CMT-Based Wire Arc Additive Manufacturing Using 316L Stainless Steel (2): Solidification Map of the Multilayer Deposit
Abstract
:1. Introduction
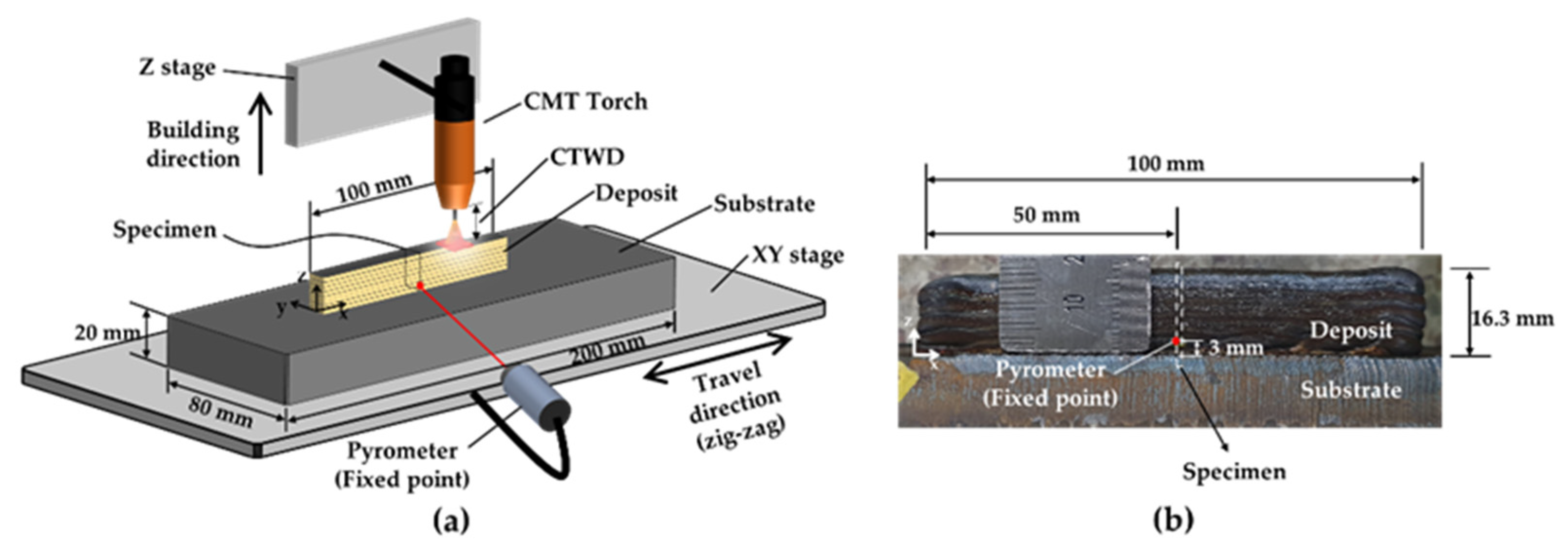
2. Experimental Setup
3. Numerical Modeling
4. Results and Discussion
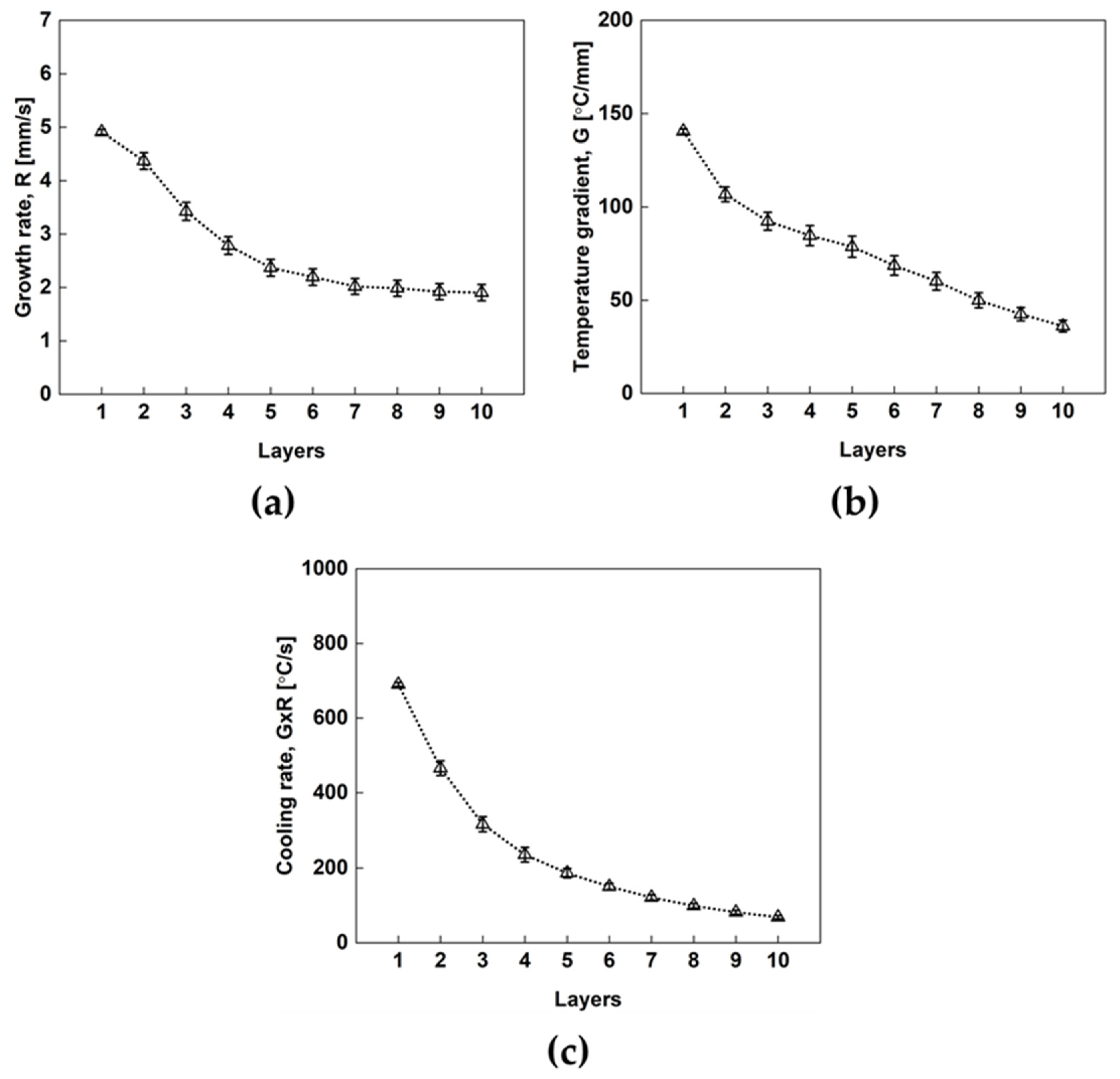
4.1. Comparison of Experimental and Simulated Temperature Profiles
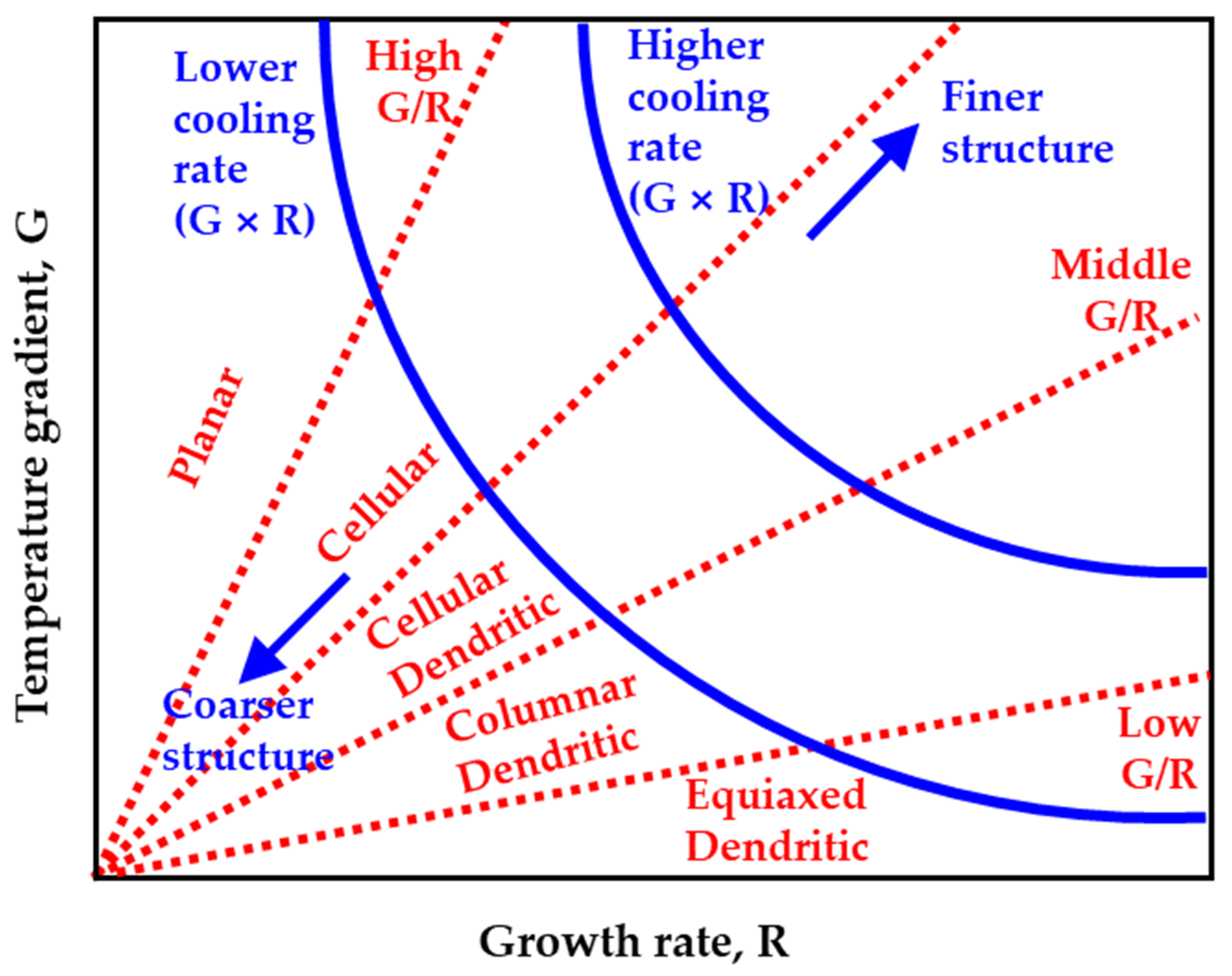
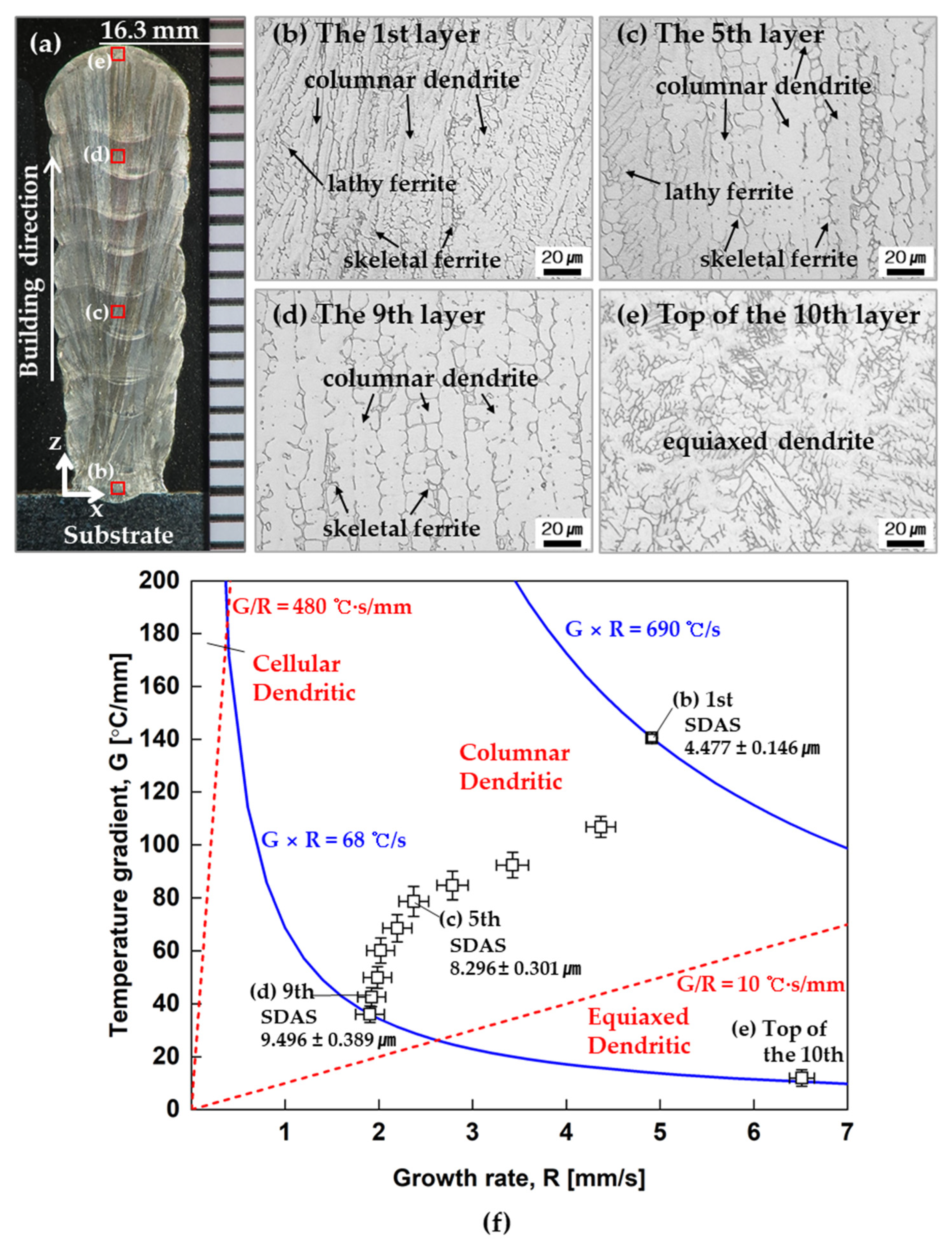
4.2. Additive Manufacturing Guideline Using Solidification Map
5. Conclusions
- (1)
- To investigate the effect of G and R on the microstructure in CMT-WAAM multilayer deposition, a mathematical model was developed to simulate the processes. Upon comparing the simulation results of the model with the experimentally measured temperatures, CMT-WAAM showed an error of 4.4%. Excluding some peaks, the simulated and experimental result were nearly identical. The developed model thus effectively simulated the thermal conditions of the multilayer processes in CMT-WAAM was suitable for calculating the solidification parameters and analyzing the heat input;
- (2)
- In the CMT-WAAM multilayer processes, a 316L SS solidification map, which consists of G and R of each layer of deposit was suggested to control and predict the microstructure. In the solidification map, which reflects the heat accumulation of deposit, G × R and G/R were ranged from 68 to 690 °C/s and 2 to 33 °Cs/mm2, respectively;
- (3)
- Through the solidification map, the effect of G and R on the microstructure of each layer was investigated. Although G/R showed variation in the solidification map, the morphology of the microstructure was not changed along with the height except for the top of the 10th layer. On the other hand, G × R decreases as the layer increases in the solidification map, but SDAS, which is affected by G × R, tends to increase;
- (4)
- By calculating G and R from the simulation model, a possible new method for predicting the microstructure shape without performing actual deposition was developed. This could provide the basis for controlling or predicting the microstructure and mechanical properties by selecting the CMT-WAAM process parameters in future research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Debroy, T.; Zhang, W.; Turner, J.; Babu, S.S. Building digital twins of 3D printing machines. Scr. Mater. 2017, 135, 119–124. [Google Scholar] [CrossRef]
- Wei, H.; Elmer, J.; DebRoy, T. Origin of grain orientation during solidification of an aluminum alloy. Acta Mater. 2016, 115, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Lippold, J.C. Welding Metallurgy and Weldability; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Ou, W.; Mukherjee, T.; Knapp, G.; Wei, Y.; DebRoy, T. Fusion zone geometries, cooling rates and solidification parameters during wire arc additive manufacturing. Int. J. Heat Mass Transf. 2018, 127, 1084–1094. [Google Scholar] [CrossRef]
- Ou, W.; Knapp, G.; Mukherjee, T.; Wei, Y.; DebRoy, T. An improved heat transfer and fluid flow model of wire-arc additive manufacturing. Int. J. Heat Mass Transf. 2020, 167, 120835. [Google Scholar] [CrossRef]
- Hejripour, F.; Binesh, F.; Hebel, M.; Aidun, D.K. Thermal modeling and characterization of wire arc additive manufactured duplex stainless steel. J. Mater. Process. Technol. 2019, 272, 58–71. [Google Scholar] [CrossRef]
- Bermingham, M.; StJohn, D.; Krynen, J.; Tedman-Jones, S.; Dargusch, M. Promoting the columnar to equiaxed transition and grain refinement of titanium alloys during additive manufacturing. Acta Mater. 2019, 168, 261–274. [Google Scholar] [CrossRef]
- Rahman Rashid, R.A.; Javed, M.A.; Barr, C.; Palanisamy, S.; Matthews, N.; Dargusch, M.S. Effect of in situ tempering on the mechanical, microstructural and corrosion properties of 316L stainless steel laser-cladded coating on mild steel. Int. J. Adv. Manuf. Technol. 2021, 117, 1–10. [Google Scholar]
- Chen, L.; Richter, B.; Zhang, X.; Ren, X.; Pfefferkorn, F.E. Modification of surface characteristics and electrochemical corrosion behavior of laser powder bed fused stainless-steel 316L after laser polishing. Addit. Manuf. 2020, 32, 101013. [Google Scholar] [CrossRef]
- Oh, W.J.; Lee, W.J.; Kim, M.S.; Jeon, J.B.; Shim, D.S. Repairing additive-manufactured 316L stainless steel using direct energy deposition. Opt. Laser Technol. 2019, 117, 6–17. [Google Scholar] [CrossRef]
- Setien, I.; Chiumenti, M.; van der Veen, S.; San Sebastian, M.; Garciandía, F.; Echeverría, A. Empirical methodology to determine inherent strains in additive manufacturing. Comput. Math. Appl. 2019, 78, 2282–2295. [Google Scholar] [CrossRef] [Green Version]
- Jayanath, S.; Achuthan, A. A computationally efficient hybrid model for simulating the additive manufacturing process of metals. Int. J. Mech. Sci. 2019, 160, 255–269. [Google Scholar] [CrossRef]
- Knapp, G.; Mukherjee, T.; Zuback, J.; Wei, H.; Palmer, T.; De, A.; DebRoy, T. Building blocks for a digital twin of additive manufacturing. Acta Mater. 2017, 135, 390–399. [Google Scholar] [CrossRef]
- Du, L.; Gu, D.; Dai, D.; Shi, Q.; Ma, C.; Xia, M. Relation of thermal behavior and microstructure evolution during multi-track laser melting deposition of Ni-based material. Opt. Laser Technol. 2018, 108, 207–217. [Google Scholar] [CrossRef]
- Wu, B.; Ding, D.; Pan, Z.; Cuiuri, D.; Li, H.; Han, J.; Fei, Z. Effects of heat accumulation on the arc characteristics and metal transfer behavior in Wire Arc Additive Manufacturing of Ti6Al4V. J. Mater. Process. Technol. 2017, 250, 304–312. [Google Scholar] [CrossRef]
- Lee, S.H. CMT-Based Wire Arc Additive Manufacturing Using 316L Stainless Steel: Effect of Heat Accumulation on the Multi-Layer Deposits. Metals 2020, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Goldak, J.; Chakravarti, A.; Bibby, M. A new finite element model for welding heat sources. Metall. Trans. B 1984, 15, 299–305. [Google Scholar] [CrossRef]
- Wu, Q.; Mukherjee, T.; Liu, C.; Lu, J.; DebRoy, T. Residual stresses and distortion in the patterned printing of titanium and nickel alloys. Addit. Manuf. 2019, 29, 100808. [Google Scholar] [CrossRef]
- Oyama, K.; Diplas, S.; M’hamdi, M.; Gunnæs, A.E.; Azar, A.S. Heat source management in wire-arc additive manufacturing process for Al-Mg and Al-Si alloys. Addit. Manuf. 2019, 26, 180–192. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Trivedi, R.; Kurz, W. Theory of Microstructural Development during Rapid Solidification in Science and Technology of the Undercooled Melt; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Caballero, A.; Ding, J.; Ganguly, S.; Williams, S. Wire+ Arc Additive Manufacture of 17-4 PH stainless steel: Effect of different processing conditions on microstructure, hardness, and tensile strength. J. Mater. Process. Technol. 2019, 268, 54–62. [Google Scholar] [CrossRef]
- Buchanan, C.; Gardner, L. Metal 3D printing in construction: A review of methods, research, applications, opportunities and challenges. Eng. Struct. 2019, 180, 332–348. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-Y.; Ji, I.; Lee, S.H. Numerical and Experimental Investigations of Laser Metal Deposition (LMD) Using STS 316L. Appl. Sci. 2020, 10, 4874. [Google Scholar] [CrossRef]
- Huang, Y.; Ansari, M.; Asgari, H.; Farshidianfar, M.H.; Sarker, D.; Khamesee, M.B.; Toyserkani, E. Rapid prediction of real-time thermal characteristics, solidification parameters and microstructure in laser directed energy deposition (powder-fed additive manufacturing). J. Mater. Process. Technol. 2019, 274, 116286. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Liu, W.; Shang, X. Characterization of stainless steel parts by laser metal deposition shaping. Mater. Des. 2014, 55, 104–119. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, G.; Yin, Z.; Wu, L. A 3D dynamic analysis of thermal behavior during single-pass multi-layer weld-based rapid prototyping. J. Mater. Process. Technol. 2011, 211, 488–495. [Google Scholar] [CrossRef]


| 316L SS | Element (wt %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cu | Ni | Cr | Mo | Fe | |
| Wire | 0.01 | 0.59 | 1.53 | 0.027 | 0.001 | 0.17 | 11.55 | 18.56 | 2.53 | Bal. |
| Substrate | 0.016 | 0.50 | 1.25 | 0.030 | 0.001 | 0.26 | 10.09 | 16.63 | 2.05 | Bal. |
| Parameters | Value |
|---|---|
| Current (A) | 120 |
| Voltage (V) | 11.2 |
| Travel speed (mm/s) | 8.33 |
| Wire feed rate (mm/min) | 3600 |
| Shielding gas (100% Ar) flow rate (L/min) | 20 |
| CTWD (mm) | 10 |
| Inter-layer time (s) | 0 |
| Pyrometer emissivity | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, S.H. CMT-Based Wire Arc Additive Manufacturing Using 316L Stainless Steel (2): Solidification Map of the Multilayer Deposit. Metals 2021, 11, 1725. https://doi.org/10.3390/met11111725
Park J, Lee SH. CMT-Based Wire Arc Additive Manufacturing Using 316L Stainless Steel (2): Solidification Map of the Multilayer Deposit. Metals. 2021; 11(11):1725. https://doi.org/10.3390/met11111725
Chicago/Turabian StylePark, Jaewoong, and Seung Hwan Lee. 2021. "CMT-Based Wire Arc Additive Manufacturing Using 316L Stainless Steel (2): Solidification Map of the Multilayer Deposit" Metals 11, no. 11: 1725. https://doi.org/10.3390/met11111725
APA StylePark, J., & Lee, S. H. (2021). CMT-Based Wire Arc Additive Manufacturing Using 316L Stainless Steel (2): Solidification Map of the Multilayer Deposit. Metals, 11(11), 1725. https://doi.org/10.3390/met11111725






