Influence of Nb on the Structure and High Temperature Performance of Billet for High-Rise Structural Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Microstructure Sample Preparation
- (1)
- The samples were polished using #120 and #2000 sandpaper. The sample surfaces were kept level during the polishing process. Finally, a polishing machine was used to polish the samples until the surfaces of the samples were bright and scratch-free.
- (2)
- After polishing, the remaining polishing agent was immediately rinsed off the surfaces of the samples with clean water, and the surfaces of the samples were rinsed with alcohol and quickly dried with a hair dryer.
- (3)
- Finally, a 4% nitric acid alcohol solution was used to corrode each sample for 1–3 s. After the corrosion was completed, the residual nitric acid alcohol solution was immediately rinsed away with clean water, and then the sample surfaces were rinsed with alcohol. A metallurgical microscope was used to observe the microstructures of the samples.
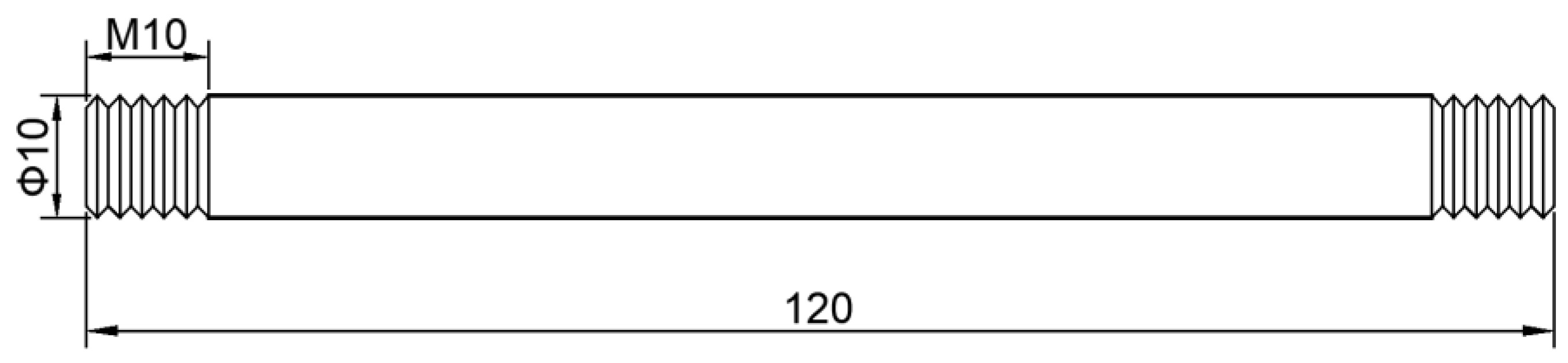
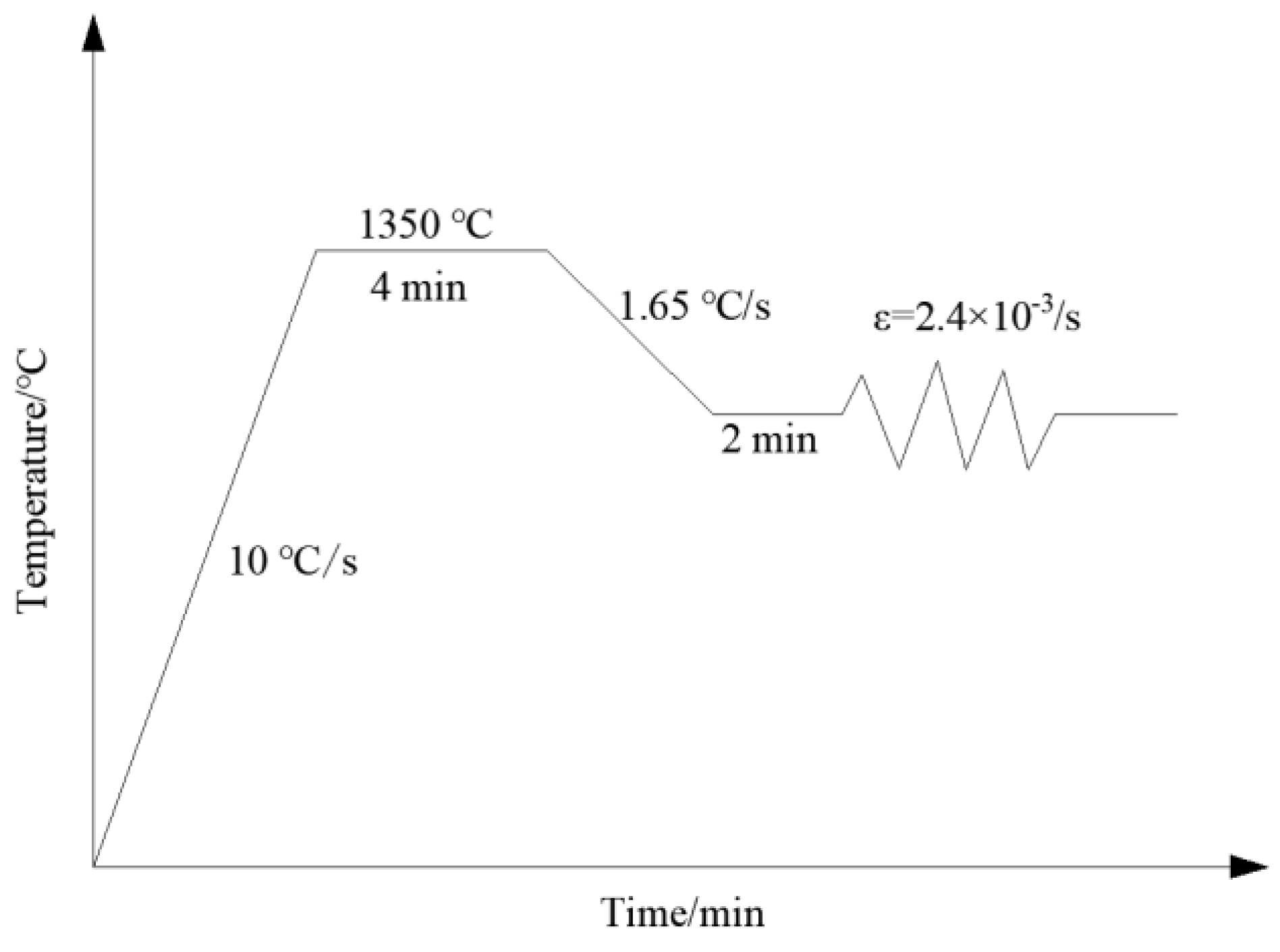
2.2. High Temperature Performance Sample Preparation
2.3. Precipitate Sample Preparation
3. Results and Discussion
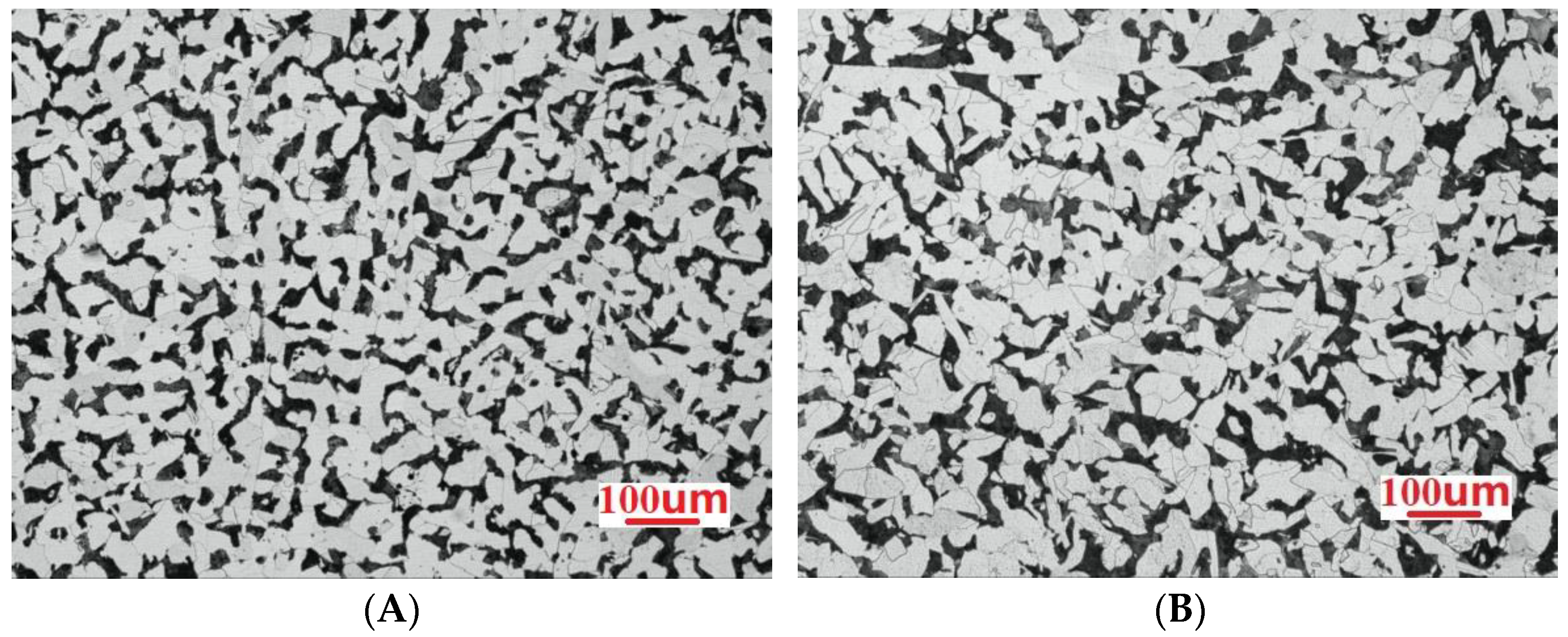
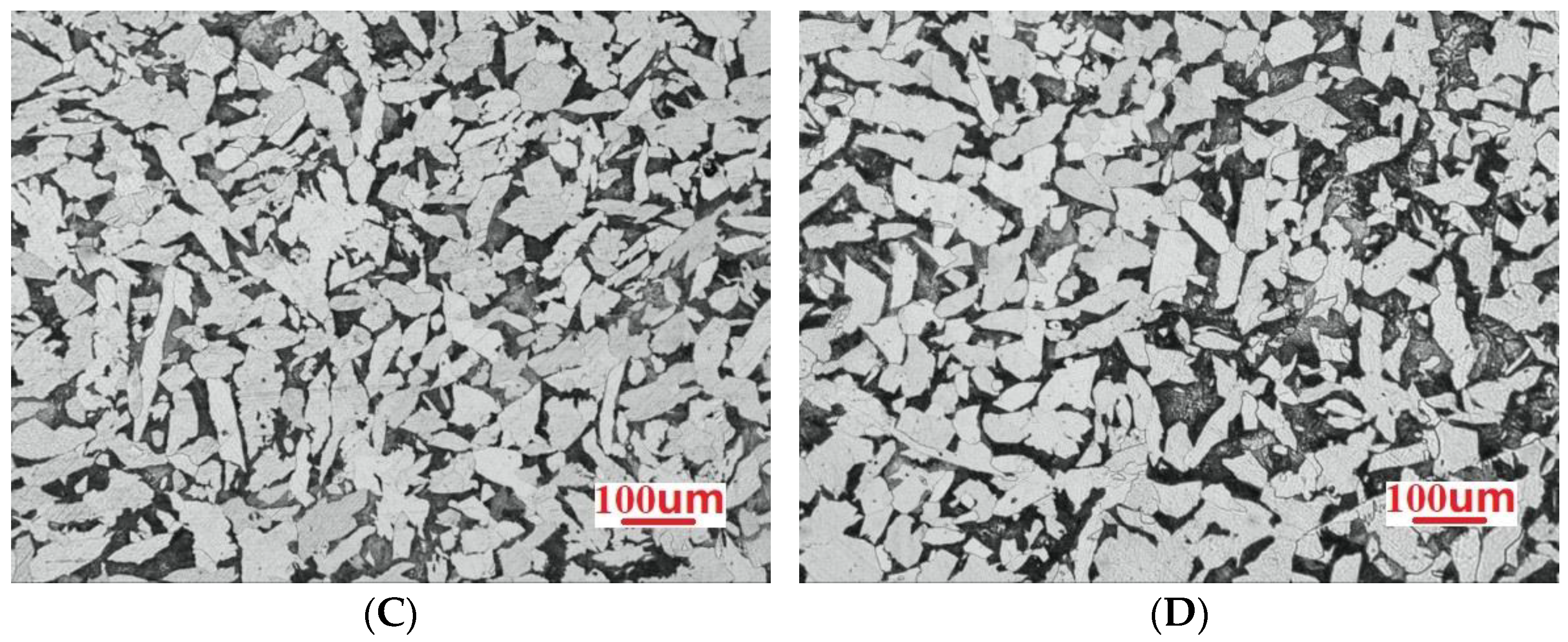
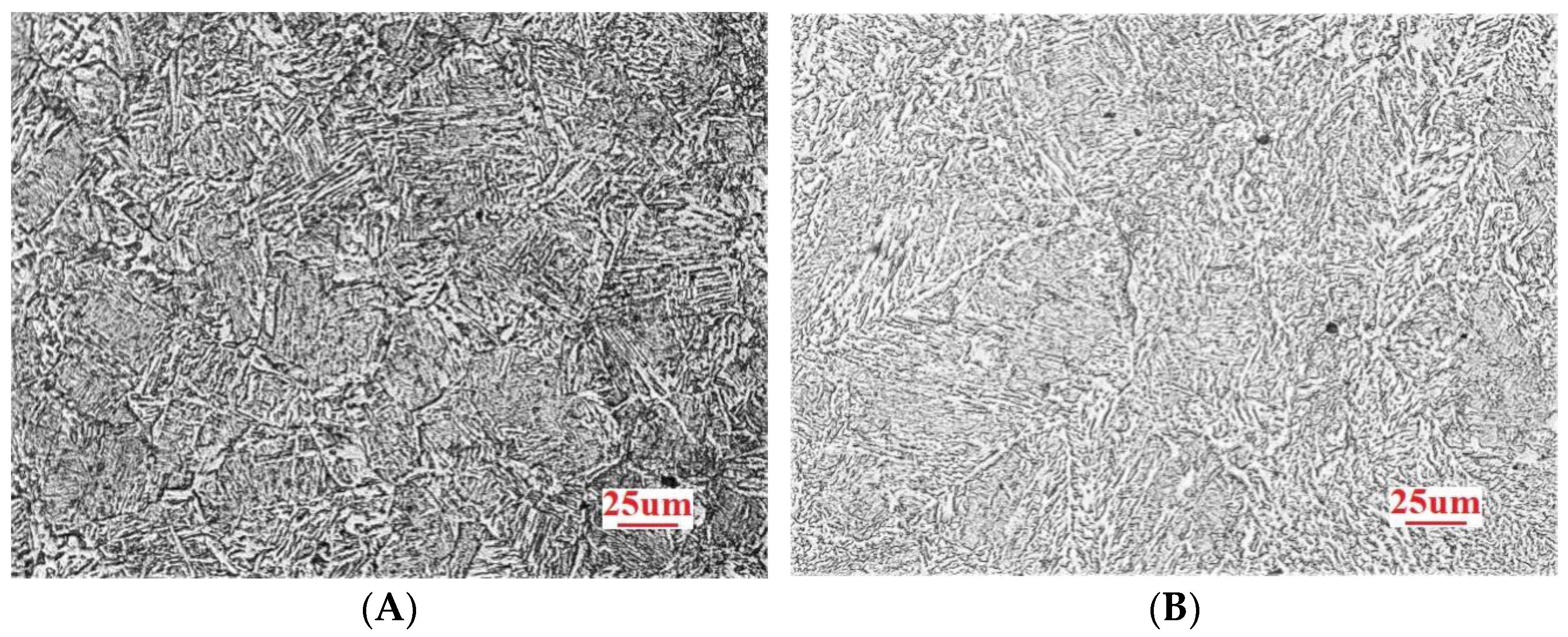
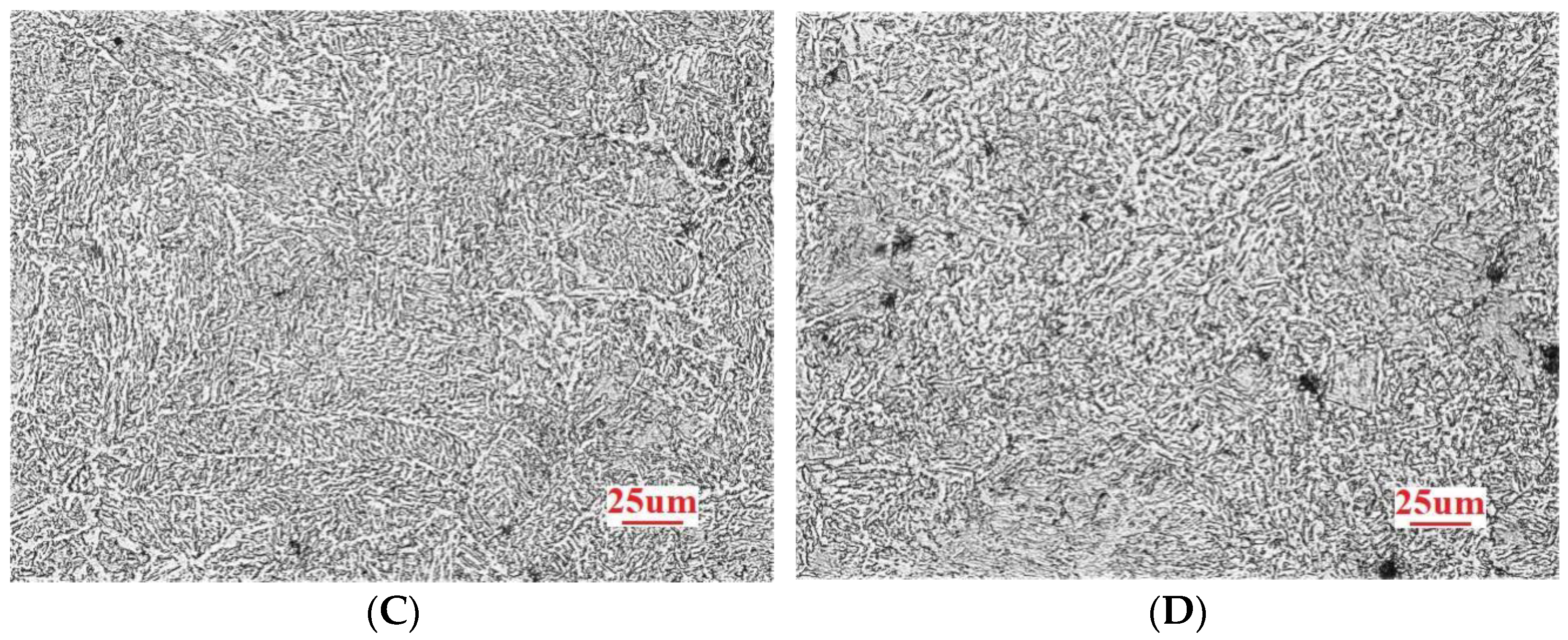
3.1. Microstructure Analysis
3.2. High Temperature Performance Analysis of Test Steel
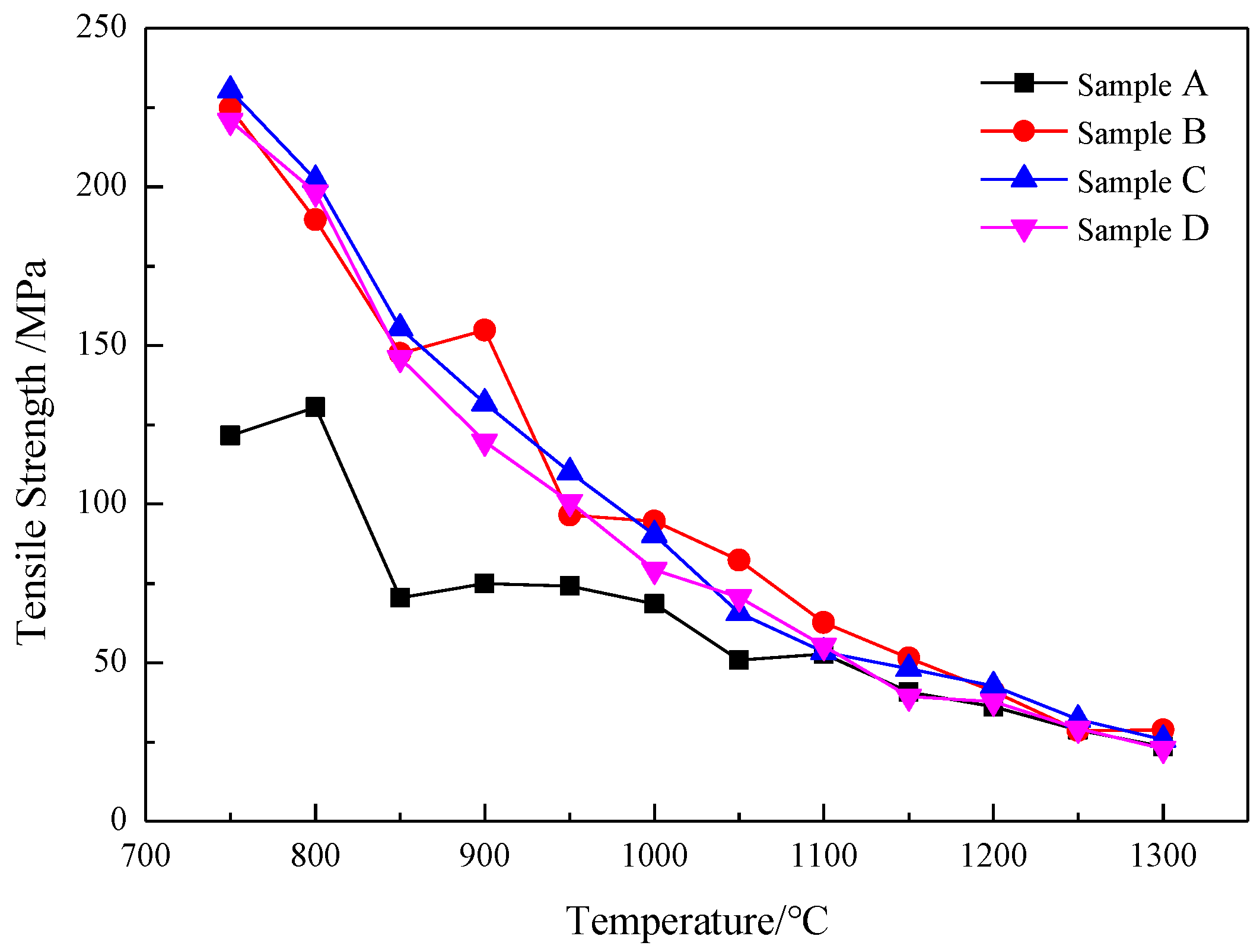
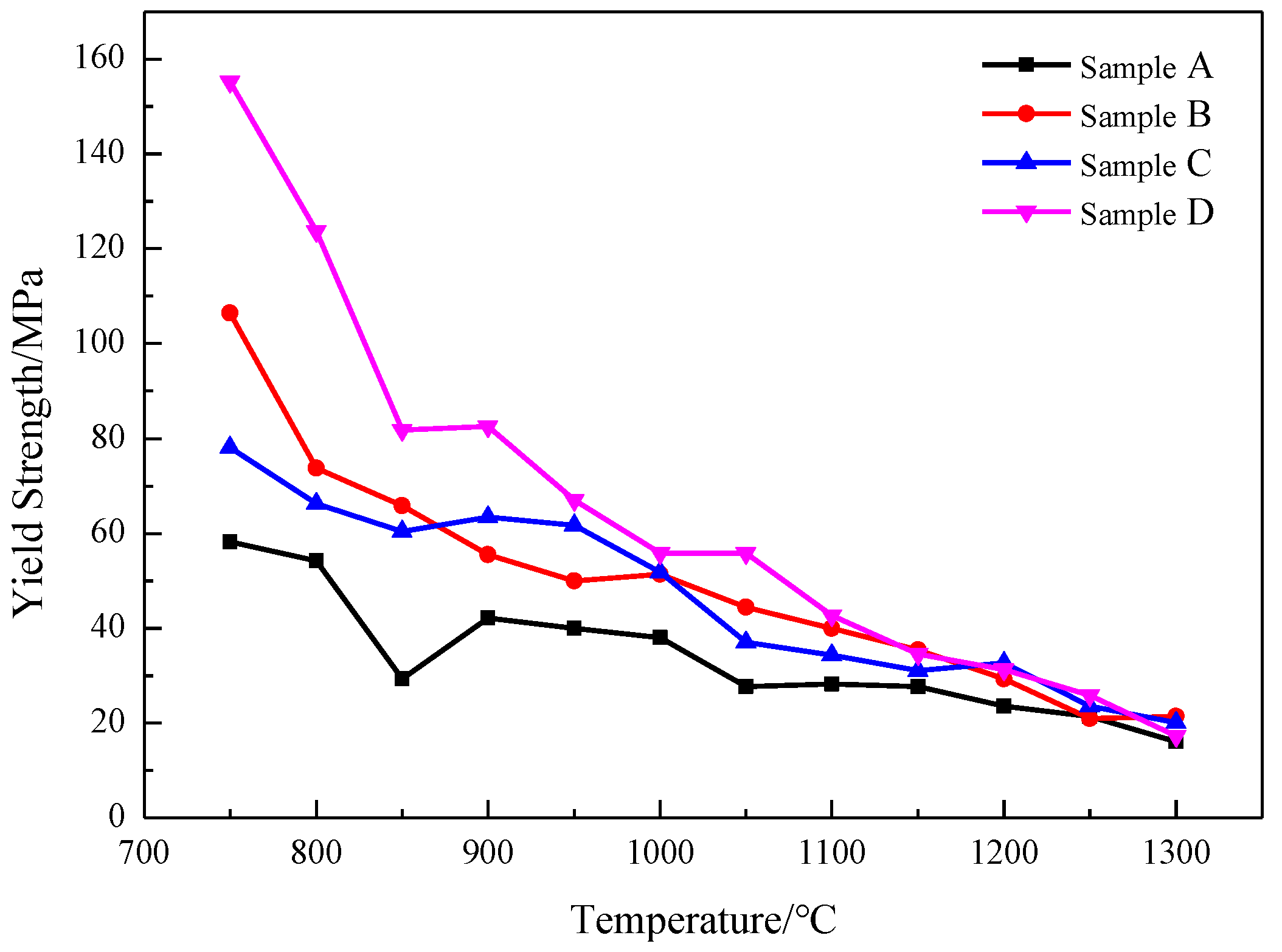
3.2.1. High Temperature Strength Curve Analysis
- (1)
- Tensile strength curve
- (2)
- Yield strength curve
3.2.2. High Temperature Thermoplastic
3.2.3. High Temperature Plastic Modulus Curve Analysis
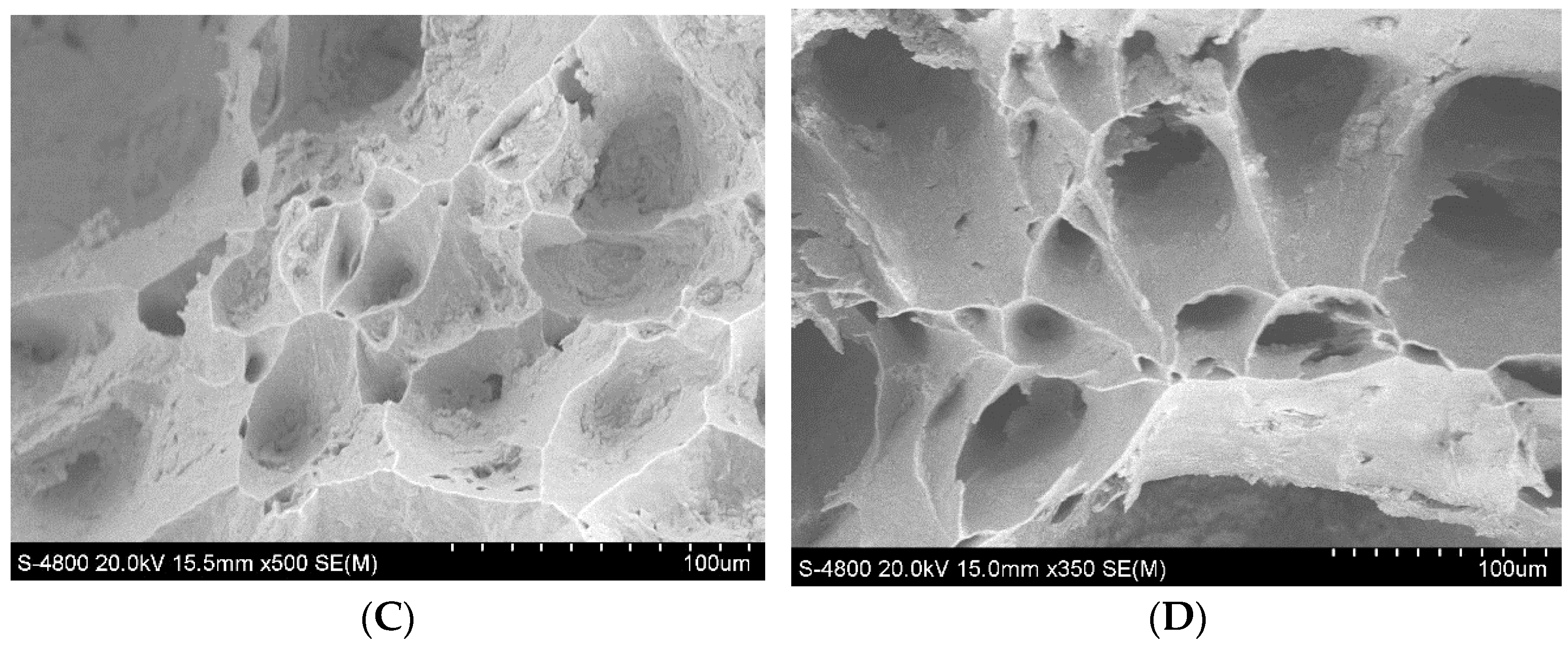
3.3. Analysis of Typical Fracture and Precipitate Morphology
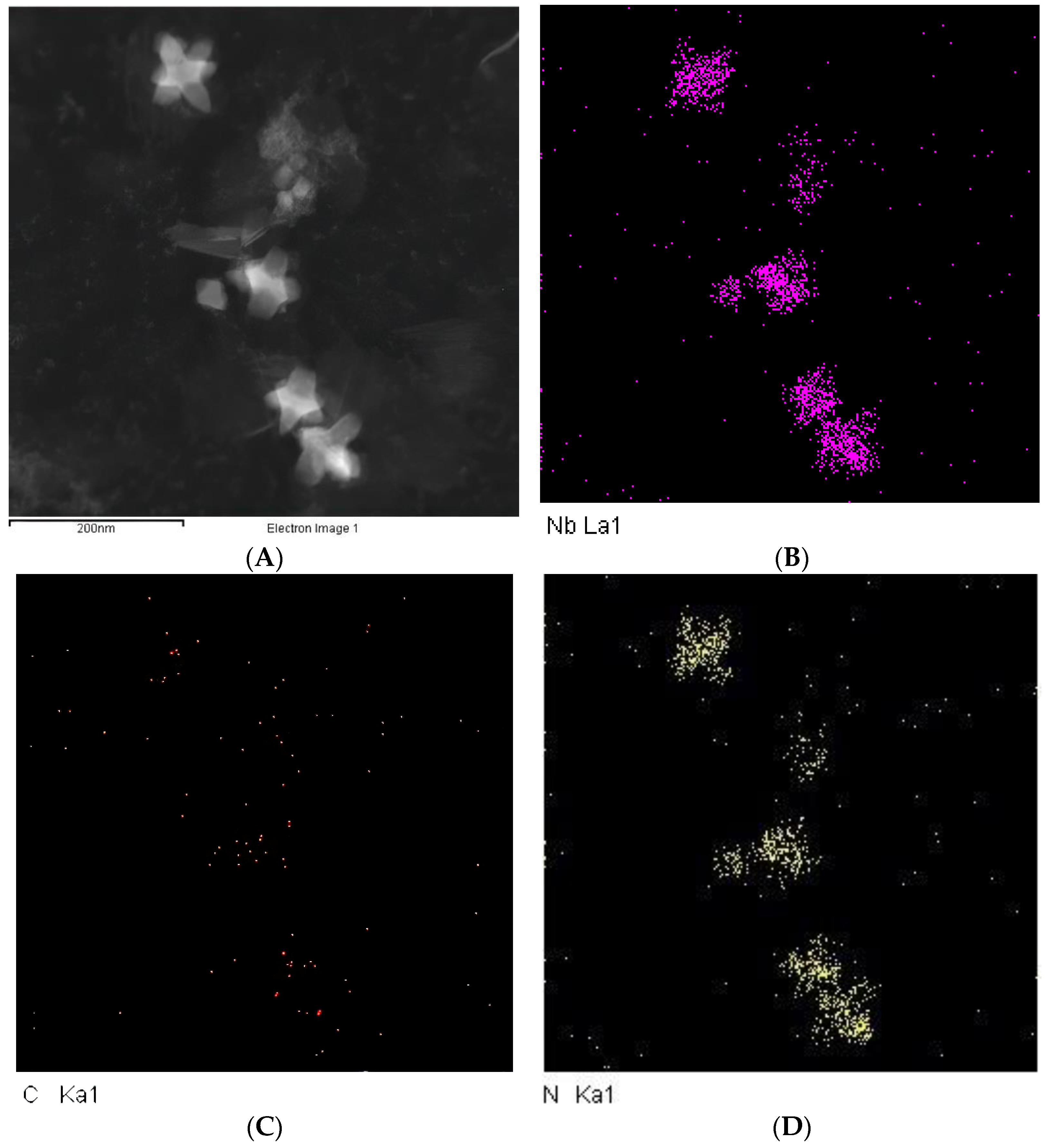
4. Analysis of Typical Precipitates
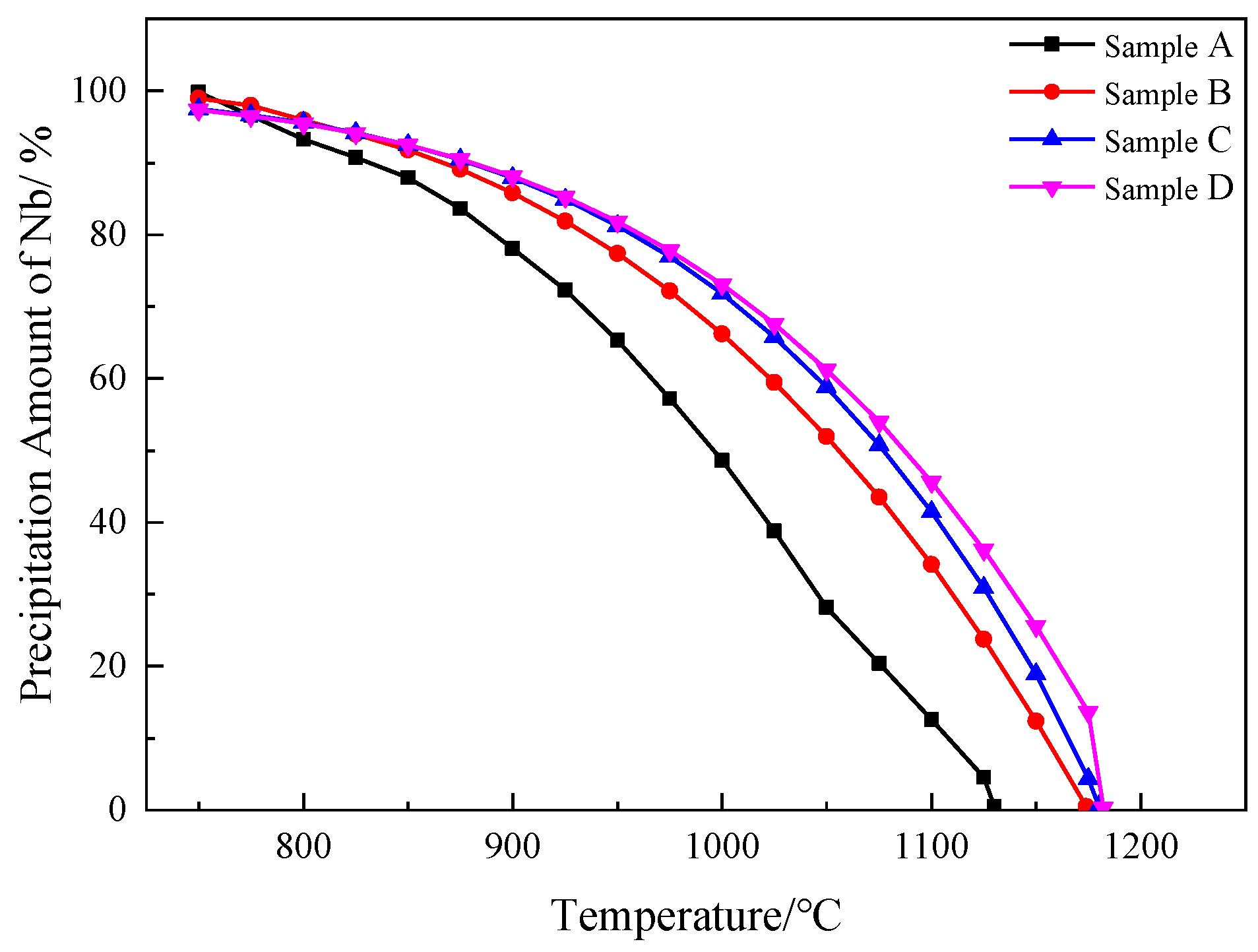
4.1. Thermodynamic Calculation of Nb Precipitation
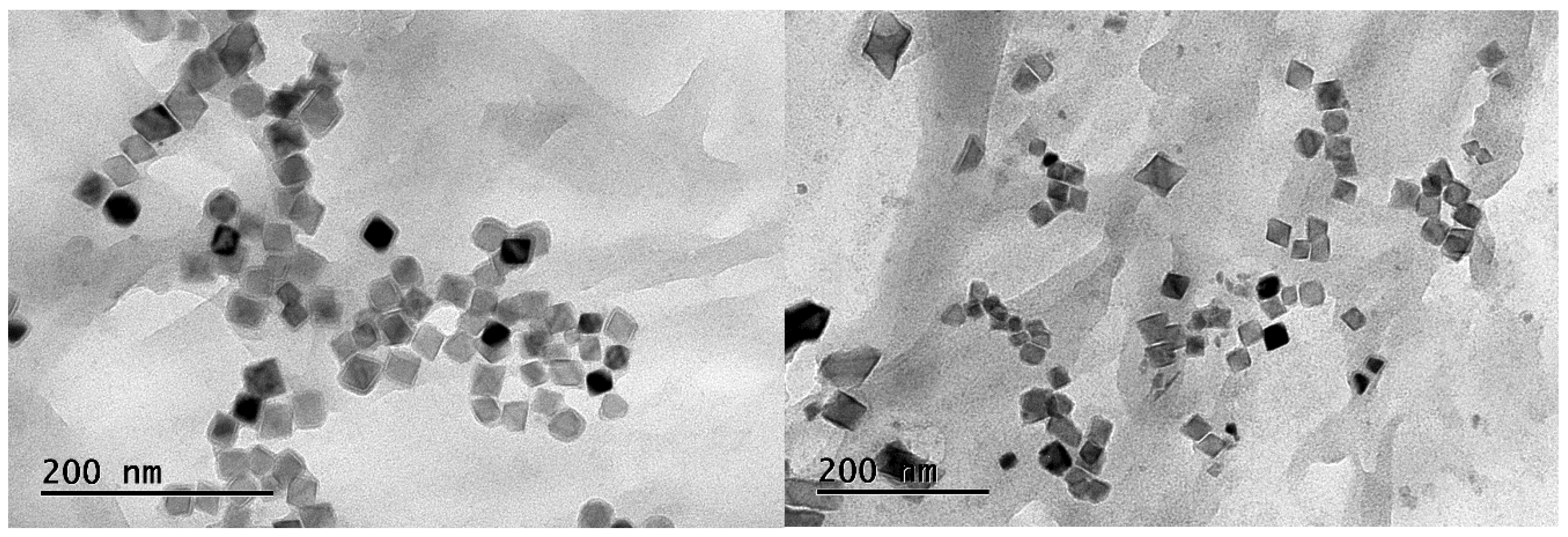
4.2. Analysis of Precipitated Phase Precipitates
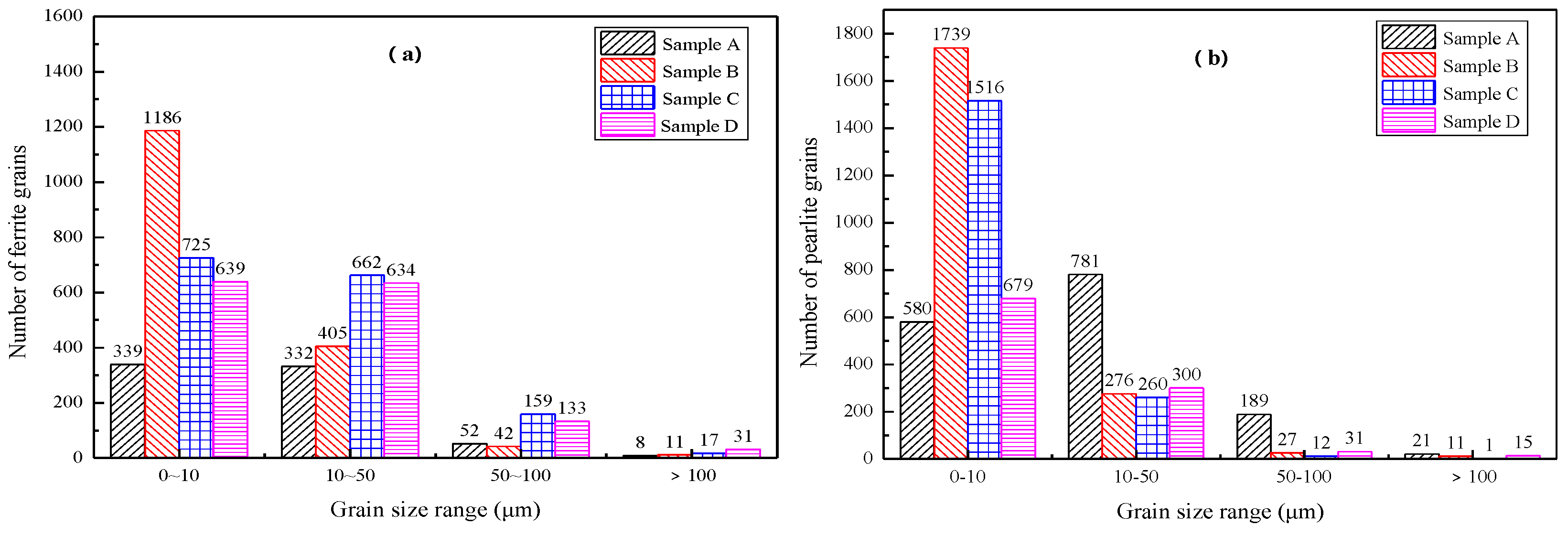
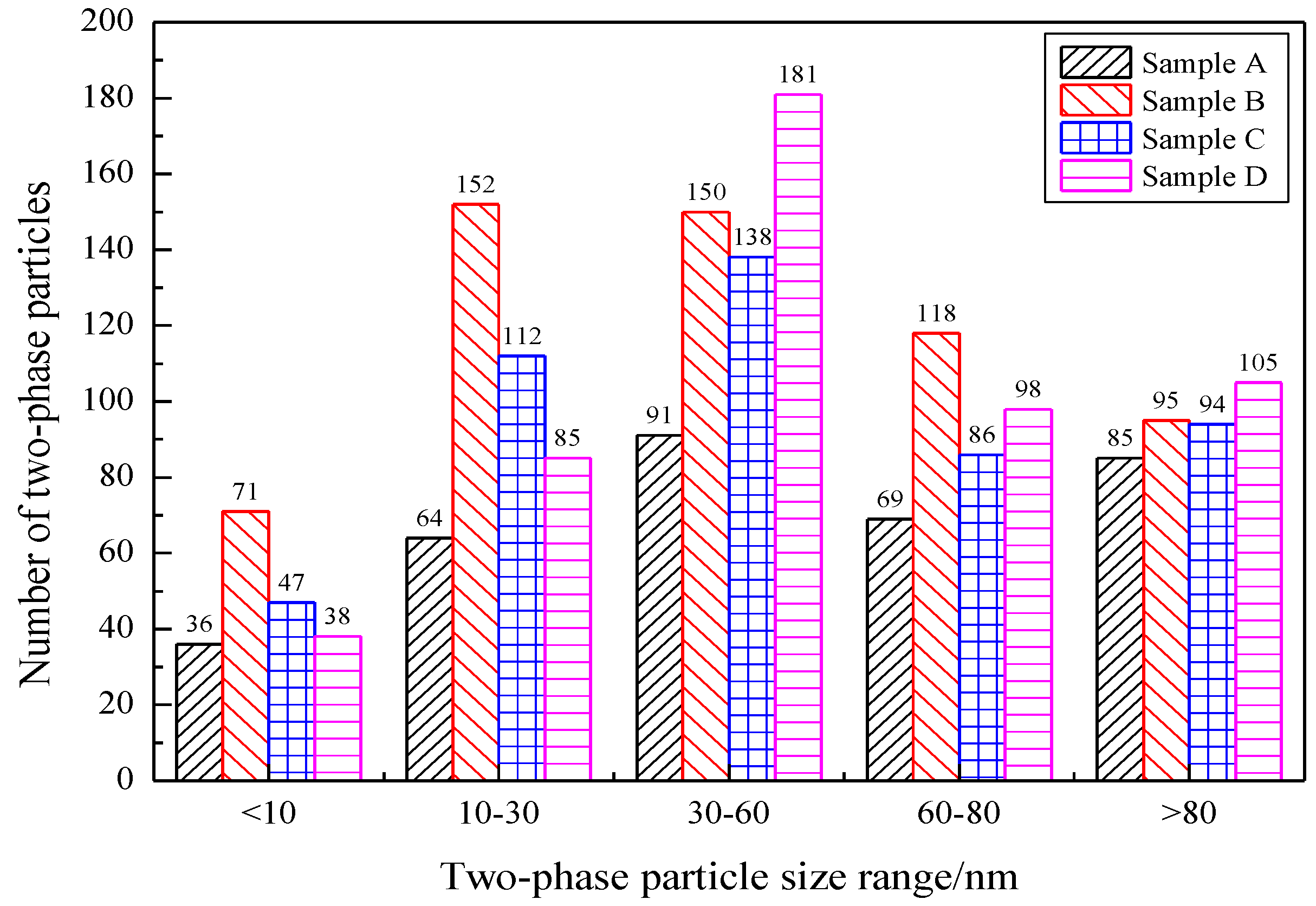
4.3. Size Distribution of Nb-Containing Two-Phase Particles in the Samples
5. Conclusions
- (1)
- Among the high-rise structural steel samples containing different amounts of Nb, the microstructures were observed to be the smallest, the distribution of the microstructures was observed to be more uniform, and the best high-temperature strength performance was obtained when the Nb content was 0.031%. In this case, the average size of ferrite was observed to be 17.36 μm. When the Nb content was further increased to 0.05%, the microstructure of the steel began to become coarse.
- (2)
- The high plastic temperature range of high-rise structural steel is from 1300 °C to 900 °C. At 850 °C, the reduction in the area of the four samples A–D reaches its minimum values, which were 55.85%, 58.34%, 62.91%, and 61.09%, respectively. At 850 ℃, the formation of pro-eutectoid ferrite and the precipitation of precipitates were the main reasons for the fracture of the sample.
- (3)
- From thermodynamic calculations, we found that almost all of the Nb compounds precipitated in the four samples at 750 °C. The increase in Nb content was found to increase the onset temperature of the Nb compounds. After heat treatment at 1100 °C and 1200 °C, the precipitated phases were mainly square and star-shaped Nb(C,N) composites.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Jia, B.; Wang, J. Influence of artificial cooling methods on post-fire mechanical properties of Q355 structural steel. Constr. Build. Mater. 2020, 252, 119092. [Google Scholar] [CrossRef]
- Outinen, J.; Mkelinen, P. Mechanical properties of structural steel at elevated temperatures and after cooling down. Fire Mater. 2004, 28, 237–251. [Google Scholar] [CrossRef]
- Ullah, S.H.; Ravi, K. Influence of stress concentration and cooling methods on post-fire mechanical behavior of ASTM A36 steels. Constr. Build. Mater. 2018, 186, 920–945. [Google Scholar]
- Sajid, H.U.; Kiran, R. Post-fire mechanical behavior of ASTM A572 steels subjected to high stress triaxialities. Eng. Struct. 2019, 191, 323–342. [Google Scholar] [CrossRef]
- DeArdo, A.J. Niobium in modern steels. Int. Mater. Rev. 2003, 48, 371–402. [Google Scholar] [CrossRef]
- Pradhan, N.; Banerjee, N.; Reddy, B.B.; Sahay, S.K.; Viswanathen, C.S.; Bhor, P.K.; Basu, D.S.; Mazumdar, S. Control of transverse cracking in special quality slabs. Ironmak. Steelmak. 2001, 28, 305–311. [Google Scholar] [CrossRef]
- Abushosha, R.; Ayyad, S.; Mintz, B. Influence of cooling rate on hot ductility of C-Mn-Al and C-Mn-Nb-Al steels. Mater. Sci. Technol. 1998, 14, 346–351. [Google Scholar] [CrossRef]
- Banks, K.; Koursaris, A.; Verdoorn, F.F.; Tuling, A. Precipitation and hot ductility of low C-V and low C-V-Nb microalloyed steels during thin slab casting. Mater. Sci. Technol. 2001, 17, 1596–1604. [Google Scholar] [CrossRef]
- Mintz, B.; Abushosha, R. The influence of vanadium on hot ductility of steel. Ironmak. Steelmak. 1993, 20, 445–452. [Google Scholar]
- Abushosha, R.; Comineli, O.; Mintz, B. Influence of Ti on hot ductility of C-Mn-Al steels. Mater. Sci. Technol. 1999, 15, 278–286. [Google Scholar] [CrossRef]
- Comineli, O.; Abushosha, R.; Mintz, B. Influence of Ti and Nb on the hot ductility of C-Mn-Nb-Al. Mater. Sci. Technol. 1999, 15, 1058–1068. [Google Scholar] [CrossRef]
- Spradbery, C.; Mintz, B. Influence of undercooling thermal cycle on hot ductility of C-Mn-Al-Ti and C-Mn-Nb-Al-Ti steels. Ironmak. Steelmak. 2005, 32, 319–324. [Google Scholar] [CrossRef]
- Abushosha, R.; Vipond, R.; Mintz, B. The influence of Ti on hot ductility of as cast steels. Mater. Sci. Technol. 1991, 7, 613–621. [Google Scholar] [CrossRef]
- Mintz, B.; Abushosha, R.; Crowther, D.N. The influence of small additions of Cu and Ni on the hot ductility of steels. Mater. Sci. Technol. 1995, 11, 474–481. [Google Scholar] [CrossRef]
- Yasumoto, K.; Maehara, Y.; Ura, S. Effect of Sulphur on Hot Ductility of Low-carbon Steel Austenite. Mater. Sci. Technol. 1985, 1, 111–116. [Google Scholar] [CrossRef]
- Ma, F.; Wen, G.; Tang, P.; Yu, X.; Li, J.; Xu, G.; Mei, F. Causes of corner transversal cracks of micro-alloyed steel in the vertical-bending continuous slab casters. Ironmak. Steelmak. 2010, 37, 73–79. [Google Scholar] [CrossRef]
- Maehara, Y.; Ohmori, Y. The precipitation of AlN and NbC and the hot ductility of low carbon steel. Mater. Sci. Eng. 1984, 62, 109–119. [Google Scholar] [CrossRef]
- Miao, C.L.; Shang, C.J.; Zhang, G.D.; Subramanian, S.V. Recrystallization and strain accumulation behaviors of high Nb-bearing line pipe steel in plate and strip rolling. Mater. Sci. Eng. A 2010, 527, 4985–4992. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Campos, S.S.; Kestenbach, H.J.; Morales, E.V. On strengthening mechanisms in commercial Nb-Ti hot strip steels. Metall. Mater. Trans. A 2001, 32, 1245–1248. [Google Scholar] [CrossRef]
- Sharma, R.C.; Lakshmanan, V.K.; Kirkaldy, J.S. Solubility of niobium carbide and niobium carbonitride in alloyed austenite and ferrite. Metall. Trans. A 1984, 15, 545–553. [Google Scholar] [CrossRef]
- Hong, S.G.; Jun, H.J.; Kang, K.B.; Park, C.G. Evolution of precipitates in the Nb–Ti–V microalloyed HSLA steels during reheating. Scr. Mater. 2003, 48, 1201–1206. [Google Scholar] [CrossRef]
- Suzuki, H.G.; Nishimura, S.; Yamaguchi, S. Characteristics of hot ductility in steels subjected to the melting and solidification. Trans. Iron Steel Inst. Jpn. 1982, 22, 48–56. [Google Scholar] [CrossRef]
- Mintz, B.; Abushosha, R. Effectiveness of hot tensile test in simulating straightening in continuous casting. Met. Sci. J. 2013, 8, 171–178. [Google Scholar] [CrossRef]
- Li, X.L.; Wu, P.; Yang, R.J.; Zhao, S.T.; Zhang, S.P.; Chen, S.; Cao, X.Z.; Wang, X.M. Nb segregation at prior austenite grain boundaries and defects in high strength low alloy steel during cooling. Mater. Des. 2017, 115, 165–169. [Google Scholar] [CrossRef]
- Yu, Q.B.; Wang, Z.D.; Liu, X.H.; Wang, G.D. Effect of microcontent Nb in solution on the strength of low carbon steels. Mater. Sci. Eng. A 2004, 379, 384–390. [Google Scholar] [CrossRef]
- Wu, H.; Ju, B.; Tang, D.; Hu, R.; Guo, A.; Kang, Q.; Wang, D. Effect of Nb addition on the microstructure and mechanical properties of an 1800MPa ultrahigh strength steel. Mater. Sci. Eng. A 2015, 622, 61–66. [Google Scholar] [CrossRef]
- Mabuchi, H.; Uemori, R.; Fujioka, M. The role of Mn depletion in intragranular ferrite transformation in the heat affected zone of welded joints with large heat input in structural steels. ISIJ Int. 1996, 36, 1406–1412. [Google Scholar] [CrossRef]
- Shim, J.H.; Byun, J.S.; Cho, Y.W.; Oh, Y.J.; Shim, J.D.; Lee, D.N. Hot deformation and acicular ferrite microstructure in C-Mn steel containing Ti2O3 inclusions. ISIJ Int. 2000, 40, 819–823. [Google Scholar] [CrossRef][Green Version]
- Chen, C.Y.; Yen, H.W.; Kao, F.H.; Li, W.C.; Huang, C.Y.; Yang, J.R.; Wang, S.H. Precipitation hardening of high-strength low-alloy steels by nanometer-sized carbides. Mater. Sci. Eng. A 2009, 499, 162–166. [Google Scholar] [CrossRef]
- Gregg, J.M.; Bhadeshiah, K.D.H. Solid-state nucleation of acicular ferrite on minerals added to molten steel. Acta Mater. 1997, 45, 739–748. [Google Scholar] [CrossRef]
- Kurebayashi, Y. Influence of carbo-nitride precipitates on austenite grain coarsening behavior during carburizing. Denki-Seiko 1996, 67, 26–33. [Google Scholar] [CrossRef]
- Suzuki, H.G.; Nishimura, S.; Yamaguchi, S. Characteristics of embrittlement in steels above 600 °C. Tetsu-Hagane 1979, 65, 2038–2046. [Google Scholar] [CrossRef][Green Version]
- Balasubramanian, K.; Kirkaldy, J.S. Thermodynamics of Fe-Ti-C and Fe-Nb-C austenites and nonstoichiometric titanium and niobium carbides. Adv. Phase Transit. 1988, 51, 37–51. [Google Scholar]
- Sun, C.; Fang, Y.; Wang, Y.; Liu, W.; Pan, H. Effect of coiling temperature on precipitation of second phase in ultra-low carbon T-3 CA steel. Iron Steel 2019, 54, 83–87. [Google Scholar]
- Sun, Y.H.; Dong, J.J.; Jin, J.N.; Fu, S.P.; Zhu, X.Z. Precipitation behavior of the second phase particles at secondary cooling temperature fluctuations zone with temperature fluctuations. Chin. J. Eng. 2016, 38, 90–95. [Google Scholar]
- Chen, J.; Shuai, T.; Liu, Z.; Wang, G. Influence of molybdenum content on transformation behavior of high performance bridge steel during continuous cooling. Mater. Des. 2013, 49, 465–470. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C.; Yu, L.; Liu, Y.; Li, H. Phase transformation behavior and microstructural control of high-cr martensitic/ferritic heat-resistant steels for power and nuclear plants: A review. J. Mater. Sci. Technol. 2015, 31, 235–242. [Google Scholar] [CrossRef]




| C | Si | Mn | P | S | Nb | Ti | |
|---|---|---|---|---|---|---|---|
| A | 0.173 | 0.206 | 1.567 | 0.014 | 0.009 | 0.006 | 0.029 |
| B | 0.175 | 0.202 | 1.555 | 0.014 | 0.008 | 0.031 | 0.026 |
| C | 0.175 | 0.210 | 1.546 | 0.014 | 0.008 | 0.050 | 0.027 |
| D | 0.173 | 0.208 | 1.557 | 0.014 | 0.008 | 0.065 | 0.026 |
| Classification | A | B | C | D |
|---|---|---|---|---|
| Ferrite | 25.69 | 17.36 | 20.73 | 22.63 |
| Pearlite | 23.96 | 16.28 | 19.12 | 21.17 |
| Group | Nb | C | N |
|---|---|---|---|
| A | 0.006 | 0.173 | 0.0060 |
| B | 0.031 | 0.175 | 0.0055 |
| C | 0.050 | 0.175 | 0.0056 |
| D | 0.065 | 0.173 | 0.0058 |
| Classification | A | B | C | D |
|---|---|---|---|---|
| Number of two-phase particles | 345 | 506 | 471 | 489 |
| Particle size > 80 nm accounted for (%) | 24.64 | 16.21 | 19.14 | 25.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhu, L.; Sun, L.; Wang, B.; Xiao, P. Influence of Nb on the Structure and High Temperature Performance of Billet for High-Rise Structural Steel. Metals 2021, 11, 1721. https://doi.org/10.3390/met11111721
Zhou J, Zhu L, Sun L, Wang B, Xiao P. Influence of Nb on the Structure and High Temperature Performance of Billet for High-Rise Structural Steel. Metals. 2021; 11(11):1721. https://doi.org/10.3390/met11111721
Chicago/Turabian StyleZhou, Jingyi, Liguang Zhu, Ligen Sun, Bo Wang, and Pengcheng Xiao. 2021. "Influence of Nb on the Structure and High Temperature Performance of Billet for High-Rise Structural Steel" Metals 11, no. 11: 1721. https://doi.org/10.3390/met11111721
APA StyleZhou, J., Zhu, L., Sun, L., Wang, B., & Xiao, P. (2021). Influence of Nb on the Structure and High Temperature Performance of Billet for High-Rise Structural Steel. Metals, 11(11), 1721. https://doi.org/10.3390/met11111721






