Copper Cathode Contamination by Nickel in Copper Electrorefining
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particle Entrapment
2.2. Electrolyte Inclusions
2.3. Electrodeposition
3. Results and Discussion
3.1. Particle Entrapment
3.2. Electrolyte Inclusion
3.3. Electrodeposition
4. Conclusions
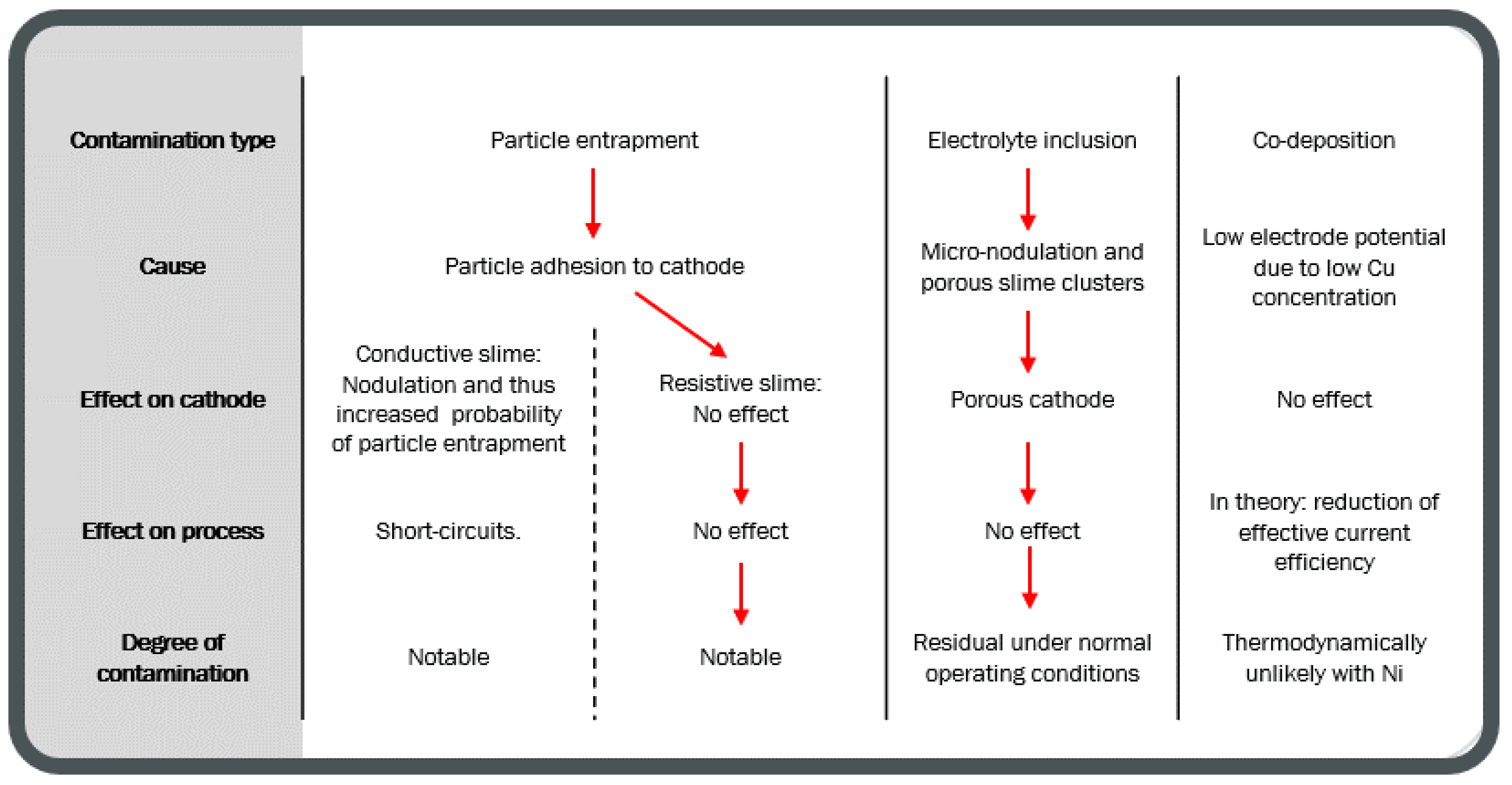
- Cathode nodulation when slime is resistive. Anode slime particle size may determine whether slime causes a nodule or not, with larger slimes causing nodules as the base cathode growth does not reach the slime level before significant Cu deposition has occurred on the slime.
- Local passivation of the cathode. Slime is resistive enough, no Cu nucleation occurs on the slime, and the cathode starts to grow elsewhere. The authors are not aware of this phenomenon occurring in industrial operations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moats, M.S.; Robinson, T.; Davenport, W.G.; Karcas, G.; Demetrio, S. Electrolytic Copper Refining—2007 World Tankhouse Operating Data. In Proceedings of the Sixth International Copper-Cobre Conference, Toronto, ON, Canada, 25–30 August 2007; Houlachi, G.E., Edwards, J.D., Robinson, T.G., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montréal, QC, Canada, 2007; Volume V, pp. 195–242. [Google Scholar]
- Ikemoto, T.; Yamashita, T.; Inoue, O. Electrolytic Refining of High Ni Copper Anode at the Tankhouse in Naoshima Smelter and Refinery. In Proceedings of the 58th Annual Conference of Metallurgists (COM) Hosting the 10th Internatioanl Copper Conference 2019, Vancouver, BC, Canada, 21 August 2019. [Google Scholar]
- Chen, T.T.; Dutrizac, J.E. A Mineralogical Overview of the Behavior of Nickel during Copper Electrorefining. Metall. Trans. B 1990, 21, 229–238. [Google Scholar] [CrossRef]
- Adams, J.F.; Fossenier, J.; Shannon, J.; Sist, C. Hydrometallurgical Strategies for Higher Impurities in Copper Refining. In Proceedings of the 58th Annual Conference of Metallurgists (COM) Hosting the 10th Internatioanl Copper Conference 2019, Vancouver, BC, Canada, 21 August 2019. [Google Scholar]
- Kalliomäki, T.; Aromaa, J.; Lundström, M. Modeling the Effect of Composition and Temperature on the Conductivity of Synthetic Copper Electrorefining Electrolyte. Minerals 2016, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Jarjoura, G.; Kipouros, G.J. Cyclic Voltametric Studies of the Effect of Nickel on Copper Anode Passivation in a Copper Sulfate Solution. Can. Metall. Q. 2005, 44, 469–482. [Google Scholar] [CrossRef]
- Jarjoura, G.; Kipouros, G.J. Effect of Nickel on Copper Anode Passivation in a Copper Sulfate Solution by Electrochemical Impedance Spectroscopy. J. Appl. Electrochem. 2006, 36, 691–701. [Google Scholar] [CrossRef]
- Jarjoura, G.; Kipouros, G.J. Electrochemical Studies on the Effect of Nickel on Copper Anode Passivation in a Copper Sulphate Solution. Can. Metall. Q. 2006, 45, 283–294. [Google Scholar] [CrossRef]
- Tetsuka, D.; Okamoto, H. Effect of Antimony, Nickel and Sulfuric Acid in Copper Electrorefining. In Proceedings of the 58th Annual Conference of Metallurgists (COM) Hosting the 10th Internatioanl Copper Conference 2019, Vancouver, BC, Canada, 21 August 2019. [Google Scholar]
- Mubarok, Z.; Filzwieser, I.; Paschen, P. Analysis of Nodulated Cathodes from Atlantic Copper and New Boliden. Erzmetall 2005, 58, 203–209. [Google Scholar]
- Zeng, W.; Wang, S.; Free, M.L. Two-Phase Flow Modeling of Copper Electrorefining Involving Impurity Particles. J. Electrochem. Soc. 2017, 164, E233. [Google Scholar] [CrossRef]
- Zeng, W.; Free, M.L.; Werner, J.; Wang, S. Simulation and Validation Studies of Impurity Particle Behavior in Copper Electrorefining. J. Electrochem. Soc. 2015, 162, E338. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Wang, S.; Free, M.L. Experimental and Simulation Studies of Electrolyte Flow and Slime Particle Transport in a Pilot Scale Copper Electrorefining Cell. J. Electrochem. Soc. 2016, 163, E111. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Wang, S.; Free, M.L. Experimental Studies of the Effects of Anode Composition and Process Parameters on Anode Slime Adhesion and Cathode Copper Purity by Performing Copper Electrorefining in a Pilot-Scale Cell. Metall. Mater. Trans. B 2016, 47, 3178–3191. [Google Scholar] [CrossRef]
- Mubarok, Z.; Antrekowitsch, H.; Mori, G. Problems in the Electrolysis of Copper Anodes with High Content of Nickel, Antimony, Tin and Lead. In Proceedings of the Sixth International Copper-Cobre Conference, Toronto, ON, Canada, 25–30 August 2007; Houlachi, G.E., Edwards, J.D., Robinson, T.G., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montréal, QC, Canada, 2007; Volume V, pp. 59–76. [Google Scholar]
- Hiskey, J.B. Mechanism and Thermodynamics of Floating Slimes Formation. In Proceedings of the T.T. Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization, Orlando, FL, USA, 11–15 March 2012; Wang, S., Dutrizac, J.E., Free, M.L., Hwang, J.Y., Kim, D., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 101–112, ISBN 978-1-118-36483-3. [Google Scholar]
- Muhlare, T.A.; Groot, D.R. The Effect of Electrolyte Additives on Cathode Surface Quality during Copper Electrorefining. J. South. Afr. Inst. Min. Metall. 2011, 111, 371–378. [Google Scholar]
- Brown, G.M.; Hope, G.A.; Schweinsberg, D.P.; Fredericks, P.M. SERS Study of the Interaction of Thiourea with a Copper Electrode in Sulphuric Acid Solution. J. Electroanal. Chem. 1995, 380, 161–166. [Google Scholar] [CrossRef]
- Brown, G.M.; Hope, G.A. In-Situ Spectroscopic Evidence for the Adsorption of SO2−4 Ions at a Copper Electrode in Sulfuric Acid Solution. J. Electroanal. Chem. 1995, 382, 179–182. [Google Scholar] [CrossRef]
- Aromaa, J.; Kekki, A.; Stefanova, A.; Forsén, O. Copper Nucleation and Growth Patterns on Stainless Steel Cathode Blanks in Copper Electrorefining. J. Solid State Electrochem. 2012, 16, 3529–3537. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Chen, T.T. Problems in the Electrolysis of Copper Anodes with High Content of Nickel, Antimony, Tin and Lead. In Proceedings of the Copper 99-Cobre 99 International Conference, Phoenix, AZ, USA, 10–13 October 1999; Dutrizac, J.E., Ramachandran, V., Eds.; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1999; Volume III, pp. 383–404. [Google Scholar]
- Girgis, M.; Ghali, E. Electrochemical Investigations on the Behaviour of Arsenic during Copper Electrodeposition. In The Electrorefining and Winning of Copper, Proceedings of the of the Symposium Sponsored by TMS Copper, Nickel, Cobalt, Precious Metals, and Eletrolytic Processes Committees, Held at the TMS 116th Annual Meeting, Denver, CO, USA, 24–26 February 1987; Hoffman, J.E., Bautista, R.G., Ettel, V.A., Kudryk, V., Wesely, R.J., Eds.; Metallurgical Society: Warrendale, PA, USA, 1987; pp. 173–193. [Google Scholar]
- Hiskey, J.B.; Maeda, Y. A Study of Copper Deposition in the Presence of Group-15 Elements by Cyclic Voltammetry and Auger-Electron Spectroscopy. J. Appl. Electrochem. 2003, 33, 393–401. [Google Scholar] [CrossRef]
- Finnish Standards Association SFS. CEN-TS-13388 Copper and Copper Alloys-Compendium of Compositions and Products; Finnish Standards Association SFS: Helsinki, Finland, 2013; pp. 1–70. [Google Scholar]
- Andersen, T.N.; Pitt, C.H.; Livingston, L.S. Nodulation of Electrodeposited Copper Due to Suspended Particulate. J. Appl. Electrochem. 1983, 13, 429–438. [Google Scholar] [CrossRef]
- Moats, M.S.; Filzwieser, A.; Wang, S.; Davenport, W.G.; Siegmund, A.; Robinson, T. Global Survey of Copper Electrorefining: 2019 World Tankhouse Operating Data. In Proceedings of the 58th Annual Conference of Metallurgists (COM) Hosting the 10th Internatioanl Copper Conference 2019, Vancouver, BC, Canada, 21 August 2019. [Google Scholar]
- Brande, P.V.; Winand, R. Nucleation and Initial Growth of Copper Electrodeposits under Galvanostatic Conditions. Surf. Coat. Technol. 1992, 52, 1–7. [Google Scholar] [CrossRef]
- Hiskey, J.B. The Historical Development of Electrolyte Additives and Their Specific Role and Influence on Cathode Quality. In Proceedings of the 58th Annual Conference of Metallurgists (COM) Hosting the 10th Internatioanl Copper Conference 2019, Vancouver, BC, Canada, 21 August 2019. [Google Scholar]
- Natter, H.; Schmelzer, M.; Hempelmann, R. Nanocrystalline Nickel and Nickel-Copper Alloys: Synthesis, Characterization, and Thermal Stability. J. Mater. Res. 1998, 13, 1186–1197. [Google Scholar] [CrossRef]
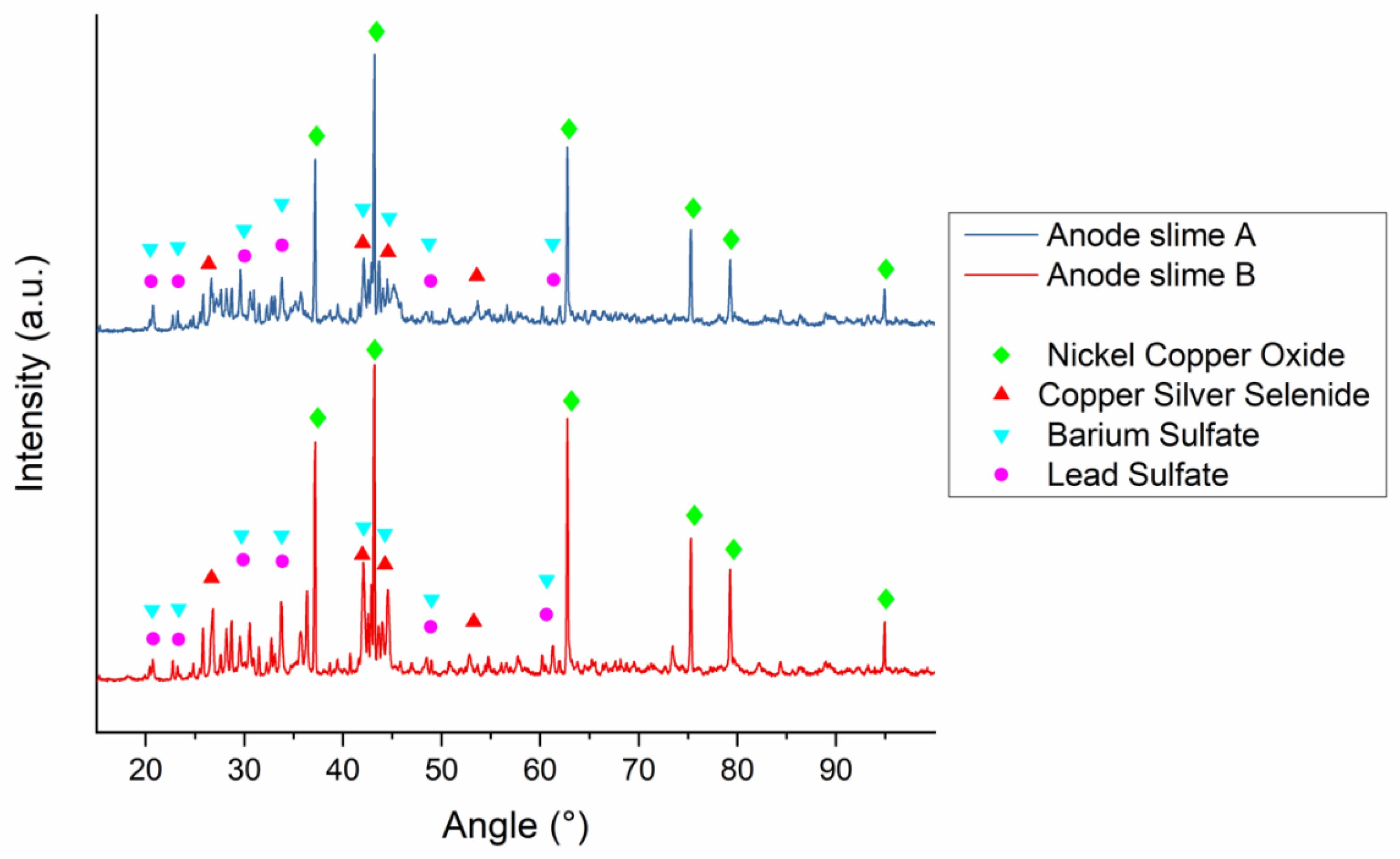
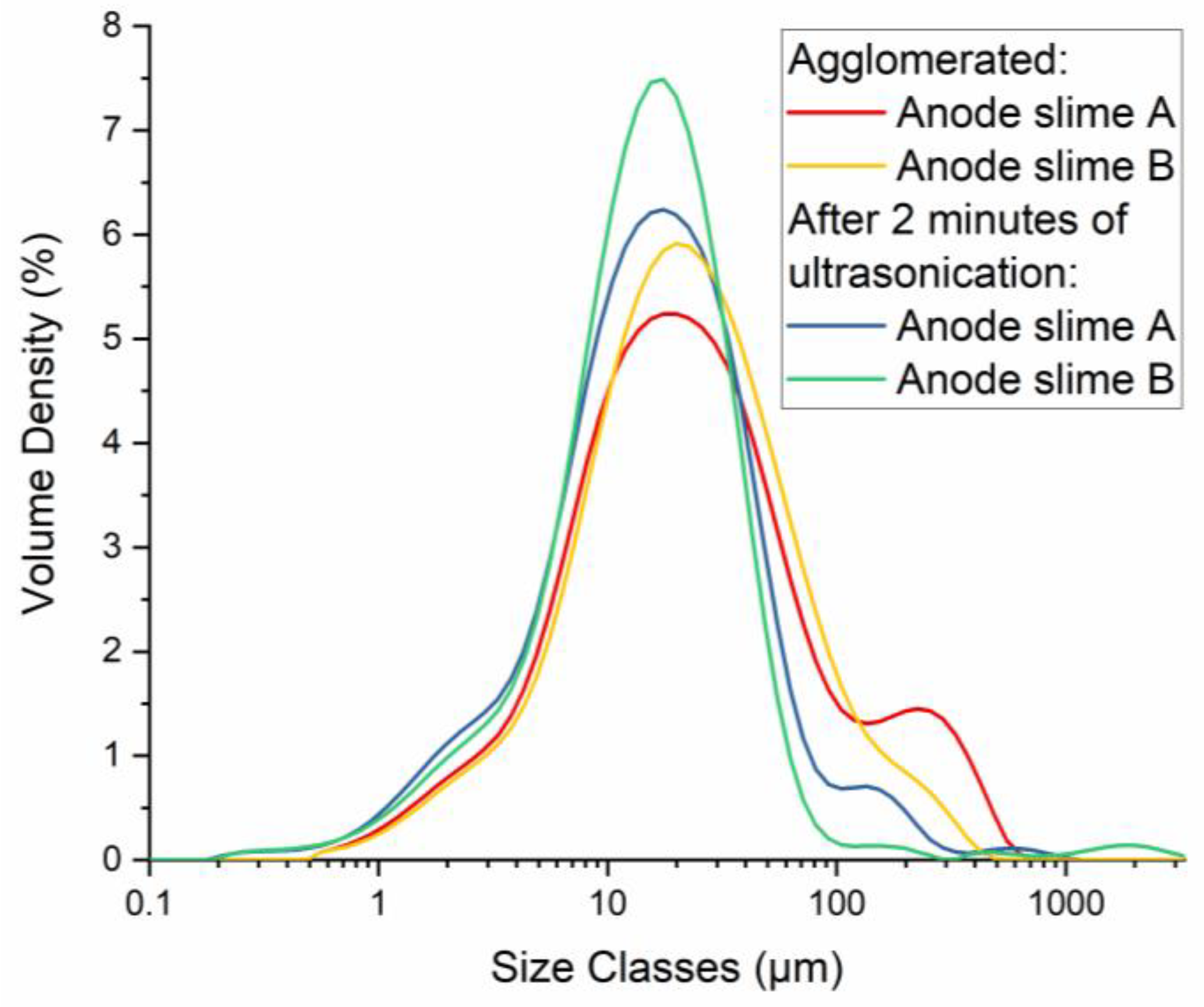
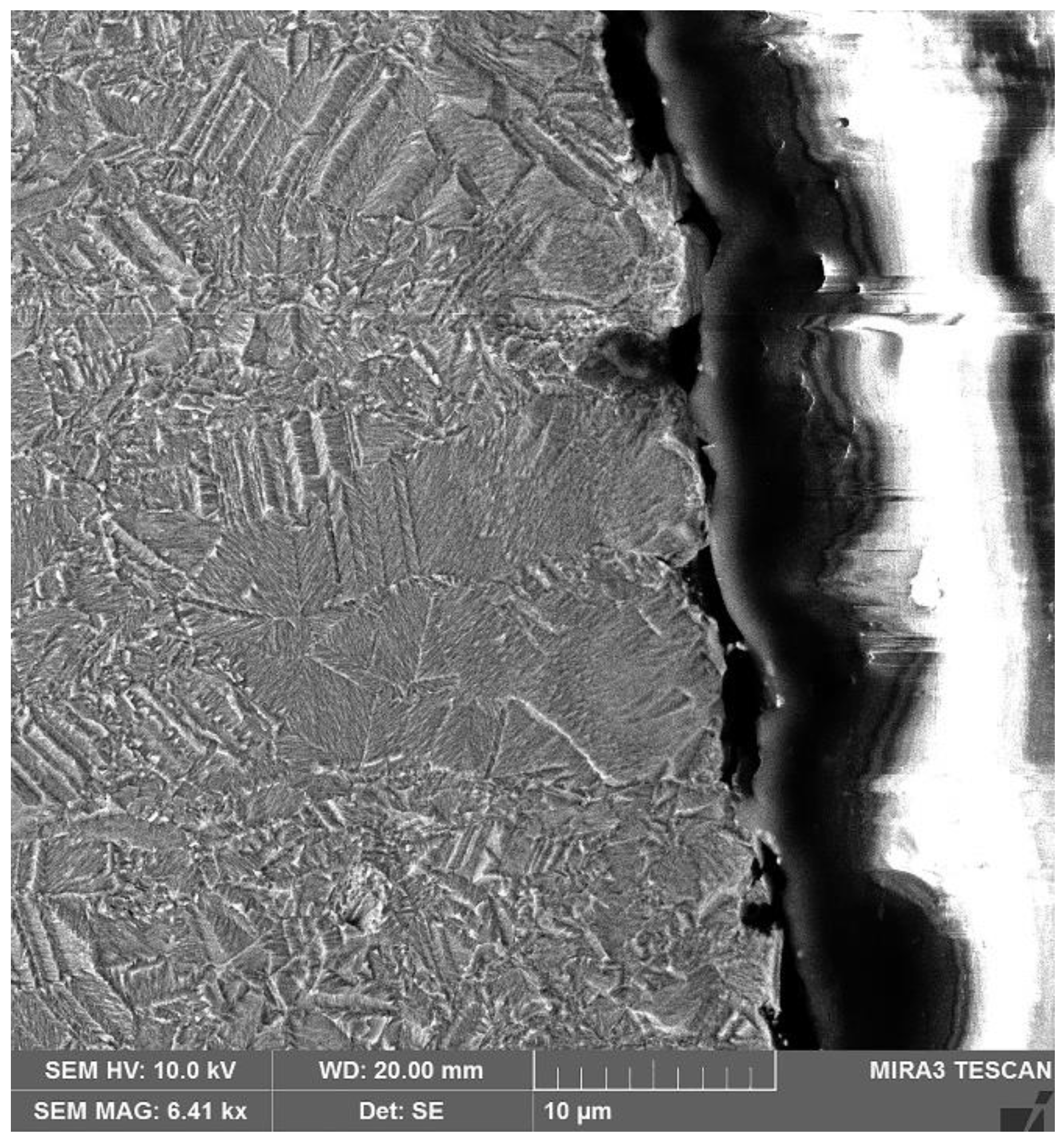
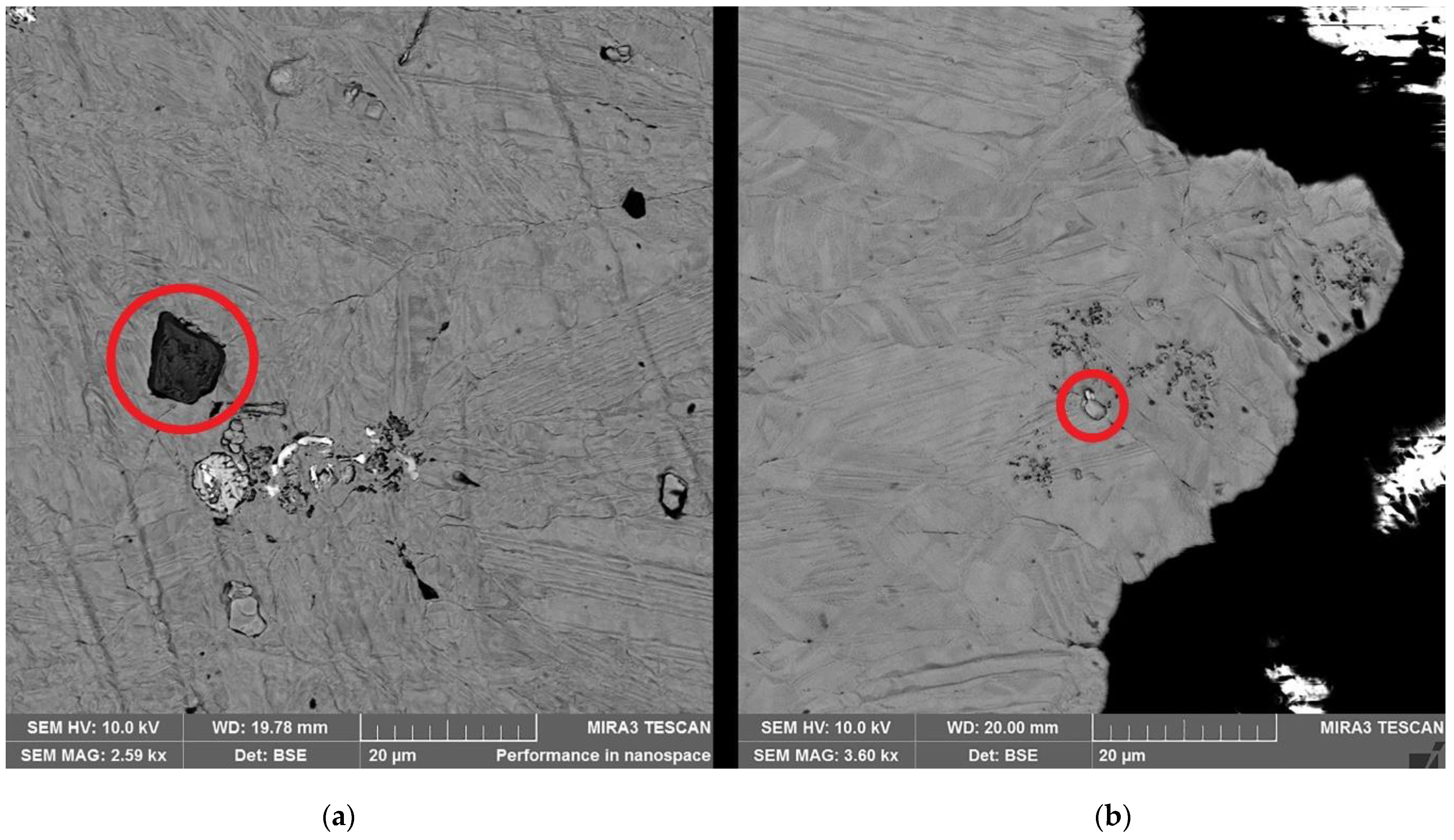
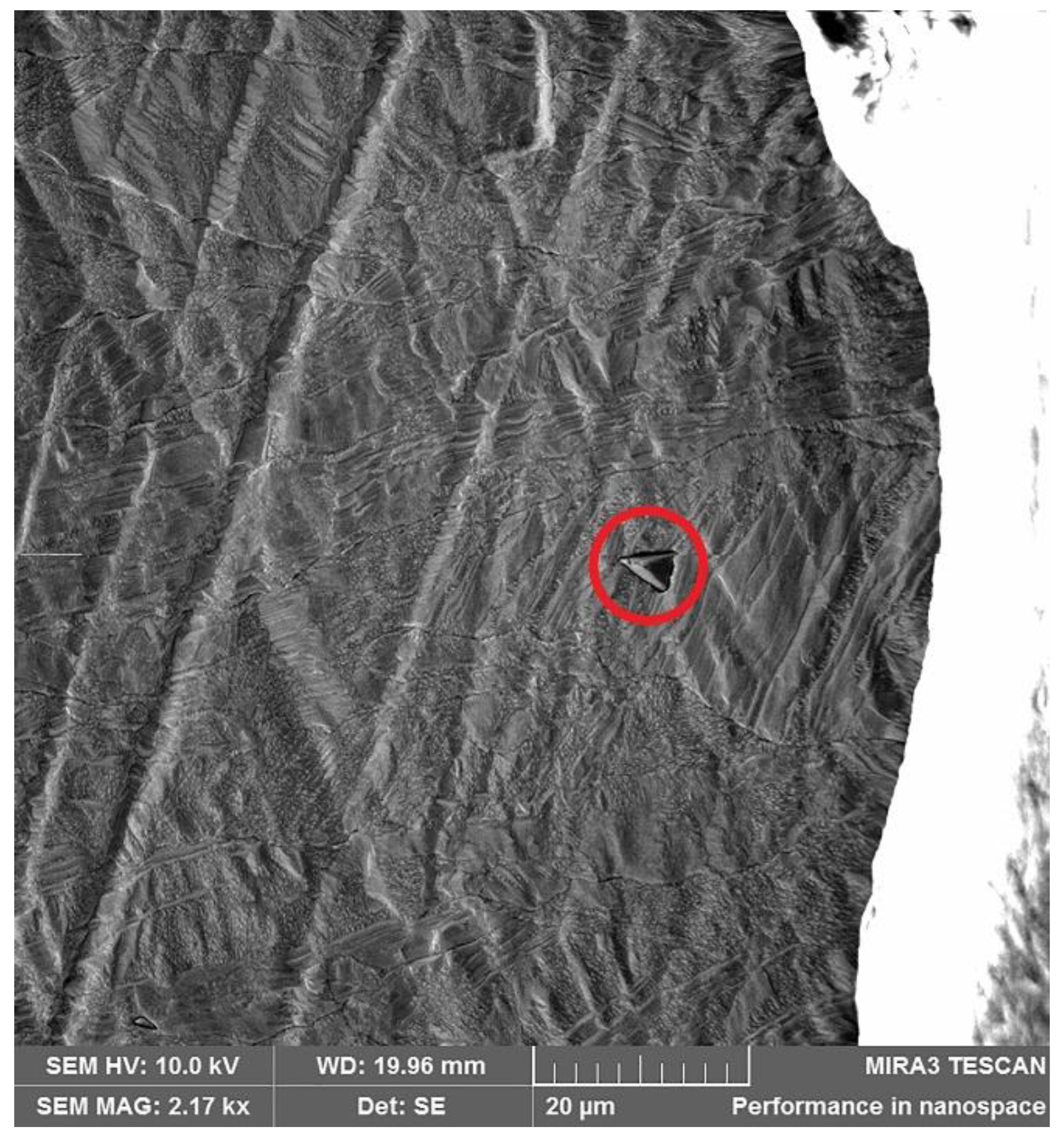
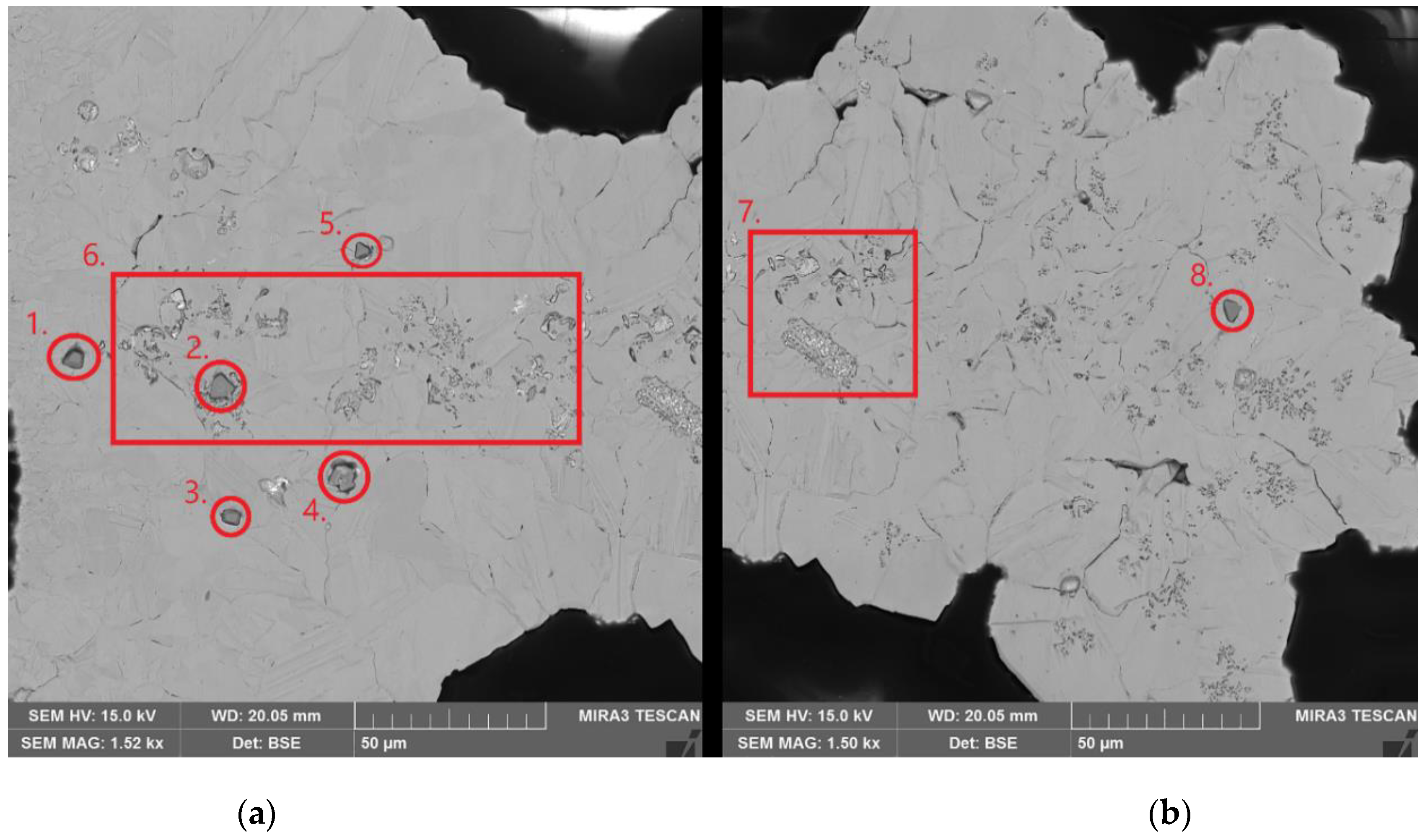


| Element | Anode Slime A (w-%) | Anode Slime B (w-%) |
|---|---|---|
| As | 5.55 | 7.62 |
| Bi | 2.59 | 3.33 |
| Cu | 15.5 | 22 |
| Fe | 0.18 | 0.21 |
| Ni | 10.3 | 20.4 |
| Pb | 4.9 | 3.93 |
| Sb | 1.53 | 1.22 |
| Sn | 0.42 | 0.35 |
| Experiment | Temperature (°C) | Solid Content (g/L) | Current Density (A/m2) |
|---|---|---|---|
| SS-1 | 60 | 20 | 250 |
| SS-2 | 65 | 20 | 250 |
| SS-3 | 70 | 20 | 250 |
| SS-4 | 60 | 40 | 250 |
| SS-5 | 65 | 40 | 250 |
| SS-6 | 70 | 40 | 250 |
| SS-7 | 60 | 60 | 250 |
| SS-8 | 65 | 60 | 250 |
| SS-9 | 70 | 60 | 250 |
| SS-10 | 60 | 20 | 350 |
| SS-11 | 65 | 20 | 350 |
| SS-12 | 70 | 20 | 350 |
| SS-13 | 60 | 40 | 350 |
| SS-14 | 65 | 40 | 350 |
| SS-15 | 70 | 40 | 350 |
| SS-16 | 60 | 60 | 350 |
| SS-17 | 65 | 60 | 350 |
| SS-18 | 70 | 60 | 350 |
| SA/SB-1 | 60 | 20 | 250 |
| SA/SB-2 | 65 | 20 | 250 |
| SA/SB-3 | 70 | 20 | 250 |
| SA/SB-4 | 60 | 20 | 350 |
| SA/SB-5 | 65 | 20 | 350 |
| SA/SB-6 | 70 | 20 | 350 |
| Experiment | Temperature (°C) | Current Density (A/m2) |
|---|---|---|
| ISS-1 | 60 | 250 |
| ISS-2 | 65 | 250 |
| ISS-3 | 70 | 250 |
| ISS-4 | 60 | 300 |
| ISS-5 | 65 | 300 |
| ISS-6 | 70 | 300 |
| ISS-7 | 60 | 350 |
| ISS-8 | 65 | 350 |
| ISS-9 | 70 | 350 |
| IES-1 | 60 | 350 |
| IES-2 | 65 | 350 |
| IES-3 | 70 | 350 |
| Element | Concentration |
|---|---|
| As | 12.00 g/L (160.2 mM) |
| Ba | 0.00012 g/L (0.9 µM) |
| Bi | 0.351 g/L (1.7 mM) |
| Cu | 35.80 g/L (563.37 mM) |
| Fe | 0.213 g/L (3.8 mM) |
| Ni | 18.40 g/L (313.5 mM) |
| Pb | 0.0227 g/L (0.1 mM) |
| S | 84.70 g/L (2641.5 mM) |
| Sb | 0.315 g/L (2.6 mM) |
| Sn | 0.00173 g/L (14.6 µM) |
| Experiment | Current Efficiency (%) | Ni (µg/g) | Fe (µg/g) | Visual Description of Cathode Quality |
|---|---|---|---|---|
| SS-1 | 73.73 | 1242 | 12 | Smooth surface. |
| SS-2 | 65.75 | 88 | - * | No nodulation but uneven growth of cathode. |
| SS-3 | 65.05 | 148 | - * | No nodulation but uneven growth of cathode. |
| SS-4 | 72.82 | 32 | - * | No nodulation but uneven growth of cathode. |
| SS-5 | 65.18 | 14 | - * | No nodulation but uneven growth of cathode. |
| SS-6 | 60.81 | 233 | - * | No nodulation but uneven growth of cathode. |
| SS-7 | 60.45 | 183 | - * | No nodulation but uneven growth of cathode. |
| SS-8 | 63.51 | 284 | 23 | No nodulation but uneven growth of cathode. |
| SS-9 | 59.63 | 180 | - * | No nodulation but uneven growth of cathode. |
| SS-10 | NA | 309 | 8 | Smooth surface. |
| SS-11 | NA | 525 | 14 | No nodulation but NiO visible on top right section of the cathode. |
| SS-12 | 166.07 | 698 | - * | No nodulation but NiO visible on top left section of the cathode. |
| SS-13 | 103.06 | 813 | 12 | Smooth surface. |
| SS-14 | 80.06 | 1128 | - * | Smooth surface. |
| SS-15 | 78.27 | 470 | 10 | Smooth surface. |
| SS-16 | NA | 914 | - * | Smooth surface. |
| SS-17 | 75.95 | 900 | - * | Smooth surface. |
| SS-18 | 71.88 | 388 | - * | Smooth surface. |
| SA-1 | 92.81 | 137 | - | Small clusters of nodules on the right side of the cathode. |
| SA-2 | 87.94 | 118 | - | Small clusters of nodules on the right side of the cathode. |
| SA-3 | 96.92 | 29 | - | Small nodules across the cathode. |
| SA-4 | 101.14 | 368 | - | Nodules across the cathode. |
| SA-5 | 99.58 | 238 | - | Nodules across the cathode. |
| SA-6 | 109.25 | 22 | - | Few nodules on the bottom of the cathode and visible slime on the surface. |
| SB-1 | 98.20 | - * | - | Smooth surface. |
| SB-2 | 102.60 | - * | - | Smooth surface. |
| SB-3 | 103.72 | - * | - | Smooth surface. |
| SB-4 | 98.15 | 126 | - | Small nodules across the cathode. |
| SB-5 | 100.16 | 125 | - | Small clusters of nodules on the right side of the cathode. |
| SB-6 | 100.32 | - * | - | Smooth surface. |
| Point | Cu (w-%) | Ni (w-%) | Fe (w-%) | O (w-%) | Se (w-%) | Sn (w-%) | Ag (w-%) | Zn (w-%) | C (w-%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.46 | 68.91 | 0.85 | 25.79 | - | - | - | - | 0.00 |
| 2 | 6.04 | 44.62 | 7.54 | 24.73 | - | 8.64 | - | 8.43 | 0.00 |
| 3 | 10.94 | 74.25 | - | 4.84 | - | - | - | - | 9.97 |
| 4 | 5.53 | 52.35 | 7.15 | 24.73 | - | 3.81 | - | 3.87 | 0.00 |
| 5 | 5.67 | 68.67 | - | 25.67 | - | - | - | - | 0.00 |
| 6 | 98.77 | - | - | 1.23 | - | - | - | - | 0.00 |
| 7 | 94.54 | - | - | 0.92 | 2.80 | - | 1.74 | - | 0.00 |
| 8 | 4.67 | 63.89 | - | 24.08 | - | - | - | - | 7.36 |
| Point # | Cu (w-%) | Ni (w-%) | Fe (w-%) | O (w-%) | S (w-%) | C (w-%) |
|---|---|---|---|---|---|---|
| 1 | 37.85 | - | 3.05 | 38.48 | 18.98 | 1.60 |
| 2 | 3.32 | 73.88 | - | 21.56 | 0.15 | 0 |
| 3 | 96.42 | 1.77 | - | 1.34 | - | 0.42 |
| 4 | 48.76 | 32.64 | - | 15.92 | 0.20 | 1.26 |
| Point | C (w-%) | Cu (w-%) | Ni (w-%) | S (w-%) | O (w-%) |
|---|---|---|---|---|---|
| 1 | 4.93 | 86.35 | - | - | 4.18 |
| 2 | 0.57 | 98.27 | - | 0.07 | 1.09 |
| 3 | 17.11 | 43.30 | 2.72 | 4.31 | 31.87 |
| Experiment | Current Efficiency (%) | Ni (µg/g) |
|---|---|---|
| ISS-1 | 95.3 | 43 |
| ISS-2 | 98.7 | 69 |
| ISS-3 | 95.7 | 271 |
| ISS-4 | 100.7 | 130 |
| ISS-5 | 93.4 | 152 |
| ISS-6 | 90.7 | 330 |
| ISS-7 | 76.6 | 80 |
| ISS-8 | 85.3 | 206 |
| ISS-9 | 99.9 | 72 |
| IES-1 | 99.0 | 51 |
| IES-2 | 100.9 | 81 |
| IES-3 | 101.1 | 72 |
| Experiment | Current Density Region (A/m2) | Ni (µg/mg) |
|---|---|---|
| HC-1 | 600–1900 | 12 |
| 420–600 | 8 | |
| 310–420 | 18 | |
| HC-2 | 600–1900 | - * |
| 420–600 | - * | |
| 310–420 | - * | |
| HC-3 | 600–1900 | - * |
| 420–600 | - * | |
| 310–420 | - * | |
| NiED-1 | 1000 | 40 |
| NiED-2 | 1000 | - * |
| NiED-3 | 1000 | - * |
| NiED-4 | 500 | - * |
| NiED-5 | 500 | - * |
| NiED-6 | 500 | - * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahlman, M.; Aromaa, J.; Lundström, M. Copper Cathode Contamination by Nickel in Copper Electrorefining. Metals 2021, 11, 1758. https://doi.org/10.3390/met11111758
Sahlman M, Aromaa J, Lundström M. Copper Cathode Contamination by Nickel in Copper Electrorefining. Metals. 2021; 11(11):1758. https://doi.org/10.3390/met11111758
Chicago/Turabian StyleSahlman, Mika, Jari Aromaa, and Mari Lundström. 2021. "Copper Cathode Contamination by Nickel in Copper Electrorefining" Metals 11, no. 11: 1758. https://doi.org/10.3390/met11111758






