Demulsification Behavior of Alkali and Organic Acid in Zinc Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.2.1. Extraction Tests
2.2.2. Saponification Test
2.3. Analytical Method
3. Results and Discussion
3.1. Zinc Extraction with D2EHPA Extractant
3.2. Demulsification
3.2.1. Demulsification by Saponification Pre-Treatment of Extractant
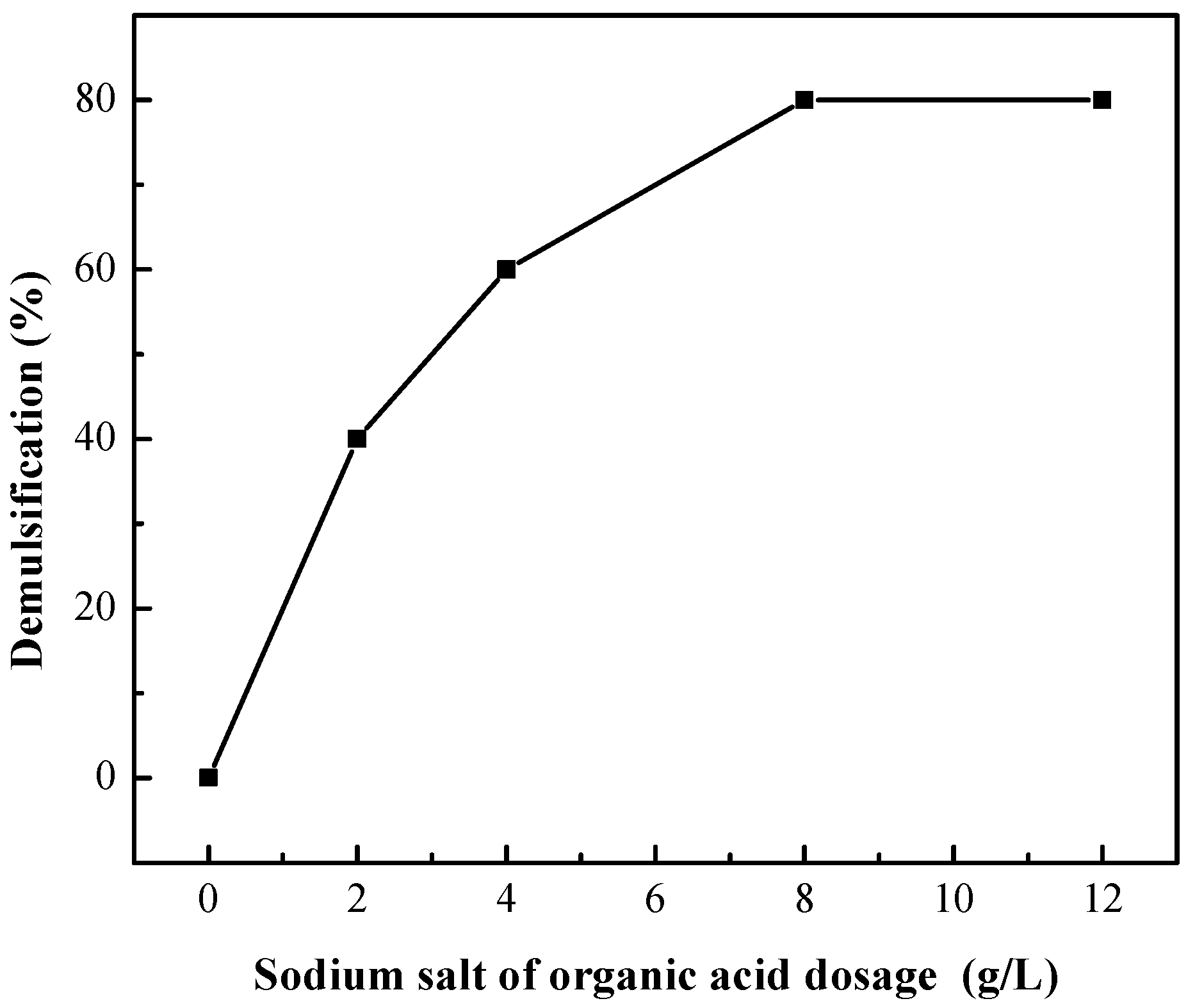
3.2.2. Demulsification by Addition of Sodium Salt of Organic Acid
3.3. Zinc Extraction with D2EHPA by Direct Addition of Alkali and Organic Acid
3.3.1. Effect of Organic Acid Dosage
3.3.2. Effect of Alkali Dosage
3.3.3. Effect of Extractant Concentration
3.4. Mechanism of Intensified Extraction with Associated Additives of Alkali and Organic Acid
3.4.1. Saturation Capacity
3.4.2. FTIR Spectroscopy
3.4.3. Demulsification with Associated Additive of Organic Acid and Strong Alkali
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rydberg, J.; Musikas, C.; Chopping, G.R. Introduction to Solvent Extraction. In Principles and Practices of Solvent Extraction; Marcel Dekker Inc.: New York, NY, USA, 1992; pp. 1–17. [Google Scholar]
- Owusu, G. Selective ext ractions Zn and Cd from Zn-Cd-Co-Ni sulphaate solution using di-2-ethylhexyl phosphoric acid extractant. Hydrometallurgy 1998, 47, 205–215. [Google Scholar] [CrossRef]
- Kongolo, O.; Mwema, M.D.; Banza, A.N.; Gock, E. Cobalt and zinc recovery from copper sulphate solution by solvent extraction. Miner. Eng. 2003, 16, 1371–1374. [Google Scholar] [CrossRef]
- Faubel, W.; Ali, S.A. Separation and purifification of fifission products from process streams of irradiated nuclear fuel. Radiochim. Acta. 1986, 40, 1–5. [Google Scholar]
- Zamani, A.A.; Amani, A.A.; Zarabadi, A.S.; Yaftian, M.R. Water soluble crown ethers: Selective masking agents for improving extraction-separation of zinc and lead cations. J. Incl. Phenom. Macro. 2009, 63, 327–334. [Google Scholar] [CrossRef]
- Ali, A.M.I.; Ahmad, I.M.; Daoud, J.A. CYANEX 272 for the extraction and recovery of zinc from aqueous waste solution using a mixer-settler unit. Separ. Purif. Technol. 2006, 47, 135–140. [Google Scholar] [CrossRef]
- Park, Y.J.; Fray, D.J. Separation of zinc and nickel ions in a strong acid through liquid–liquid extraction. J. Hazard. Mater. 2009, 163, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, B.M.; Sonia, D.F.R.; Fernando, S.M.; Jeaneth, D.S.B. Selective extraction of zinc(II) over iron(II) from spent hydrochloric acid pickling effluents by liquid–liquid extraction. J. Hazard. Mater. 2008, 150, 669–678. [Google Scholar]
- Abdelhamid, M.; Djafer, B. The solvent extraction of zinc, cadmium and chromium from phosphoric acid solutions by tri-nbutyl phosphate in kerosene diluent. Separ. Purif. Technol. 2007, 56, 220–224. [Google Scholar]
- Tang, B.; Zhu, Y.C.; Bai, X.M.; Cai, B.; Chen, L.Q.; Zhang, J.H. Study on separation of zinc from iron in waste sulfuric acid pickle liquor by solvent extraction. Min. Metall. Eng. 2003, 5, 47–49. (In Chinese) [Google Scholar]
- Yang, Y.; Shi, X.; Li, Q.; Jiang, T.; Wang, W.; Nie, H. Research on Control of pH in Solvent Extraction of Zinc by D2EHPA (P204). In Proceedings of the 19th International Biohydrometallurgy Symposium, Changsha, China, 18–22 September 2011. [Google Scholar]
- Luo, F.; Li, D.; Wei, P. Synergistic extraction of zinc(II) and cadmium(II) with mixtures of primary amine N1923 and neutral organophosphorous derivatives. Hydrometallurgy 2004, 3, 31–40. [Google Scholar] [CrossRef]
- Keshavarz, A.E.; Moradkhani, D.; Oradkhani, D.; Darvishi, D.; Askari, B.M.; Behnian, C.D. Synergistic effect of MEHPA on co-extraction of zinc and cadmium with DEHPA. Miner. Eng. 2004, 17, 89–92. [Google Scholar] [CrossRef]
- Principe, F.; Demopoulos, G.P. Comparative study of iron(III) separation from zinc sulphate–sulphuric acid solutions using the organophosphorus extractants, OPAP and D2EHPA: Part I: Extraction. Hydrometallurgy 2004, 74, 93–102. [Google Scholar] [CrossRef]
- Rice, N.M.; Smitth, M.R. The recovery of zinc, cadmium and mercury(II) by solvent extraction. J. Appl. Chem. Biotechnol. 1975, 25, 379–402. [Google Scholar] [CrossRef]
- Forrest, C.; Orrest, C.; Hughes, M.A. The separation of Zn from Cu by DEHPA-an equilibrium study. Hydrometallurgy 1978, 3, 327–342. [Google Scholar] [CrossRef]
- Kolarik, Z. Critical evaluation of some equilibrium constants involving acidic organophosphorus extractant. Pure Appl. Chem. 1982, 54, 2593–2674. [Google Scholar] [CrossRef]
- Vahidi, E.; Rashchi, F.; Moradkhani, D. Recovery of zinc from an industrial zinc leach residue by solvent extraction using D2EHPA. Miner. Eng. 2009, 22, 204–206. [Google Scholar] [CrossRef]
- Pereira, D.D.; Rocha, S.D.; Mansur, M.B. Recovery of zinc sulphate from industrial effluents by liquid–liquid extraction using D2EHPA (di-2-ethylhexyl phosphoric acid). Sep. Purif. Technol. 2007, 53, 89–96. [Google Scholar] [CrossRef]
- Chen, S.; Lai, X. A study on extracting zinc out of low acid waste solution form copper leaching process by using D2EHPA agent. Copp. Eng. 2007, 20, 21–23. (In Chinese) [Google Scholar]
- Tang, J. Technology and Mechanism of Recovery Zinc from Zn-Bearing Waste Stream by Solvent Extraction. Master’s Thesis, Central South University, Changsha, China, 2008. [Google Scholar]
- Liu, X. Research on Mechanism and Prevention of Interfacial Emulsion Formation in Copper Solvent Extraction Process. Ph.D. Thesis, Central South University, Changsha, China, 2001. [Google Scholar]
- Brown, E.N.; White, S.R.; Sottos, N.R. Microcapsule induced toughening in a self-healing polymer composite. J. Mater. Sci. 2004, 39, 1703–1710. [Google Scholar] [CrossRef]
- Biniak, S.; Szymanski, G.; Siedlewski, J.; Światkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Shi, X. Technology and Mechanism on Solvent Extraction of Zinc from High Concentration Zinc Solution by P204. Master’s Thesis, Central South University, Changsha, China, 2011. [Google Scholar]




| Alkali Dosage (g/L) | Extraction (%) | pH Value of Raffinate | Extraction Phenomenon |
|---|---|---|---|
| 0 | 23.92 | 1.334 | - |
| 4.8 | 30.85 | 1.491 | - |
| 9.6 | 37.95 | 1.711 | - |
| 14.4 | 44.71 | 2.018 | - |
| 19.2 | 53.47 | 2.511 | - |
| 24 | 56.08 | 3.240 | emulsification |
| 28 | 65.78 | 6.555 | emulsification |
| 32 | 79.89 | 6.726 | emulsification |
| Alkali Dosage (g/L) | Saponification Ratio (%) | Extraction (%) | The pH of Raffinate | Extraction Phenomenon |
|---|---|---|---|---|
| 0 | 0 | 23.92 | 1.334 | - |
| 4.8 | 20 | 30.16 | 1.534 | - |
| 9.6 | 40 | 36.07 | 1.719 | - |
| 14.4 | 60 | 41.51 | 2.012 | - |
| 19.2 | 80 | 50.19 | 2.548 | - |
| 24 | 100 | 57.47 | 3.082 | - |
| 28 | 100 | 60.40 | 5.573 | emulsification |
| 32 | 100 | 61.87 | 6.087 | emulsification |
| Alkali Dosage (g/L) | Saponification Ratio (%) | Salt Dosage (g/L) | Demulsification Ratio (%) | Extraction (%) | pH of Raffinate |
|---|---|---|---|---|---|
| 19.2 | 80 | 8 | 80 | 54.40 | 2.636 |
| 24 | 100 | 10 | 70 | 60.12 | 3.641 |
| 28 | – | 12 | 50 | 63.90 | 6.428 |
| 32 | – | 13 | 50 | 63.96 | 6.672 |
| Organic Acid Dosage (mL/L). | Extraction (%) | pH of Raffinate | Extraction Phenomenon |
|---|---|---|---|
| 20 | - | - | emulsification |
| 40 | - | - | emulsification |
| 52 | 61.79 | 3.063 | - |
| 100 | 56.00 | 2.935 | - |
| Alkali Dosage (g/L) | Extraction (%) | pH of Raffinate | Extraction Phenomenon |
|---|---|---|---|
| 8 | 34.32 | 2.013 | - |
| 24 | 52.48 | 2.793 | - |
| 32 | 55.18 | 2.902 | - |
| 40 | 61.79 | 3.063 | - |
| 48 | - | - | emulsification |
| Extractant (%) | Alkali Dosage (g/L) | Organic Acid Dosage (mL/L) | Extraction % | pH of Raffinate |
|---|---|---|---|---|
| 20 | 40 | 52 | 61.79 | 3.063 |
| 48 | 64 | 61.93 | 3.188 | |
| 56 | 76 | 62.62 | 3.298 | |
| 30 | 40 | 52 | 79.39 | 2.692 |
| 56 | 72 | 86.88 | 3.159 | |
| 64 | 84 | 87.64 | 3.287 | |
| 40 | 40 | 52 | 91.34 | 2.350 |
| 48 | 64 | 96.64 | 2.770 | |
| 64 | 84 | 98.29 | 3.242 | |
| 80 | 104 | 99.61 | 3.370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Meng, F.; Li, K.; Zhong, Q.; Xu, B.; Li, Q.; Yang, Y. Demulsification Behavior of Alkali and Organic Acid in Zinc Extraction. Metals 2021, 11, 1833. https://doi.org/10.3390/met11111833
Jiang T, Meng F, Li K, Zhong Q, Xu B, Li Q, Yang Y. Demulsification Behavior of Alkali and Organic Acid in Zinc Extraction. Metals. 2021; 11(11):1833. https://doi.org/10.3390/met11111833
Chicago/Turabian StyleJiang, Tao, Feiyu Meng, Ke Li, Qaing Zhong, Bin Xu, Qian Li, and Yongbin Yang. 2021. "Demulsification Behavior of Alkali and Organic Acid in Zinc Extraction" Metals 11, no. 11: 1833. https://doi.org/10.3390/met11111833
APA StyleJiang, T., Meng, F., Li, K., Zhong, Q., Xu, B., Li, Q., & Yang, Y. (2021). Demulsification Behavior of Alkali and Organic Acid in Zinc Extraction. Metals, 11(11), 1833. https://doi.org/10.3390/met11111833





