A Novel Mg–CaMgSn Master Alloy for Grain Refinement in Mg–Al-Based Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
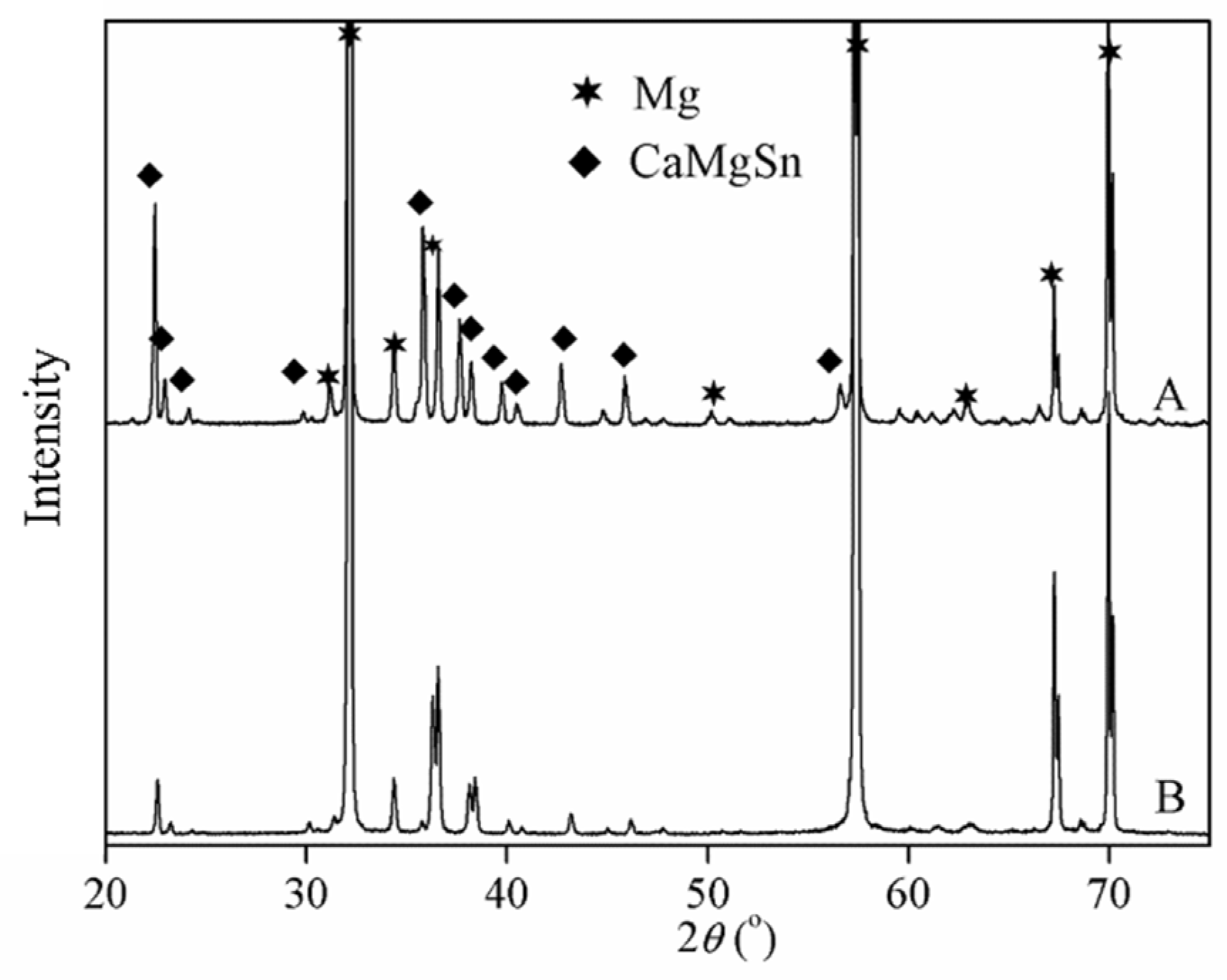
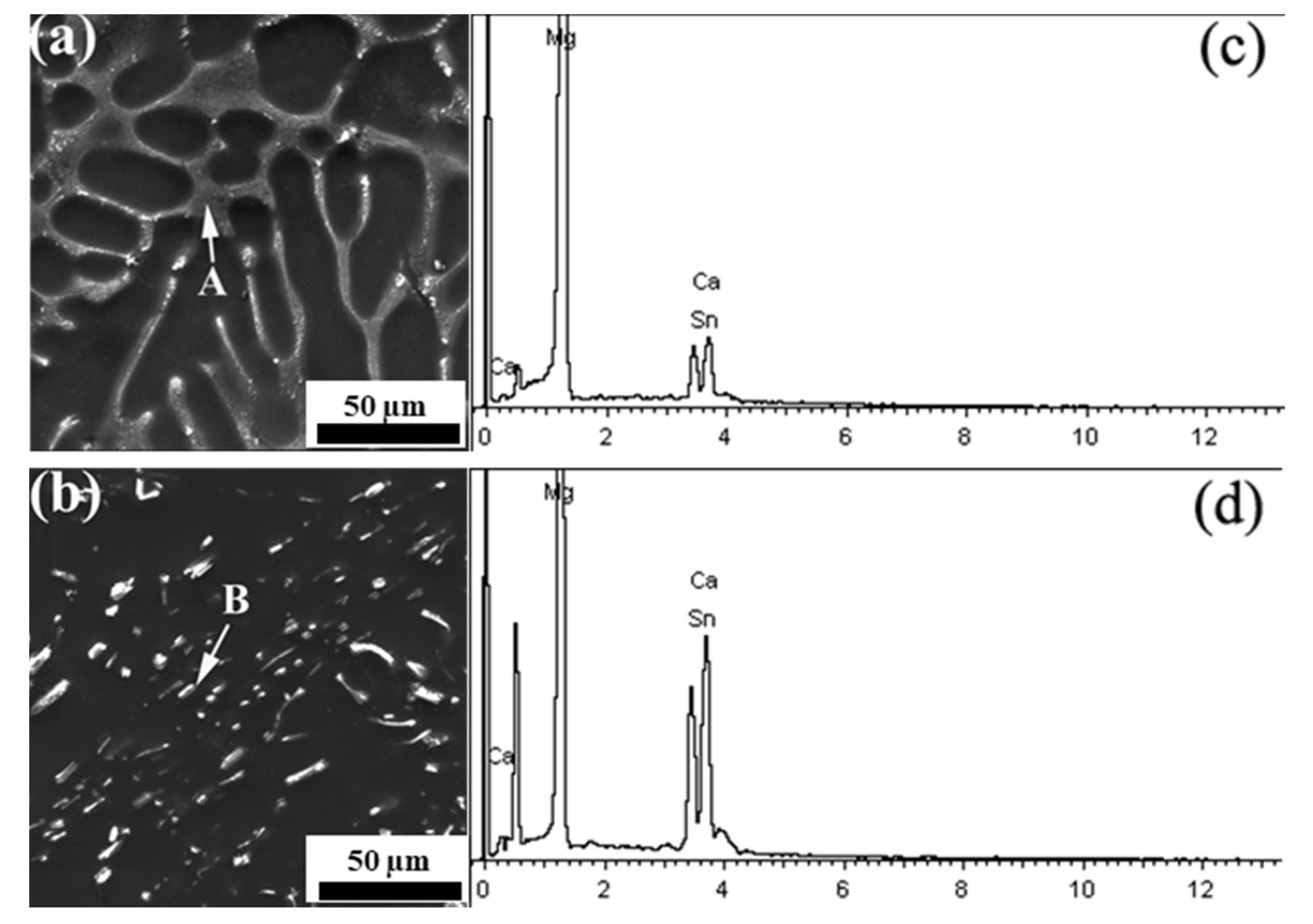
3.1. Characterization of Mg–3CaMgSn Master Alloy
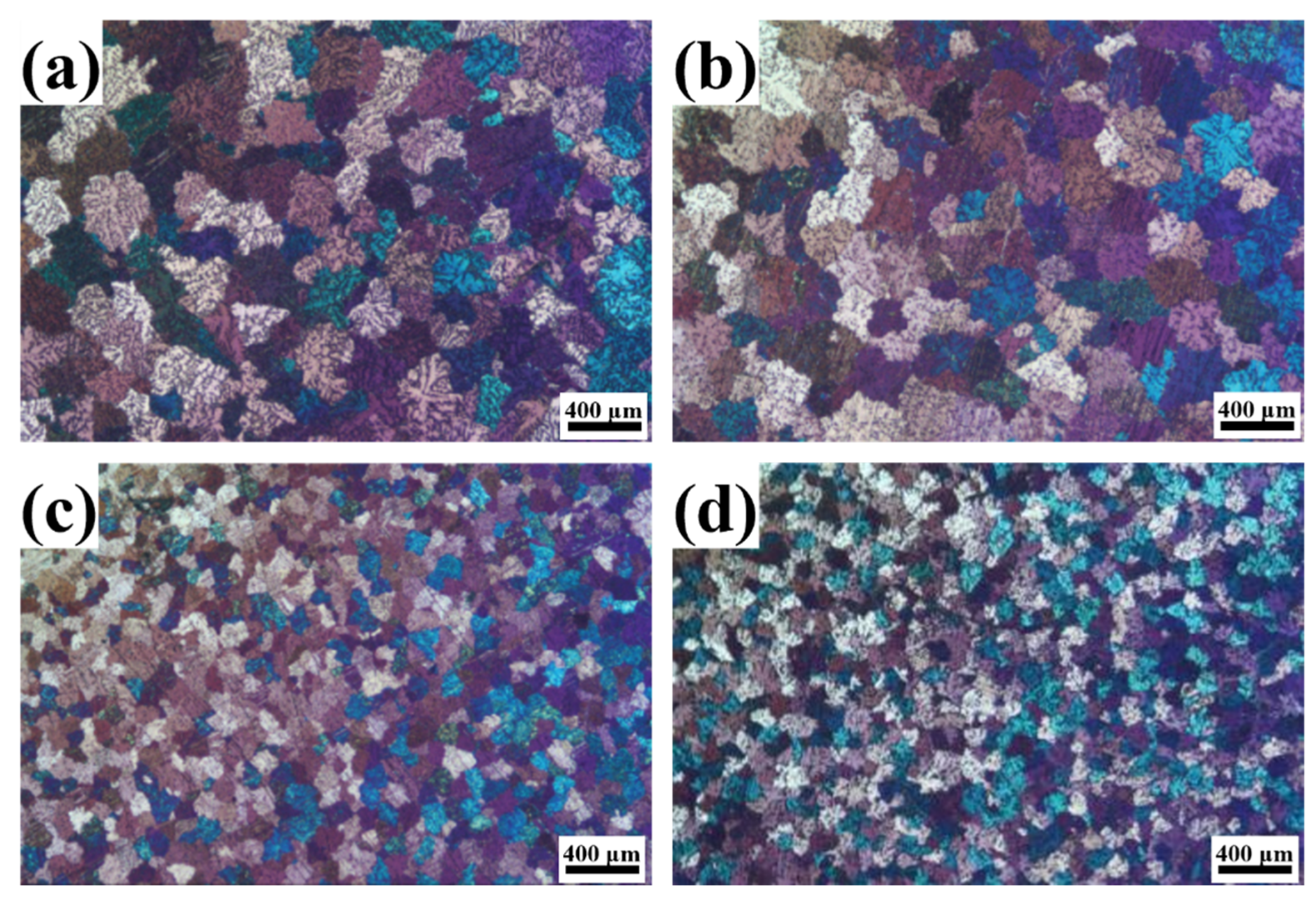
3.2. Grain Refinement Effect
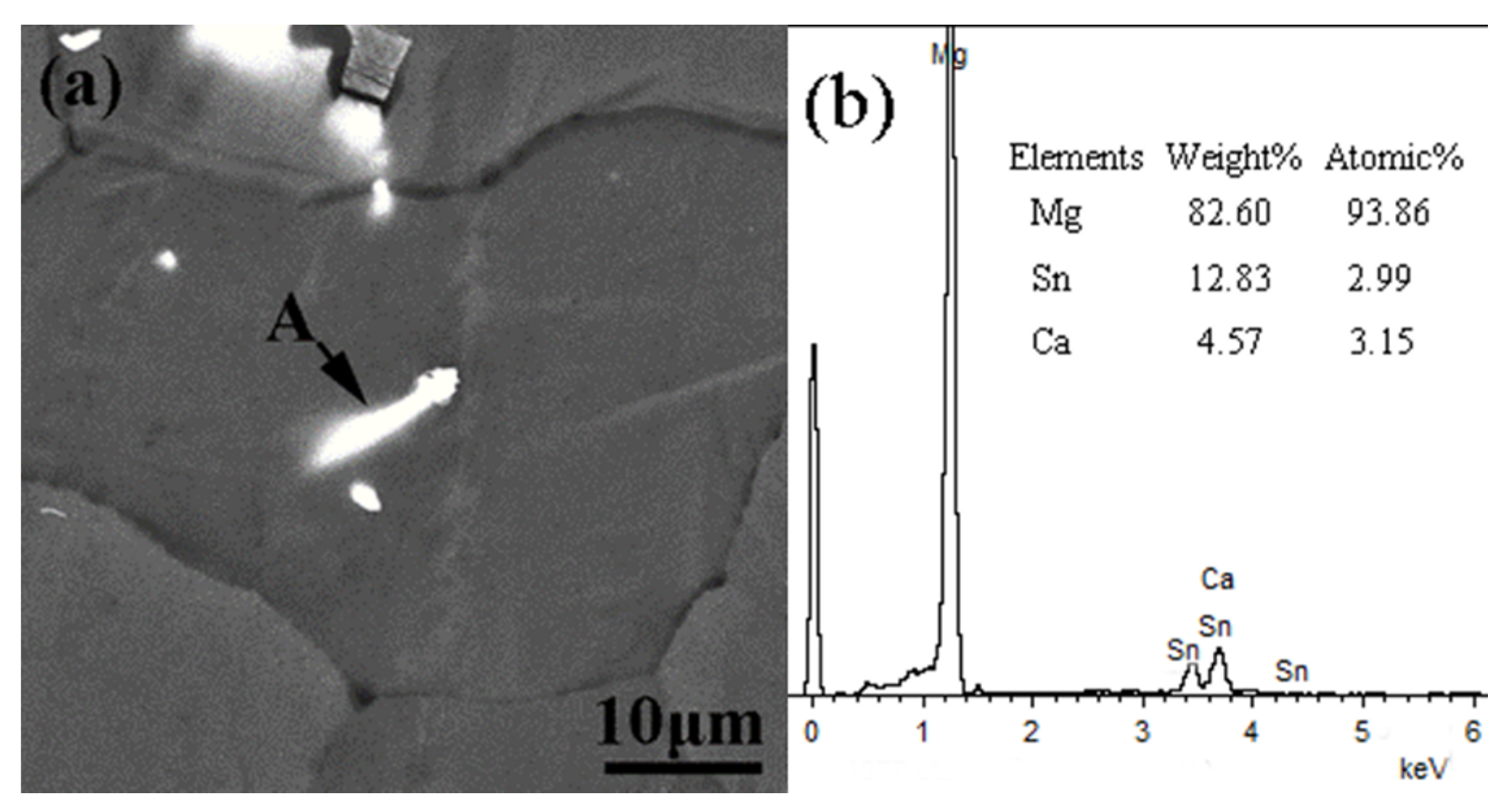
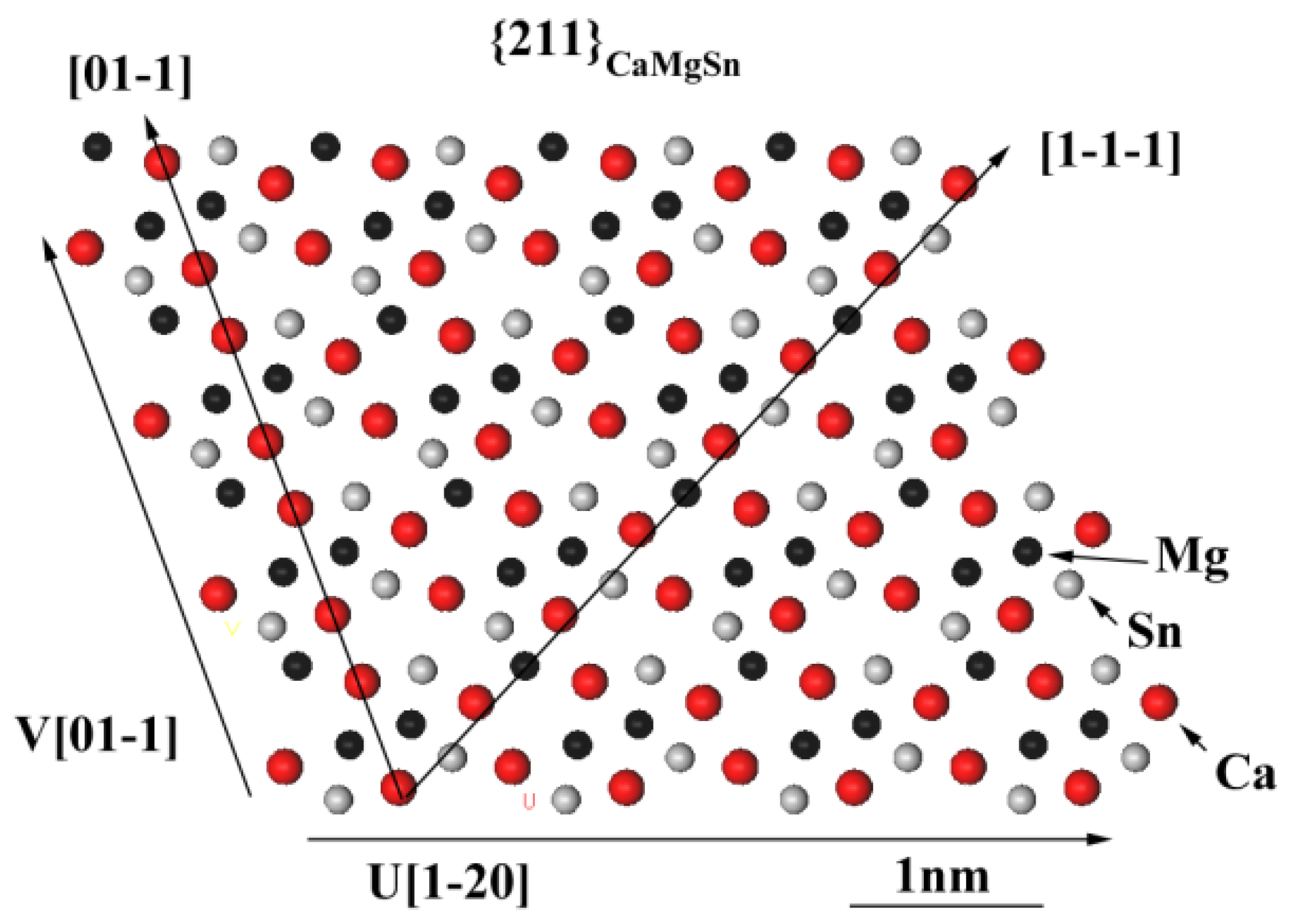
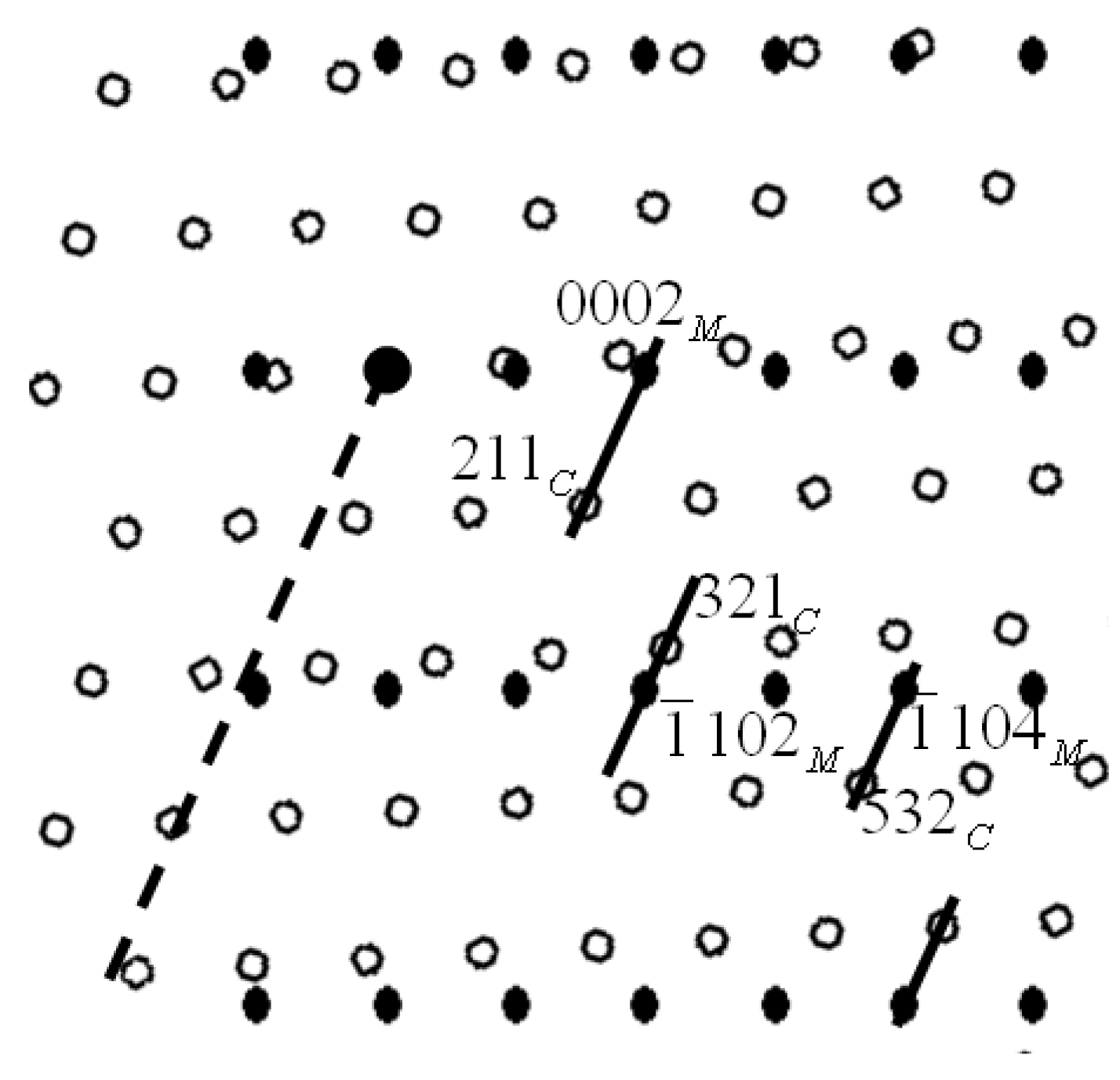
3.3. Grain Refinement Mechanism
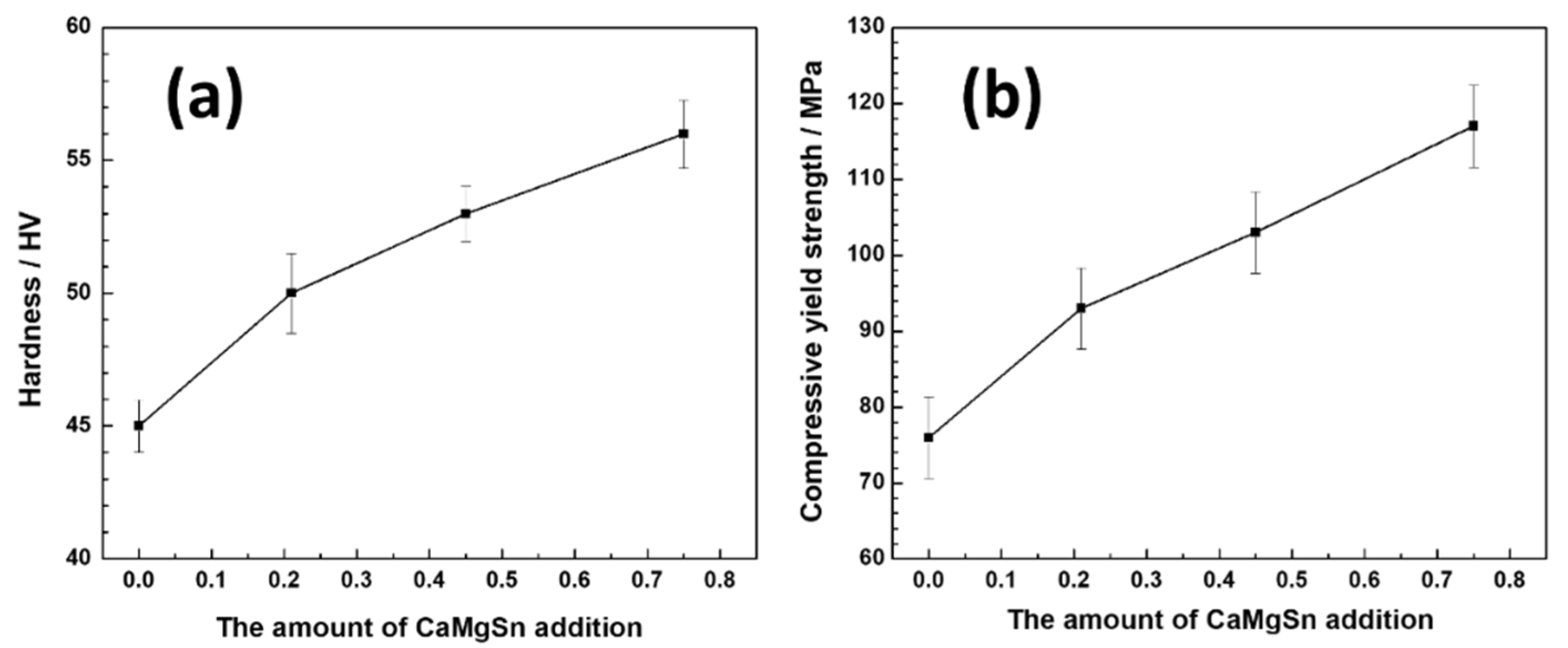
3.4. Hardness and Mechanical Properties
4. Conclusions
- (1)
- The addition of Mg–3CaMgSn master alloy provided significant grain refining efficiency for AZ31. With the addition of 0.45 wt.% CaMgSn particles, the grain size was reduced from 270 to 93 µm in as-cast AZ31 alloy samples;
- (2)
- The added CaMgSn particles survived in molten Mg and acted as heterogeneous nucleation cites for α-Mg during solidification;
- (3)
- The crystallographic matching relationship between CaMgSn and α-Mg was evaluated by an edge-to-edge matching model. Based on the calculated interatomic spacing misfits along the matching planes, it was concluded that the {211}CaMgSn plane of CaMgSn potentially matched the Mg plane of the Mg matrix for the heterogeneous nucleation cites of α-Mg.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloys 2013, 1, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Luo, A.A. Magnesium: Current and potential automotive applications. JOM 2002, 54, 42–48. [Google Scholar] [CrossRef]
- Taub, A.; De Moor, E.; Luo, A.; Matlock, D.K.; Speer, J.G.; Vaidya, U. Materials for Automotive Lightweighting. Annu. Rev. Mater. Res. 2019, 49, 327–359. [Google Scholar] [CrossRef]
- Zhang, J.; Jian, Y.; Zhao, X.; Meng, D.; Pan, F.; Han, Q. The tribological behavior of a surface-nanocrystallized magnesium alloy AZ31 sheet after ultrasonic shot peening treatment. J. Magnes. Alloys 2020, 9, 1187–1200. [Google Scholar] [CrossRef]
- Ge, M.Z.; Xiang, J.Y.; Tang, Y.; Ye, X.; Fan, Z.; Lu, Y.L.; Zhang, X.H. Wear behavior of Mg-3Al-1Zn alloy subjected to laser shock peening. Surf. Coat. Technol. 2018, 337, 501–509. [Google Scholar] [CrossRef]
- Gopi, K.R.; Shivananda Nayaka, H. Tribological and corrosion properties of AM70 magnesium alloy processed by equal channel angular pressing. J. Mater. Res. 2017, 32, 2153–2160. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, P.; She, J.; Jiang, B.; Tang, A.; Pan, F.; Han, Q. A study of the corrosion behavior of AZ31 Mg alloy in depth direction after surface nanocrystallization. Surf. Coat. Technol. 2020, 396, 125968. [Google Scholar] [CrossRef]
- Op’t Hoog, C.; Birbilis, N.; Estrin, Y. Corrosion of pure Mg as a function of grain size and processing route. Adv. Eng. Mater. 2008, 10, 579–582. [Google Scholar] [CrossRef]
- Yang, J.; Peng, J.; Nyberg, E.A.; Pan, F.S. Effect of Ca addition on the corrosion behavior of Mg-Al-Mn alloy. Appl. Surf. Sci. 2016, 369, 92–100. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, J.H.; Huang, Y.; Pruncu, C.; Jiang, J. Evolution of twinning and shear bands in magnesium alloys during rolling at room and cryogenic temperature. Mater. Des. 2020, 193, 108793. [Google Scholar] [CrossRef]
- Luo, A. Processing, microstructure, and mechanical behavior of cast magnesium metal matrix composites. Metall. Mater. Trans. A 1995, 26, 2445–2455. [Google Scholar] [CrossRef]
- Luo, A. Heterogeneous and Grain Refinement in Cast Mg (AZ9 l)/SiC $ Metal Matrix Composites. Can. Metall. Q. 1996, 35, 375–383. [Google Scholar] [CrossRef]
- Fu, H.M.; Zhang, M.X.; Qiu, D.; Kelly, P.M.; Taylor, J.A. Grain refinement by AlN particles in Mg-Al based alloys. J. Alloys Compd. 2009, 478, 809–812. [Google Scholar] [CrossRef]
- Yano, E.; Tamura, Y.; Motegi, T.; Sato, E. Effect of carbon powder on grain refinement of an AZ91E magnesium alloy. Mater. Trans. 2003, 44, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Chen, Y.; Han, H. Grain refinement of AZ91D magnesium alloy by a new Mg-50%Al4C3 master alloy. J. Alloys Compd. 2015, 624, 266–269. [Google Scholar] [CrossRef]
- Lu, L.; Dahle, A.K.; Stjohn, D.H. Grain refinement efficiency and mechanism of aluminium carbide in Mg-Al alloys. Scr. Mater. 2005, 53, 517–522. [Google Scholar] [CrossRef]
- Fu, H.M.; Qiu, D.; Zhang, M.X.; Wang, H.; Kelly, P.M.; Taylor, J.A. The development of a new grain refiner for magnesium alloys using the edge-to-edge model. J. Alloys Compd. 2008, 456, 390–394. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, W.; Qiu, D.; Zhang, M.X.; Pan, F. Grain refinement of Ca addition in a twin-roll-cast Mg-3Al-1Zn alloy. Mater. Chem. Phys. 2012, 133, 611–616. [Google Scholar] [CrossRef]
- Jiang, Z.T.; Jiang, B.; Zhang, J.Y.; Dai, J.H.; Yang, Q.S.; Qin, Y.A.; Pan, F.S. Effect of Al2Ca intermetallic compound addition on grain refinement of AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 1284–1293. [Google Scholar] [CrossRef]
- Chang, H.W.; Qiu, D.; Taylor, J.A.; Easton, M.A.; Zhang, M.X. The role of Al2Y in grain refinement in Mg-Al-Y alloy system. J. Magnes. Alloys 2013, 1, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Nayyeri, G.; Mahmudi, R.; Salehi, F. The microstructure, creep resistance, and high-temperature mechanical properties of Mg-5Sn alloy with Ca and Sb additions, and aging treatment. Mater. Sci. Eng. A 2010, 527, 5353–5359. [Google Scholar] [CrossRef]
- Nayyeri, G.; Mahmudi, R. Effects of Ca additions on the microstructural stability and mechanical properties of Mg-5%Sn alloy. Mater. Des. 2011, 32, 1571–1576. [Google Scholar] [CrossRef]
- Li, W.; Huang, X.; Huang, W. Effects of Ca, Ag addition on the microstructure and age-hardening behavior of a Mg-7Sn (wt%) alloy. Mater. Sci. Eng. A 2017, 692, 75–80. [Google Scholar] [CrossRef]
- Baghani, A.; Khalilpour, H.; Miresmaeili, S.M. Microstructural evolution and creep properties of Mg-4Sn alloys by addition of calcium up to 4 wt.%. Trans. Nonferrous Met. Soc. China 2020, 30, 896–904. [Google Scholar] [CrossRef]
- Hort, N.; Huang, Y.; Leil, T.A.; Maier, P.; Kainer, K.U. Microstructural investigations of the Mg-Sn-xCa system. Adv. Eng. Mater. 2006, 8, 359–364. [Google Scholar] [CrossRef]
- Kozlov, A.; Ohno, M.; Arroyave, R.; Liu, Z.K.; Schmid-Fetzer, R. Phase equilibria, thermodynamics and solidification microstructures of Mg-Sn-Ca alloys, Part 1: Experimental investigation and thermodynamic modeling of the ternary Mg-Sn-Ca system. Intermetallics 2008, 16, 299–315. [Google Scholar] [CrossRef]
- Ali, Y.; Qiu, D.; Jiang, B.; Pan, F.; Zhang, M. Current research progress in grain refinement of cast magnesium alloys: A review article. J. Alloys Compd. 2015, 619, 639–651. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, L.; Pan, F. Comparison about effects of Ce, Sn and Gd additions on as-cast microstructure and mechanical properties of Mg-3.8Zn-2.2Ca (wt%) magnesium alloy. J. Mater. Sci. 2009, 44, 4577–4586. [Google Scholar] [CrossRef]
- Chen, T.J.; Wang, R.Q.; Huang, H.J.; Ma, Y.; Hao, Y. Grain refining technique of AM60B magnesium alloy by MgCO3. Trans. Nonferrous Met. Soc. China 2012, 22, 1533–1539. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Easton, M.A.; Taylor, J.A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model. Acta Mater. 2005, 53, 1427–1438. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Qian, M.; Taylor, J.A. Crystallography of grain refinement in Mg-Al based alloys. Acta Mater. 2005, 53, 3261–3270. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Crystallographic features of phase transformations in solids. Prog. Mater. Sci. 2009, 54, 1101–1170. [Google Scholar] [CrossRef]
- ICDD. JCPDS International Centre for Diffraction Data; PCPDFWIN v. 2.3; ICDD: Swarthmore, PA, USA, 2002. [Google Scholar]
- Zhang, M.X.; Kelly, P.M. Edge-to-edge matching and its applications: Part II. Application to Mg-Al, Mg-Y and Mg-Mn alloys. Acta Mater. 2005, 53, 1085–1096. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Edge-to-edge matching model for predicting orientation relationships and habit planes—The improvements. Scr. Mater. 2005, 52, 963–968. [Google Scholar] [CrossRef]
- Suresh, M.; Srinivasan, A.; Ravi, K.R.; Pillai, U.T.; Pai, B.C. Influence of boron addition on the grain refinement and mechanical properties of AZ91 Mg alloy. Mater. Sci. Eng. A 2009, 525, 207–210. [Google Scholar] [CrossRef]
- Chen, T.J.; Wang, R.Q.; Ma, Y.; Hao, Y. Grain refinement of AZ91D magnesium alloy by Al–Ti–B master alloy and its effect on mechanical properties. Mater. Des. 2012, 34, 637–648. [Google Scholar] [CrossRef]
- Saha, S.; Ravindran, C. Effects of zinc oxide addition on the microstructure and mechanical properties of AZ91E Mg alloy. Int. J. Met. 2015, 9, 39–48. [Google Scholar] [CrossRef]

| Master Alloy | Ca | Sn | Mg |
|---|---|---|---|
| Mg–3CaMgSn | 0.61 | 1.88 | Bal. |
| Potential Matching Plane | fd/% | Potential Matching Plane | fd/% | Potential Matching Plane | fd/% |
|---|---|---|---|---|---|
| 9.1 | 15.9 | 2.3 | |||
| 46.6 | 55.8 | 37.5 | |||
| 39.9 | 48.2 | 31.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhou, G.; Jiang, B.; Luo, A.; Zhao, X.; Tang, A.; Pan, F. A Novel Mg–CaMgSn Master Alloy for Grain Refinement in Mg–Al-Based Alloys. Metals 2021, 11, 1722. https://doi.org/10.3390/met11111722
Zhang J, Zhou G, Jiang B, Luo A, Zhao X, Tang A, Pan F. A Novel Mg–CaMgSn Master Alloy for Grain Refinement in Mg–Al-Based Alloys. Metals. 2021; 11(11):1722. https://doi.org/10.3390/met11111722
Chicago/Turabian StyleZhang, Jianyue, Guanyu Zhou, Bin Jiang, Alan Luo, Xuzhe Zhao, Aitao Tang, and Fusheng Pan. 2021. "A Novel Mg–CaMgSn Master Alloy for Grain Refinement in Mg–Al-Based Alloys" Metals 11, no. 11: 1722. https://doi.org/10.3390/met11111722
APA StyleZhang, J., Zhou, G., Jiang, B., Luo, A., Zhao, X., Tang, A., & Pan, F. (2021). A Novel Mg–CaMgSn Master Alloy for Grain Refinement in Mg–Al-Based Alloys. Metals, 11(11), 1722. https://doi.org/10.3390/met11111722







