Adaptation of the Rist Operating Diagram as a Graphical Tool for the Direct Reduction Shaft
Abstract
1. Introduction
- The gas composition is more complex than in the blast furnace. Particular attention will be paid to considering the hydrocarbons, including methane.
- The carburization phenomena in DRI will impact the operating line of the diagram.
2. Modeling
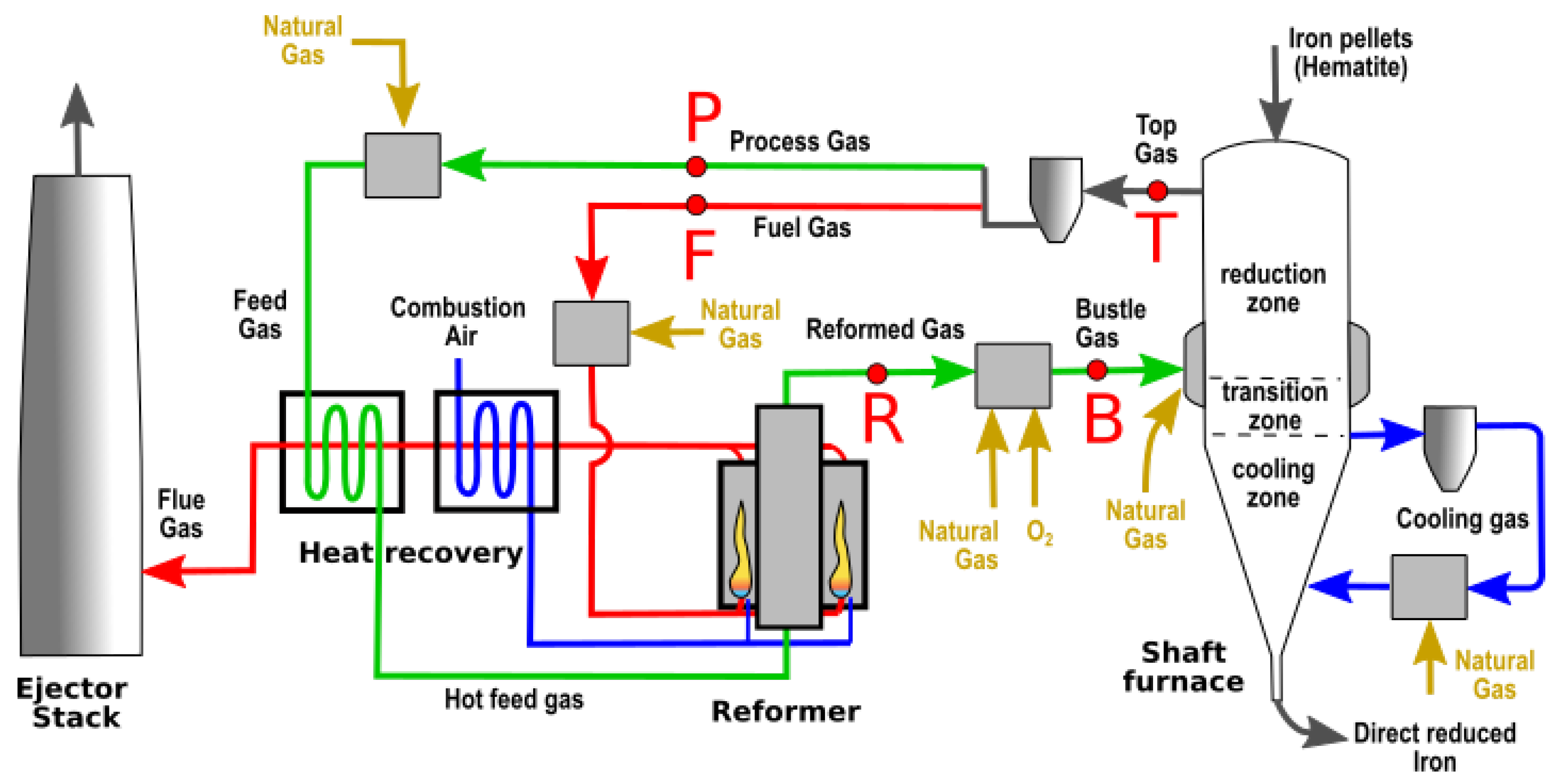
2.1. Description of the Midrex Process
- The shaft furnace is a vertical gas–solid countercurrent reactor with a downward flow of iron oxides and an upward flow of a hot reducing gas. The iron pellets, consisting mainly of hematite (%Fe2O3 > 95%), are then both reduced and carburized. The direct-reduced iron (DRI) leaving the shaft achieves a high rate of metallization (92–96% iron metal, with residual iron oxides being Wustite) and a moderate level of carburization (2–2.5% carbon in the total mass).
- The preparation of the reducing gas follows several steps. First, a fraction of the recycled top gas, named the process gas (P), is mixed with injected natural gas. The corresponding mixture, called the feed gas, is preheated in the recovery heat device and then injected into the reformer. Cracking occurs between CH4, CO2, and H2O in the tubes of the reformer. The resulting reformed gas (R) is mainly composed of CO, CO2, H2, and H2O, with a low rate of remaining CH4 (few %).
- The reformed gas (R) is mixed with additional natural gas and pure oxygen and injected in the shaft furnace at a temperature around 950 °C. Additional natural gas is also injected in the shaft, in the bottom area, inside the loop of the cooling gas and in the transition zone with the bustle gas.
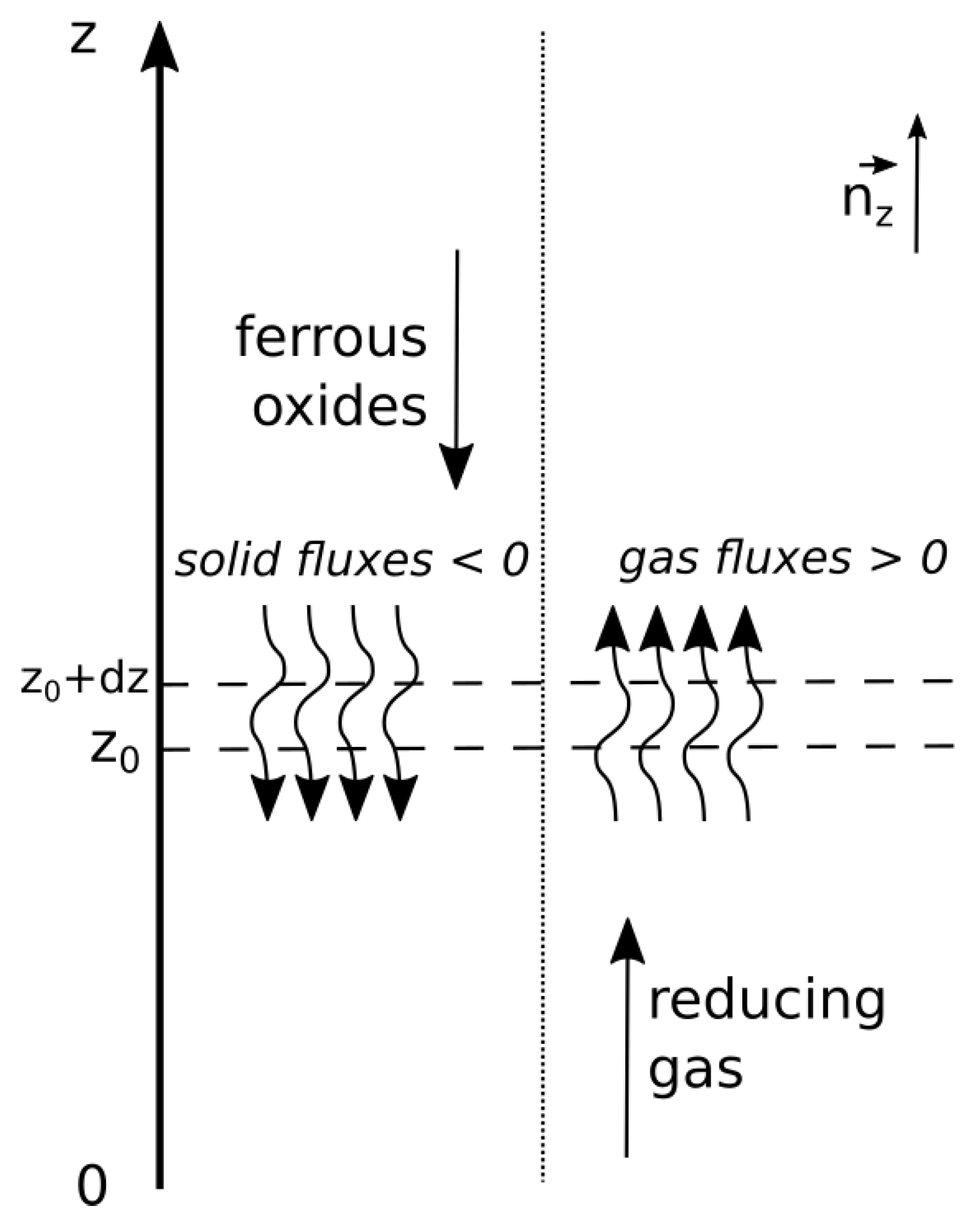
2.2. Description of the Local Mass Balance in the Shaft Furnace
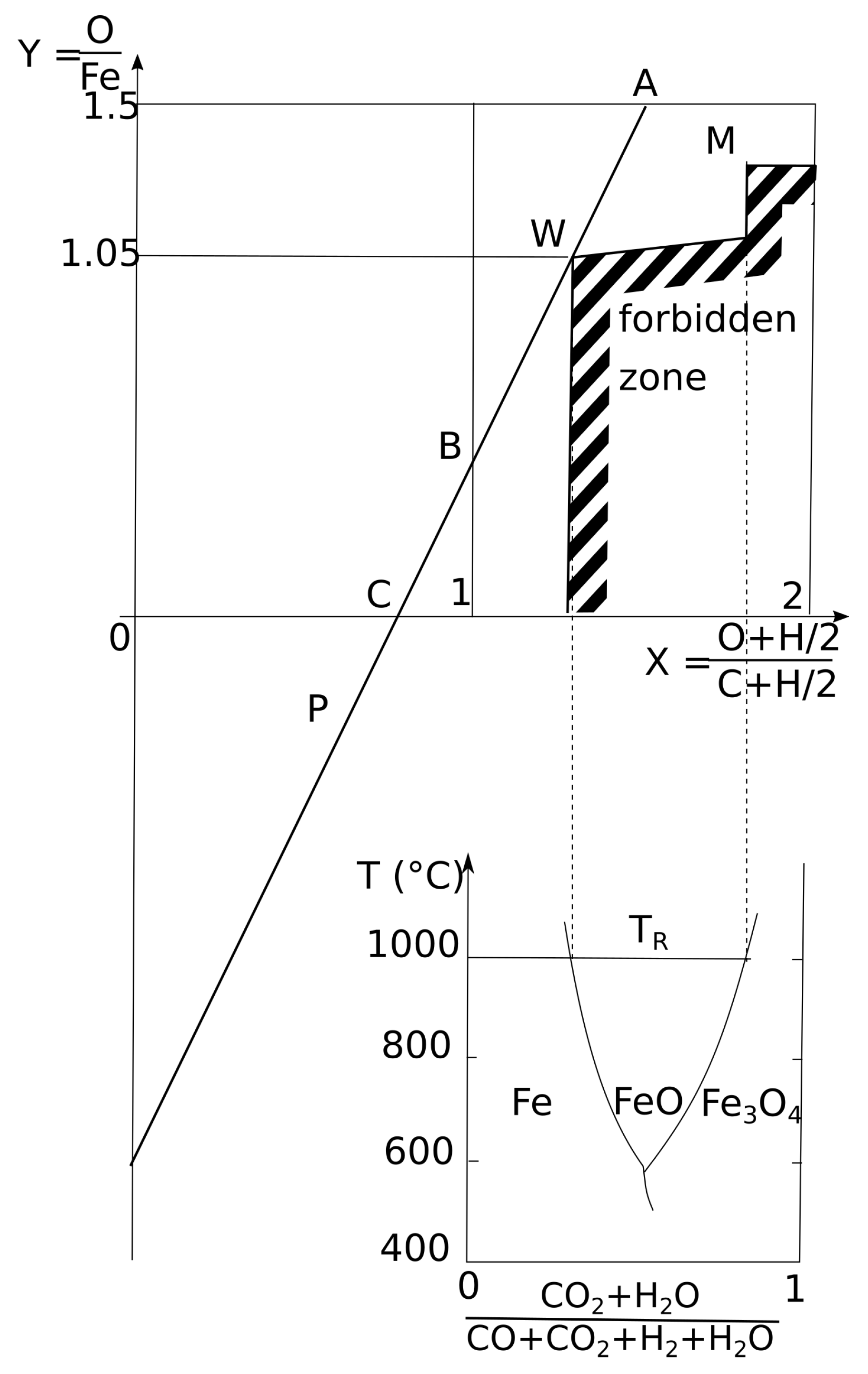
2.3. Presentation of the Operating Diagram
2.4. Application to the Description of the Shaft Furnace in the Midrex NGTM Process
- The more complex composition of the reducing gas, including the presence of hydrocarbons,
- the in situ reforming of hydrocarbons in the shaft furnace,
- the phenomena of carburization of DRI by gas, through the Boudouard and Beggs reactions.
2.4.1. Simplified Case: No Carburization Phenomena
2.4.2. Real Case: Carburization Is Taken into Account
2.5. Plotting of the Thermodynamic Forbidden Zone
3. Results and Discussion
3.1. Graphical Description of a Direct-Reduction Shaft Working Point in ArcelorMittal Contrecoeur
3.2. Influence of In Situ Reforming
3.3. Definition of the Ideal Working Point
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Greek letters | |
| specific consumption | |
| molar flux (mol·m−3·s−1) | |
| Latin letters | |
| volumetric fraction of the molecule j in a mix gas | |
| local molar concentration of the atomic element i (mol·m−3) | |
| number of atoms i in the molecule j | |
| mass flow rate (kg·s−1) | |
| volumetric flow rate (m3·s−1) | |
| molar volume of the gas (m3·mol−1) | |
| w | mass fraction |
| gas oxidation degree (derived definition) | |
| gas oxidation degree (original definition) | |
| y | burden oxidation degree |
| superscript | |
| gas | related to gaseous element |
| s | related to solid element (iron bearing material) |
| subscript | |
| i | atomic element (C, H, O, or Fe) |
References
- Usui, T.; Ohmi, M.; Yamamura, E. Analysis of Rate of Hydrogen Reduction of Porous Wustite Pellets Basing. ISIJ Int. 1990, 30, 347–355. [Google Scholar] [CrossRef]
- Vannucci, M.; Colla, V.; Corbo, G.; Fera, S. A simplified approach to the simulation of direct reduction of iron ore. Metall. Res. Technol. 2010, 107, 195–204. [Google Scholar] [CrossRef]
- Parisi, D.R.; Laborde, M.A. Modeling of counter current moving bed gas-solid reactor used in direct reduction of iron ore. Chem. Eng. J. 2004, 104, 35–43. [Google Scholar] [CrossRef]
- Shams, A.; Moazeni, F. Modeling and Simulation of the MIDREX Shaft Furnace: Reduction, Transition and Cooling Zones. JOM 2015, 67, 2681–2689. [Google Scholar] [CrossRef]
- Hamadeh, H.; Mirgaux, O.; Patisson, F. Detailed Modeling of the Direct Reduction of Iron Ore in a Shaft Furnace. Materials 2018, 11, 1865. [Google Scholar] [CrossRef]
- Rist, A.; Bonnivard, G. Reduction d’un lit d’oxydes de fer par un gaz, première partie. Rev. Métall. 1963, 60, 23–37. [Google Scholar] [CrossRef]
- Rist, A.; Meysson, N. Étude graphique de la marche du haut fourneau avec vent humide et injections aux tuyères. Rev. Métall. 1965, 62, 995–1040. [Google Scholar] [CrossRef]
- Rist, A.; Bonnivard, G. Réduction d’un lit d’oxydes de fer par un gaz, deuxième partie. Rev. Métall. 1966, 63, 296–312. [Google Scholar] [CrossRef]
- Rist, A.; Dancoisne, P.; Jon, R. Connaissance du haut fourneau. Rev. Métall. 1967, 64, 97–118. [Google Scholar] [CrossRef]
- Cordier, J.; Guerbois, J.; Hazard, P.; Malvaux, R.; Reveil, C. Les injections au haut fourneau. Rev. Métall. 1962, 59, 791–815. [Google Scholar] [CrossRef]
- Rist, A.; Meysson, N. Recherche graphique de la mise au mille minimale du haut fourneau a faible température de vent. Rev. Métall. 1964, 61, 121–146. [Google Scholar] [CrossRef]
- Meysson, N.; Maaref, A.; Rist, A. Emploi de boulettes prereduites au haut fourneau etude graphique. Rev. Métall. 1965, 62, 1161. [Google Scholar]
- Nicolle, R.; Capelani, R.; Guillot, J.B.; Rist, A. La réduction dans le haut fourneau et la recherche des très faibles mises au mille de coke. Rev. Métall. 1980, 77, 769–779. [Google Scholar] [CrossRef]
- Rist, A. Analogue Diagrams for Process Metallurgists. ISIJ Int. 1992, 32, 1034–1043. [Google Scholar] [CrossRef][Green Version]
- Takenaka, Y.; Kimura, Y.; Narita, K.; Kaneko, D. Mathematical model of direct reduction shaft furnace and its application to actual operations of a model plant. Comput. Chem. Eng. 1986, 10, 67–75. [Google Scholar] [CrossRef]
- Quatravaux, T.; Barros, J.; Maurice, Y.; Pierret, H.; Chen, E.; Tsvik, G. Laboratory scale study of reduction kinetics of iron oxides on direct reduction conditions. In Proceedings of the METEC & 2nd Estad 2015, Düsseldorf, Germany, 15–19 June 2015. [Google Scholar]



| Gas Composition (% mol) | H2 | CO | H2O | CO2 | CH4 + N2 |
|---|---|---|---|---|---|
| Reducing gas | 52.90 | 30.0 | 4.7 | 4.8 | 8.1 |
| Top gas (T) | 37.0 | 18.9 | 21.2 | 14.3 | 8.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quatravaux, T.; Barros, J.; Gardin, P.; Lucena, G. Adaptation of the Rist Operating Diagram as a Graphical Tool for the Direct Reduction Shaft. Metals 2021, 11, 1953. https://doi.org/10.3390/met11121953
Quatravaux T, Barros J, Gardin P, Lucena G. Adaptation of the Rist Operating Diagram as a Graphical Tool for the Direct Reduction Shaft. Metals. 2021; 11(12):1953. https://doi.org/10.3390/met11121953
Chicago/Turabian StyleQuatravaux, Thibault, Jose Barros, Pascal Gardin, and Gabriel Lucena. 2021. "Adaptation of the Rist Operating Diagram as a Graphical Tool for the Direct Reduction Shaft" Metals 11, no. 12: 1953. https://doi.org/10.3390/met11121953
APA StyleQuatravaux, T., Barros, J., Gardin, P., & Lucena, G. (2021). Adaptation of the Rist Operating Diagram as a Graphical Tool for the Direct Reduction Shaft. Metals, 11(12), 1953. https://doi.org/10.3390/met11121953




