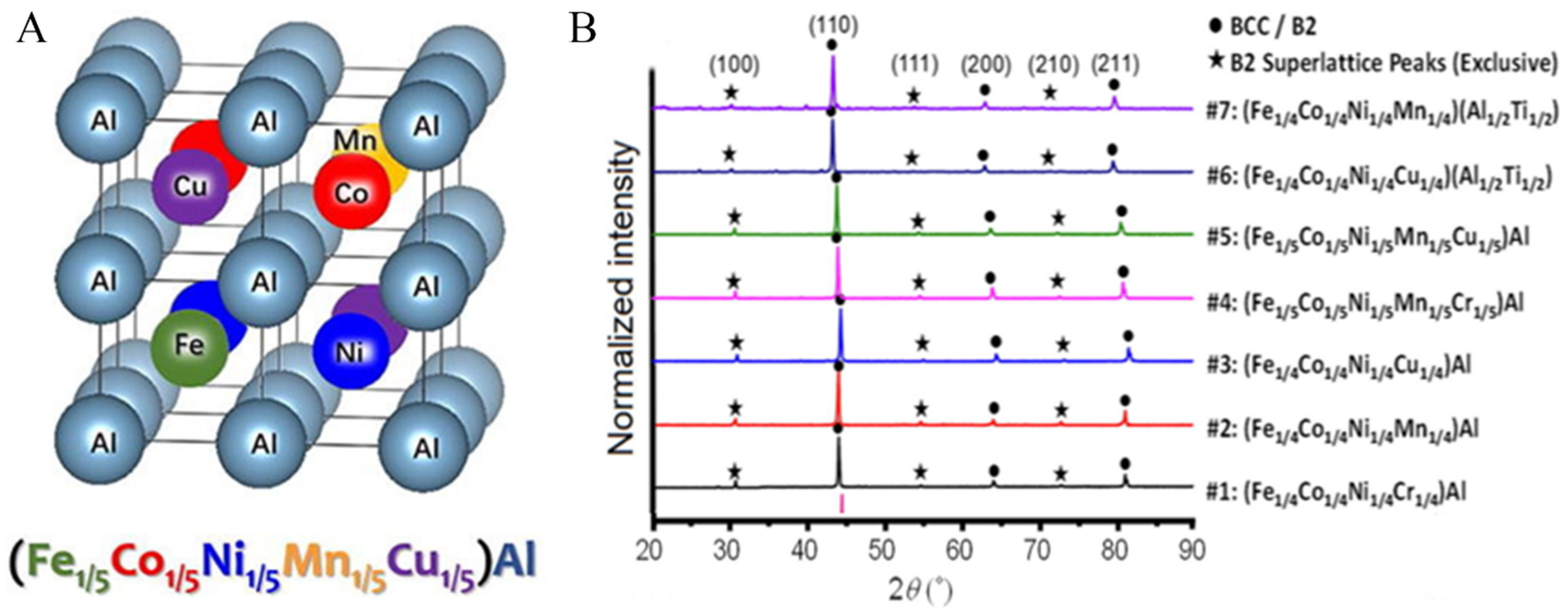
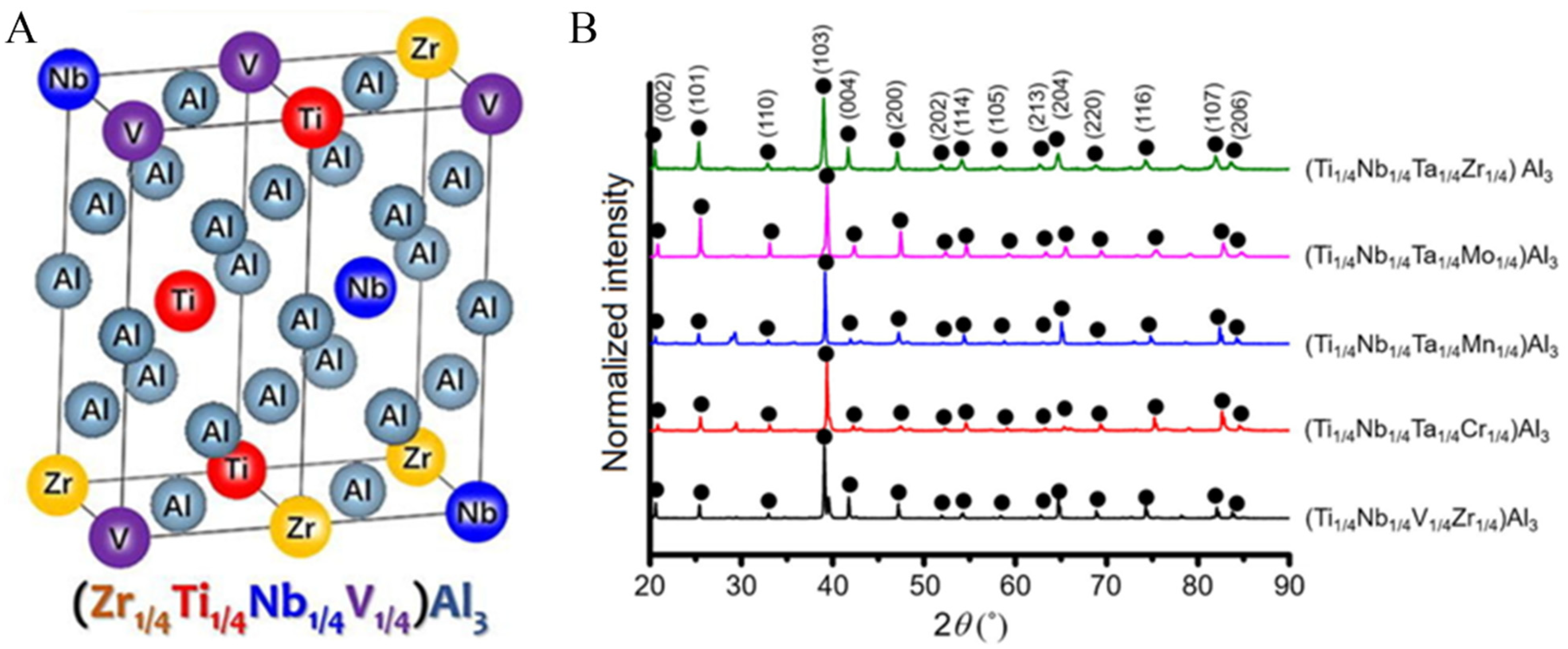
- Zhou, N.; Jiang, S.; Huang, T.; Qin, M.; Hu, T.; Luo, J. Single-phase high-entropy intermetallic compounds (HEICs): Bridging high-entropy alloys and ceramics. Sci. Bull. 2019, 64, 856–864. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Li, R.; Li, Y.; Wen, Y.; Zhong, Y.; Ren, W.; Shen, Z.; Zheng, T.; Peng, J.; Liang, X.; et al. Hierarchical crack buffering triples ductility in eutectic herringbone high-entropy alloys. Science 2021, 373, 912–918. [Google Scholar] [CrossRef]
- Takeyama, M.; Liu, C.T. Effect of grain size on yield strength of Ni3Al and other alloys. J. Mater. Res. 1988, 3, 665–674. [Google Scholar] [CrossRef]
- Mckamey, C.G.; Devan, J.H.; Tortorelli, P.F.; Sikka, V.K. A review of recent developments in Fe3Al-based alloys. J. Mater. Res. 1991, 6, 1779–1805. [Google Scholar] [CrossRef] [Green Version]
- Aoki, K. Ductilization of L12 Intermetallic Compound Ni3Al by Microalloying with Boron. Mater. Trans. Jim 1990, 31, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Cotton, J.D.; Noebe, R.D.; Kaufman, M.J. The effects of chromium on NiAl intermetallic alloys: Part I. microstructures and mechanical properties. Intermetallics 1993, 1, 3–20. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kaneno, Y.; Takasugi, T. Room-temperature tensile property and fracture behavior of recrystallized B2-type CoZr intermetallic compound. Scr. Mater. 2005, 52, 39–44. [Google Scholar] [CrossRef]
- George, E.P.; Liu, C.T.; Padgett, R.A. Comparison of grain boundary compositions in B-doped and B-free Ni3Al. Scr. Metall. 1989, 23, 979–982. [Google Scholar] [CrossRef]
- Chiba, A.; Hanada, S.; Watanabe, S. Ductilization of Ni3Al by macroalloying with Pd. Acta Metall. Et Mater. 1991, 39, 1799–1805. [Google Scholar] [CrossRef]
- Liu, C.T.; George, E.P.; Oliver, W.C. Grain-boundary fracture and boron effect in Ni3Si alloys. Intermetallics 1996, 4, 77–83. [Google Scholar] [CrossRef]
- Takasugi, T.; Ma, C.L.; Hanada, S. Environmental embrittlement and grain boundary segregation of boron and carbon in Ni3(Si, Ti) alloys. Scr. Metall. Et Mater. 1995, 29, 1587–1591. [Google Scholar] [CrossRef]
- Takasugi, T.; Izumi, O. Electronic and structural studies of grain boundary strength and fracture in L12 ordered alloys—I. On binary A3B alloys. Acta Metall. 1985, 33, 1247–1258. [Google Scholar] [CrossRef]
- Matsuda, M.; Nishimoto, T.; Matsunaga, K.; Morizono, Y.; Tsurekawa, S.; Nishida, M. Deformation structure in ductile B2-type Zr–Co–Ni alloys with martensitic transformation. J. Mater. Sci. 2011, 46, 4221–4227. [Google Scholar] [CrossRef]
- Mckamey, C.G.; Horton, J.A.; Liu, C.T. Effect of chromium on properties of Fe3Al. J. Mater. Res. 1989, 4, 1156–1163. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Luan, J.H.; Han, B.; Wei, J.; Kai, J.J.; Liu, C.T. Nanoparticles-strengthened high-entropy alloys for cryogenic applications showing an exceptional strength-ductility synergy. Scr. Mater. 2019, 164, 30–35. [Google Scholar] [CrossRef]
- Song, W.; Ingendahl, T.; Bleck, W. Control of Strain Hardening Behavior in High-Mn Austenitic Steels. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 546–556. [Google Scholar] [CrossRef]
- Welsch, E.; Ponge, D.; Hafez Haghighat, S.M.; Sandlöbes, S.; Choi, P.; Herbig, M.; Zaefferer, S.; Raabe, D. Strain hardening by dynamic slip band refinement in a high-Mn lightweight steel. Acta Mater. 2016, 116, 188–199. [Google Scholar] [CrossRef]
- Jia, Z.; Tao, Y.; Sun, L.; Zhao, Y.; Liu, C.T. A Novel multinary intermetallic as an active electrocatalyst for hydrogen evolution. Adv. Mater. 2020, 32, 2000385. [Google Scholar] [CrossRef]
- Yao, C.; Ma, Y. Superconducting materials: Challenges and opportunities for large-scale applications. iScience 2021, 24, 102541. [Google Scholar] [CrossRef]
- Hughes, J.P.; Clipsham, J.; Chavushoglu, H.; Rowley-Neale, S.J.; Banks, C.E. Polymer electrolyte electrolysis: A review of the activity and stability of non-precious metal hydrogen evolution reaction and oxygen evolution reaction catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110709. [Google Scholar] [CrossRef]
- Sai Ram, B.; Paul, A.K.; Kulkarni, S.V. Soft magnetic materials and their applications in transformers. J. Magn. Magn. Mater. 2021, 537, 168210. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloys 2021. [Google Scholar] [CrossRef]





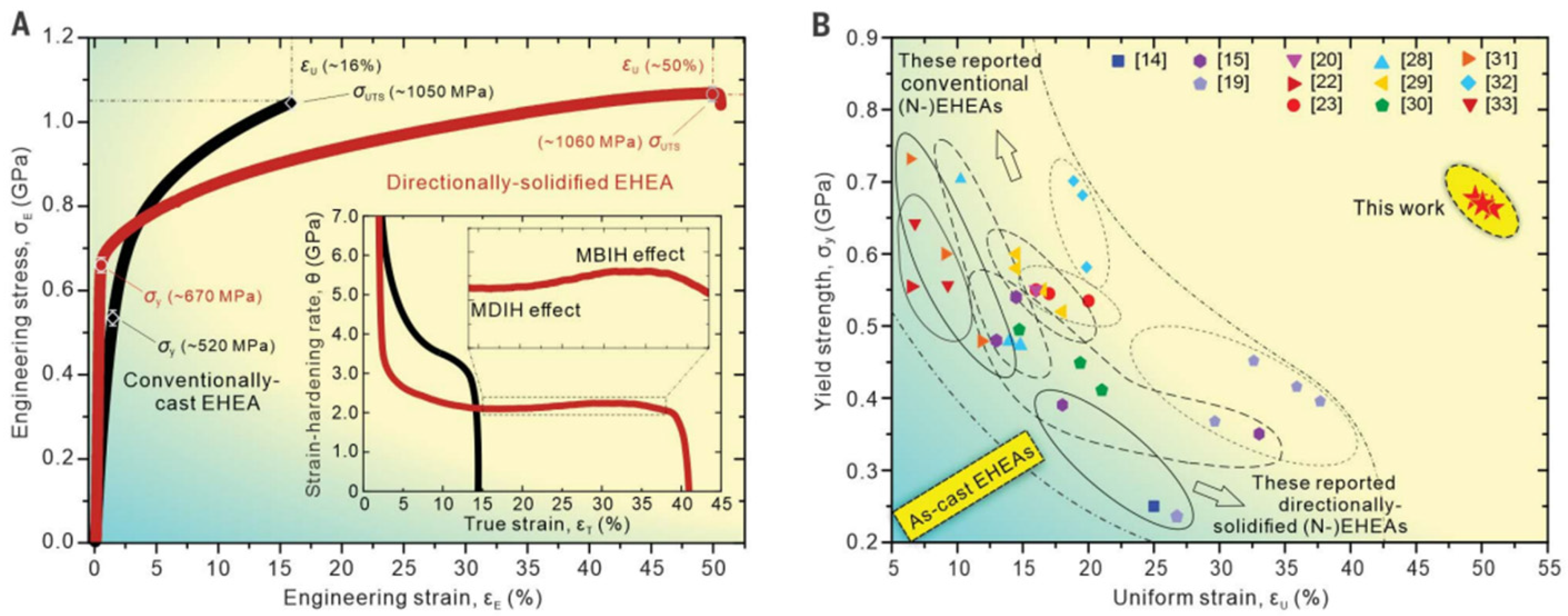


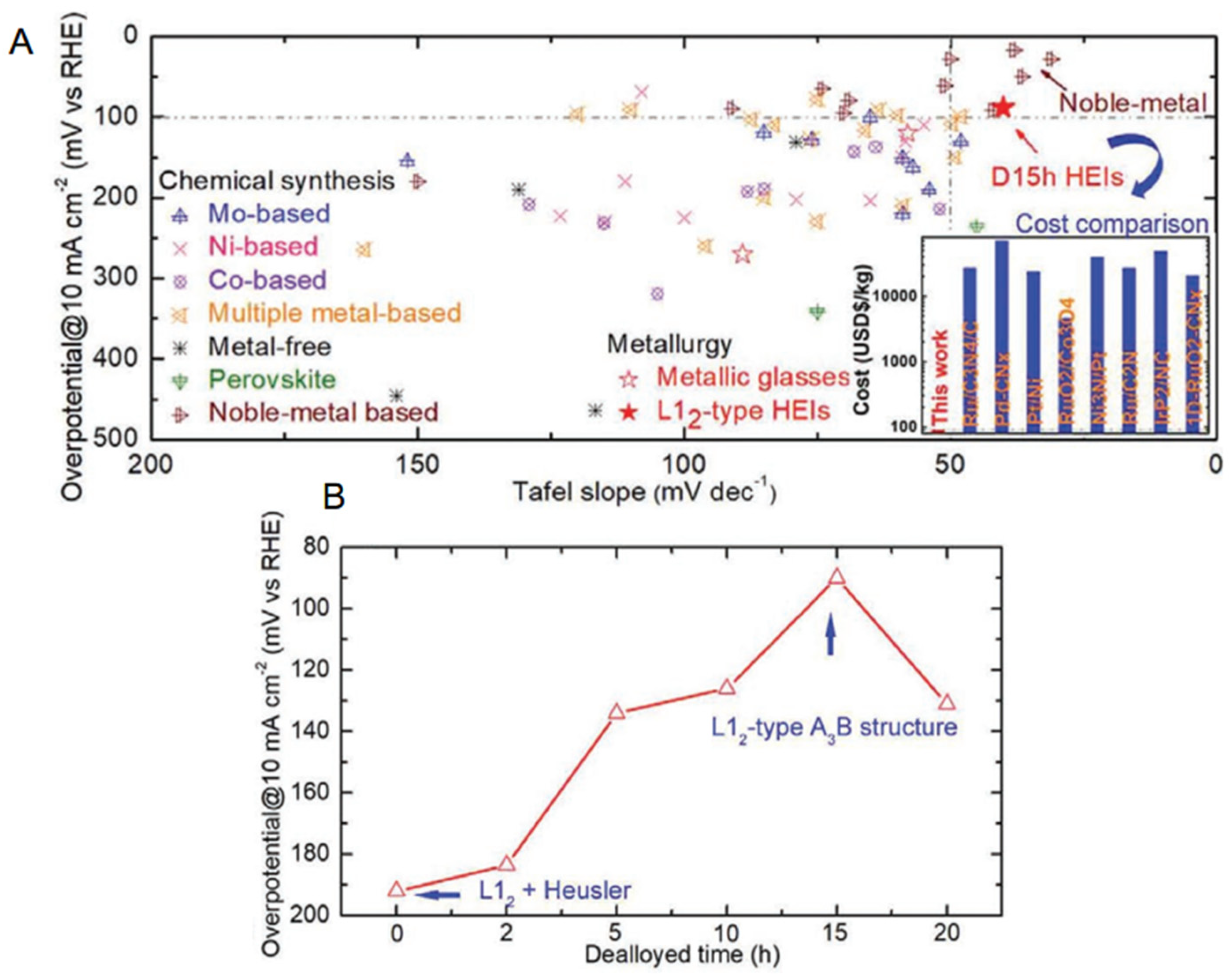

| Composition (at.%) | Aging Condition | Strengthening Phase | YS (MPa) | UTS (MPa) | EL(%) | Ref. |
|---|---|---|---|---|---|---|
| Fe25Co25Ni25Cr25 | 800 °C/1 h | Nil | ~276 | ~705 | ~39 | [12] |
| Fe20Co20Ni20Cr20Mn20 | 800 °C/1 h | Nil | ~265 | ~460 | ~47 | [12] |
| Al7Co23.26Cr23.26Fe23.26Ni23.26 | 550 °C/150 h | L12 | ~285 | ~540 | ~50 | [113] |
| 630 °C/50 h | B2 + L12 | ~490 | ~835 | ~48 | [113] | |
| (Fe25Co25Ni25Cr25)94Ti2Al4 | 700 °C/18 h | L12 + L21 | ~551 | ~982 | ~42 | [82] |
| 800 °C/18 h | L12 + L21 | ~645 | ~1094 | ~39 | [82] | |
| 900 °C/18 h | L21 | ~301 | ~715 | ~46 | [82] | |
| Coldroll +700 °C/4 h | L12 + L21 | ~1005 | ~1273 | ~17 | [69] | |
| Al3.7Cr18.5Fe18.5Co18.5Ni37Cu37 | 700 °C/20 h | L12 | ~719 | ~1048 | ~30.4 | [95] |
| 800 °C/1 h | L12 | ~460 | ~732 | ~31.7 | [95] | |
| Al3.64Co40.9Cr27.27Fe27.27Ni40.9Ti5.45 | 750 °C/50 h | L12 | ~640 | ~830 | ~10 | [114] |
| Al3.31Co27Cr18Fe18Ni27.27Ti5.78 | Hotforging | L12 | ~952 | ~1306 | ~20.5 | [85] |
| Al8Co17Cr17Cu8Fe17Ni33 | 700 °C/5 h | L12 | ~365 | ~365 | ~0.1 | [115] |
| 1150 °C/5 h | L12 | ~215 | ~489 | ~59 | [115] | |
| Al10Co25Cr8Fe15Ni36Ti6 | 900 °C/5 h | L12 | ~568 | ~786 | ~12 | [86] |
| 900 °C/50 h | L12 | ~596 | ~1039 | ~20 | [86] | |
| Ni47.9Al10.2Co16.9Cr7.4Fe8.9Ti5.8Mo0.9Nb1.2 W0.4C0.4 | 800 °C/20 h | L12 | ~847 | Nil | Nil | [116] |
| Composition (at.%) | Processing Technic | Phase | YS (MPa) | UTS (MPa) | EL(%) | Microstructure Characteristic |
|---|---|---|---|---|---|---|
| Ni43.9Co22.4Fe8.8Al10.7Ti11.7B2.5 [142] | arc melting and thermomechanical processing | L12 + 0.13 vol%FCC | 1040 | 1600 | 25 | disordered interfaces |
| Al19Fe20Co20Ni41 [146] | Conventional casting | 55 vol%L12 + B2 | 520 | 1050 | 16 | eutectic herringbone |
| Al19Fe20Co20Ni41 [146] | directional solidification | 59 vol%L12 + B2 | 670 | 1060 | 50 | eutectic herringbone |
| Fe12.5Co12.5Ni12.5Cu12.5Al50 [144] | anneal at 1100 °C for 10 h | B2 | - | |||
| Fe10Co10Ni10Mn10Cu10Al50 [144] | anneal at 1100 °C for 10 h | B2 | ||||
| Ti6.25Nb6.25V6.25Zr6.25Al75 [144] | anneal at 1300 °C for 10 h | D022 | ||||
| FeCoNiAlTi [163] | dealloying for 15 h | L12 | dendritic-like structure | |||
| Ni62Co34Fe15Al12Ti21 [140] | L12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, X.; Singh, A.P.; Xu, H.; Kong, F.; Yang, F. The Evolution of Intermetallic Compounds in High-Entropy Alloys: From the Secondary Phase to the Main Phase. Metals 2021, 11, 2054. https://doi.org/10.3390/met11122054
Liu J, Wang X, Singh AP, Xu H, Kong F, Yang F. The Evolution of Intermetallic Compounds in High-Entropy Alloys: From the Secondary Phase to the Main Phase. Metals. 2021; 11(12):2054. https://doi.org/10.3390/met11122054
Chicago/Turabian StyleLiu, Junqi, Xiaopeng Wang, Ajit Pal Singh, Hui Xu, Fantao Kong, and Fei Yang. 2021. "The Evolution of Intermetallic Compounds in High-Entropy Alloys: From the Secondary Phase to the Main Phase" Metals 11, no. 12: 2054. https://doi.org/10.3390/met11122054
APA StyleLiu, J., Wang, X., Singh, A. P., Xu, H., Kong, F., & Yang, F. (2021). The Evolution of Intermetallic Compounds in High-Entropy Alloys: From the Secondary Phase to the Main Phase. Metals, 11(12), 2054. https://doi.org/10.3390/met11122054








