Production and Characterization of a 316L Stainless Steel/β-TCP Biocomposite Using the Functionally Graded Materials (FGMs) Technique for Dental and Orthopedic Applications
Abstract
:1. Introduction
2. Materials and Methods
- ρv = green density (g/cm3),
- ρsint = sintered density (g/cm3),
- ρH2O = water density (g/cm3),
- m = dry weight (g),
- msub = suspended weight (g),
- mu = saturated weight (g),
- V = volume (cm3), and
- Pa = apparent porosity (%).
- R = hemolysis rate (%),
- A = sample absorbance (%),
- C1 = negative control absorbance (%), and
- C2 = positive control absorbance (%).
3. Results and Discussion
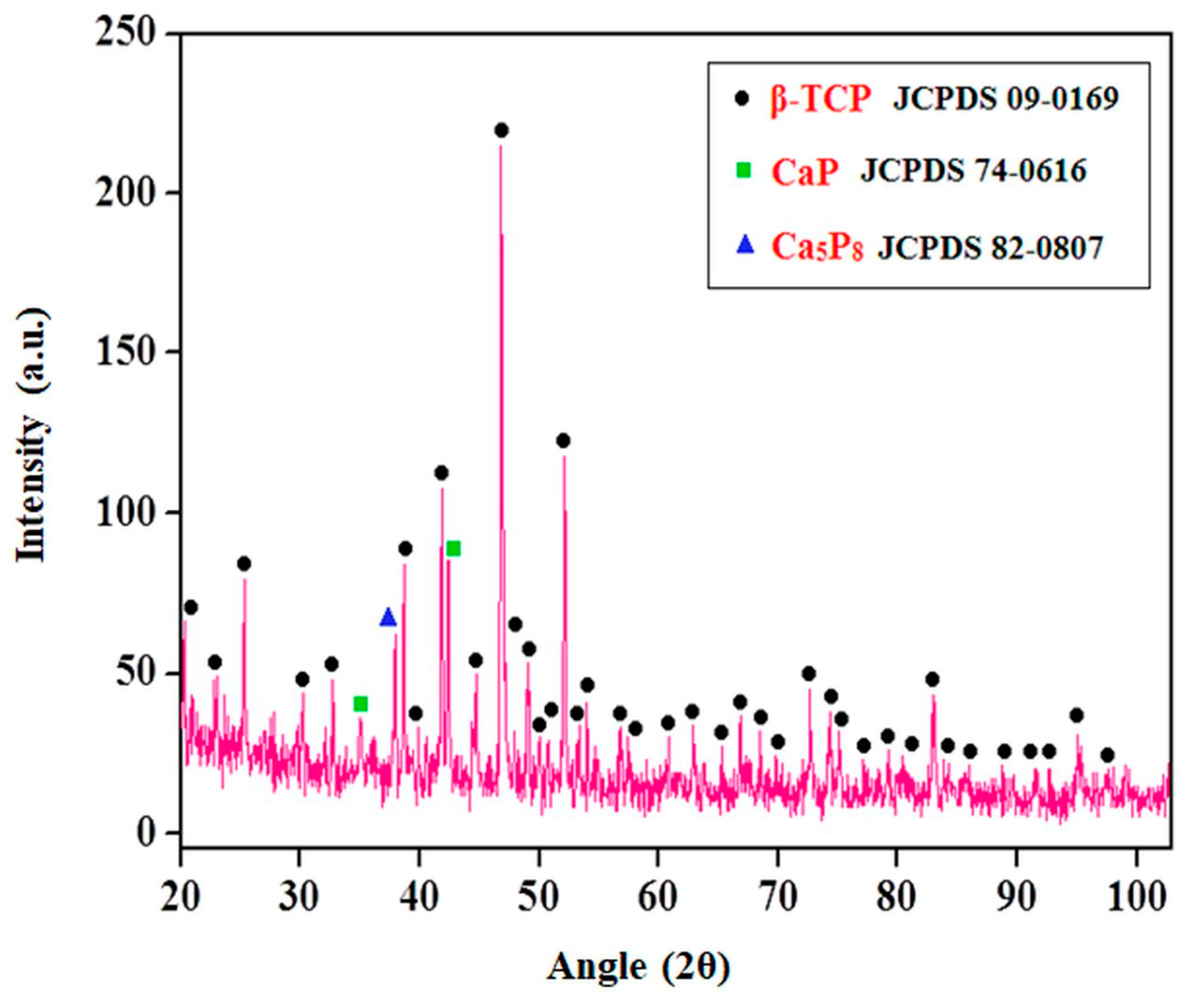
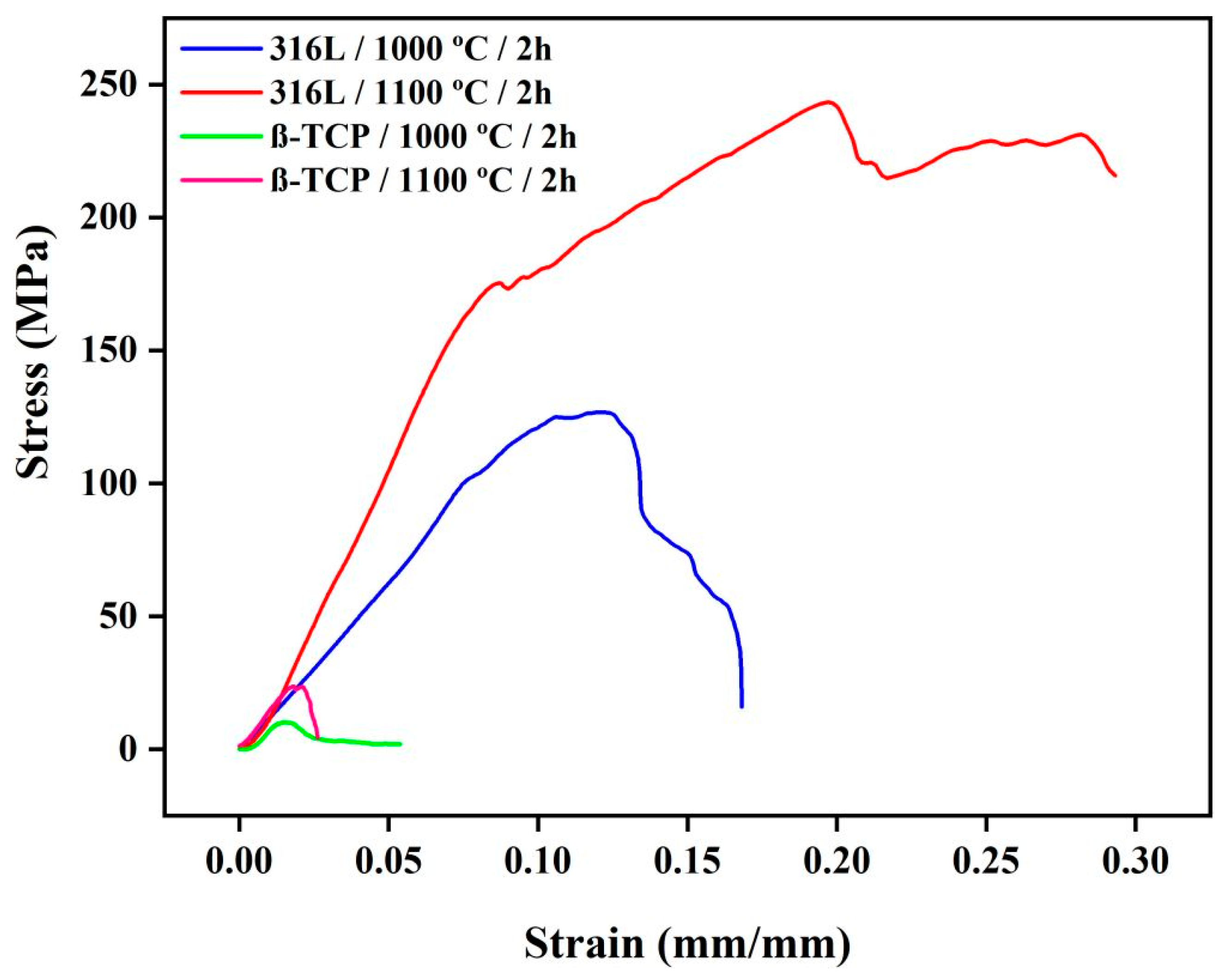
3.1. Evaluation of Best Sintering Temperature
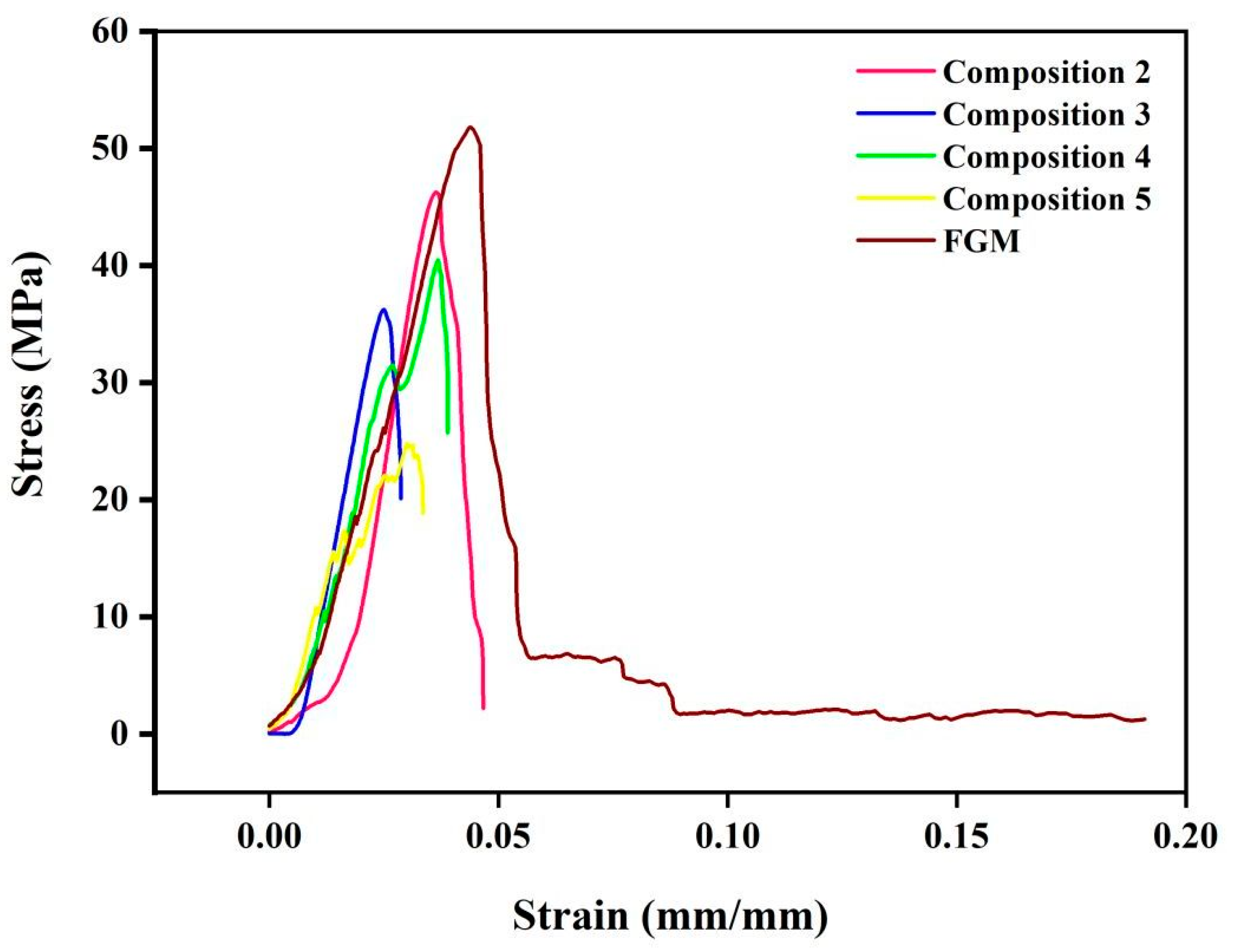
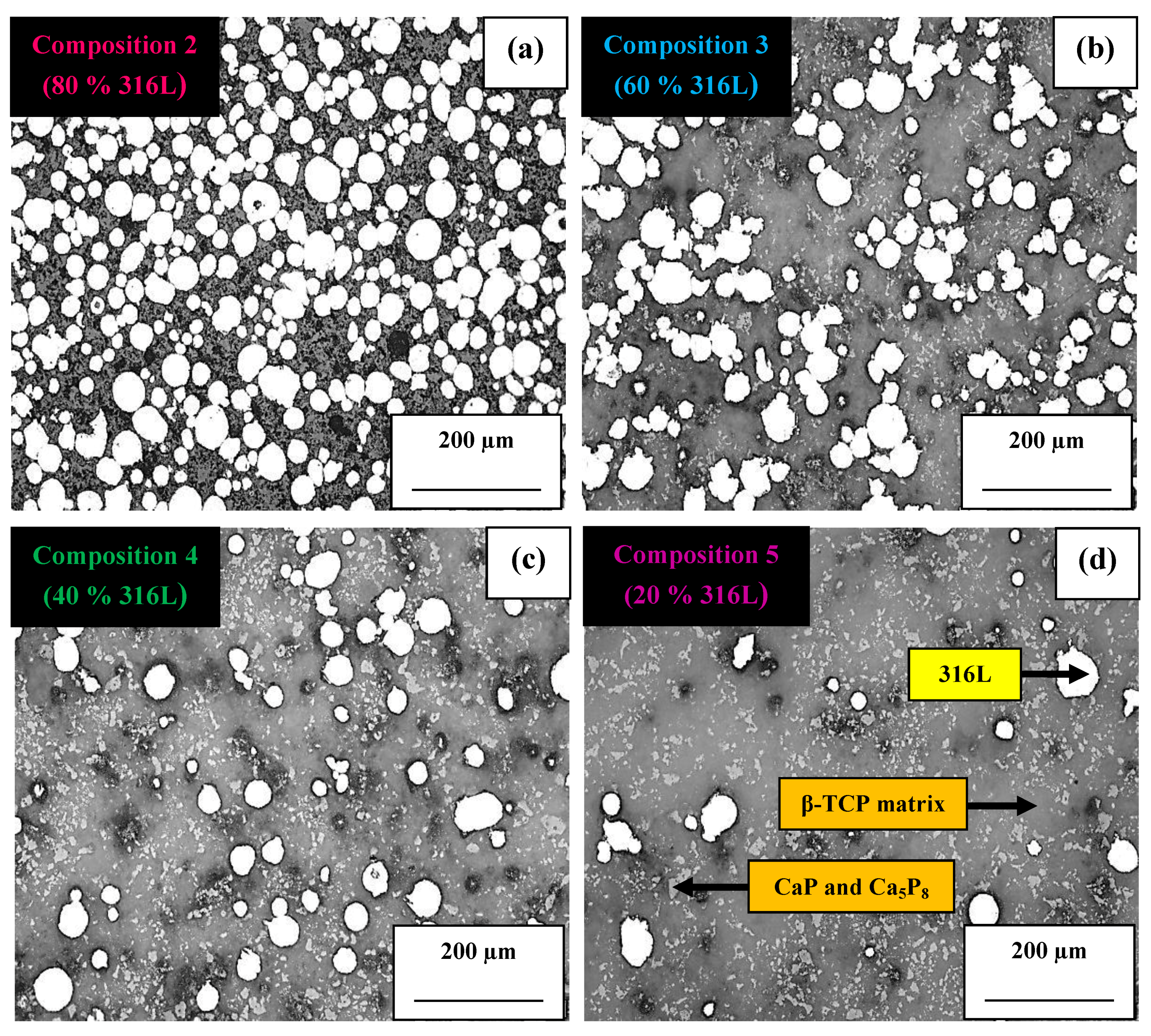
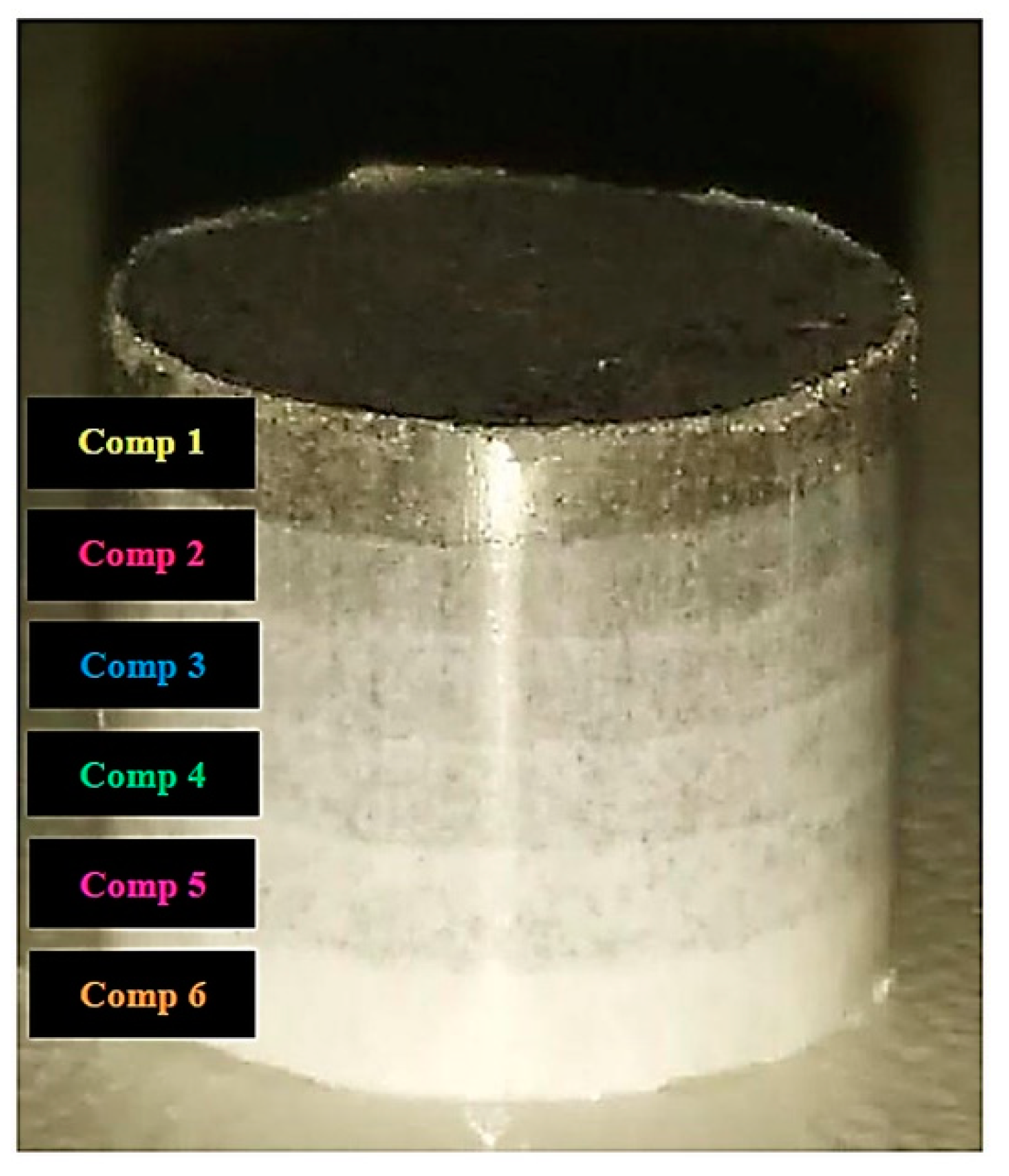
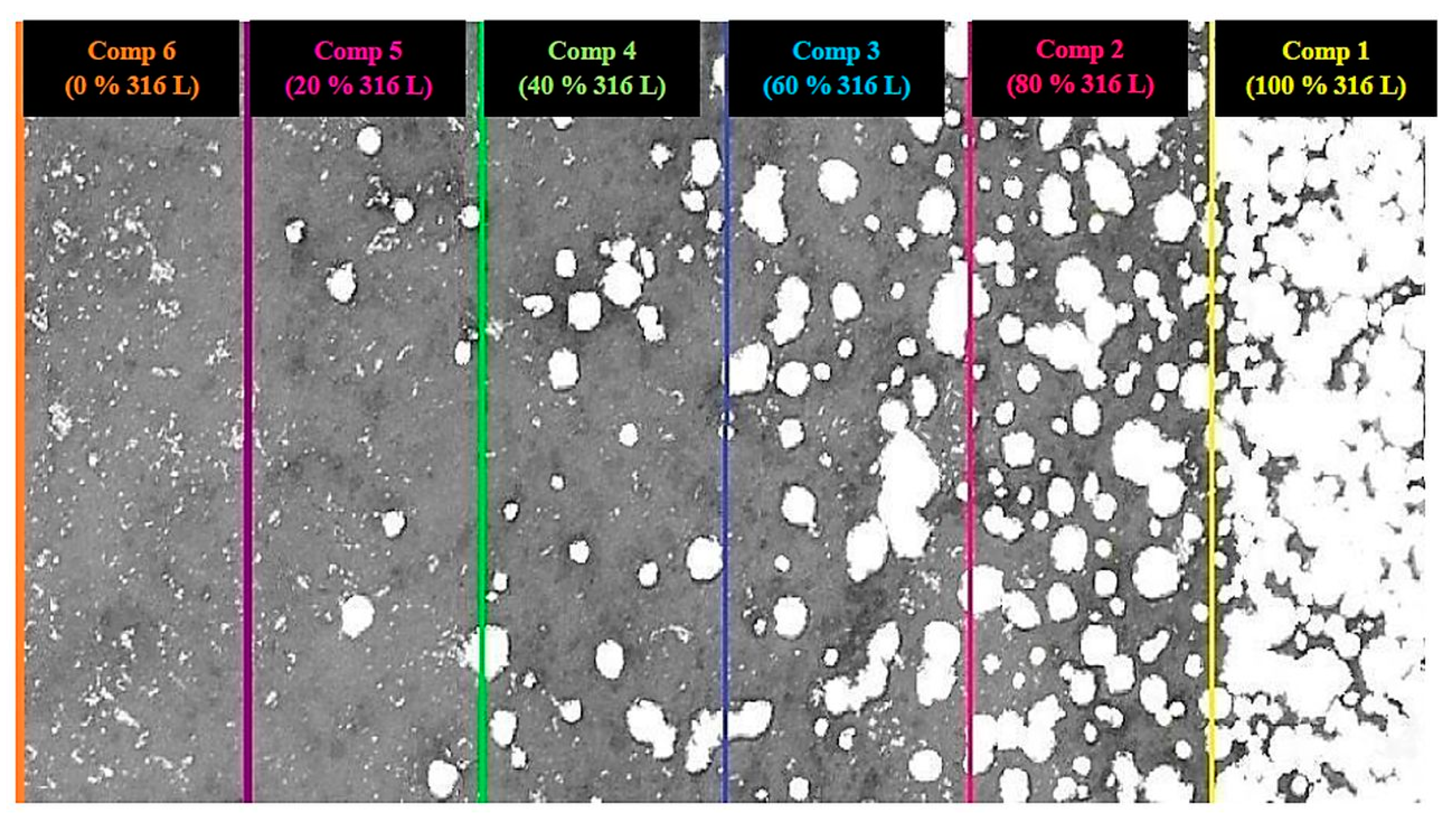
3.2. Microstructural and Mechanical Analysis of Blended Compositions and FGM
3.3. Wettability and Hemocompatibility Analysis of Individual Compositions and FGM
4. Conclusions
- Tests performed to determine the ideal sintering condition indicated that the best microstructural and mechanical results were obtained for 1100 °C/2 h; the condition of 1100 °C/4 h was considered unsatisfactory.
- The blended compositions and the FGM composite presented microstructural and mechanical properties as good as those of pure compositions (316L stainless steel and β-TCP).
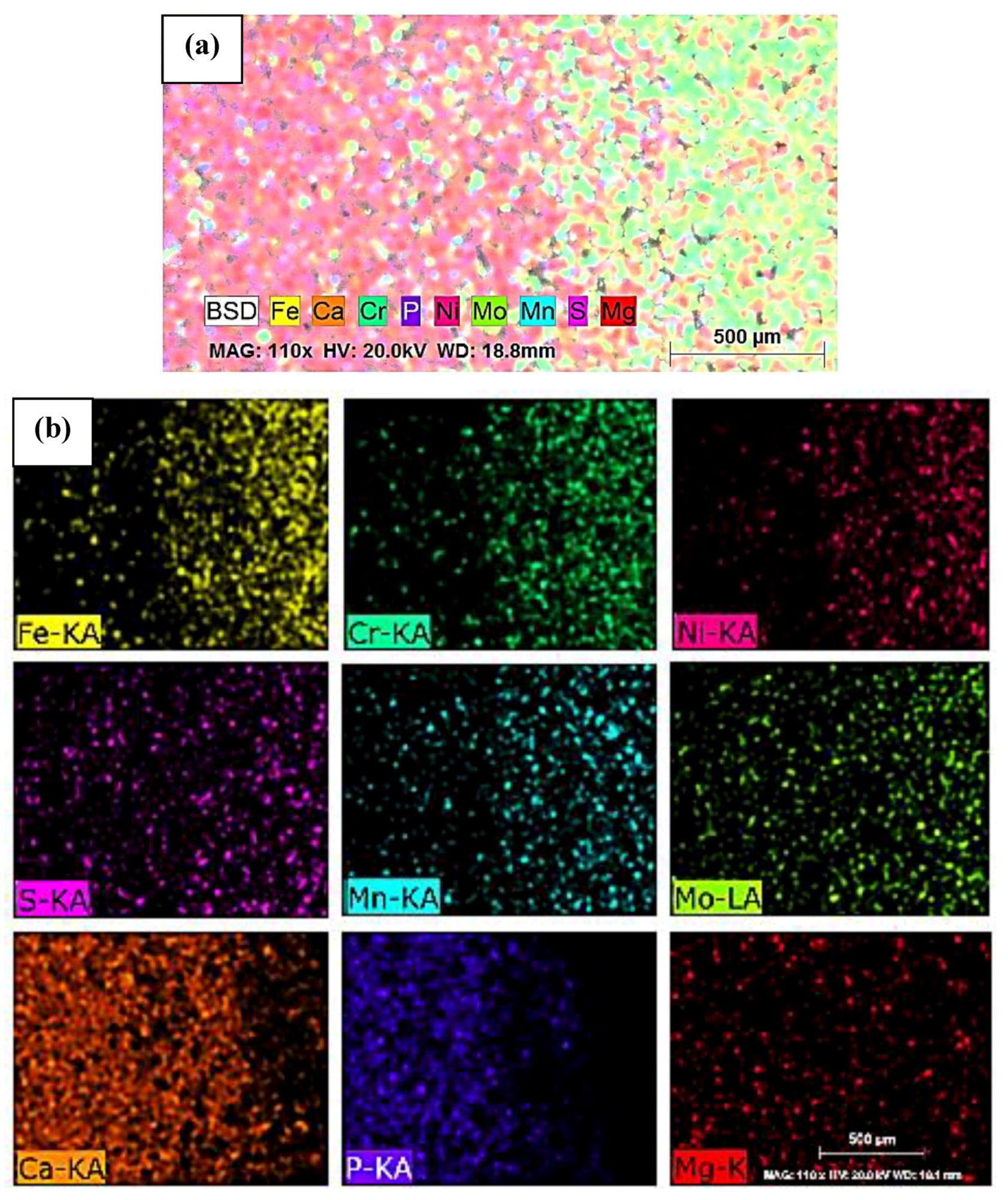
- SEM analysis of FGM composite showed good layering of each composition. Through mapping by EDS, it was observed that its chemical composition was homogeneously distributed throughout the composite.
- For wettability and hemocompatibility tests, both individual compositions and FGM composite presented hydrophilic behavior and high hemocompatibility. It showed that 316L stainless steel/β-TCP composites produced by FGM are possible. It is because the powder metallurgy route did not insert contaminations in the composite that would make it unviable.
- Finally, the FGM composite obtained in this study showed good microstructural and mechanical characteristics, indicating that this material could be considered as a strong candidate for dental and orthopedic applications, but additional experiments to analyze the integration between bone and implant must be performed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitić, Ž.; Stolić, A.; Stojanović, S.; Najman, S.; Ignjatović, N.; Nikolić, G.; Trajanović, M. Instrumental methods and techniques for structural and physicochemical characterization of biomaterials and bone tissue: A review. Mater. Sci. Eng. C 2017, 79, 930–949. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Kolahreez, D.; Ramakrishna, S.; Williams, D. Key terminology in biomaterials and biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 45–50. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzańska-Danikiewicz, A.D.; Dobrzański, L.B. Effect of biomedical materials in the implementation of a long and healthy life policy. Processes 2021, 9, 865. [Google Scholar] [CrossRef]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Li, G.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 77, 90–105. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Sivakumar, S.; Adam Khan, M.; Ebenezer, G.; Chellaganesh, D.; Vignesh, V. A review: Machinability studies on human implant materials. Mater. Today Proc. 2021, 46, 7338–7343. [Google Scholar] [CrossRef]
- Woźniak, A.; Staszuk, M.; Reimann, Ł.; Bialas, O.; Brytan, Z.; Voinarovych, S.; Kyslytsia, O.; Kaliuzhnyi, S.; Basiaga, M.; Admiak, M. The influence of plasma-sprayed coatings on surface properties and corrosion resistance of 316L stainless steel for possible implant application. Arch. Civ. Mech. Eng. 2021, 21, 148. [Google Scholar] [CrossRef]
- Xiao, F.; Zong, C.; Wang, W.; Liu, X.Z.; Osaka, A.; Ma, X.C. Low-temperature fabrication of titania layer on 3D-printed 316L stainless steel for enhancing biocompatibility. Surf. Coat. Technol. 2019, 367, 91–99. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, P.; Li, J.; Liu, C.; Wang, L. Functional Gradient Metallic Biomaterials: Techniques, Current Scenery, and Future Prospects in the Biomedical Field. Front. Bioeng. Biotechnol. 2021, 8, 616845. [Google Scholar] [CrossRef]
- Essa, K.; Jamshidi, P.; Zou, J.; Attallah, M.M.; Hassanin, H. Porosity control in 316L stainless steel using cold and hot isostatic pressing. Mater. Des. 2018, 138, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Mehboob, H.; Chang, S.H. Evaluation of the development of tissue phenotypes: Bone fracture healing using functionally graded material composite bone plates. Compos. Struct. 2014, 117, 105–113. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef]
- Tanaka, T.; Komaki, H.; Chazono, M.; Kitasato, S.; Kakuta, A.; Akiyama, S.; Marumo, K. Basic research and clinical application of beta-tricalcium phosphate (β-TCP). Morphologie 2017, 101, 164–172. [Google Scholar] [CrossRef]
- Grigoraviciute-Puroniene, I.; Tsuru, K.; Garskaite, E.; Stankeviciute, Z.; Beganskiene, A.; Ishikawa, K.; Kareiva, A. A novel wet polymeric precipitation synthesis method for monophasic β-TCP. Adv. Powder Technol. 2017, 28, 2325–2331. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Y.; Yin, H.; Ruan, Y.; Sun, Y.; Lin, K. Effects of Mn-doping on the structural evolution of b-Tricalcium Phosphate by Rietveld refinement and Raman spectroscopy. Ceram. Int. 2019, 45, 11073–11078. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Honeycomb blocks composed of carbonate apatite, β-tricalcium phosphate, and hydroxyapatite for bone regeneration: Effects of composition onbiological responses. Mater. Today Bio 2019, 4, 100031. [Google Scholar] [CrossRef]
- Carson, J.S.; Bostrom, M.P.G. Synthetic bone scaffolds and fracture repair. Injury 2007, 38, S33–S37. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, K.Y.; Shim, D.S. Effects of process parameters on properties of porous foams formed by laser-assisted melting of steel powder (AISI P21)/foaming agent (ZrH2) mixture. Opt. Laser Technol. 2018, 98, 326–338. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Paternoster, C.; Mostaed, E.; Tolouei, R.; Casati, R.; Vedani, M.; Mantovani, D. Synthesis, mechanical properties and corrosion behavior of powder metallurgy processed Fe/Mg2Si composites for biodegradable implant applications. Mater. Sci. Eng. C 2017, 81, 511–521. [Google Scholar] [CrossRef]
- Salimi, E. Functionally graded calcium phosphate bioceramics: An overview of preparation and properties. Ceram. Int. 2020, 46, 19664–19668. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Zhang, Z.; Liu, W. Size-dependent vibration and stability of multi-span viscoelastic functionally graded material nanopipes conveying fluid using a hybrid method. Compos. Struct. 2017, 179, 590–600. [Google Scholar] [CrossRef]
- Höganäs AB. Datasheet: 3045HOG 316L Austenitic Stainless Steel for Laser Powder Bed Fusion; Höganäs AB: Hoganas, Sweden, 2020; pp. 2–3. Available online: https://www.hoganas.com/globalassets/download-media/sharepoint/brochures-and-datasheets---all-documents/additeve-manufacturing_am-316l_20-53_3045hog.pdf (accessed on 10 January 2021).
- Almeida, J.A.M.; Kuffner, B.H.B.; Silva, G.; Capellato, P.; Sachs, D. Analysis of the influence of β-TCP particle size on deagglomeration processes. Res. Soc. Dev. 2020, 9, e175943067. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Feng, Y.; Yuan, S.; Zhang, X.; Wang, G. Melting behavior of Ag nanoparticles and their clusters. Appl. Therm. Eng. 2017, 111, 1457–1463. [Google Scholar] [CrossRef]
- Kowsar, S.; Soheilifard, R. The effect of the degradation pattern of biodegradable bone plates on the healing process using a biphasic mechano-regulation theory. Biomech. Model. Mechanobiol. 2021, 20, 309–321. [Google Scholar] [CrossRef]
- Mehboob, H.; Chang, S.H. Optimal design of a functionally graded biodegradable composite bone plate by using the Taguchi method and finite element analysis. Compos. Struct. 2015, 119, 166–173. [Google Scholar] [CrossRef]
- Salavati, H.; Mohammadi, H.; Yusefi, A.; Berto, F. Fracture assessment of V-notched specimens with end holes made of tungsten-copper functionally graded material under mode I loading. Theor. Appl. Fract. Mech. 2018, 97, 357–367. [Google Scholar] [CrossRef]
- Mohammadi, H.; Salavati, H.; Alizadeh, Y.; Berto, F. Prevalent mode II fracture assessment of inclined U-notched specimens made of tungsten-copper functionally graded material. Theor. Appl. Fract. Mech. 2017, 89, 90–99. [Google Scholar] [CrossRef]
- Valarezo, A.; Bolelli, G.; Choi, W.B.; Sampath, S.; Cannillo, V.; Lusvarghi, L.; Rosa, R. Damage tolerant functionally graded WC-Co/Stainless Steel HVOF coatings. Surf. Coat. Technol. 2010, 205, 2197–2208. [Google Scholar] [CrossRef]
- Thomas, G.; Vincent, R.; Matthews, G.; Dance, B.; Grant, P.S. Interface topography and residual stress distributions in W coatings for fusion armour applications. Mater. Sci. Eng. A 2008, 477, 35–42. [Google Scholar] [CrossRef]
- Lin, X.; Yue, T.M. Phase formation and microstructure evolution in laser rapid forming of graded SS316L/Rene88DT alloy. Mater. Sci. Eng. A 2005, 402, 294–306. [Google Scholar] [CrossRef]
- Torres, P.; Abrantes, J.; Kaushal, A.; Pina, S.; Dobelin, N.; Bohner, M.; Ferreira, J. Influence of Mg-doping, calcium pyrophosphate impurities and cooling rate on the allotropic α ↔ β-tricalcium phosphate phase transformations. J. Eur. Ceram. Soc. J. 2015, 36, 817–827. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef]
- Cardoso, H.A.I.; Motisuke, M.; Zavaglia, C.A.C. Analysis of the influence of two different milling processes on the properties of beta-TCP precursor powder and cement. Ceramica 2012, 58, 225–228. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. ASTM C20-00: Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water; ASTM International: West Conshohocken, PA, USA, 2010; Volume 15.01, pp. 1–3. [Google Scholar] [CrossRef]
- ASTM International. ASTM E384: Standard Test Method for Microindentation Hardness of Materials. In American Society for Testing Materials Handbook; ASTM International: West Conshohocken, PA, USA, 2017; Volume 03.01, pp. 281–293. [Google Scholar] [CrossRef]
- ASTM International. ASTM E92-17: Standard Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials BT—Standard Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2017; Volume 03.01, pp. 1–27. [Google Scholar] [CrossRef]
- ASTM International. ASTM E9-19: Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature; ASTM International: West Conshohocken, PA, USA, 2019; Volume 03.01, pp. 1–9. [Google Scholar] [CrossRef]
- ASTM International. ASTM F756-17: Standard Practice for Assessment of Hemolytic Properties of Materials; ASTM International: West Conshohocken, PA, USA, 2017; Volume 13.01, p. 5. [Google Scholar] [CrossRef]
- Nasr, M.N.A.; Ng, E.G.; Elbestawi, M.A. Modelling the effects of tool-edge radius on residual stresses when orthogonal cutting AISI 316L. Int. J. Mach. Tools Manuf. 2007, 47, 401–411. [Google Scholar] [CrossRef]
- Choi, J.P.; Lee, G.Y.; Song, J.-I.; Lee, W.S.; Lee, J.S. Sintering behavior of 316L stainless steel micro-nanopowder compact fabricated by powder injection molding. Powder Technol. 2015, 279, 196–202. [Google Scholar] [CrossRef]
- Mehdikhani, B.; Borhani, G.H. Densification and Mechanical Behavior of β-Tricalcium Phosphate Bioceramics. Int. Lett. Chem. Phys. Astron. 2014, 36, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Ziętala, M.; Durejko, T.; Polański, M.; Kunce, I.; Płociński, T.; Zieliński, W.; Łazińska, M.; Stępniowski, W.; Czujko, T.; Kurzydłowski, K.J.; et al. The microstructure, mechanical properties and corrosion resistance of 316L stainless steel fabricated using laser engineered net shaping. Mater. Sci. Eng. A 2016, 677, 1–10. [Google Scholar] [CrossRef]
- Cao, M.; Jiang, F.; Wang, C.; Cui, H.; Guo, C.; Chang, Y.; Wang, Z. Preparation, microstructure and mechanical property of double-layered metal-ceramic hollow spheres. Mater. Sci. Eng. A 2020, 780, 139188. [Google Scholar] [CrossRef]
- Singh, R.P.; Batra, U. Structure and properties of silver-doped calcium phosphate nanopowders. Bull. Mater. Sci. 2016, 39, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Kostov-Kytin, V.V.; Dyulgerova, E.; Ilieva, R.; Petkova, V. Powder X-ray diffraction studies of hydroxyapatite and β-TCP mixtures processed by high energy dry milling. Ceram. Int. 2018, 44, 8664–8671. [Google Scholar] [CrossRef]
- Nascimento, R.M.; Faita, F.L.; Agostini, D.L.S.; Job, A.E.; Guimarães, F.E.G.; Bechtold, I.H. Production and characterization of natural rubber-Ca/P blends for biomedical purposes. Mater. Sci. Eng. C 2014, 39, 29–34. [Google Scholar] [CrossRef]
- Amanov, A.; Lee, S.W.; Pyun, Y.S. Low friction and high strength of 316L stainless steel tubing for biomedical applications. Mater. Sci. Eng. C 2017, 71, 176–185. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Metsger, D.S.; Rieger, M.R.; Foreman, D.W. Mechanical properties of sintered hydroxyapatite and tricalcium phosphate ceramic. J. Mater. Sci. Mater. Med. 1999, 10, 9–17. [Google Scholar] [CrossRef]
- Chicot, D.; Tricoteaux, A.; Lesage, J.; Leriche, A.; Descamps, M.; Rguiti-Constantin, E. Mechanical Properties of Porosity-Free Beta Tricalcium Phosphate (β-TCP) Ceramic by Sharp and Spherical Indentations. New J. Glas. Ceram. 2013, 03, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Pillar, R.M. Metallic Biomaterials. In Biomedical Materials; Narayan, R., Ed.; Springer: Toronto, ON, Canada, 2021; pp. 1–48. ISBN 9783030492052. [Google Scholar]
- Marzuki, M.; Mazni Ismail, N.; Ihsan Abdul Latiff, M. Preparation and Microstructural Characterization of Five-layered Aluminium-Aluminium Oxide Functionally Graded Material. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012031. [Google Scholar] [CrossRef]
- Al-Qureshi, H.A.; Soares, M.R.F.; Hotza, D.; Alves, M.C.; Klein, A.N. Analyses of the fundamental parameters of cold die compaction of powder metallurgy. J. Mater. Process. Technol. 2008, 199, 417–424. [Google Scholar] [CrossRef]
- Pasha, A.; Rajaprakash, B.M. Fabrication and mechanical properties of functionally graded materials: A review. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Pang, D.; He, L.; Wei, L.; Zheng, H.; Deng, C. Preparation of a beta-tricalcium phosphate nanocoating and its protein adsorption behaviour by quartz crystal microbalance with dissipation technique. Colloids Surf. B Biointerfaces 2018, 162, 1–7. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Shang, Y.; Yuan, Y.; Li, D.; Li, Y.; Chen, J. Effects of scanning speed on in vitro biocompatibility of 316L stainless steel parts elaborated by selective laser melting. Int. J. Adv. Manuf. Technol. 2017, 92, 4379–4385. [Google Scholar] [CrossRef]
- Krishna Samanta, S.; Chanda, A. Study on the Structure and Properties of Crystalline Pure and Doped β-Tri Calcium Phosphate Ceramics. Mater. Today Proc. 2018, 5, 2330–2338. [Google Scholar] [CrossRef]
- ASTM International. ASTM F 756-00: Standard Practice for Assessment of Hemolytic Properties of Materials; ASTM International: West Conshohocken, PA, USA, 2000; Volume 13.01, p. 5. [Google Scholar]
- Esmaeili, A.; Ghaffari, S.A.; Nikkhah, M.; Malek Ghaini, F.; Farzan, F.; Mohammadi, S. Biocompatibility assessments of 316L stainless steel substrates coated by Fe-based bulk metallic glass through electro-spark deposition method. Colloids Surf. B Biointerfaces 2021, 198, 111469. [Google Scholar] [CrossRef]
- Mihalcea, E.; Vergara-Hernández, H.J.; Jimenez, O.; Olmos, L.; Chávez, J.; Arteaga, D. Design and characterization of Ti6Al4V/20CoCrMo−highly porous Ti6Al4V biomedical bilayer processed by powder metallurgy. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2021, 31, 178–192. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, P. Microstructure and mechanical behaviour of Ti-Cu foams synthesized via powder metallurgy technique. Mater. Res. Express 2021, 8, 035402. [Google Scholar] [CrossRef]
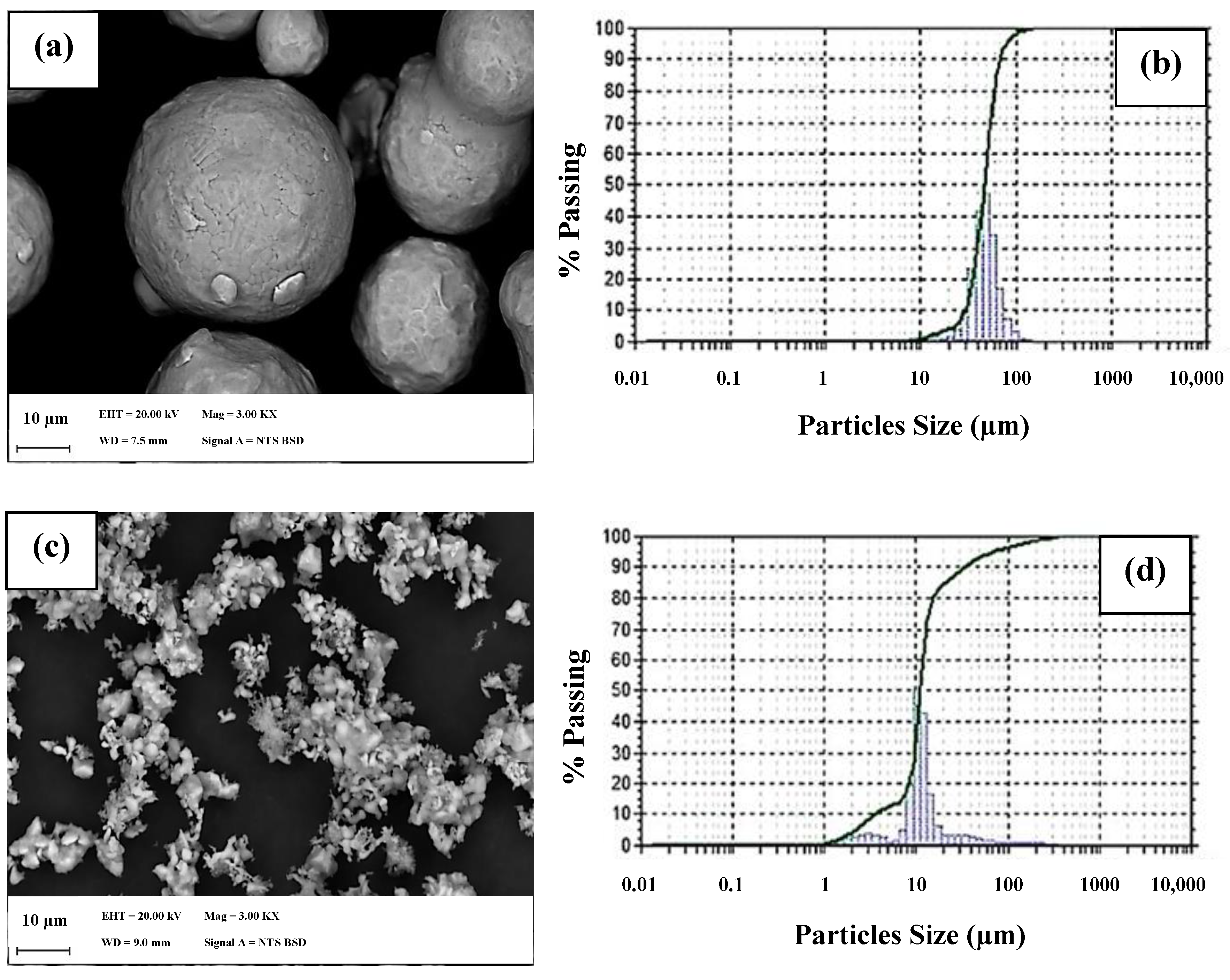

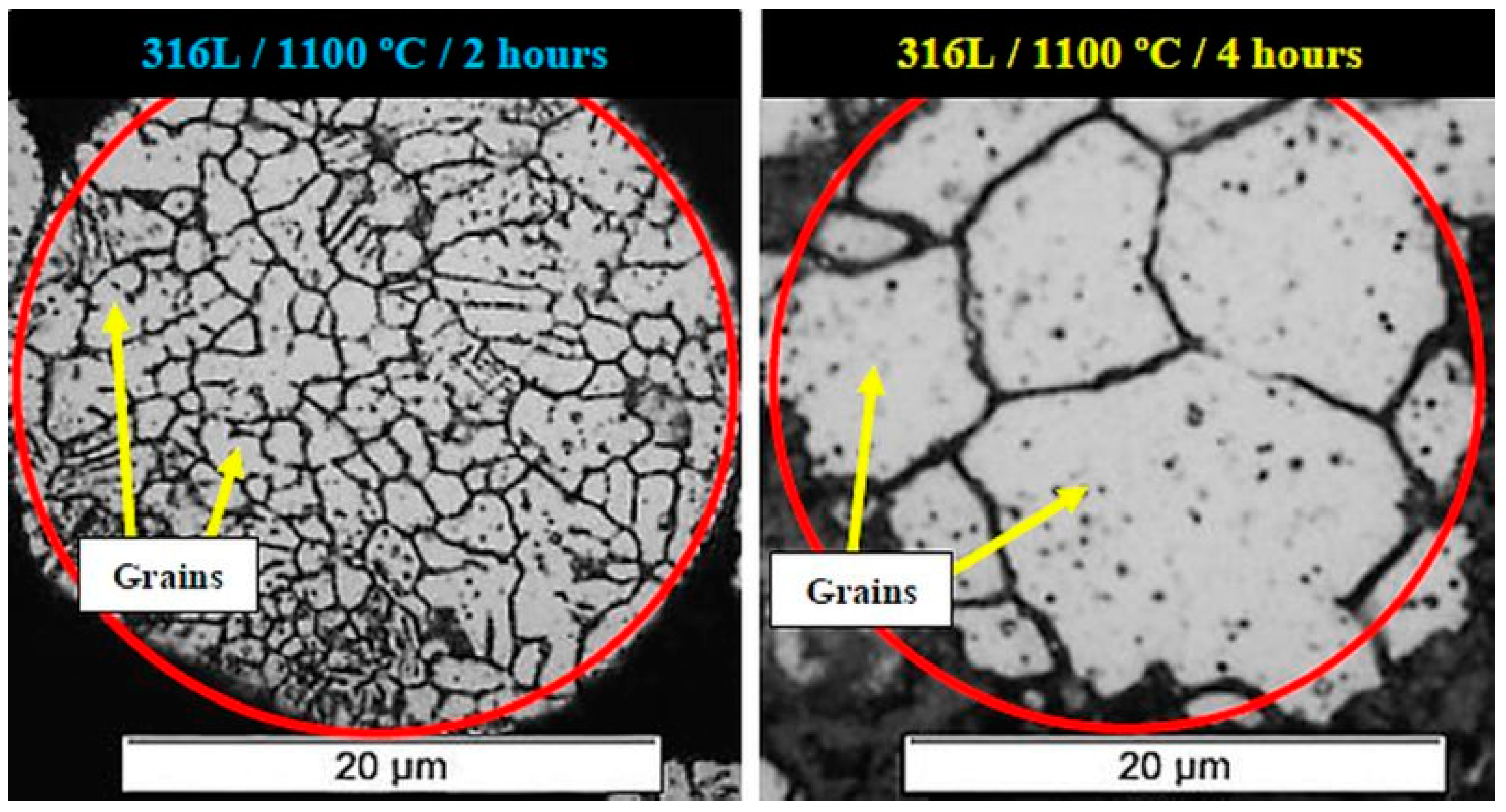
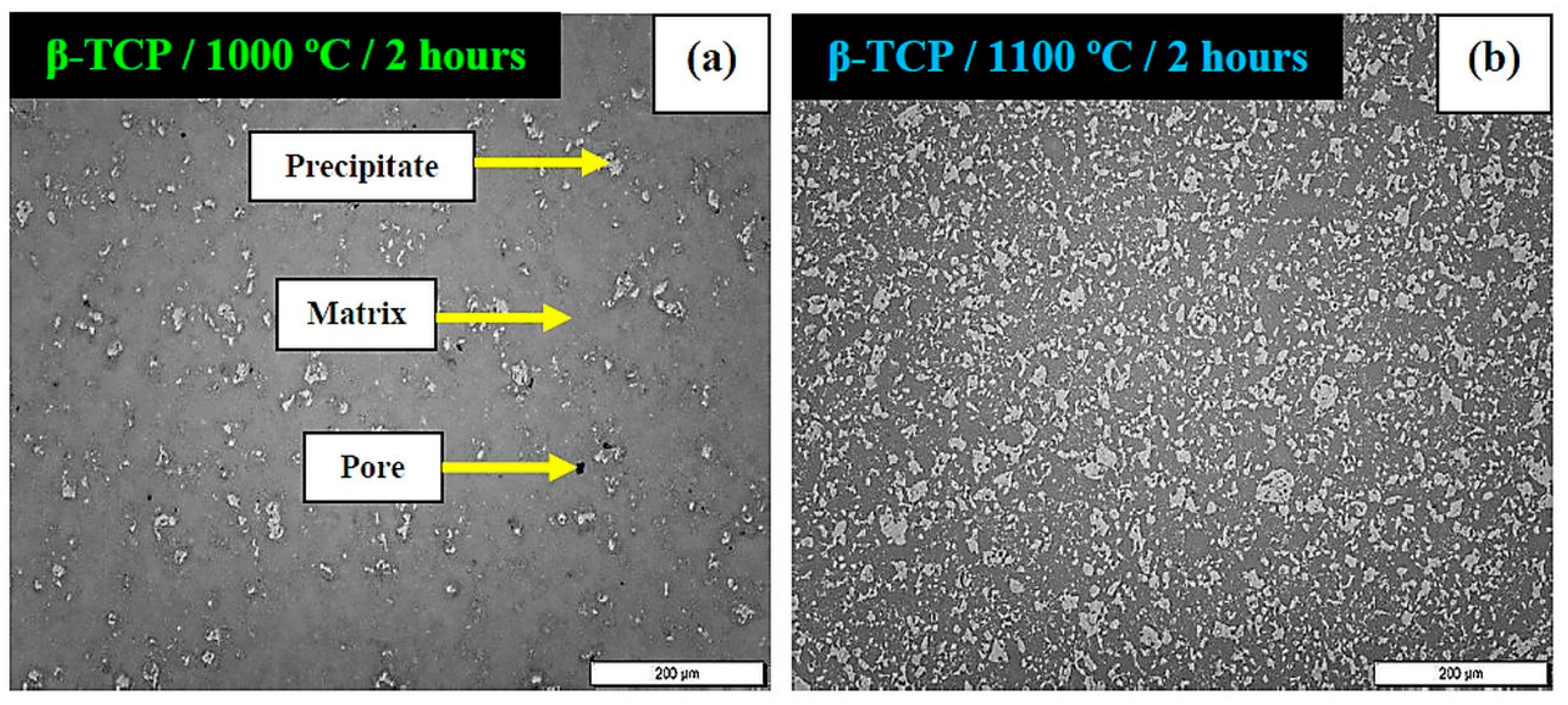
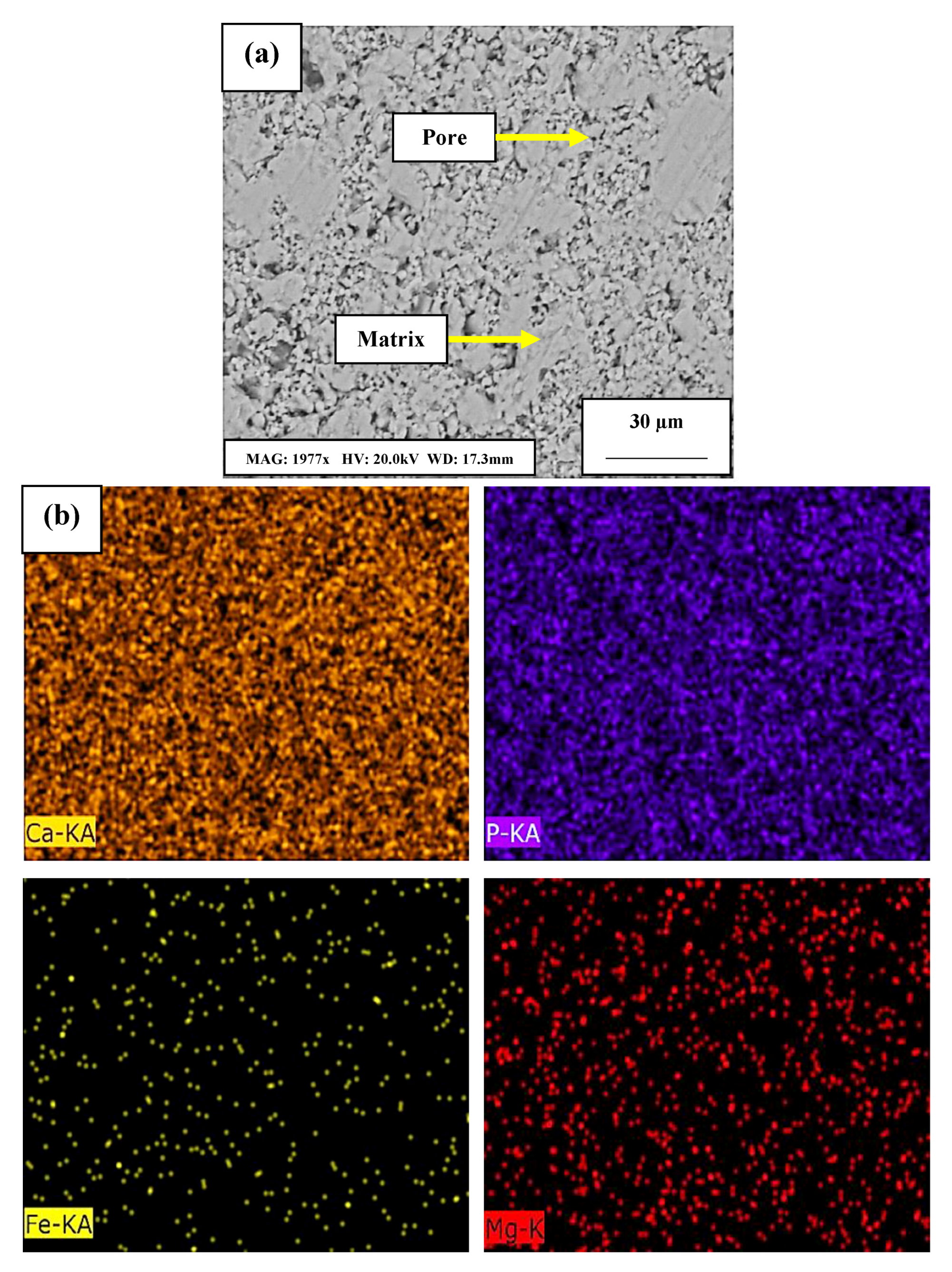

| Chemical Element | Content % |
|---|---|
| Cr | 17 |
| Ni | 12 |
| Mo | 2.5 |
| Mn | 1.5 |
| Si | 0.8 |
| C | 0.01 |
| O | 0.06 |
| N | 0.10 |
| Fe | Balance |
| Layer | Composition | Constitution (v/v%) |
|---|---|---|
| 1 | Pure | 100% 316L |
| 2 | Blended | 80% 316L–20% β-TCP |
| 3 | Blended | 60% 316L–40% β-TCP |
| 4 | Blended | 40% 316L–60% β-TCP |
| 5 | Blended | 20% 316L–80% β-TCP |
| 6 | Pure | 100% β-TCP |
| Composition | Green Density (g/cm3) | Sintered Density (g/cm3) | Apparent Porosity (%) | Microhardness (HV) | Young’s Modulus (GPa) | Yield Strength (MPa) | Ultimate Compressive Strength (MPa) | Contact Angle (θ) | Hemolysis Rate (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 100% 316L | 1000 °C 2 h | 5.46 | 5.92 | 25.28 | 131 | 1.26 | 115.48 | 126.81 | - | - |
| 1100 °C 2 h | 5.46 | 6.06 | 24.49 | 203 | 2.59 | 172.52 | 243.43 | 85.90 | 2.35 | ||
| 1100 °C 4 h | - | - | - | 115 | - | - | - | - | - | ||
| 2 | 80% 316L 20% β-TCP | - | 4.53 | 4.85 | 22.12 | 185.17 | 2.27 | 45.86 | 46.24 | 68.73 | 4.47 |
| 3 | 60% 316L 40% β-TCP | - | 3.76 | 3.98 | 21.58 | 161.80 | 2.08 | 35.98 | 36.23 | 71.30 | 2.62 |
| 4 | 40% 316L 60% β-TCP | - | 3.01 | 3.28 | 24.59 | 141.50 | 1.87 | 31.28 | 40.44 | 76.00 | 4.08 |
| 5 | 20% 316L 80% β-TCP | - | 2.54 | 2.83 | 25.68 | 122.20 | 1.73 | 17.18 | 24.81 | 63.33 | 2.42 |
| 6 | 100% β-TCP | 1000 °C 2 h | 2.24 | 2.46 | 27.22 | 43.52 | 1.12 | 10.03 | 10.20 | - | - |
| 1100 °C 2 h | 2.24 | 2.48 | 26.32 | 112.53 | 1.63 | 23.54 | 23.68 | 44.47 | 4.42 | ||
| FGM | - | - | 3.54 | 3.78 | 25.03 | - | 1.74 | 51.17 | 51.82 | 66.03 | 4.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuffner, B.H.B.; Capellato, P.; Ribeiro, L.M.S.; Sachs, D.; Silva, G. Production and Characterization of a 316L Stainless Steel/β-TCP Biocomposite Using the Functionally Graded Materials (FGMs) Technique for Dental and Orthopedic Applications. Metals 2021, 11, 1923. https://doi.org/10.3390/met11121923
Kuffner BHB, Capellato P, Ribeiro LMS, Sachs D, Silva G. Production and Characterization of a 316L Stainless Steel/β-TCP Biocomposite Using the Functionally Graded Materials (FGMs) Technique for Dental and Orthopedic Applications. Metals. 2021; 11(12):1923. https://doi.org/10.3390/met11121923
Chicago/Turabian StyleKuffner, Bruna Horta Bastos, Patricia Capellato, Larissa Mayra Silva Ribeiro, Daniela Sachs, and Gilbert Silva. 2021. "Production and Characterization of a 316L Stainless Steel/β-TCP Biocomposite Using the Functionally Graded Materials (FGMs) Technique for Dental and Orthopedic Applications" Metals 11, no. 12: 1923. https://doi.org/10.3390/met11121923
APA StyleKuffner, B. H. B., Capellato, P., Ribeiro, L. M. S., Sachs, D., & Silva, G. (2021). Production and Characterization of a 316L Stainless Steel/β-TCP Biocomposite Using the Functionally Graded Materials (FGMs) Technique for Dental and Orthopedic Applications. Metals, 11(12), 1923. https://doi.org/10.3390/met11121923









