The Microstructure in an Al–Ti Alloy Melt: The Wulff Cluster Model from a Partial Structure Factor
Abstract
1. Introduction
2. Methods
2.1. Theoretical Methods
2.2. Experimental Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kilmametov, A.R.; Ivanisenko, Y.; Mazilkin, A.A.; Straumal, B.B.; Gornakova, A.S.; Fabrichnaya, O.B.; Kriegel, M.J.; Rafaja, D.; Hahn, H. The α→ω and β→ω phase transformations in Ti–Fe alloys under high-pressure torsion. Acta Mater. 2018, 144, 337–351. [Google Scholar] [CrossRef]
- Hennig, R.G.; Trinkle, D.R.; Bouchet, J.; Srinivasan, S.G.; Albers, R.C.; Wilkins, J.W. Impurities block the α to ω martensitic transformation in titanium. Nat. Mater. 2005, 4, 129–133. [Google Scholar] [CrossRef]
- Pang, J.; Blackwood, D.J. Corrosion of titanium alloys in high temperature near anaerobic seawater. Corros. Sci. 2016, 105, 17–24. [Google Scholar] [CrossRef]
- Sedmák, P.; Pilch, J.; Heller, L.; Kopeček, J.; Wright, J.; Sedlák, P.; Frost, M.; Šittner, P. Grain-resolved analysis of localized deformation in nickel-titanium wire under tensile load. Science 2016, 353, 559–562. [Google Scholar] [CrossRef]
- Wang, S.Q.; Patel, V.K.; Bhole, S.D.; Wen, G.D.; Chen, D.L. Microstructure and mechanical properties of ultrasonic spot welded Al/Ti alloy joints. Mater. Des. 2015, 78, 33–41. [Google Scholar] [CrossRef]
- Lahiri, A.; Das, R. Spectroscopic studies of the ionic liquid during the electrodeposition of Al–Ti alloy in 1-ethyl-3-methylimidazolium chloride melt. Mater. Chem. Phys. 2012, 132, 34–38. [Google Scholar] [CrossRef]
- Chen, G.; Peng, Y.; Zheng, G.; Qi, Z.; Wang, M.; Yu, H.; Dong, C.; Liu, C.T. Polysynthetic twinned TiAl single crystals for high-temperature applications. Nat. Mater. 2016, 15, 876–881. [Google Scholar] [CrossRef]
- Srirangam, P.; Kramer, M.J.; Shankar, S. Effect of strontium on liquid structure of Al–Si hypoeutectic alloys using high-energy X-ray diffraction. Acta Mater. 2011, 59, 503–513. [Google Scholar] [CrossRef]
- Georgarakis, K.; Louzguine-Luzgin, D.V.; Antonowicz, J.; Vaughan, G.; Yavari, A.R.; Egami, T.; Inoue, A. Variations in atomic structural features of a supercooled Pd–Ni–Cu–P glass forming liquid during in situ vitrification. Acta Mater. 2011, 59, 708–716. [Google Scholar] [CrossRef]
- Bian, X.; Guo, J.; Lv, X.; Qin, X.; Wang, C. Prediction of glass-forming ability of metallic liquids. Appl. Phys. Lett. 2007, 91, 221910. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Meyer, A. Self-diffusion in liquid copper as seen by quasielastic neutron scattering. Phys. Rev. B 2010, 81, 12102. [Google Scholar] [CrossRef]
- Susskind, H.; Becker, W. Random packing of spheres in non-rigid containers. Nature 1966, 212, 1564–1565. [Google Scholar] [CrossRef]
- Bernal, J.D. A geometrical approach to the structure of liquids. Nature 1959, 183, 141–147. [Google Scholar] [CrossRef]
- Huang, L.; Wang, C.Z.; Ho, K.M. Structure and dynamics of liquid Ni 36 Zr 64 by ab initio molecular dynamics. Phys. Rev. B 2011, 83, 184103. [Google Scholar] [CrossRef]
- Schenk, T.; Holland-Moritz, D.; Simonet, V.; Bellissent, R.; Herlach, D.M. Icosahedral short-range order in deeply undercooled metallic melts. Phys. Rev. Lett. 2002, 89, 75507. [Google Scholar] [CrossRef]
- Lou, H.; Wang, X.; Cao, Q.; Zhang, D.; Zhang, J.; Hu, T.; Mao, H.; Jiang, J. Negative expansions of interatomic distances in metallic melts. Proc. Natl. Acad. Sci. USA 2013, 110, 10068–10072. [Google Scholar] [CrossRef]
- Hui, L.; Xiufang, B.; Guanghou, W. Molecular dynamics study of the local order and defects in quenched states. Phys. Rev. B 2003, 67, 94202. [Google Scholar] [CrossRef]
- Hui, L.; Pederiva, F. Anomalies in liquid structure of Ni 3 Al alloys during a rapid cooling process. Phys. Rev. B 2003, 68, 54210. [Google Scholar] [CrossRef]
- Vasisht, V.V.; Saw, S.; Sastry, S. Liquid–liquid critical point in supercooled silicon. Nat. Phys. 2011, 7, 549–553. [Google Scholar] [CrossRef]
- Jakse, N.; Pasturel, A. Local order of liquid and supercooled zirconium by ab initio molecular dynamics. Phys. Rev. Lett. 2003, 91, 195501. [Google Scholar] [CrossRef]
- Ceschini, L.; Boromei, I.; Morri, A.; Seifeddine, S.; Svensson, I.L. Effect of Fe content and microstructural features on the tensile and fatigue properties of the Al–Si10–Cu2 alloy. Mater. Des. (1980–2015) 2012, 36, 522–528. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Doty, H.W.; Kaufman, M.J. The effects of Mn additions on the microstructure and mechanical properties of Al–Si–Cu casting alloys. Mater. Sci. Eng. A 2008, 488, 496–504. [Google Scholar] [CrossRef]
- Xue, X.; Bian, X.; Geng, H.; Qin, X. Structural evolution of medium range and short-range order with temperature in Cu-25 wt.% Sn. Mater. Sci. Eng. A 2003, 363, 134–139. [Google Scholar] [CrossRef]
- Song, L.; Tian, X.; Shao, A.; Li, L.; Zhang, Y.; Li, H.; Lin, X. The structure of metallic melts in binary homogenous alloys: A thermodynamical understanding from the Wulff cluster model. Phys. Chem. Chem. Phys. 2020, 22, 23237–23245. [Google Scholar] [CrossRef]
- Song, L.; Tian, X.; Yang, Y.; Qin, J.; Li, H.; Lin, X. Probing the microstructure in pure Al & Cu melts: Theory meets experiment. Front. Chem. 2020, 8, 607. [Google Scholar]
- Song, L.; Tian, X.; Shao, A.; Hua, M.; Li, L.; Li, H.; Lin, X. The structure of metallic melts in eutectic alloys based on the Wulff cluster model: Theory meets experiment. Phys. Chem. Chem. Phys. 2021, 23, 3606–3614. [Google Scholar] [CrossRef]
- Tyson, W.R.; Miller, W.A. Surface free energies of solid metals: Estimation from liquid surface tension measurements. Surf. Sci. 1977, 62, 267–276. [Google Scholar] [CrossRef]
- Tran, R.; Xu, Z.; Radhakrishnan, B.; Winston, D.; Sun, W.; Persson, K.A.; Ong, S.P. Surface energies of elemental crystals. Sci. Data 2016, 3, 160080. [Google Scholar] [CrossRef]
- Einstein, T.L. Equilibrium shape of crystals. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 215–264. [Google Scholar]
- Hui, L.; Pederiva, F.; Wang, B.L.; Wang, J.L.; Wang, G.H. How does the nickel nanowire melt? Appl. Phys. Lett. 2005, 86, 11913. [Google Scholar] [CrossRef]
- Hacene, M.; Anciaux Sedrakian, A.; Rozanska, X.; Klahr, D.; Guignon, T.; Fleurat Lessard, P. Accelerating VASP electronic structure calculations using graphic processing units. J. Comput. Chem. 2012, 33, 2581–2589. [Google Scholar] [CrossRef]
- Hutchinson, M.; Widom, M. VASP on a GPU: Application to exact-exchange calculations of the stability of elemental boron. Comput. Phys. Commun. 2012, 183, 1422–1426. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Harrison, M.J.; Woodruff, D.P.; Robinson, J.; Sander, D.; Pan, W.; Kirschner, J. Adsorbate-induced surface reconstruction and surface-stress changes in Cu (100)/O: Experiment and theory. Phys. Rev. B 2006, 74, 165402. [Google Scholar] [CrossRef]
- Bonzel, H.P.; Yu, D.K.; Scheffler, M. The three-dimensional equilibrium crystal shape of Pb: Recent results of theory and experiment. Appl. Phys. A 2007, 87, 391–397. [Google Scholar] [CrossRef][Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, R.G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 84106. [Google Scholar] [CrossRef]
- Mathew, K.; Kolluru, V.C.; Mula, S.; Steinmann, S.N.; Hennig, R.G. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 2019, 151, 234101. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Fang, Q.; Chen, H.; Fan, T.; Liu, B.; Liu, F.; Tang, P. Effects of finite temperature on the surface energy in Al alloys from first-principles calculations. Appl. Surf. Sci. 2019, 479, 499–505. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Van Veldhuizen, D.A.; Zydallis, J.B.; Lamont, G.B. Considerations in engineering parallel multiobjective evolutionary algorithms. IEEE Trans. Evol. Comput. 2003, 7, 144–173. [Google Scholar] [CrossRef]
- Engel, G.E.; Wilke, S.; König, O.; Harris, K.; Leusen, F. PowderSolve–a complete package for crystal structure solution from powder diffraction patterns. J. Appl. Crystallogr. 1999, 32, 1169–1179. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Tian, X.L.; Zhan, C.W.; Hou, J.X.; Chen, X.C.; Sun, J.J. Nanocrystal model for liquid metals and amorphous metals. J. Mater. Sci. Technol. 2010, 26, 69–74. [Google Scholar] [CrossRef]


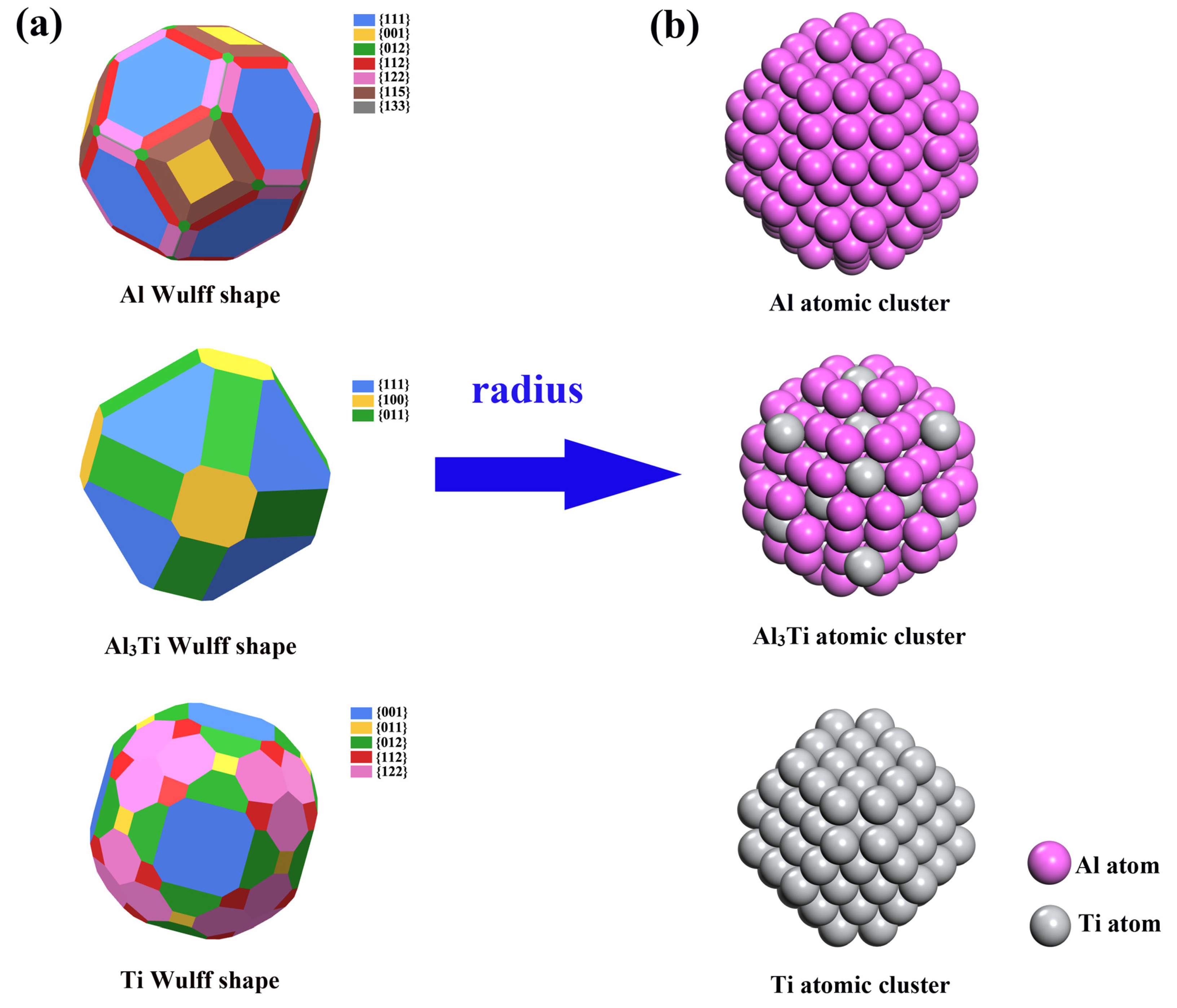
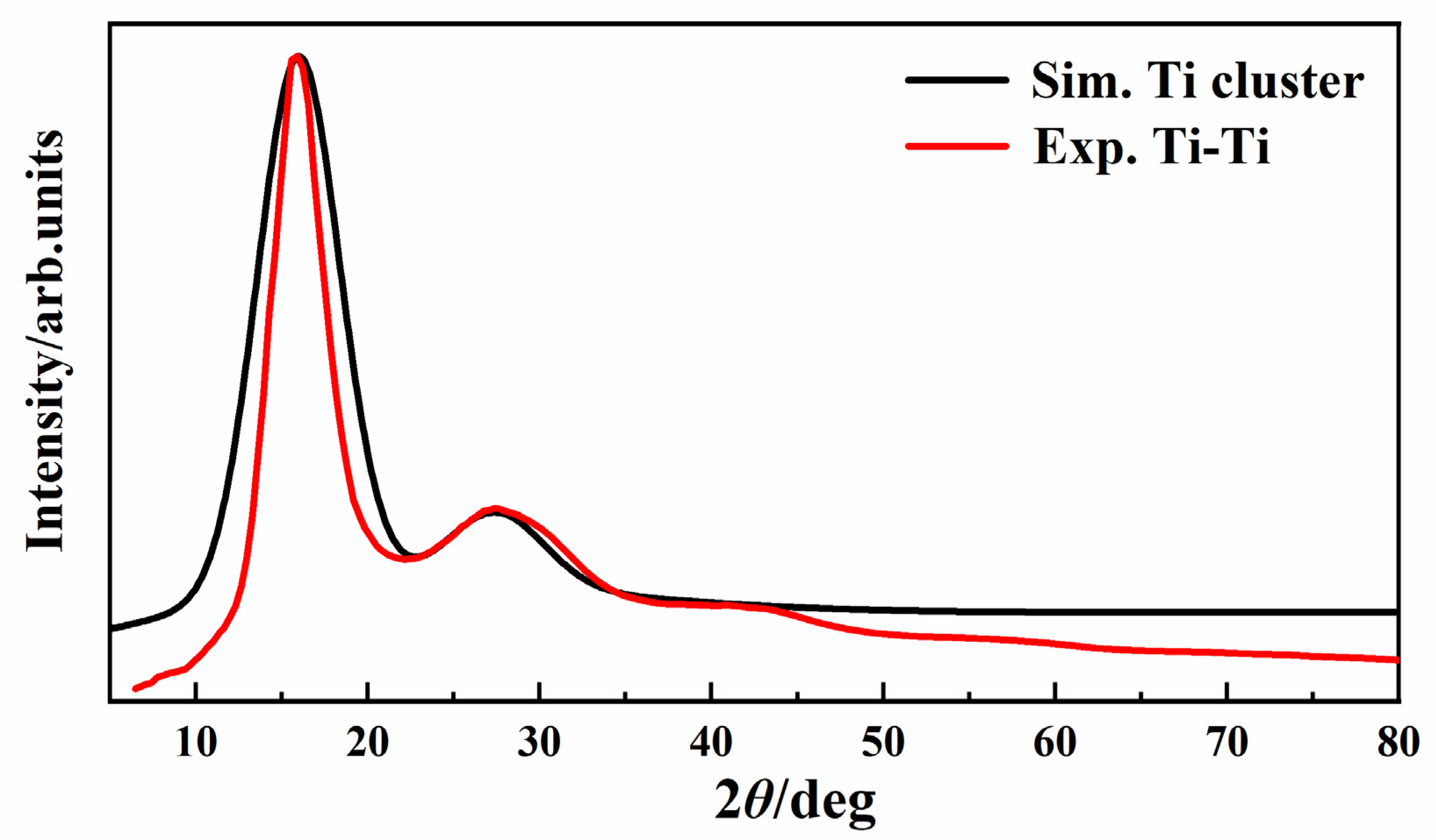
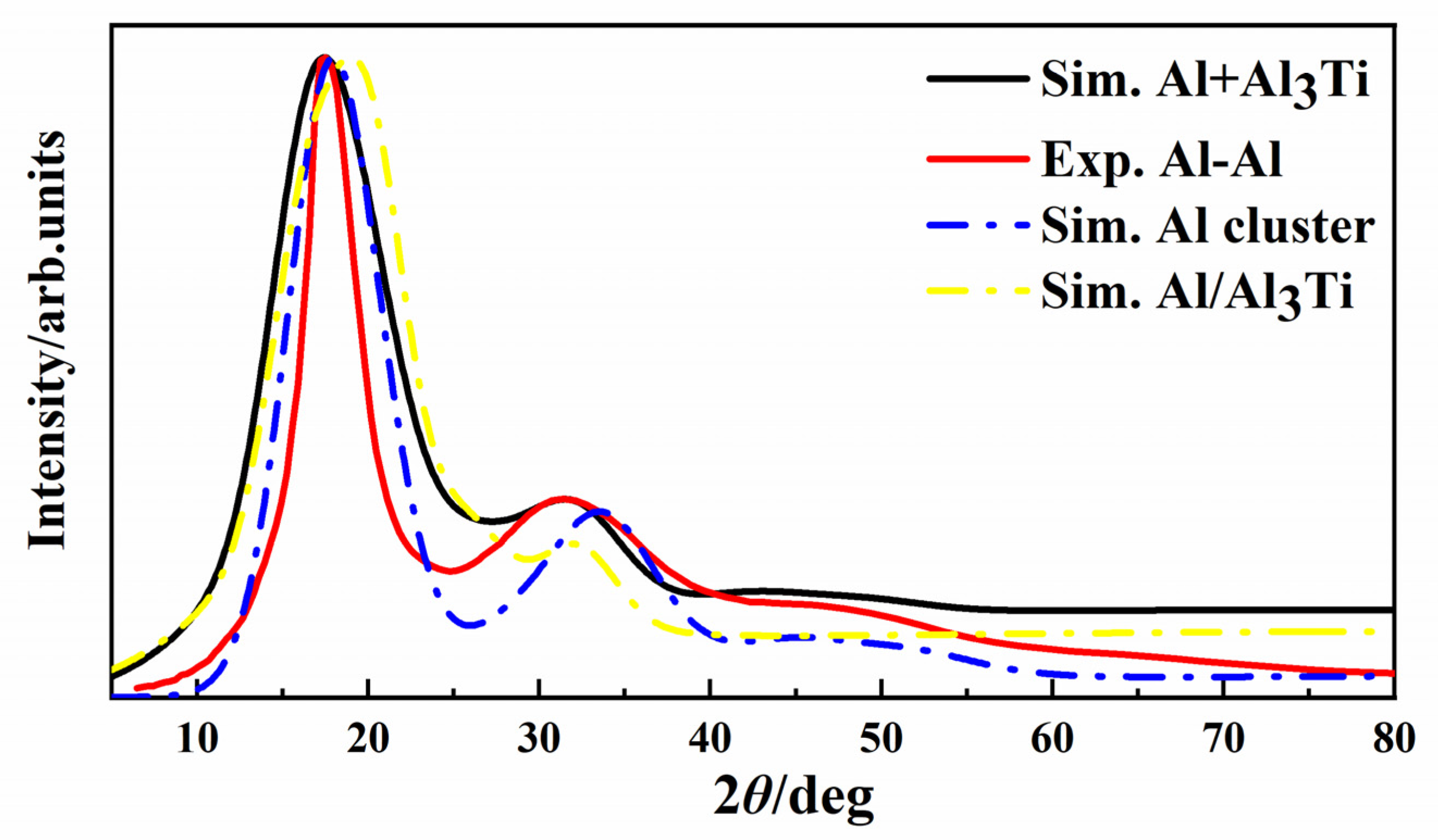
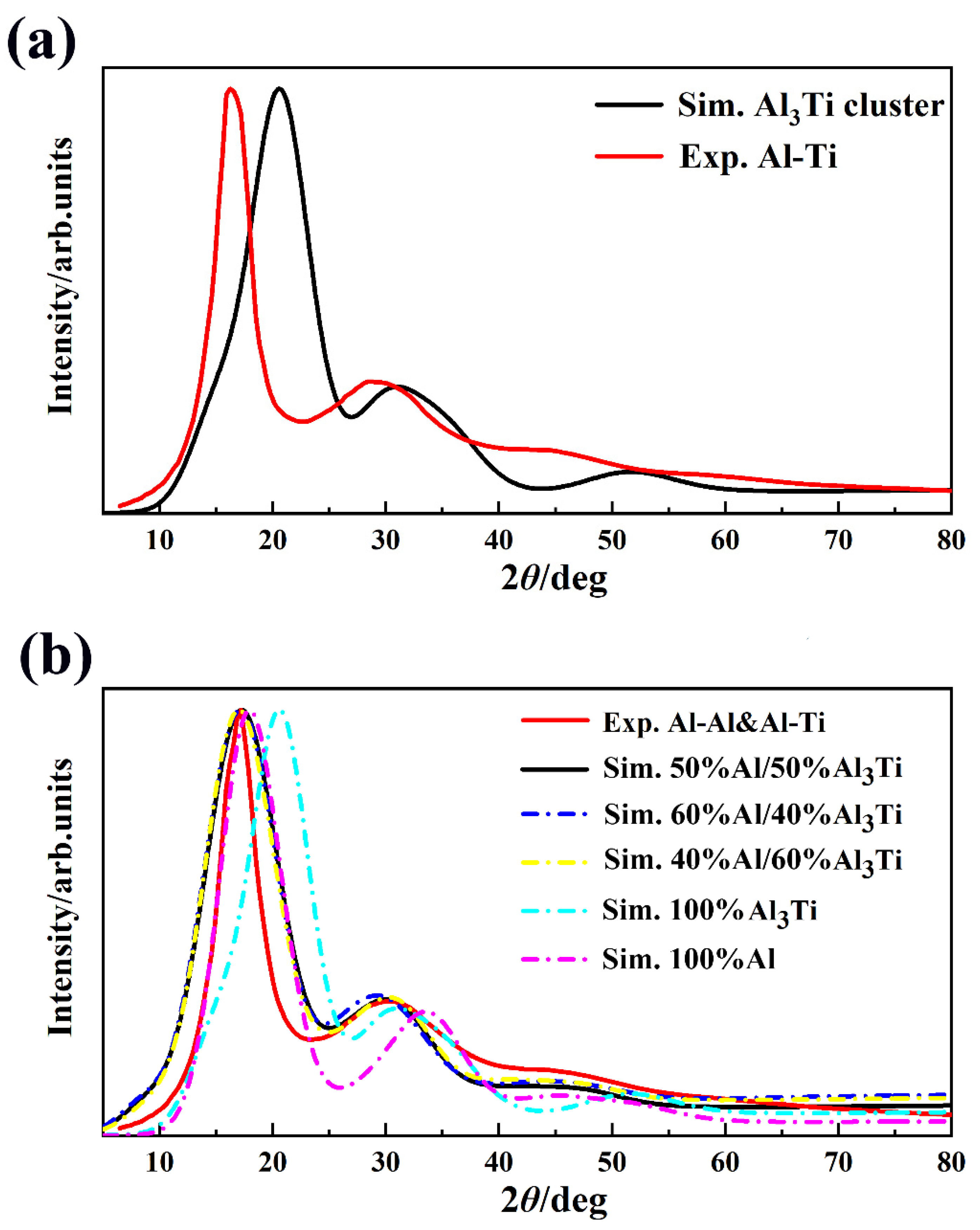
| Types | γ (J/m2) | Types | γ (J/m2) | Types | γ (J/m2) |
|---|---|---|---|---|---|
| Ti(100) | 1.56 | Al(100) | 0.96 | Al3Ti (100)-1 | 1.85 |
| Ti(110) | 1.73 | Al(110) | 1.03 | Al3Ti (100)-2 | 1.23 |
| Ti(111) | 1.82 | Al(111) | 0.87 | Al3Ti (110)-1 | 1.93 |
| Ti(210) | 1.69 | Al(210) | 1.03 | Al3Ti (110)-2 | 1.15 |
| Ti(211) | 1.73 | Al(211) | 0.96 | Al3Ti (111) | 1.09 |
| Ti(221) | 1.69 | Al(221) | 0.96 | Al3Ti (210)-1 | 1.93 |
| Ti(310) | 1.72 | Al(310) | 1.03 | Al3Ti (210)-2 | 1.91 |
| Ti(311) | 1.81 | Al(311) | 0.99 | Al3Ti (211)-1 | 1.56 |
| Ti(511) | 1.78 | Al(511) | 0.98 | Al3Ti (211)-2 | 1.28 |
| Al3Ti (221) | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Song, L.; Shao, A.; Hua, M.; Li, H.; Tian, X. The Microstructure in an Al–Ti Alloy Melt: The Wulff Cluster Model from a Partial Structure Factor. Metals 2021, 11, 1799. https://doi.org/10.3390/met11111799
Lin X, Song L, Shao A, Hua M, Li H, Tian X. The Microstructure in an Al–Ti Alloy Melt: The Wulff Cluster Model from a Partial Structure Factor. Metals. 2021; 11(11):1799. https://doi.org/10.3390/met11111799
Chicago/Turabian StyleLin, Xiaohang, Lin Song, Anchen Shao, Minghao Hua, Hui Li, and Xuelei Tian. 2021. "The Microstructure in an Al–Ti Alloy Melt: The Wulff Cluster Model from a Partial Structure Factor" Metals 11, no. 11: 1799. https://doi.org/10.3390/met11111799
APA StyleLin, X., Song, L., Shao, A., Hua, M., Li, H., & Tian, X. (2021). The Microstructure in an Al–Ti Alloy Melt: The Wulff Cluster Model from a Partial Structure Factor. Metals, 11(11), 1799. https://doi.org/10.3390/met11111799







