Microstructural Evolution and Electrochemical Behavior of Solution Treated, Hot Rolled and Aged MgDyZnZr Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloy Fabrication and Heat Treatments
2.2. Vickers Hardness
2.3. Microstructural Characterizations
2.4. Electrochemical Tests
3. Results and Discussion
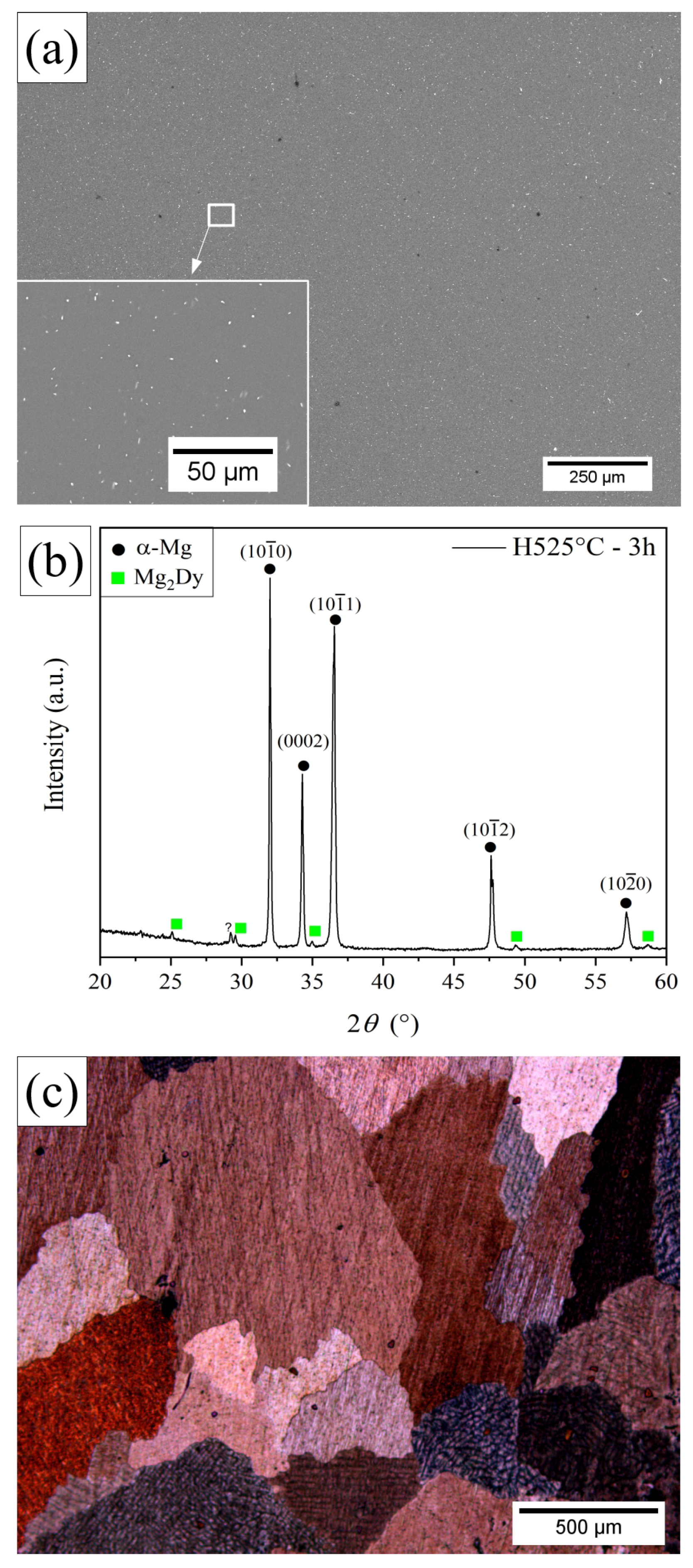
3.1. Microstructural Characterization of the H525 °C-3 h Specimen
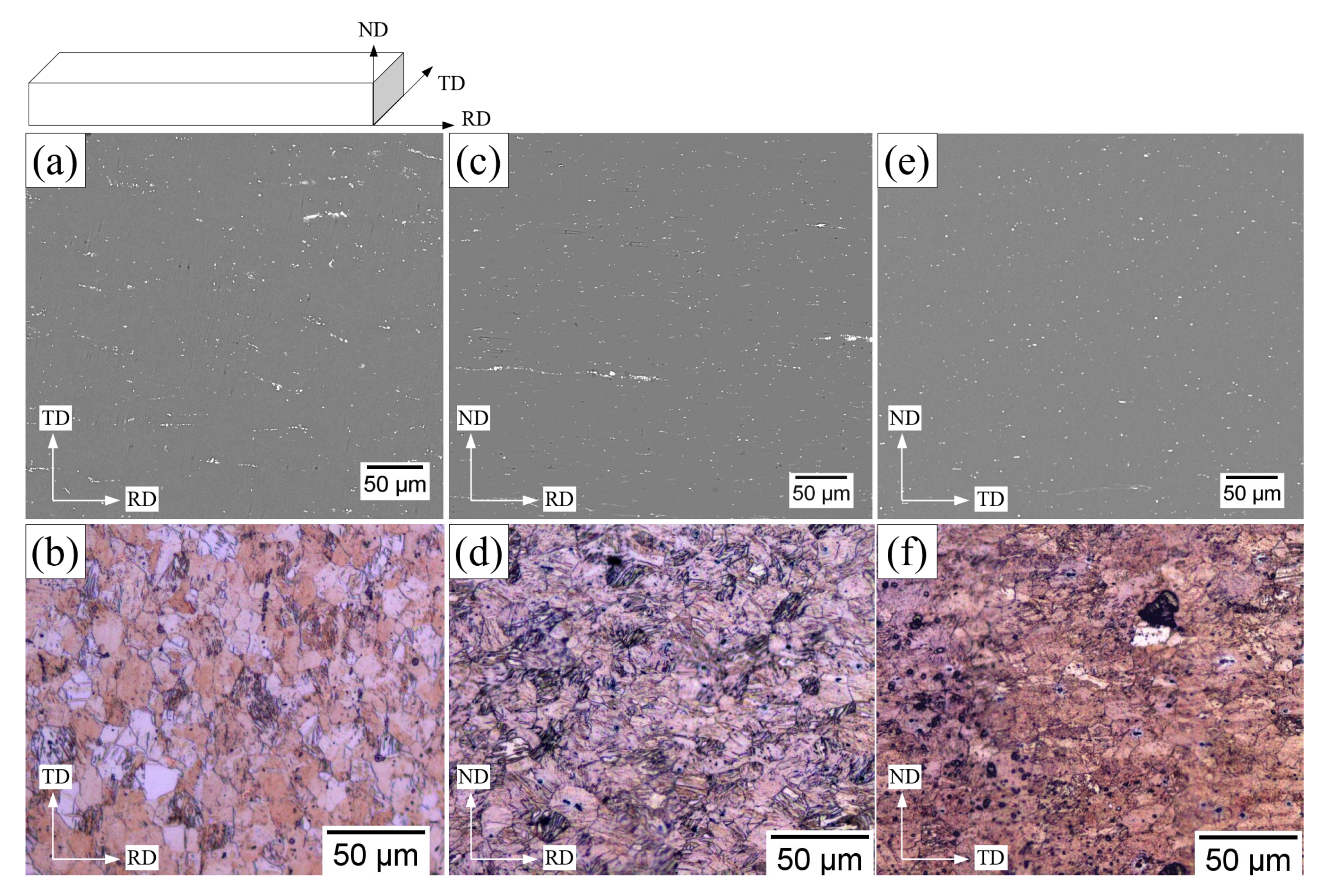
3.2. Microstructural Characterization of Rolled Specimen
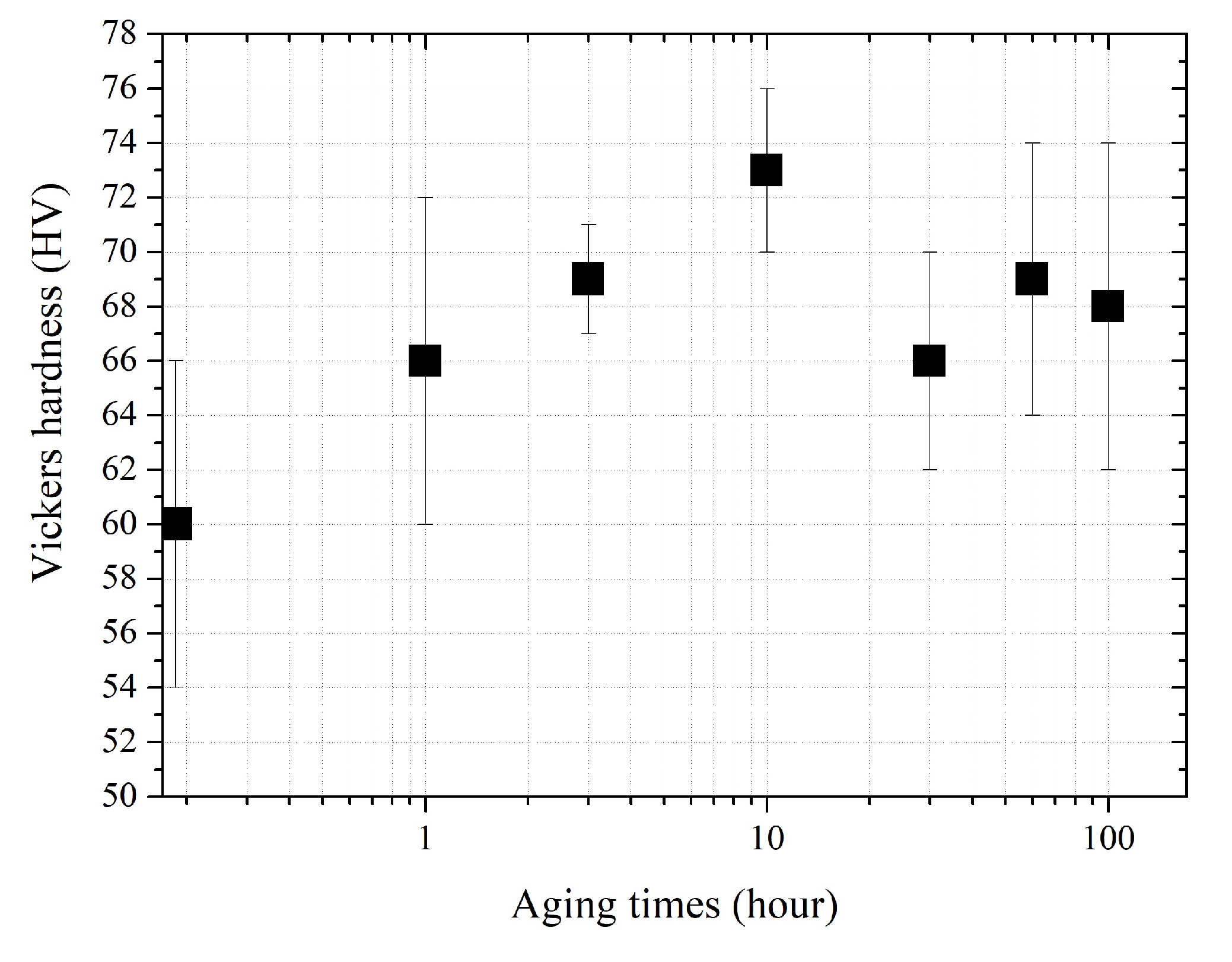
3.3. Aging Hardening Behavior of the Alloy
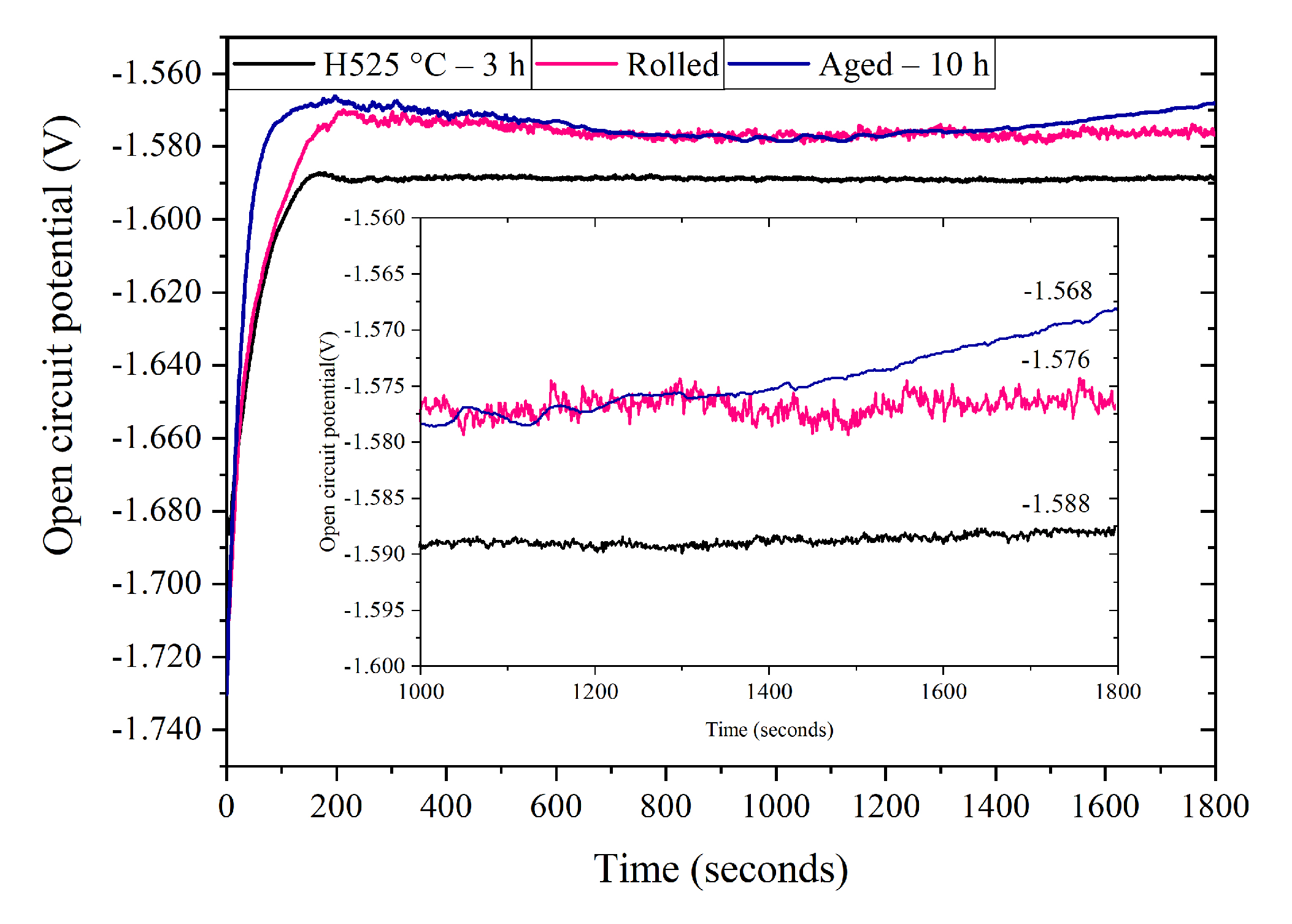
3.4. Electrochemical Characterization
4. Conclusions
- -
- Homogenization heat treatments at 525 °C for 3 h successfully avoided any inhomogeneity that otherwise occurred during the melting process.
- -
- The hot rolling process with multiple passes promoted a homogeneous grain-refined microstructure for all rolling directions with a basal {0002} texture.
- -
- A combination of hot rolling and aging heat treatments at 250 °C for 10 h promoted an alloy with a small grain size with thin, dispersed precipitates.
- -
- These precipitates dispersed after aging at 250 °C for 10 h collaborated for an increase in the strength of the alloy as shown by the hardness increase.
- -
- These characteristics of grain refinement and thin, dispersed precipitates led to a better corrosion resistance of aging at 250 °C for 10 h specimens as shown by a lower corrosion rate.
Author Contributions
Funding
Conflicts of Interest
References
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Liu, D.; Yang, D.; Li, X.; Hu, S. Mechanical properties, corrosion resistance and biocompatibilities of degradable Mg-RE alloys: A review. J. Mater. Res. Technol. 2019, 8, 1538–1549. [Google Scholar] [CrossRef]
- Nayak, S.; Bhushan, B.; Jayaganthan, R.; Gopinath, P.; Agarwal, R.; Lahiri, D. Strengthening of Mg based alloy through grain refinement for orthopaedic application. J. Mech. Behav. Biomed. Mater. 2016, 59, 57–70. [Google Scholar] [CrossRef]
- Lowe, T.; Valiev, R. Frontiers for bulk nanostructured metals in biomedical applications. In Advanced Biomaterials and Biodevices; Tiwari, A., Nordin, A.N., Eds.; Wiley-Blackwell: Oxford, UK, 2014; pp. 1–52. [Google Scholar]
- Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Fu, P.; Ding, W. Effects of extrusion and heat treatment on the mechanical properties and biocorrosion behaviors of a Mg–Nd–Zn–Zr alloy. J. Mech. Behav. Biomed. Mater. 2012, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, Y.; Peng, Q.; Feyerabend, F.; Kainer, K.U.; Willumeit, R.; Hort, N. Mechanical and corrosion properties of binary Mg–Dy alloys for medical applications. Mater. Sci. Eng. B 2011, 176, 1827–1834. [Google Scholar] [CrossRef]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haley, T.J.; Koste, L.; Komesu, N.; Efros, M.; Upham, H. Pharmacology and toxicology of dysprosium, holmium, and erbium chlorides. Toxicol. Appl. Pharmacol. 1966, 8, 37–43. [Google Scholar] [CrossRef]
- Zaichick, S.; Zaichick, V.; Karandashev, V.; Nosenko, S. Accumulation of rare earth elements in human bone within the lifespan. Metallomics 2011, 3, 186–194. [Google Scholar] [CrossRef]
- Zaichick, V. Chemical elements of human bone tissue investigated by nuclear analytical and related methods. Biol. Trace Elem. Res. 2013, 153, 84–99. [Google Scholar] [CrossRef]
- Yang, L.; Ma, L.; Huang, Y.; Feyerabend, F.; Blawert, C.; Höche, D.; Willumeit-Römer, R.; Zhang, E.; Kainer, K.U.; Hort, N. Influence of Dy in solid solution on the degradation behavior of binary Mg-Dy alloys in cell culture medium. Mater. Sci. Eng. C 2017, 75, 1351–1358. [Google Scholar] [CrossRef]
- Bottrill, M.; Kwok, L.; Long, N.J. Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 2006, 35, 557. [Google Scholar] [CrossRef]
- Du, Z.; Yang, X.; Ling, G.; Liu, H. Thermodynamic assessment of the Dy–Mg system. Z. für Met. 2004, 95, 1115–1119. [Google Scholar] [CrossRef]
- Ma, N.; Peng, Q.; Pan, J.; Li, H.; Xiao, W. Effect of microalloying with rare-earth on recrystallization behaviour and damping properties of Mg sheets. J. Alloys Compd. 2014, 592, 24–34. [Google Scholar] [CrossRef]
- Liu, H.H.; Ning, Z.L.; Yi, J.Y.; Qian, M.; Sun, H.C.; Huang, Y.J.; Sun, J.F. Effect of Dy addition on microstructure and mechanical properties of Mg-4Y-3Nd-0.4 Zr alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 797–803. [Google Scholar] [CrossRef]
- Gao, X.; He, S.M.; Zeng, X.; Peng, L.; Ding, W.J.; Nie, J.F. Microstructure evolution in a Mg–15Gd–0.5Zr (wt.%) alloy during isothermal aging at 250 °C. Mater. Sci. Eng. A 2006, 431, 322–327. [Google Scholar] [CrossRef]
- Apps, P.; Karimzadeh, H.; King, J.; Lorimer, G. Precipitation reactions in magnesium-rare earth alloys containing yttrium, gadolinium or dysprosium. Scr. Mater. 2003, 48, 1023–1028. [Google Scholar] [CrossRef]
- Saito, K.; Yasuhara, A.; Nishijima, M.; Hiraga, K. Structural changes of precipitates by aging of an Mg-4 at% Dy solid solution studied by atomic-scaled transmission electron microscopy. Mater. Trans. 2011, 52, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, M.; Hiraga, K.; Yamasaki, M.; Kawamura, Y. Characterization of β′ phase precipitates in an Mg-5 at% Gd alloy aged in a peak hardness condition, studied by high-angle annular detector dark-field scanning transmission electron microscopy. Mater. Trans. 2006, 47, 2109–2112. [Google Scholar] [CrossRef] [Green Version]
- Honma, T.; Ohkubo, T.; Hono, K.; Kamado, S. Chemistry of nanoscale precipitates in Mg–2.1 Gd–0.6 Y–0.2 Zr (at.%) alloy investigated by the atom probe technique. Mater. Sci. Eng. A 2005, 395, 301–306. [Google Scholar] [CrossRef]
- Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar] [CrossRef]
- Beausir, B.; Fundenberger, J. Analysis Tools for Electron and X-ray Diffraction, ATEX Software. 2017. Available online: www.atex-software.eu (accessed on 11 August 2021).
- ASTM E112. Standard test methods for determining average grain size. Heyn Linear Intercept Procedure. 2013.
- Ding, Y.; Lin, J.; Wen, C.; Zhang, D.; Li, Y. Mechanical properties, corrosion, and biocompatibility of Mg–Zr–Sr–Dy alloys for biodegradable implant applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2425–2434. [Google Scholar] [CrossRef]
- Honma, T.; Ohkubo, T.; Kamado, S.; Hono, K. Effect of Zn additions on the age-hardening of Mg–2.0 Gd–1.2 Y–0.2 Zr alloys. Acta Mater. 2007, 55, 4137–4150. [Google Scholar] [CrossRef]
- Nie, J.F.; Muddle, B.C. Characterisation of strengthening precipitate phases in a Mg–Y–Nd alloy. Acta Mater. 2000, 48, 1691–1703. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Feyerabend, F.; Willumeit, R.; Kainer, K.U.; Hort, N. Influence of ageing treatment on microstructure, mechanical and bio-corrosion properties of Mg–Dy alloys. J. Mech. Behav. Biomed. Mater. 2012, 13, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Guangli, B.; Jiang, J.; Zhang, F.; Daqing, F.; Yuandong, L.; Ying, M.; Yuan, H. Microstructure evolution and corrosion properties of Mg-Dy-Zn alloy during cooling after solution treatment. J. Rare Earths 2016, 34, 931–937. [Google Scholar]
- Wan, Y.; Jiang, S.; Liu, C.; Wang, B.; Chen, Z. Effect of Nd and Dy on the microstructure and mechanical property of the as extruded Mg–1Zn–0.6Zr alloy. Mater. Sci. Eng. A 2015, 625, 158–163. [Google Scholar] [CrossRef]
- Hou, R.Q.; Ye, C.Q.; Chen, C.D.; Dong, S.G.; Lv, M.Q.; Zhang, S.; Pan, J.S.; Song, G.L.; Lin, C.J. Localized corrosion of binary Mg–Ca alloy in 0.9 wt% sodium chloride solution. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Z.; Qu, R.; Wang, L.; Li, Q.; Guan, S. Effect of rare earth and Mn elements on the corrosion behavior of extruded AZ61 system in 3.5 wt% NaCl solution and salt spray test. J. Magnes. Alloy. 2013, 1, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Ding, W. Biocorrosion properties of as-extruded Mg–Nd–Zn–Zr alloy compared with commercial AZ31 and WE43 alloys. Mater. Lett. 2012, 66, 209–211. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D. Structural and corrosion characterization of biodegradable Mg–RE (RE = Gd, Y, Nd) alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 1215–1225. [Google Scholar] [CrossRef]
- Willbold, E.; Gu, X.; Albert, D.; Kalla, K.; Bobe, K.; Brauneis, M.; Janning, C.; Nellesen, J.; Czayka, W.; Tillmann, W.; et al. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Acta Biomater. 2015, 11, 554–562. [Google Scholar] [CrossRef]
- Leleu, S.; Rives, B.; Caussé, N.; Pébère, N. Corrosion rate determination of rare-earth Mg alloys in a Na2SO4 solution by electrochemical measurements and inductive coupled plasma-optical emission spectroscopy. J. Magnes. Alloy. 2019, 7, 47–57. [Google Scholar] [CrossRef]
- Srinivasan, A.; Blawert, C.; Huang, Y.; Mendis, C.; Kainer, K.; Hort, N. Corrosion behavior of Mg–Gd–Zn based alloys in aqueous NaCl solution. J. Magnes. Alloy. 2014, 2, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Baril, G.; Pebere, N. The corrosion of pure magnesium in aerated and deaerated sodium sulphate solutions. Corros. Sci. 2001, 43, 471–484. [Google Scholar] [CrossRef]
- Anik, M.; Celikten, G. Analysis of the electrochemical reaction behavior of alloy AZ91 by EIS technique in H3PO4/KOH buffered K2SO4 solutions. Corros. Sci. 2007, 49, 1878–1894. [Google Scholar] [CrossRef]
- Chu, J.; Tong, L.; Jiang, Z.; Zou, D.; Wang, Q.; Liu, S.; Zhang, H. A comparison study of Ce/La and Ca microalloying on the bio-corrosion behaviors of extruded Mg-Zn alloys. J. Magnes. Alloy. 2020, 8, 1269–1280. [Google Scholar] [CrossRef]
- González-Murguía, J.L.; Veleva, L.; Rodríguez-Gattorno, G.; Figueroa-Torres, M.Z.; Feliu, S. Mg–Ca0.3 Electrochemical Activity Exposed to Hank’s Physiological Solution and Properties of Ag-Nano-Particles Deposits. Metals 2021, 11, 1357. [Google Scholar] [CrossRef]
- Dinodi, N.; Shetty, A.N. Alkyl carboxylates as efficient and green inhibitors of magnesium alloy ZE41 corrosion in aqueous salt solution. Corros. Sci. 2014, 85, 411–427. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Zhang, C.; Zhang, B.; Zhang, T.; Li, Y.; Wu, K.; Wang, F. Significantly improved corrosion resistance of Mg–15Gd-2Zn-0.39Zr alloys: Effect of heat-treatment. J. Mater. Sci. Technol. 2019, 35, 1644–1654. [Google Scholar] [CrossRef]
- Alateyah, A.; Aljohani, T.A.; Alawad, M.O.; El-Hafez, H.A.; Almutairi, A.; Alharbi, E.; Alhamada, R.; El-Garaihy, B.; El-Garaihy, W. Improved Corrosion Behavior of AZ31 Alloy through ECAP Processing. Metals 2021, 11, 363. [Google Scholar] [CrossRef]


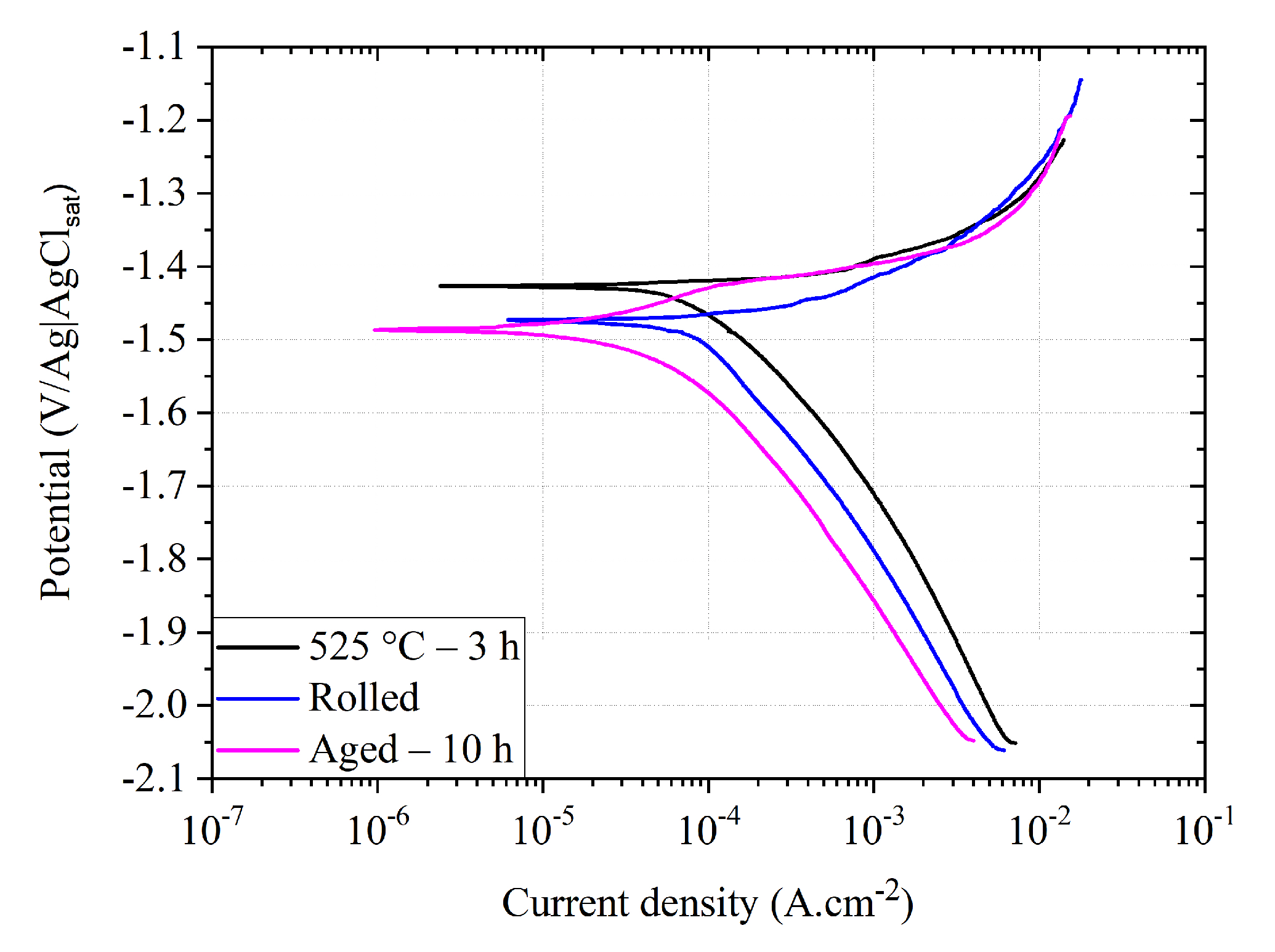

| Condition | E (V/Ag|AgCl) | i (A cm) | CR (mm Year) |
|---|---|---|---|
| H525 °C-3 h | −1.423 ± 0.011 | 85.2 ± 3.0 | 2.0 ± 0.1 |
| Rolled | −1.475 ± 0.020 | 74.4 ± 3.1 | 1.7 ± 0.1 |
| Aged-10 h | −1.479 ± 0.021 | 35.4 ± 9.7 | 0.9 ± 0.2 |
| Alloy (wt.%) | Condition | Solution | CR (mm Year) | Ref. |
|---|---|---|---|---|
| Mg–3.4Dy–0.2Zn–0.4Zr | 525 °C-3 h | 0.9 wt.% NaCl | 2.0 | This work |
| Mg–3.4Dy–0.2Zn–0.4Zr | Rolled | 0.9 wt.% NaCl | 1.7 | This work |
| Mg–3.4Dy–0.2Zn–0.4Zr | Aged-10 h | 0.9 wt.% NaCl | 0.9 | This work |
| Mg–3.1Nd–0.2Zn–0.4Zr | Extruded–Aged 10 h | SBF | 0.18 | [5] |
| WE43 | Extruded | SBF | 0.125 | [34] |
| Mg–5Dy | 520 °C—24 h | 0.9 wt.% NaCl | 0.5 | [27] |
| Mg–10Dy | 520 °C—24 h | 0.9 wt.% NaCl | 0.9 | [27] |
| Mg–3Gd–1Y | As-cast | 9 g L NaCl | 3.16 | [35] |
| Mg–4Nd | 520 °C—20 h | 9 g L NaCl | 0.62 | [35] |
| Mg–1Y | Rolled | SBF | 1.65 | [33] |
| Mg–2.13Nd | As-cast | SBF | 1.25 | [36] |
| Mg–1.27Ce | As-cast | SBF | 1.84 | [36] |
| Mg–0.69La | As-cast | SBF | 2.15 | [36] |
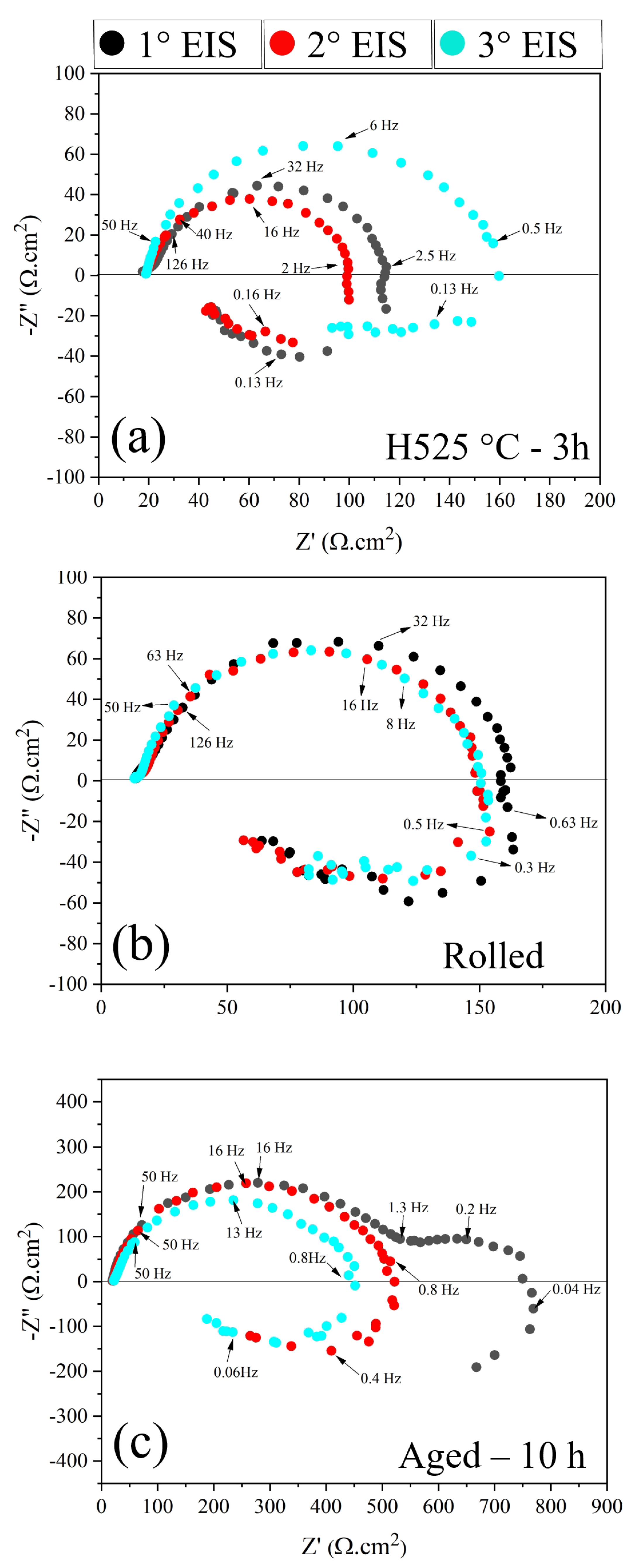
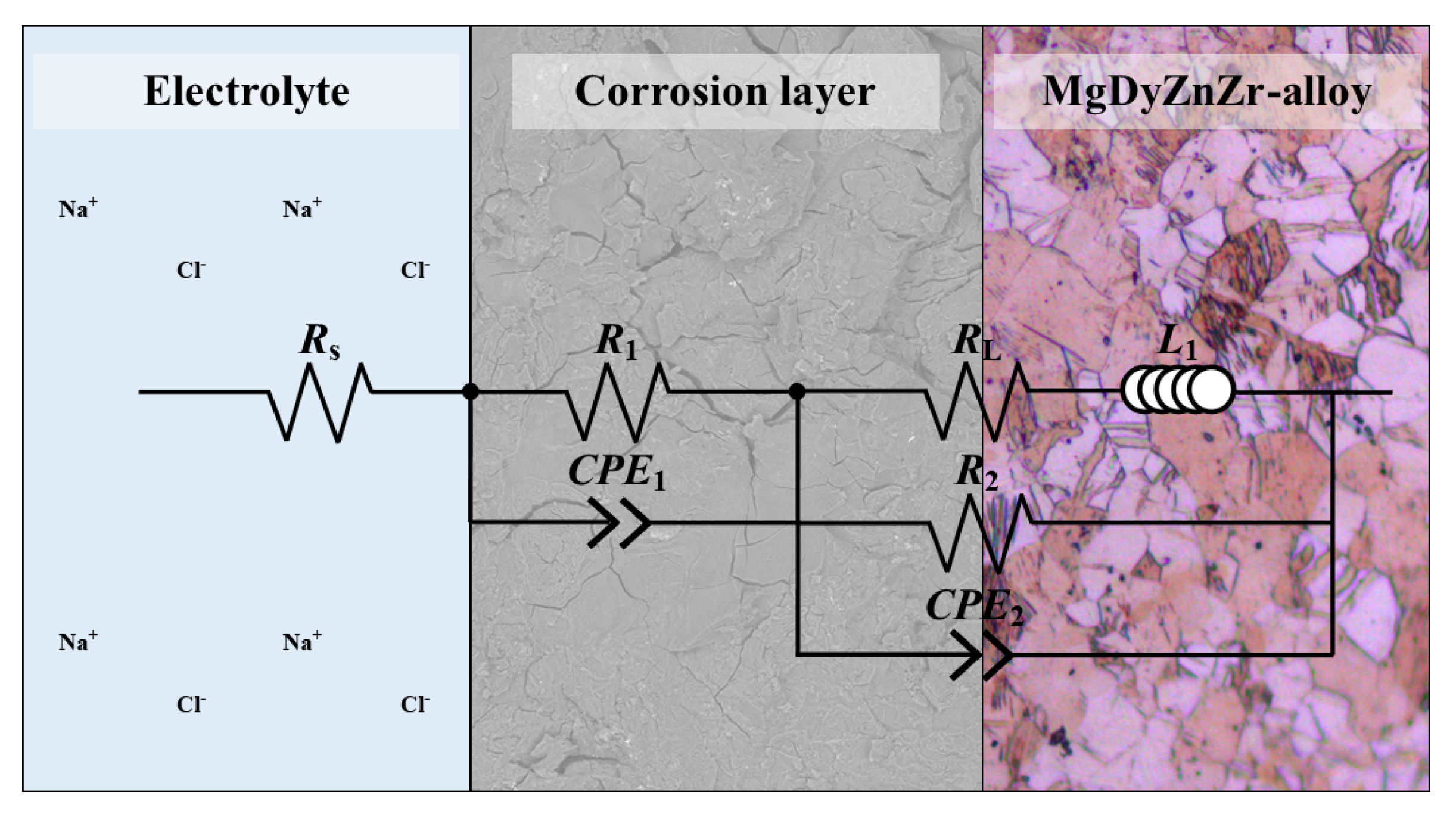
| Condition | EIS | R (·cm) | R (·cm) | CPE (·s·cm) | n | R (·cm) | CPE (·s·cm) | n | R (·cm) |
|---|---|---|---|---|---|---|---|---|---|
| H525 °C-3 h | 1° | 15.99 ± 1.06 | 89.25 ± 2.13 | 4.5 × 10 ± 3.89 × 10 | 0.98 ± 0.01 | 9.18 ± 2.73 | 1.88 × 10 ± 2.17 × 10 | 0.60 ± 0.04 | 8.06 ± 0.02 |
| H525 °C-3 h | 2° | 1.02 ± 0.08 | 84.86 ± 1.42 | 7.17 × 10 ± 4.45 × 10 | 0.93 ± 0.01 | 18.20 ± 0.20 | 5.30 × 10 ± 4.75 × 10 | 0.70 ± 0.03 | 12.99 ± 0.14 |
| H525 °C-3 h | 3° | 0.52 ± 0.09 | 142.4 ± 1.30 | 9.53 × 10 ± 2.98 × 10 | 0.93 ± 0.01 | 18.62 ± 0.11 | 2.59 × 10 ± 2.76 × 10 | 0.70 ± 0.02 | 13.76 ± 0.12 |
| Rolled | 1° | 12.79 ± 0.16 | 144.6 ± 1.21 | 2.13 × 10 ± 4.07 × 10 | 0.95 ± 0.02 | 8.95 ± 1.00 | 6.86 × 10 ± 1.30 × 10 | 0.74 ± 0.05 | 8.21 ± 0.83 |
| Rolled | 2° | 12.87 ± 0.13 | 135.5 ± 0.73 | 3.40 × 10 ± 3.26 × 10 | 0.95 ± 0.02 | 6.46 ± 0.24 | 2.82 × 10 ± 3.90 × 10 | 0.60 ± 0.04 | 6.03 ± 0.20 |
| Rolled | 3° | 12.27 ± 0.14 | 137.6 ± 0.94 | 4.78 × 10 ± 1.24 × 10 | 0.95 ± 0.01 | 4.32 ± 0.19 | 2.28 × 10 ± 4.60 × 10 | 0.60 ± 0.01 | 4.17 ± 0.17 |
| Aged-10 h | 1° | 19.74 ± 0.11 | 17,361.0 ± 1607 | 3.66 × 10 ± 5.54 × 10 | 0.95 ± 0.03 | 477.2 ± 7.86 | 8.81 × 10 ± 2.08 × 10 | 0.91 ± 0.01 | 462.4 ± 7.21 |
| Aged-10 h | 2° | 21.25 ± 0.15 | 402.3 ± 20.0 | 1.44 × 10 ± 6.15 × 10 | 0.95 ± 0.02 | 106.9 ± 20.6 | 2.58 × 10 ± 3.59 × 10 | 0.70 ± 0.05 | 65.54 ± 5.97 |
| Aged-10 h | 3° | 21.66 ± 0.20 | 335.3 ± 20.8 | 2.28 × 10 ± 1.46 × 10 | 0.95 ± 0.03 | 98.09 ± 23.7 | 1.25 × 10 ± 1.80 × 10 | 0.66 ± 0.04 | 61.66 ± 6.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas, B.X.; Antonini, L.A.; Cury, P.L.C.T.; da Silva, V.L.F.; Chaia, N.; Tomachuk, C.R.; Mathieu, S.; Coelho, G.C.; dos Santos, C.; Nunes, C.A. Microstructural Evolution and Electrochemical Behavior of Solution Treated, Hot Rolled and Aged MgDyZnZr Alloy. Metals 2021, 11, 1855. https://doi.org/10.3390/met11111855
de Freitas BX, Antonini LA, Cury PLCT, da Silva VLF, Chaia N, Tomachuk CR, Mathieu S, Coelho GC, dos Santos C, Nunes CA. Microstructural Evolution and Electrochemical Behavior of Solution Treated, Hot Rolled and Aged MgDyZnZr Alloy. Metals. 2021; 11(11):1855. https://doi.org/10.3390/met11111855
Chicago/Turabian Stylede Freitas, Bruno Xavier, Leonardo A. Antonini, Paula L. C. T. Cury, Viviane L. F. da Silva, Nabil Chaia, Célia R. Tomachuk, Stéphane Mathieu, Gilberto C. Coelho, Claudinei dos Santos, and Carlos A. Nunes. 2021. "Microstructural Evolution and Electrochemical Behavior of Solution Treated, Hot Rolled and Aged MgDyZnZr Alloy" Metals 11, no. 11: 1855. https://doi.org/10.3390/met11111855
APA Stylede Freitas, B. X., Antonini, L. A., Cury, P. L. C. T., da Silva, V. L. F., Chaia, N., Tomachuk, C. R., Mathieu, S., Coelho, G. C., dos Santos, C., & Nunes, C. A. (2021). Microstructural Evolution and Electrochemical Behavior of Solution Treated, Hot Rolled and Aged MgDyZnZr Alloy. Metals, 11(11), 1855. https://doi.org/10.3390/met11111855







