Flexible Powder Production for Additive Manufacturing of Refractory Metal-Based Alloys
Abstract
:1. Introduction
2. Materials and Methods
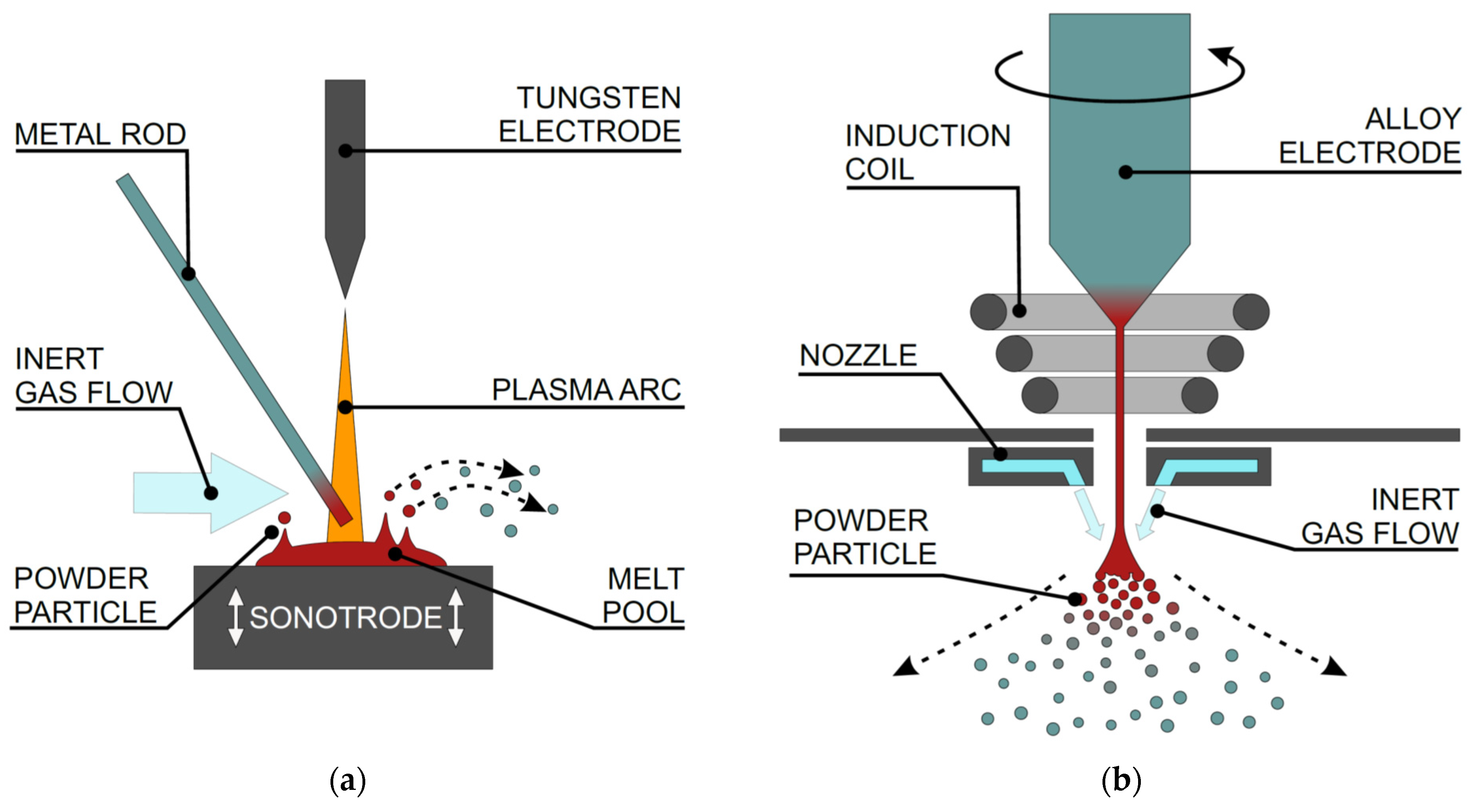
2.1. Sample Synthesis
2.2. Material Analysis
3. Results
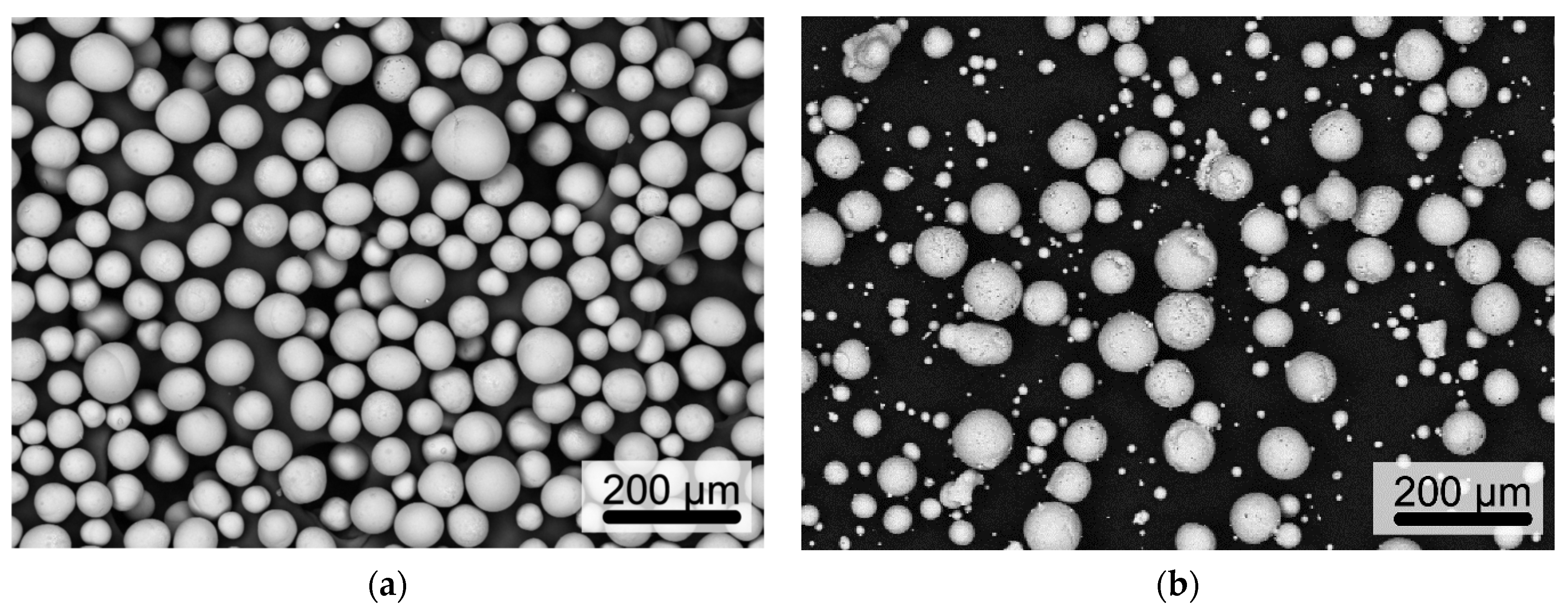
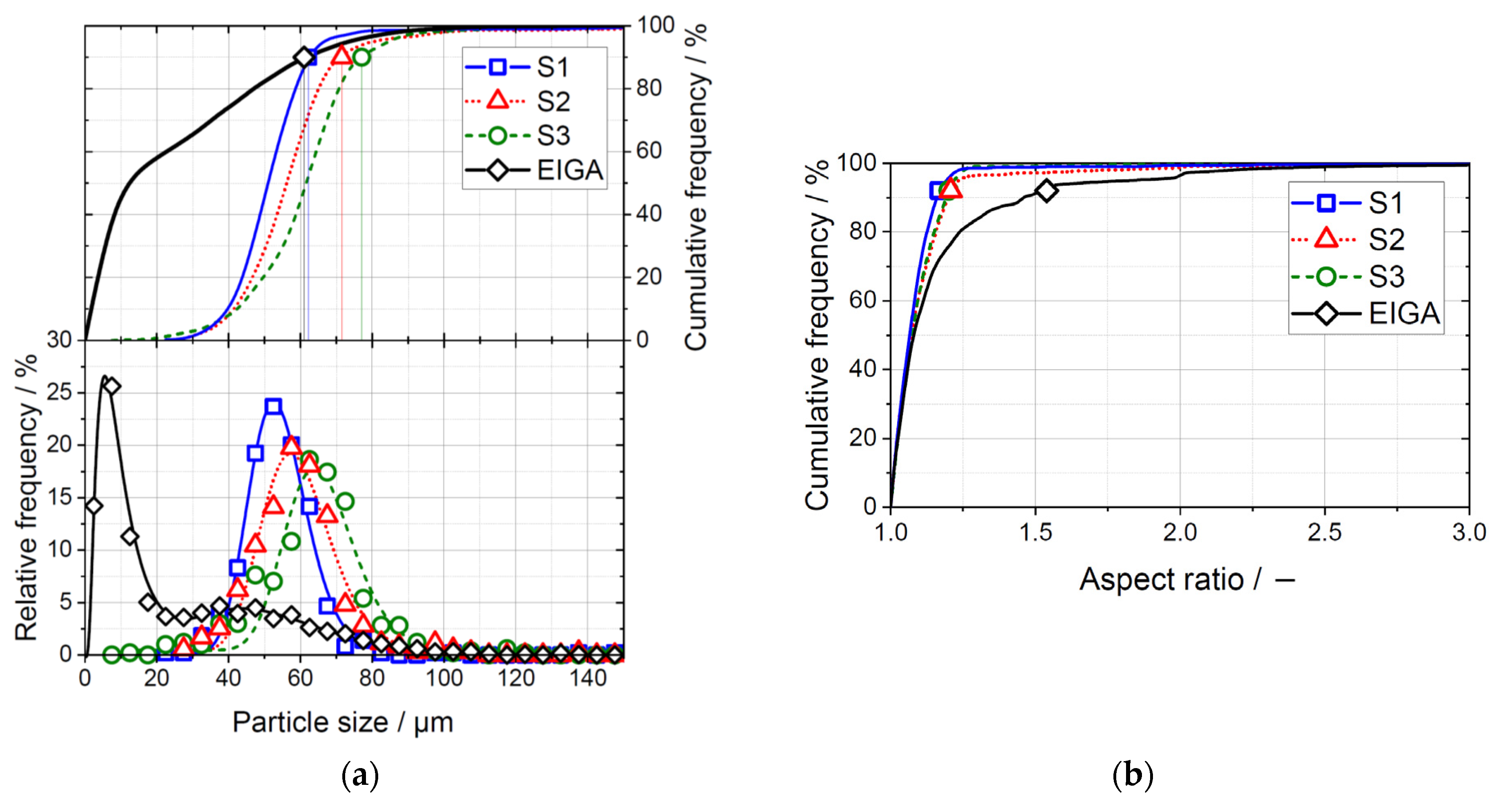
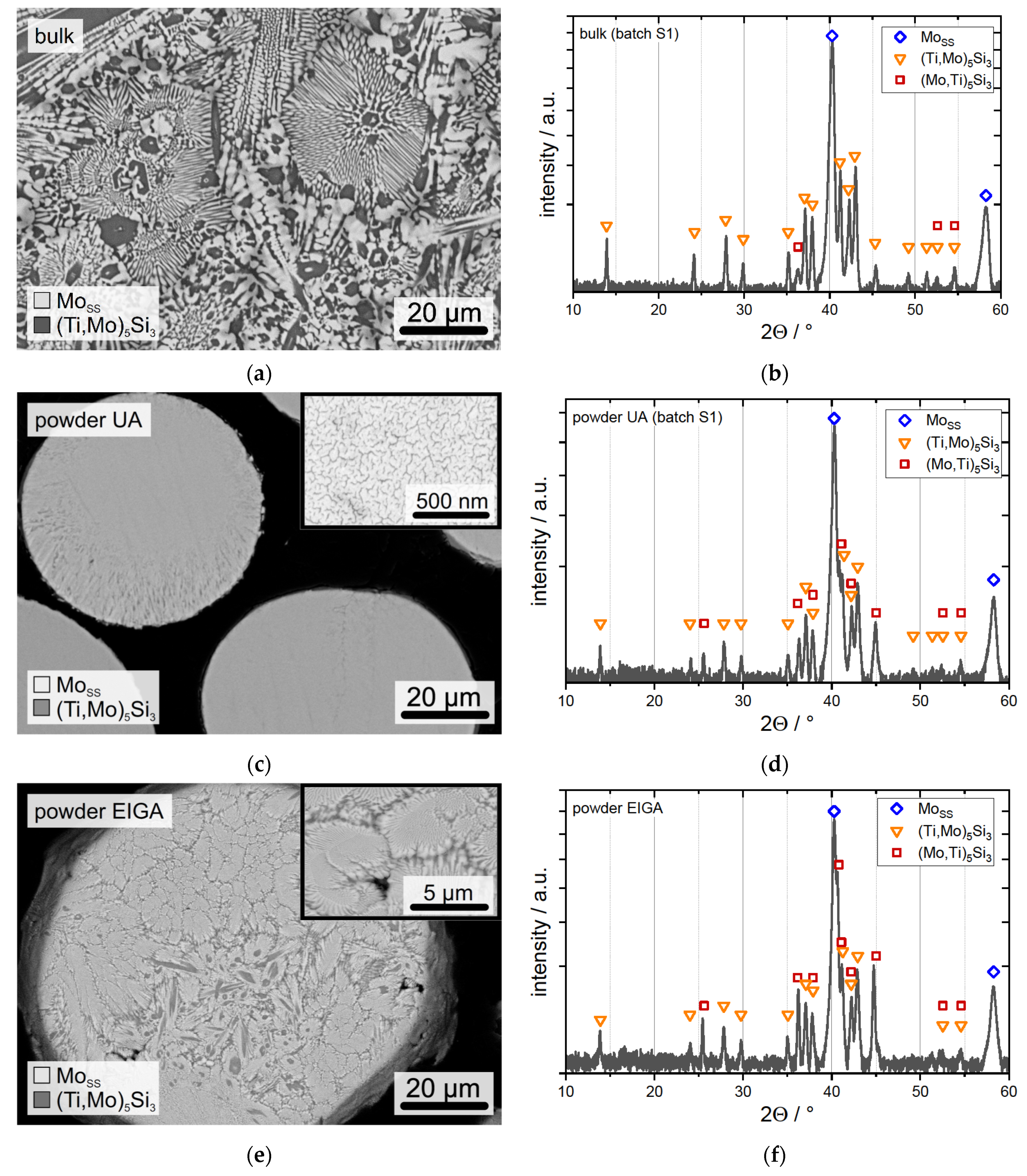
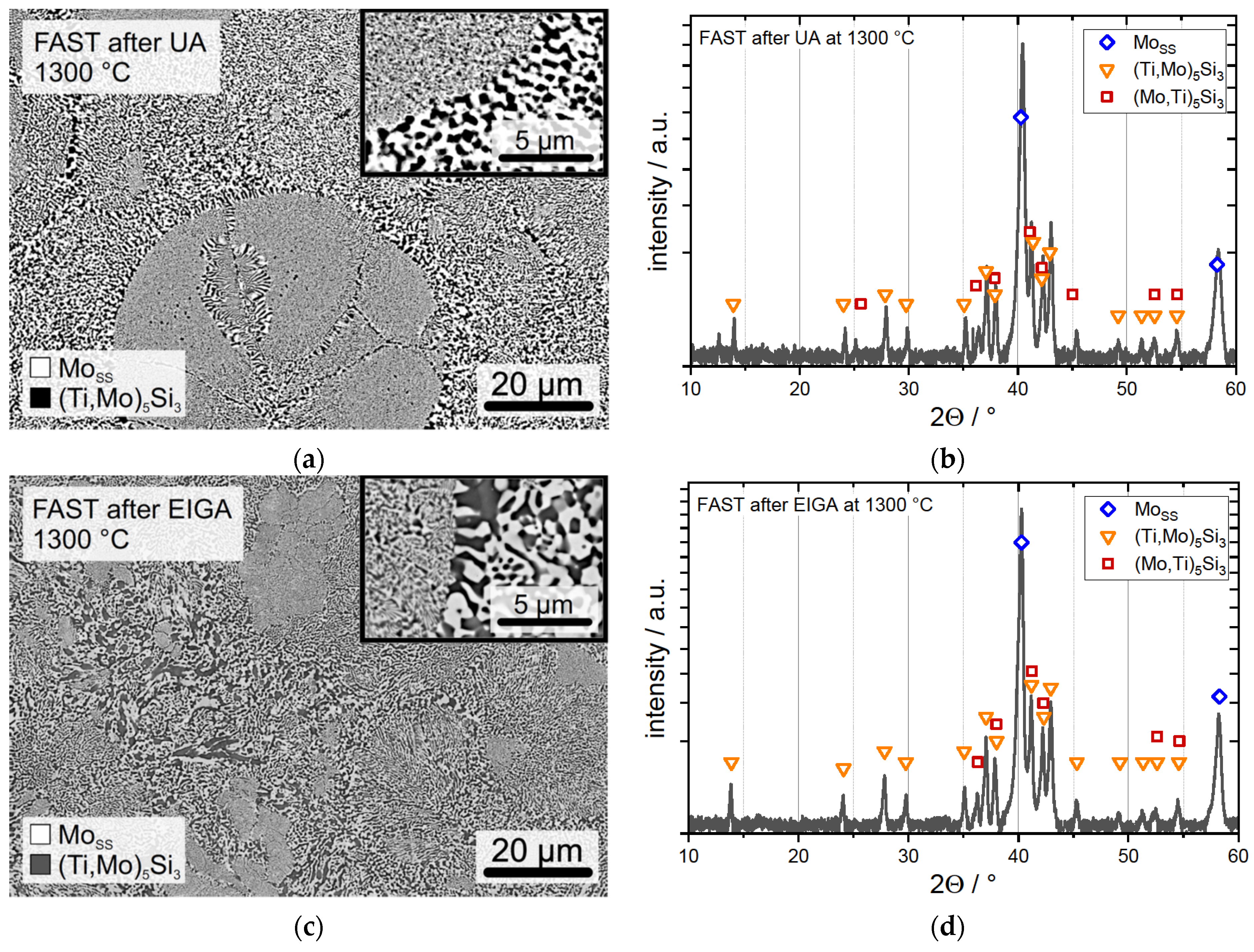
3.1. UA Powder
3.2. EIGA Powder
3.3. Powder Consolidation
4. Discussion
5. Conclusions
- Powders of Mo-20Si-52.8Ti (at.%) were produced for the first time with an UA process at laboratory-scale, and an EIGA process for upscaling to large-scale industrial quantities was used.
- Both processes can be adapted to process refractory metal alloys of various compositions.
- Quick adaptation to various alloy compositions makes UA well suited for flexible alloy powder development.
- With both processes, powders were produced that fulfil the necessary requirements for AM.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Additive manufacturing of metallic components by selective electron beam melting—A review. Int. Mater. Rev. 2016, 61, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, J.; Sevvel, P.; Solomon, I.J. Metallic materials fabrication by selective laser melting: A review. Mater. Today Proc. 2021, 37, 252–256. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Seifi, M. Metal Additive Manufacturing: A Review of Mechanical Properties. Annu. Rev. Mater. Res. 2016, 46, 151–186. [Google Scholar] [CrossRef] [Green Version]
- Vock, S.; Klöden, B.; Kirchner, A.; Weißgärber, T.; Kieback, B. Powders for powder bed fusion: A review. Prog. Addit. Manuf. 2019, 4, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Gombola, C.; Kauffmann, A.; Geramifard, G.; Blankenburg, M.; Heilmaier, M. Microstructural Investigations of Novel High Temperature Alloys Based on NiAl-(Cr,Mo). Metals 2020, 10, 961. [Google Scholar] [CrossRef]
- Jehanno, P.; Kestler, H.; Venskutonis, A.; Boning, M.; Heilmaier, M.; Bewlay, B.; Jackson, M. Assessment of a powder metallurgical processing route for refractory metal silicide alloys. Met. Mater. Trans. A 2005, 36, 515–523. [Google Scholar] [CrossRef]
- Gerling, R.; Clemens, H.; Schimansky, F. Powder Metallurgical Processing of Intermetallic Gamma Titanium Aluminides. Adv. Eng. Mater. 2004, 6, 23–38. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Krüger, M.; Heilmaier, M. Mo-Silicide Alloys for High-Temperature Structural Applications. Mater. Perform. Charact. 2021, 10, 20200183. [Google Scholar] [CrossRef]
- Schliephake, D.; Kauffmann, A.; Cong, X.; Gombola, C.; Azim, M.; Gorr, B.; Christ, H.-J.; Heilmaier, M. Constitution, oxidation and creep of eutectic and eutectoid Mo-Si-Ti alloys. Intermetallics 2019, 104, 133–142. [Google Scholar] [CrossRef]
- ALD Brochure. Available online: https://www.ald-vt.com/wp-content/uploads/2018/01/Metal_Additive_Manufacturing.pdf (accessed on 12 October 2021).
- Obert, S.; Kauffmann, A.; Seils, S.; Schellert, S.; Weber, M.; Gorr, B.; Christ, H.-J.; Heilmaier, M. On the chemical and microstructural requirements for the pesting-resistance of Mo–Si–Ti alloys. J. Mater. Res. Technol. 2020, 9, 8556–8567. [Google Scholar] [CrossRef]
- Saage, H.; Krüger, M.; Sturm, D.; Heilmaier, M.; Schneibel, J.; George, E.; Heatherly, L.; Somsen, C.; Eggeler, G.; Yang, Y. Ductilization of Mo–Si solid solutions manufactured by powder metallurgy. Acta Mater. 2009, 57, 3895–3901. [Google Scholar] [CrossRef]
- Krüger, M.; Franz, S.; Saage, H.; Heilmaier, M.; Schneibel, J.; Jéhanno, P.; Böning, M.; Kestler, H. Mechanically alloyed Mo–Si–B alloys with a continuous α-Mo matrix and improved mechanical properties. Intermetallics 2008, 16, 933–941. [Google Scholar] [CrossRef]
- Schmelzer, J.; Rittinghaus, S.-K.; Weisheit, A.; Stobik, M.; Paulus, J.; Gruber, K.; Wessel, E.; Heinze, C.; Krüger, M. Printability of gas atomized Mo-Si-B powders by laser metal deposition. Int. J. Refract. Met. Hard Mater. 2019, 78, 123–126. [Google Scholar] [CrossRef]
- Zhou, W.; Tsunoda, K.; Nomura, N.; Yoshimi, K. Effect of hot isostatic pressing on the microstructure and fracture toughness of laser additive-manufactured MoSiBTiC multiphase alloy. Mater. Des. 2020, 196, 109132. [Google Scholar] [CrossRef]
- Fichtner, D.; Schmelzer, J.; Yang, W.; Heinze, C.; Krüger, M. Additive manufacturing of a near-eutectic Mo–Si–B alloy: Processing and resulting properties. Intermetallics 2021, 128, 107025. [Google Scholar] [CrossRef]
- Biamino, S.; Penna, A.; Ackelid, U.; Sabbadini, S.; Tassa, O.; Fino, P.; Pavese, M.; Gennaro, P.; Badini, C. Electron beam melting of Ti–48Al–2Cr–2Nb alloy: Microstructure and mechanical properties investigation. Intermetallics 2011, 19, 776–781. [Google Scholar] [CrossRef]
- Baudana, G.; Biamino, S.; Klöden, B.; Kirchner, A.; Weißgärber, T.; Kieback, B.; Pavese, M.; Ugues, D.; Fino, P.; Badini, C. Electron Beam Melting of Ti-48Al-2Nb-0.7Cr-0.3Si: Feasibility investigation. Intermetallics 2016, 73, 43–49. [Google Scholar] [CrossRef]
- Schwerdtfeger, J.; Körner, C. Selective electron beam melting of Ti–48Al–2Nb–2Cr: Microstructure and aluminium loss. Intermetallics 2014, 49, 29–35. [Google Scholar] [CrossRef]
- Wisutmethangoon, S.; Plookphol, T.; Sungkhaphaitoon, P. Production of SAC305 powder by ultrasonic atomization. Powder Technol. 2011, 209, 105–111. [Google Scholar] [CrossRef]
- Sheikhaliev, S.M.; Popel, S.I. Production of metal powders by ultrasonic atomization of melts. Powder Met. Met. Ceram. 1983, 22, 793–798. [Google Scholar] [CrossRef]
- Lierke, E.; Grießhammer, G. The formation of metal powders by ultrasonic atomization of molten metals. Ultrasonics 1967, 5, 224–228. [Google Scholar] [CrossRef]
- Żrodowski, Ł.; Wróblewski, R.; Choma, T.; Morończyk, B.; Ostrysz, M.; Leonowicz, M.; Łacisz, W.; Błyskun, P.; Wróbel, J.; Cieślak, G.; et al. Novel Cold Crucible Ultrasonic Atomization Powder Production Method for 3D Printing. Materials 2021, 14, 2541. [Google Scholar] [CrossRef]
- Alavi, S.H.; Harimkar, S.P. Ultrasonic vibration-assisted laser atomization of stainless steel. Powder Technol. 2017, 321, 89–93. [Google Scholar] [CrossRef]
- Eisenmenger, W. Dynamic properties of the surface tension of water and aqueous solutions of surface active agents with standing capillary waves in the frequency range from 10 kc/s to 1.5 Mc/s. Acta Acust. United Acust. 1959, 9, 327–340. [Google Scholar]
- Söllner, K. The mechanism of the formation of fogs by ultrasonic waves. Trans. Faraday Soc. 1936, 32, 1532–1536. [Google Scholar] [CrossRef]
- Reimann, U.; Pohlman, R. Optimierung der Vernebelung von Flüssigkeiten mit Ultraschall unter Berücksichtigung der Probleme bei höheren Frequenzen. Forsch. Ing. 1976, 42, 1–7. [Google Scholar] [CrossRef]
- Ahsan, M.N.; Pinkerton, A.; Moat, R.; Shackleton, J. A comparative study of laser direct metal deposition characteristics using gas and plasma-atomized Ti–6Al–4V powders. Mater. Sci. Eng. A 2011, 528, 7648–7657. [Google Scholar] [CrossRef]
- Helmer, H.E.; Körner, C.; Singer, R.F. Additive manufacturing of nickel-based superalloy Inconel 718 by selective electron beam melting: Processing window and microstructure. J. Mater. Res. 2014, 29, 1987–1996. [Google Scholar] [CrossRef]
- Iebba, M.; Astarita, A.; Mistretta, D.; Colonna, I.; Liberini, M.; Scherillo, F.; Pirozzi, C.; Borrelli, R.; Franchitti, S.; Squillace, A. Influence of Powder Characteristics on Formation of Porosity in Additive Manufacturing of Ti-6Al-4V Components. J. Mater. Eng. Perform. 2017, 26, 4138–4147. [Google Scholar] [CrossRef]
- ASTM F2924-14(2021). Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium with Powder Bed Fusion; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- Sun, P.; Fang, Z.Z.; Zhang, Y.; Xia, Y. Review of the Methods for Production of Spherical Ti and Ti Alloy Powder. JOM 2017, 69, 1853–1860. [Google Scholar] [CrossRef] [Green Version]
- Rock, C.; Lara-Curzio, E.; Ellis, B.; Ledford, C.; Leonard, D.N.; Kannan, R.; Kirka, M.; Horn, T. Additive Manufacturing of Pure Mo and Mo + TiC MMC Alloy by Electron Beam Powder Bed Fusion. JOM 2020, 72, 4202–4213. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, X.; Tsunoda, K.; Kikuchi, K.; Nomura, N.; Yoshimi, K.; Kawasaki, A. Powder fabrication and laser additive manufacturing of MoSiBTiC alloy. Intermetallics 2019, 104, 33–42. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinrichs, F.; Kauffmann, A.; Schliephake, D.; Seils, S.; Obert, S.; Ratschbacher, K.; Allen, M.; Pundt, A.; Heilmaier, M. Flexible Powder Production for Additive Manufacturing of Refractory Metal-Based Alloys. Metals 2021, 11, 1723. https://doi.org/10.3390/met11111723
Hinrichs F, Kauffmann A, Schliephake D, Seils S, Obert S, Ratschbacher K, Allen M, Pundt A, Heilmaier M. Flexible Powder Production for Additive Manufacturing of Refractory Metal-Based Alloys. Metals. 2021; 11(11):1723. https://doi.org/10.3390/met11111723
Chicago/Turabian StyleHinrichs, Frauke, Alexander Kauffmann, Daniel Schliephake, Sascha Seils, Susanne Obert, Karin Ratschbacher, Melissa Allen, Astrid Pundt, and Martin Heilmaier. 2021. "Flexible Powder Production for Additive Manufacturing of Refractory Metal-Based Alloys" Metals 11, no. 11: 1723. https://doi.org/10.3390/met11111723
APA StyleHinrichs, F., Kauffmann, A., Schliephake, D., Seils, S., Obert, S., Ratschbacher, K., Allen, M., Pundt, A., & Heilmaier, M. (2021). Flexible Powder Production for Additive Manufacturing of Refractory Metal-Based Alloys. Metals, 11(11), 1723. https://doi.org/10.3390/met11111723









