Experimental and Numerical Investigations on Charging Carbon Composite Briquettes in a Blast Furnace
Abstract
1. Introduction
2. Experimental
2.1. CCB Sample
2.2. Non-Isothermal Reaction Tests
3. Model Development
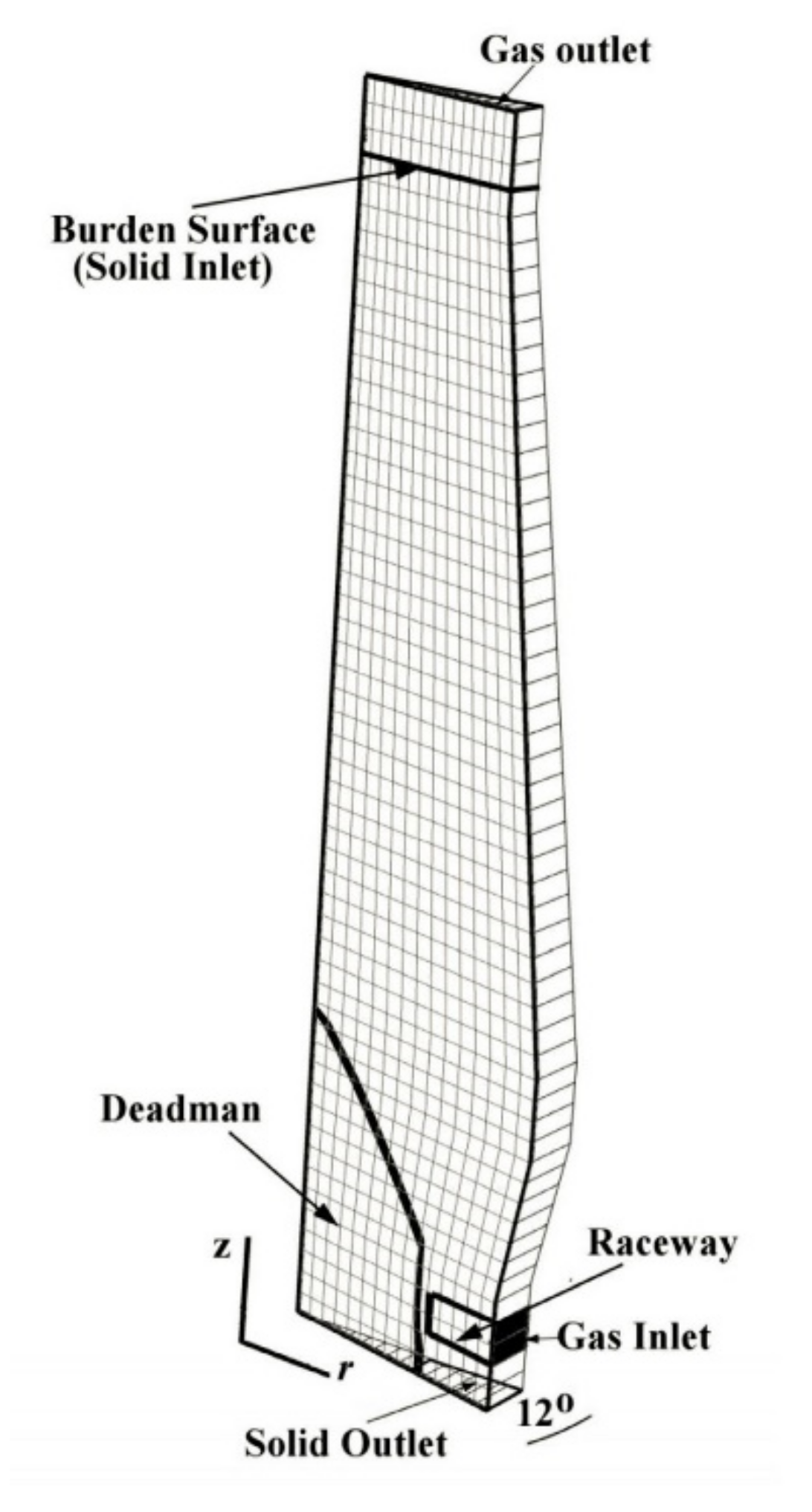
3.1. Description of BF Operation with CCB Charging
3.2. BF Model
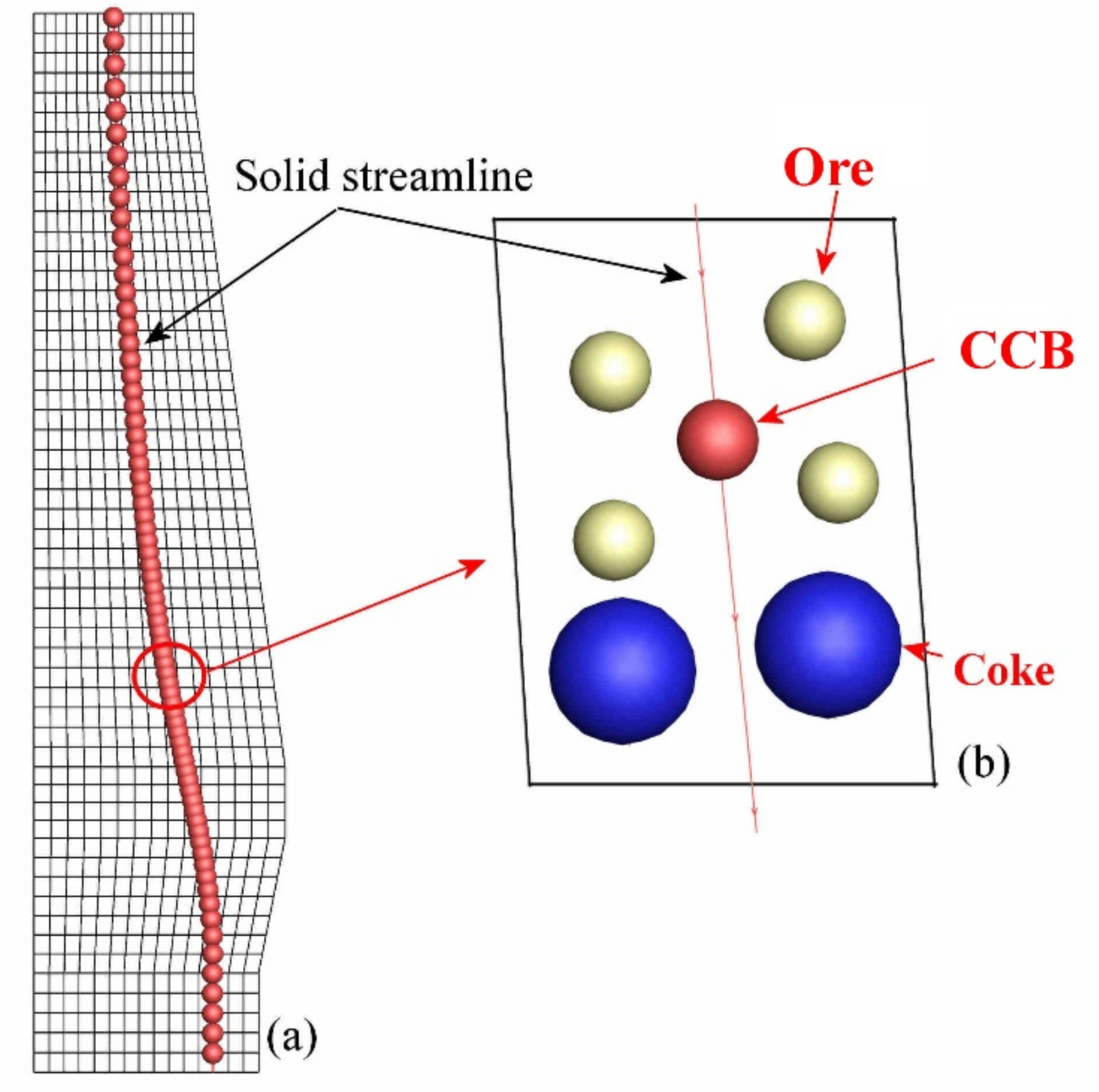
3.3. CCB Model
3.4. Solution Strategy
4. Results and Discussion
4.1. Determination of Parameter in CCB Model
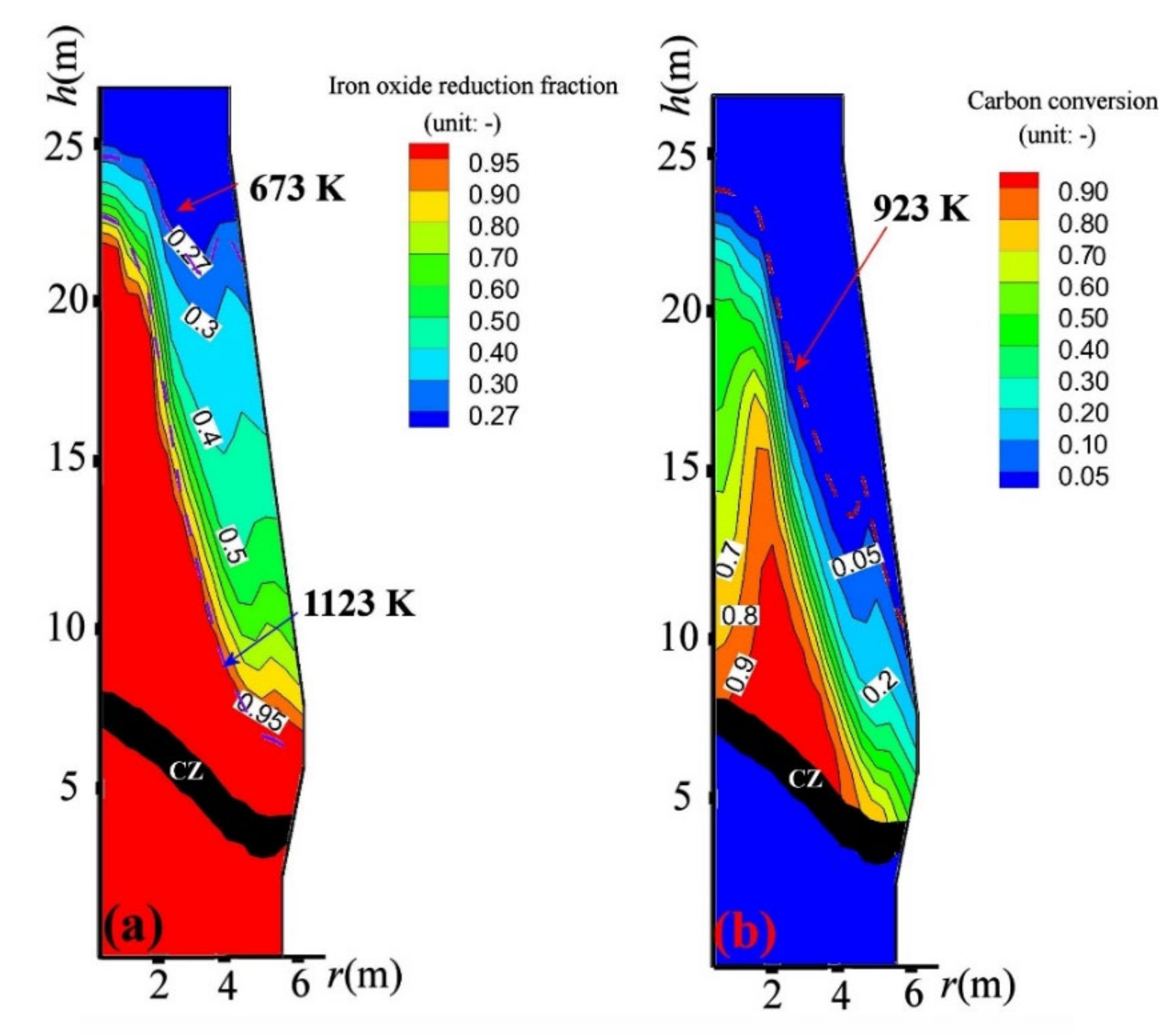
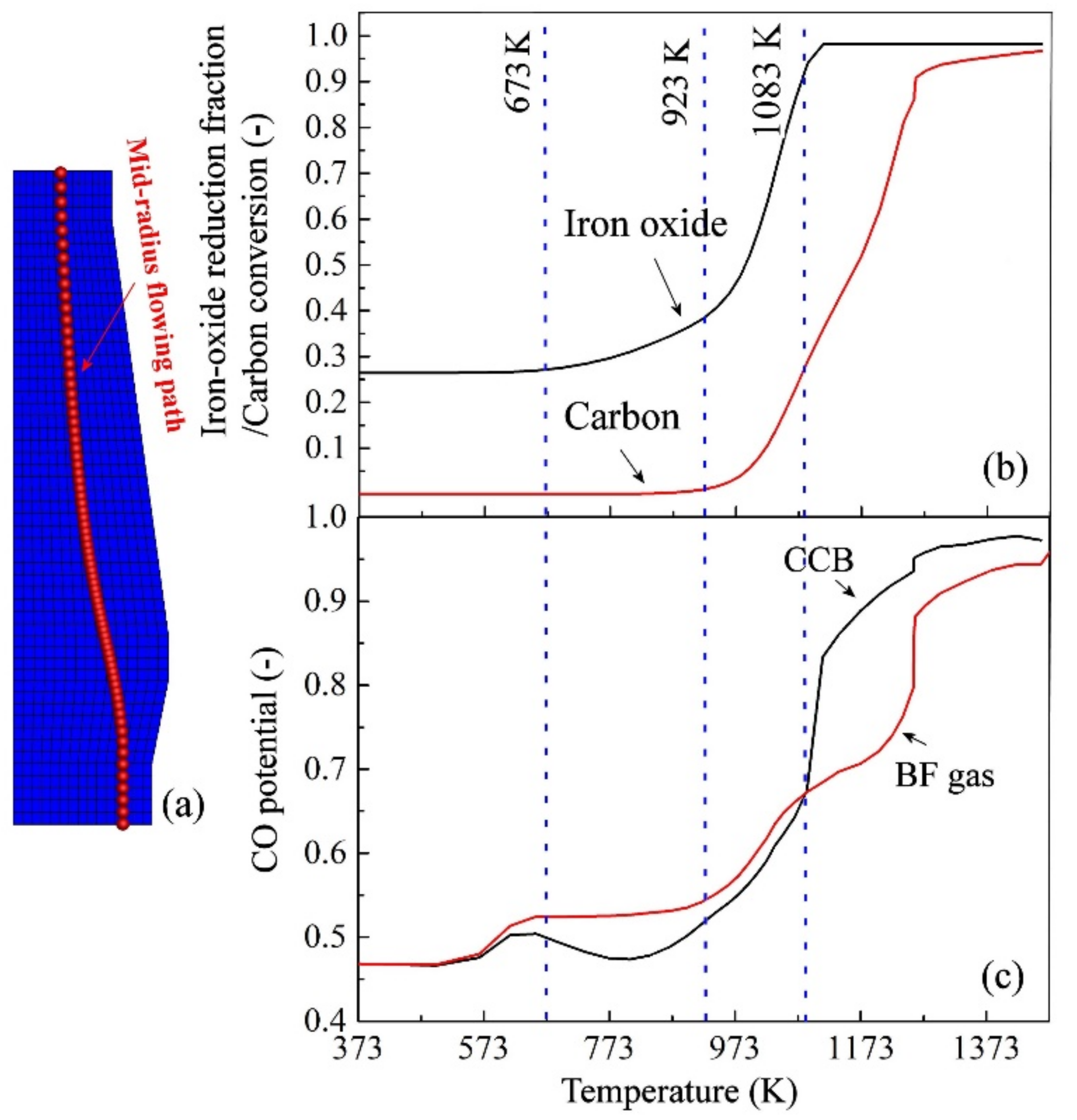
4.2. CCB Behavior in BF
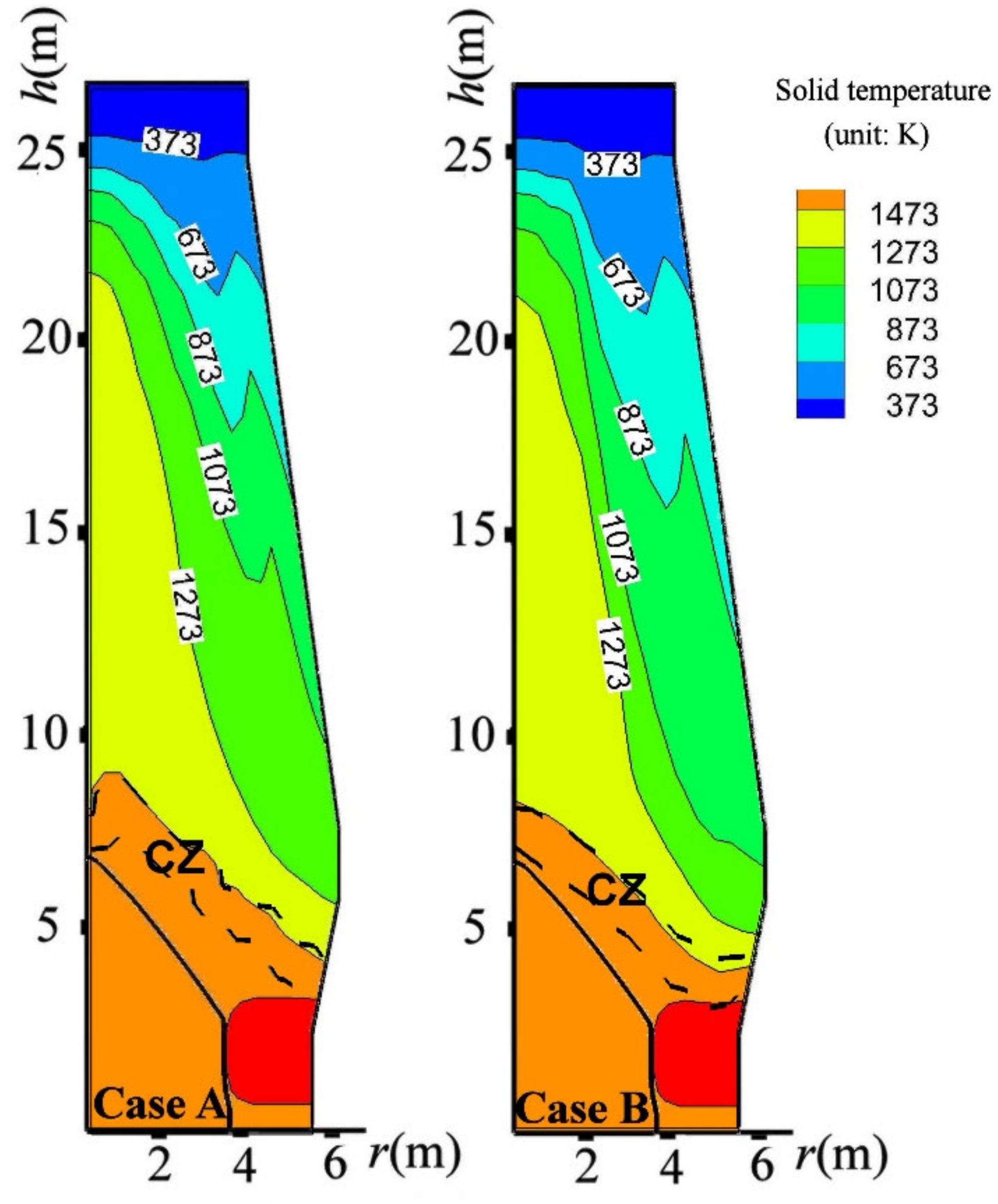
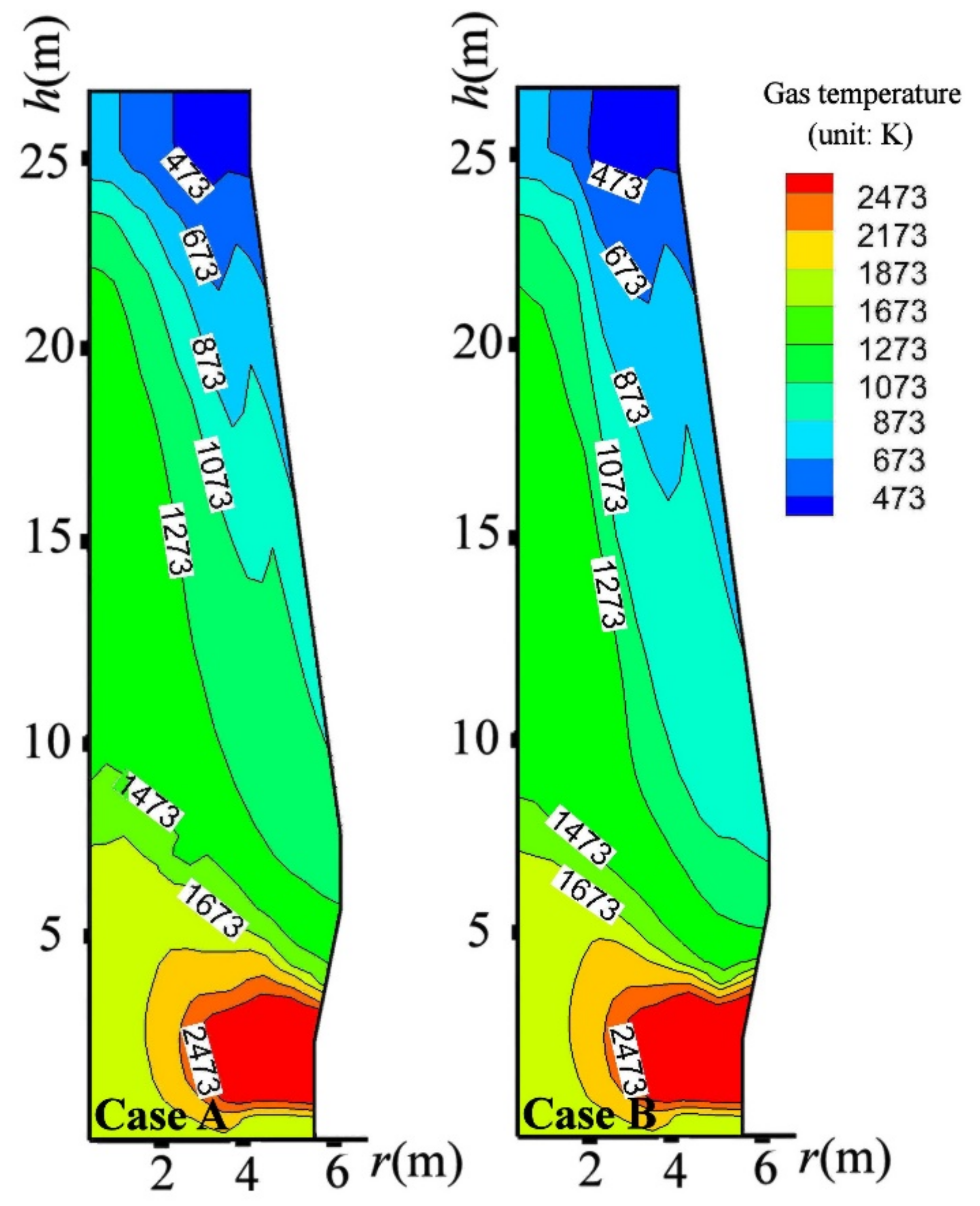
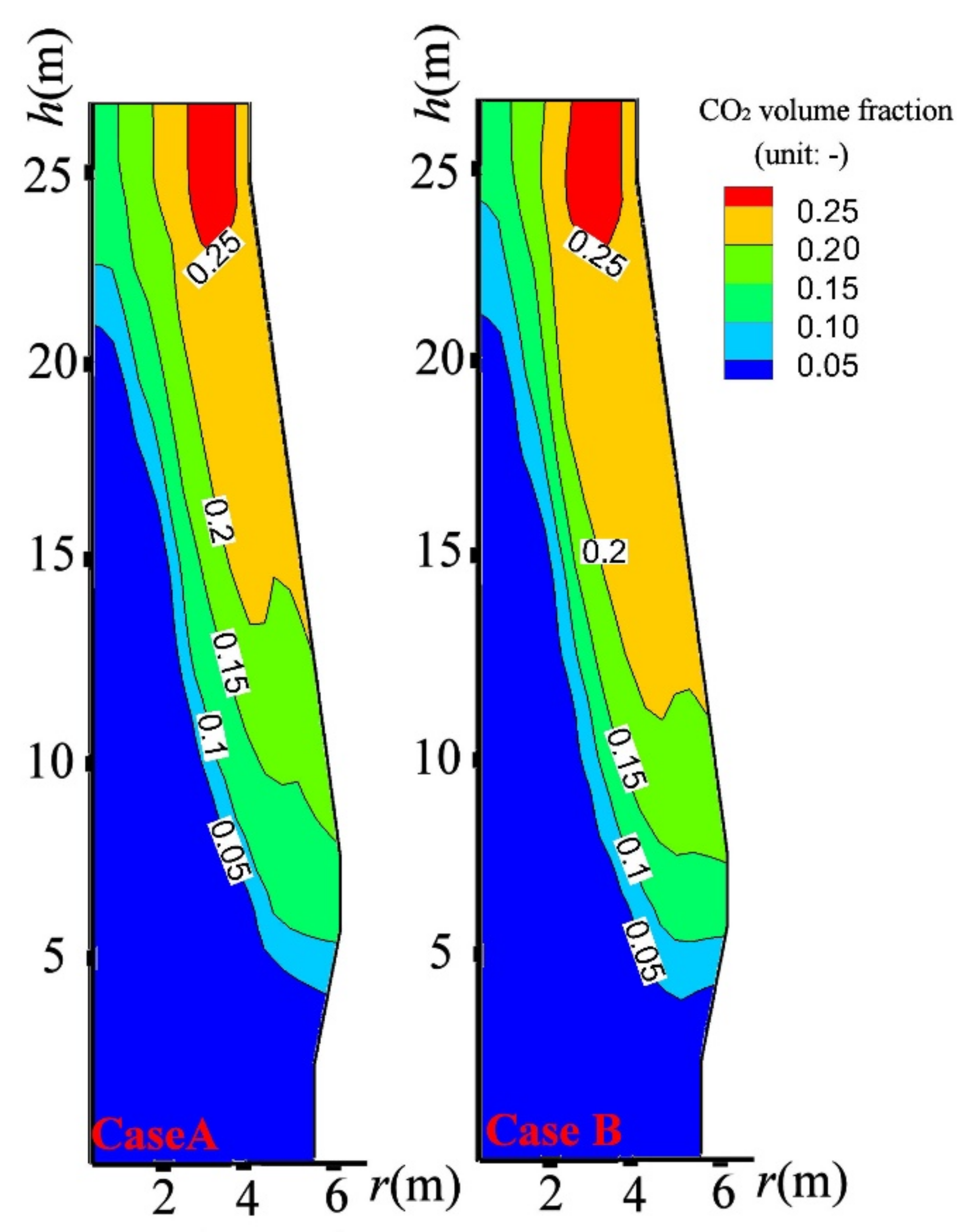
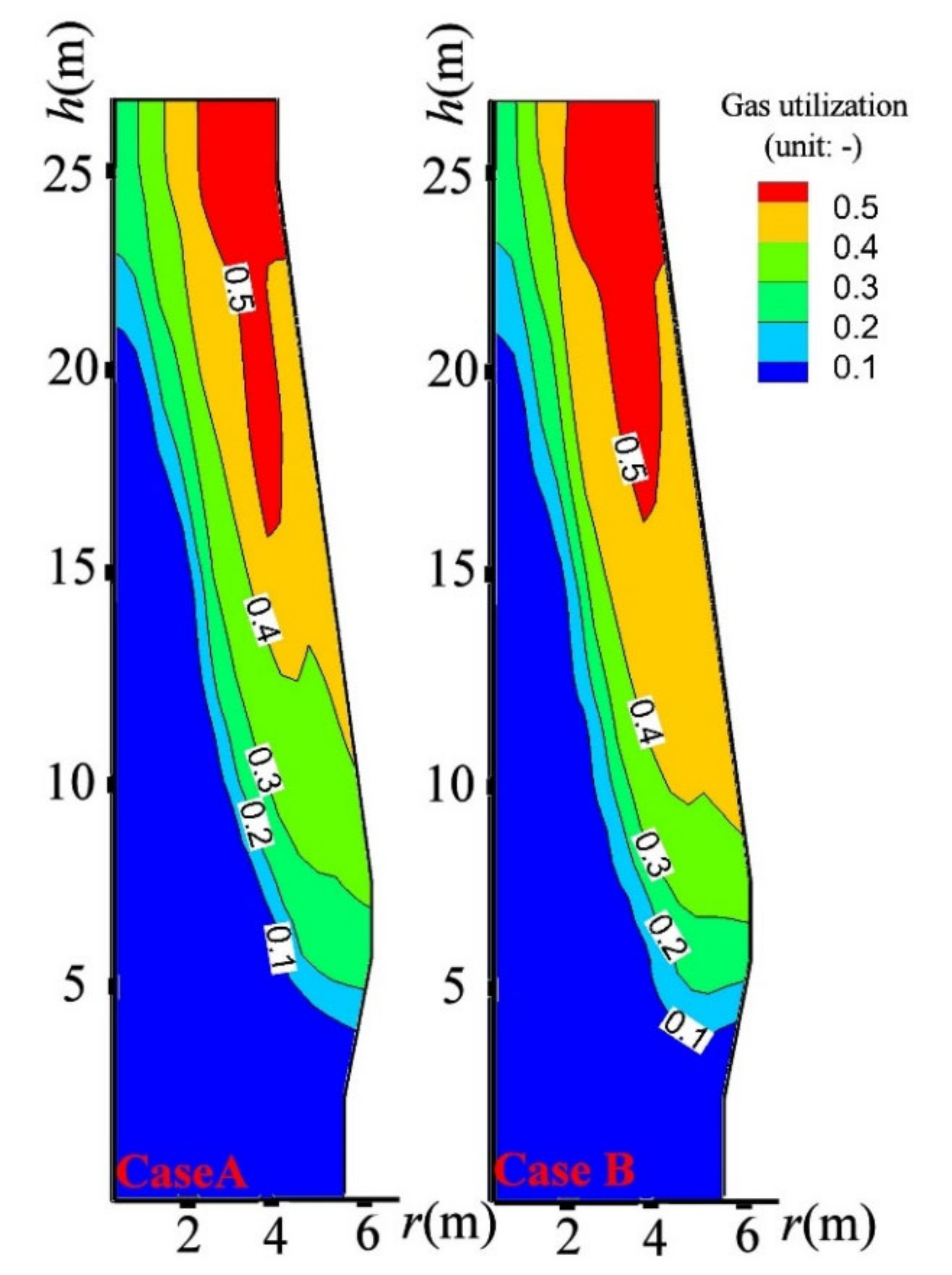
4.3. Influence on BF in-Furnace State
4.4. Coke-Saving Analysis
5. Conclusions
- The CCB reaction behavior in BF could be modeled using the previously proposed model by the current authors. Under ags = 1900 m2·m−3, the agreement between experimental measurements and model predictions was satisfying.
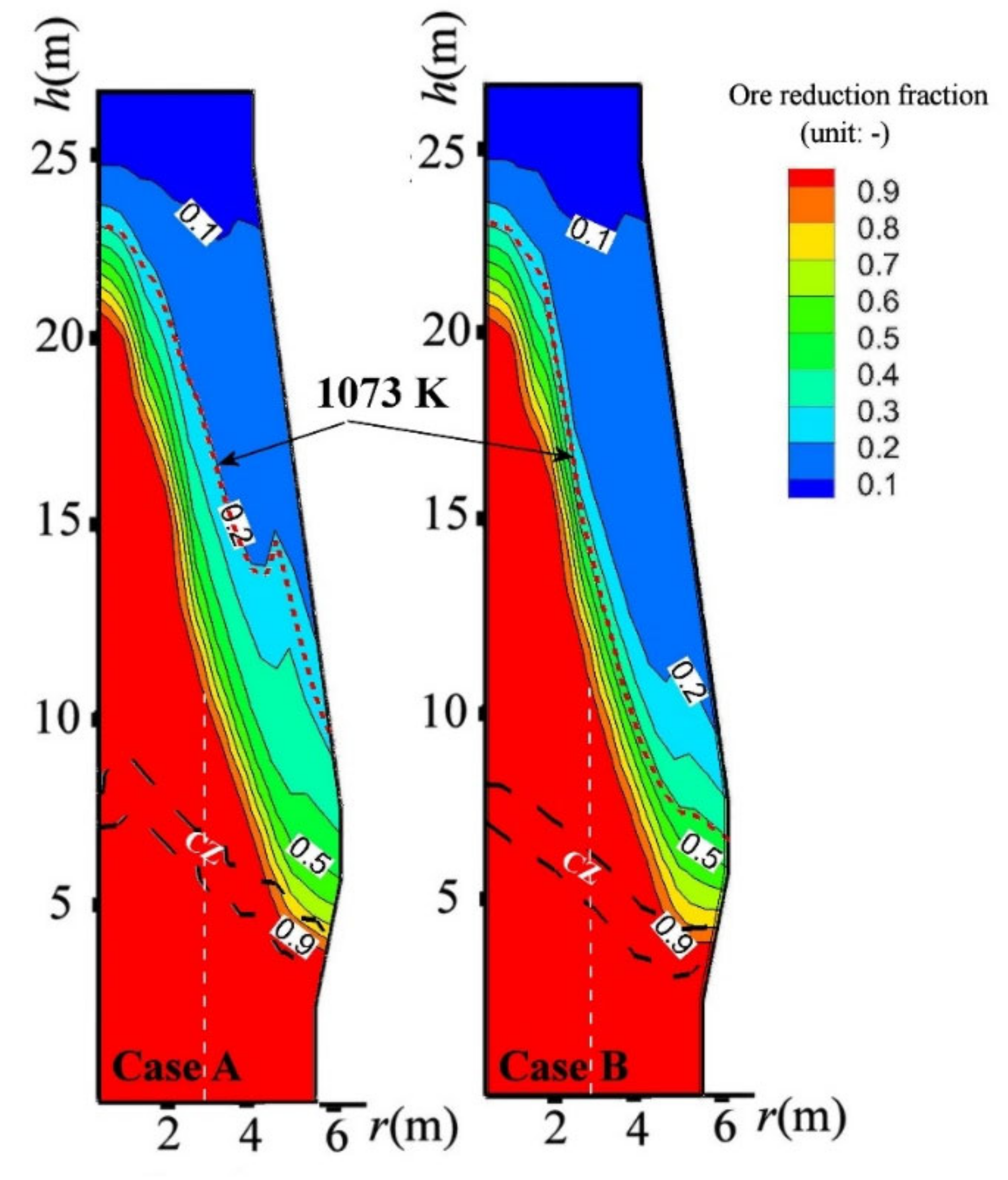
- In the CCB charging operation, the CCB reached a full iron-oxide reduction and a carbon conversion of 85% above CZ.
- By charging CCB, the thermal state in the BF upper part was significantly changed; however, the BF thermal state in the BF lower part was inconsiderably influenced.
- By charging CCB, the ore reduction was retarded before the temperature reached 1073 K and was prompted after; and the local gas utilization tended to increase above the CZ.
- By the CCB reduction above the CZ, the BF top gas temperature was decreased by 8 K, the BF top gas utilization was increased by 1.3%, the BF productivity was decreased by 17 tHM/day, 101 kg sinter and 52.8 kg could be saved to produce one-ton hot metal. Considering the energy consumption of sintering and coking, charging the CCB could have a significant energy-saving and CO2-emission-reducing effect for BF iron making.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| ags | specific surface of iron-oxide particles in CCB, (m2·m−3) |
| Cp | heat capacity, (J·kg−1·K−1) |
| d | diameter of CCB, (m) |
| D, Deff | gas diffusivity, effective gas diffusivity, (m2·s−1) |
| E | enthalpy source, (J·m−3·s−1)) |
| fi | reduction fraction or carbon conversion of reaction i in Table 5, (-) |
| H | total enthalpy (J·kg−1) |
| ΔHi | reaction heat of reaction i in Table 2, (J·kmol−1) |
| ki | reaction rate constant of reaction i in Table 5, (m·s−1, kg·s−1atm−1) |
| Ki | equilibrium constant of reaction i in Table 5, (-) |
| m | mass supply/consumption rate of the given element, (kg·s−1) |
| M | molar weight, (kg·kmol−1) |
| P | pressure, (pa) |
| Pr | Prandtl number, (-) |
| R | gas constant, (8.314 J·mol−1·K−1) |
| Re | Reynolds number, (-) |
| Ri | chemical reaction rate of reaction i in Table 2, (kmol·m−3⋅s−1) |
| r | radial direction, (m) |
| ri | chemical reaction rate of reaction i in Table 5, (mol·m−3⋅s−1) |
| S | source, units vary |
| Sc | Schmidt number (-) |
| T | temperature, (K) |
| t | time, (s) |
| Vcell | cell volume, (m3) |
| y | mass fraction, (-) |
| Greek letter | |
| α | CCB porosity, (-) |
| general dependent variable | |
| general difusion coeffcient | |
| ρ | density, (kg·m−3) |
| thermal conductivity, (W·m−1·K−1) | |
| µ | fluid viscosity, (kg·m−1·s−1) |
| Vector | |
| superficial gas velocity, (m·s−1) | |
| solid physical velocity, (m·s−1) | |
| gas flow resistance, (N·m−3) | |
| Subscript | |
| 0 | initial |
| CCB | CCB variable |
| coke | coke variable |
| ore | ore variable |
| g | gas variable |
| l | liquid variable |
| s | solid variable |
| e | environment variable |
| species or element name | variable of assigned species or element |
References
- Wang, Y.; Zuo, H.; Zhao, J. Recent progress and development of ironmaking in China as of 2019: An overview. Ironmak. Steelmak. 2020, 47, 640–649. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Liu, Z.; Wang, G.; Yang, T. Status, technological progress, and development directions of the ironmaking industry in China. Ironmak. Steelmak. 2019, 46, 937–941. [Google Scholar] [CrossRef]
- Naito, M.a.K.; Takeda, K.; Matsui, Y. Ironmaking technology for the last 100 years: Deployment to advanced technologies from introduction of technological know-how, and Evolution to Next-generation Process. ISIJ Int. 2015, 55, 7–35. [Google Scholar] [CrossRef]
- An, R.; Yu, B.; Ru, L.; Wei, Y.M. Potential of energy savings and CO2 emission reduction in China’s iron and steel industry. Appl. Energy 2018, 226, 862–880. [Google Scholar] [CrossRef]
- Tan, X.; Li, H.; Guo, J.; Gu, B.; Zeng, Y. Energy-saving and emission-reduction technology selection and CO2 emission reduction potential of China’s iron and steel industry under energy substitution policy. J. Clean. Prod. 2019, 222, 823–834. [Google Scholar] [CrossRef]
- Ariyama, T.; Murai, R.; Ishii, J.; Sato, M. reduction of CO2 emissions from integrated steel works and its subjects for a future study. ISIJ Int. 2005, 45, 1371–1378. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chakraborti, N.; Sen, P.K. Energy optimization studies for integrated steel plant employing diverse steel-making route: Models and evolutionary algorithm-based approach. Miner. Process. Extr. Metall. Rev. 2020, 42, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, J.L.; Yang, T.J. Low Carbon Operation of Super-Large Blast Furnaces in China. ISIJ Int. 2015, 55, 1146–1156. [Google Scholar] [CrossRef]
- Ahmed, H. New trends in the application of carbon-bearing materials in blast furnace iron-making. Minerals 2018, 8, 561. [Google Scholar] [CrossRef]
- Sharma, T. Composite pre-reduced pellet (CPR)—A new way for fines utilisation. Miner. Process. Extr. Metall. Rev. 1993, 12, 115–124. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Viswanathan, N.; Bjorkman, B. Composite pellets—A potential raw material for iron-making. Steel Res. Int. 2014, 85, 293–306. [Google Scholar] [CrossRef]
- Kasai, A.; Toyota, H.; Nozawa, K.; Kitayama, S. Reduction of reducing agent rate in blast furnace operation by carbon composite iron ore hot briquette. ISIJ Int. 2011, 51, 1333–1335. [Google Scholar] [CrossRef]
- Yokoyama, H.; Higuchi, K.; Ito, T.; Oshio, A. Decrease in carbon consumption of a commercial blast furnace by using carbon composite iron ore. Tetsu Hagane 2012, 100, 601–609. [Google Scholar] [CrossRef]
- Andersson, A.; Gullberg, A.; Kullerstedt, A.; Wedholm, A.; Wikstrm, J.; Ahmed, H.; Kvist, L.S. Recycling of blast furnace sludge to the blast furnace via cold-bonded briquettes: Evaluation of feasibility and influence on operation. ISIJ Int. 2019, 59, 1786–1795. [Google Scholar] [CrossRef]
- Nikai, I.; Garbers-Craig, A.M. Use of iron ore fines in cold-bonded self-reducing composite pellets. Miner. Process. Extr. Metall. Rev. 2016, 37, 42–48. [Google Scholar] [CrossRef][Green Version]
- Ueda, S.; Yanagiya, K.; Watanabe, K.; Murakami, T.; Inoue, R.; Ariyama, T. Reaction model and reduction behavior of carbon iron ore composite in blast furnace. ISIJ Int. 2009, 49, 827–836. [Google Scholar]
- Mourao, M.B.; Cyro, T. Self-reducing pellets for ironmaking: Reaction rate and processing. Miner. Process. Extr. Metall. Rev. 2003, 24, 183–202. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueda, S.; Inoue, R.; Ariyama, T. Enhancement of reactivity of carbon iron ore composite using redox reaction of iron oxide powder. ISIJ Int. 2010, 50, 524–530. [Google Scholar] [CrossRef]
- Kasai, A.; Matsui, Y. Lowering of thermal reserve zone temperature in blast furnace by adjoining carbonaceous material and iron ore. ISIJ Int. 2004, 44, 2073–2078. [Google Scholar] [CrossRef]
- Kawanari, M.; Matsumoto, A.; Ashida, R.; Miura, K. Enhancement of reduction rate of iron ore by utilizing iron ore/carbon composite consisting of fine iron ore particles and highly thermoplastic carbon material. ISIJ Int. 2011, 51, 1227–1233. [Google Scholar]
- Kemppainen, A.; Iljana, M.; Heikkinen, E.P.; Paananen, T.; Mattila, O.; Fabritius, T. Reduction behavior of cold-bonded briquettes under simulated blast furnace conditions. ISIJ Int. 2014, 54, 1539–1545. [Google Scholar] [CrossRef]
- Singh, M.; Björkman, B. Testing of cement-bonded briquettes under laboratory and blast furnace conditions Part 1—Effect of processing parameters. Ironmak. Steelmak. 2017, 34, 41–53. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Nishio, M.; Tsubouchi, N.; Akiyama, T. Reduction behavior and crushing strength of carbon-containing pellet prepared from COG tar. Fuel Process. Technol. 2016, 142, 287–295. [Google Scholar] [CrossRef]
- Tang, H.; Liu, S.; Tao, R. Preparation of High-Carbon Metallic Briquette for Blast Furnace Application. ISIJ Int. 2019, 59, 12–20. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Y.; Rong, T. Experimental and numerical investigation of reaction behavior of carbon composite briquette in blast furnace. Metals 2020, 10, 49. [Google Scholar] [CrossRef]
- Kuang, S.; Li, Z.; Yu, A. Review on modeling and simulation of blast furnace. Steel Res. Int. 2018, 89, 1700071. [Google Scholar] [CrossRef]
- Okosun, T.; Silaen, A.K.; Zhou, C.Q. Review on computational modeling and visualization of the ironmaking blast furnace at purdue university northwest. Steel Res. Int. 2019, 90, 1900046. [Google Scholar] [CrossRef]
- Sahai, Y. Computer/mathematical modeling in materials processing operations. Miner. Process. Extr. Metall. Rev. 1992, 9, 1–26. [Google Scholar] [CrossRef]
- Ueda, S.; Natsui, S.; Nogami, H.; Yagi, J.I.; Ariyama, T. Recent progress and future perspective on mathematical modeling of blast furnace. ISIJ Int. 2010, 50, 914–923. [Google Scholar] [CrossRef]
- Chu, M.; Nogami, H.; Yagi, J.I. Numerical analysis on charging carbon composite agglomerates into blast furnace. ISIJ Int. 2004, 44, 510–517. [Google Scholar] [CrossRef]
- Yu, X.; Shen, Y. Computational fluid dynamics study of the flow and thermochemical behaviors of a carbon composite briquette in an ironmaking blast furnace. Energy Fuels 2019, 33, 11603–11616. [Google Scholar] [CrossRef]
- Tankg, H.; Rong, T.; Fan, K. Numerical investigation of applying high-carbon metallic briquette in blast furnace ironmaking. ISIJ Int. 2019, 59, 810–819. [Google Scholar] [CrossRef]
- Kuang, S.B.; Li, Z.Y.; Yan, D.L.; Qi, Y.H.; Yu, A.B. Numerical study of hot charge operation in ironmaking blast furnace. Miner. Eng. 2014, 63, 45–56. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Y.; Rong, T.; Guo, Z. Reaction model and reaction behavior of carbon composite briquette in blast furnace. Powder Technol. 2021, 377, 832–842. [Google Scholar] [CrossRef]
- Cham. PHOENICS User Document; CHAM Ltd.: London, UK, 2000. [Google Scholar]
- Tanaka, Y.; Ueno, T.; Okumura, K.; Hayashi, S. Reaction behavior of coal rich composite iron ore hot briquettes under load at high temperatures until 1400 °C. ISIJ Int. 2011, 51, 1240–1246. [Google Scholar] [CrossRef]
- Gavel, D.J. A review on nut coke utilization in the ironmaking blast furnaces. Mater. Sci. Technol. 2017, 33, 381–387. [Google Scholar] [CrossRef]


| Carbon | Fe3O4 | FeO | Metallic Iron | Gangue |
|---|---|---|---|---|
| 20.30 | 29.70 | 39.70 | 1.57 | 8.73 |
| Variable | Value |
|---|---|
| Productivity (tHM·day−1) | 6250 |
| Blast temperature (K) | 1523 |
| Blast rate (Nm3·min−1) | 4800 |
| Oxygen enrichment (mol%) | 4.0 |
| Top absolute pressure (Pa) | 2.8 × 105 |
| PC injection rate (kg·tHM−1) | 180 |
| Ore rate (kg·tHM−1) | 1680 |
| Coke rate (kg·tHM−1) | 335 |
| Batch weight of ore (ton) | 76 |
| Batch weight of coke (ton) | 15 |
| Solid inlet temperature (K) | 300 |
| Ore particle property | Composition: TFe: 55.8 wt.%, FeO: 6.8 wt.%, CaO: 4.60, SiO2: 4.97 wt.%, Al2O3: 2.19 wt.%, TiO2: 2.0 wt.% Porosity: 0.35; Bulk density: 1750 kg/m3; Average particle size: 20 mm. |
| Coke particle property | Composition: Fixed Carbon: 90 wt.%, and Ash: 10 wt.%; Porosity: 0.50; Bulk density: 500 kg/m3; Average particle size: 40 mm. |
| PC property | Composition: C: 80.0 wt.%, H: 4.0 wt.%, O: 3.5 wt.%, N: 2.0 wt.%, and S: 0.32 wt.%; H2O: 4.0 wt.%, and Ash: 7.0 wt.%. |
| Liquid phase (molten iron and slag) property | [%C]: 4.0 wt.%, Temperature: 1753 K, Average heat capacity: 1000 J/kg, and Slag rate: approximately 400 kg/tHM |
| Reaction | Reaction Rate (kmol·m−3s−1) | Explanation | |
|---|---|---|---|
| 1 | 3Fe2O3 (ore, s) + CO(g) = 2Fe3O4(ore, s) + CO2(g) | R1 | stepwise reduction of ore (sinter, pellet and lump ore) by CO |
| 2 | Fe3O4 (ore, s) + CO(g)=3 FeO(ore, s) + CO2(g) | R2 | |
| 3 | FeO (ore, s) + CO(g) = Fe(ore, s) + CO2(g) | R3 | |
| 4 | C (coke) + CO2 (g) = 2CO (g) | R4 | coke solution-loss reaction |
| 5 | C (coke) + 1/2O2 (g) = CO (g) | R5 | coke combustion |
| 6 | 3Fe2O3 (CCB, s) + CO (g) = 2Fe3O4 (CCB, s) + CO2 (g) | R6 | CCB reactions |
| 7 | Fe3O4(CCB, s) + CO(g) = 3FeO(CCB, s) + CO2(g) | R7 | |
| 8 | FeO (CCB, s) + CO (g) = Fe (CCB, s) + CO2 (g) | R8 | |
| 9 | C (CCB) + CO2 (g) = 2CO (g) | R9 | |
| 10 | Fe (ore,s) = Fe (l) | R10 | melting reactions of ore |
| 11 | FeO (ore,s) = FeO (l) | R11 | |
| 12 | Gangue (ore,s) = Slag (l) | R12 | |
| 13 | Fe (CCB,s) = Fe (l) | R13 | melting reactions of CCB |
| 14 | FeO (CCB,s) = FeO (l) | R14 | |
| 15 | Gangue (CCB,s) = Slag (l) | R15 | |
| 16 | FeO (l) + C (s) = Fe (l) + CO (g) | R16 | direct reduction of molten FeO |
| Equation | ||
|---|---|---|
| Mass | 1 | |
| Momentum | ||
| Energy | ||
| Species | ||
| 0 |
| Equation | |||
|---|---|---|---|
| Continuity | 1 | 0 | |
| Momentum | |||
| Energy | |||
| Species | 0 | 0 | |
| 0 | |||
| 0 | |||
| 0 | |||
| 0 | |||
| 0 | |||
| 0 | |||
| 0 | |||
| 0 | |||
| 0 | |||
| 0 |
| No | Reaction | Reaction Rate/(mol·m−3·s−1) |
|---|---|---|
| 1 | 3Fe2O3(fine, s) + CO(g) = 2Fe3O4(fine, s) + CO2(g) | , (i = 1,2,3), , , , |
| 2 | Fe3O4(fine, s) + CO(g) = 3FeO(fine, s) + CO2(g) | |
| 3 | FeO(fine, s) + CO(g) = Fe(fine, s) + CO2(g) | |
| 4 | C(fine,s) + CO2(g) = 2CO(g) |
| Condition | Variable | Case A | Case B |
|---|---|---|---|
| Solid inlet conditions | Ore supply rate (kg/s) | 4.05 | 3.64 |
| CCB supply rate (kg/s) | 0 | 0.41 | |
| Coke supply rate (kg/s) | 0.81 | - | |
| Solid temperature (K) | 300 | ||
| Gas inlet conditions | Gas supply rate (kg/s) | 3.88 | |
| Gas composition (mass fraction, -) | CO: 20, O2:13, N2:67 | ||
| Gas temperature (K) | 2350 | ||
| Index | Case A | Case B |
|---|---|---|
| Productivity (tHM·day−1) | 6250 | 6233 |
| Top gas temperature (K) | 463 | 455 |
| Top gas utilization (%) | 51.3 | 52.6 |
| Fuel rate (kg·tHM−1) | PC: 180 Coke:335, CCB carbon: 0 | PC: 180.5, Coke:282.8, CCB carbon gasified above CZ: 30 above CZ:30.0 PC:180.5, and CCB carbon gasified above CZ:30.0 |
| Item | Case A | Case B |
|---|---|---|
| Combustion | 175.0 | 175.5 |
| Carbon solution loss in upper BF | 56.8 | 42.5 |
| Direct reduction of molten FeO in lower BF | 49.2 | 10.8 |
| Carburization of molten iron | 45.0 | 45.0 |
| Other reactions | 9.0 | 9.0 |
| Total | 335.0 | 282.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, Z.; Tang, H.; Guo, Z. Experimental and Numerical Investigations on Charging Carbon Composite Briquettes in a Blast Furnace. Metals 2021, 11, 1669. https://doi.org/10.3390/met11111669
Wang Q, Liu Z, Tang H, Guo Z. Experimental and Numerical Investigations on Charging Carbon Composite Briquettes in a Blast Furnace. Metals. 2021; 11(11):1669. https://doi.org/10.3390/met11111669
Chicago/Turabian StyleWang, Qiang, Zhu Liu, Huiqing Tang, and Zhancheng Guo. 2021. "Experimental and Numerical Investigations on Charging Carbon Composite Briquettes in a Blast Furnace" Metals 11, no. 11: 1669. https://doi.org/10.3390/met11111669
APA StyleWang, Q., Liu, Z., Tang, H., & Guo, Z. (2021). Experimental and Numerical Investigations on Charging Carbon Composite Briquettes in a Blast Furnace. Metals, 11(11), 1669. https://doi.org/10.3390/met11111669






