Continuous Cooling Transformation of Under-Cooled Austenite of SXQ500/550DZ35 Hydropower Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
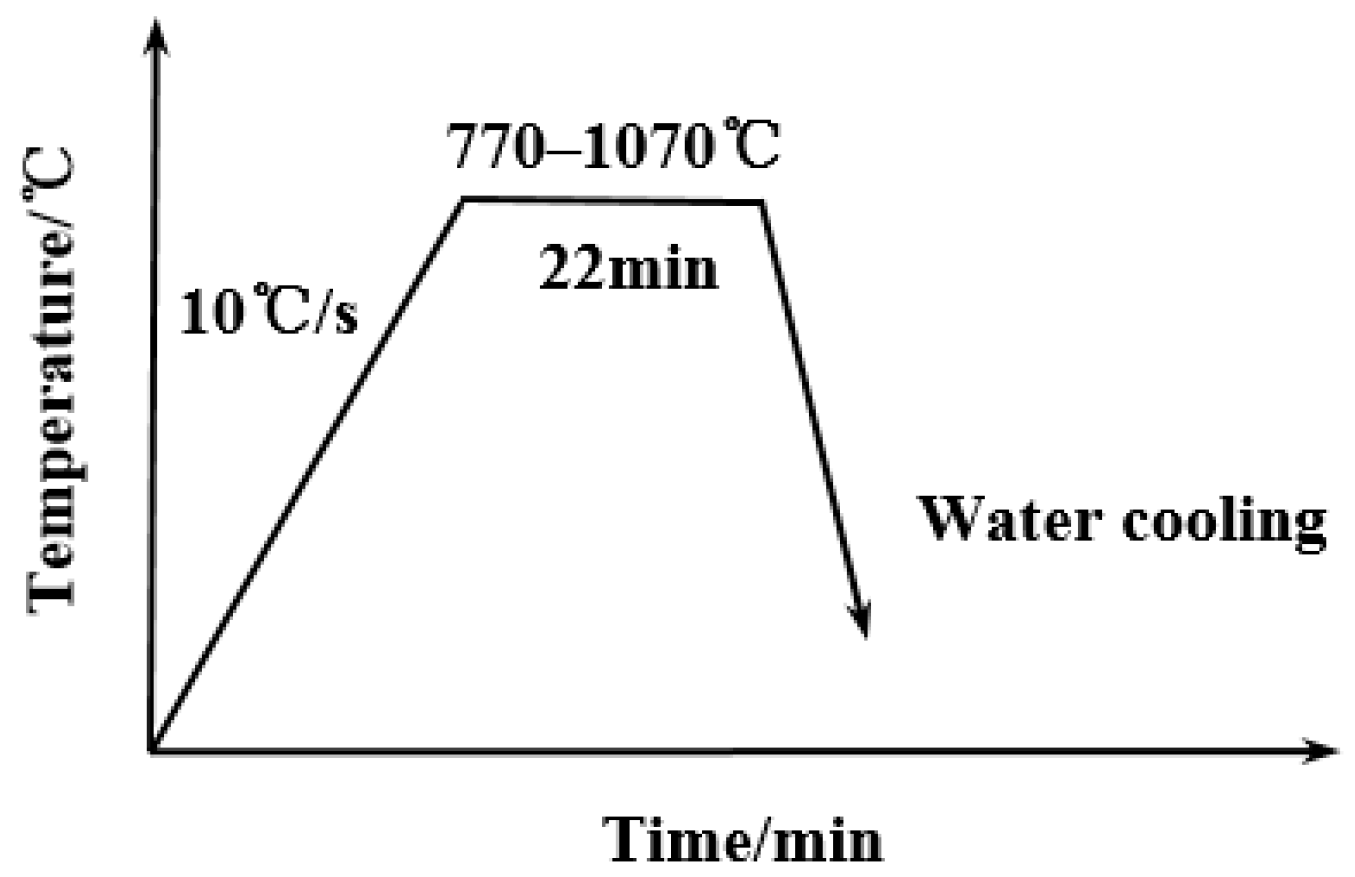
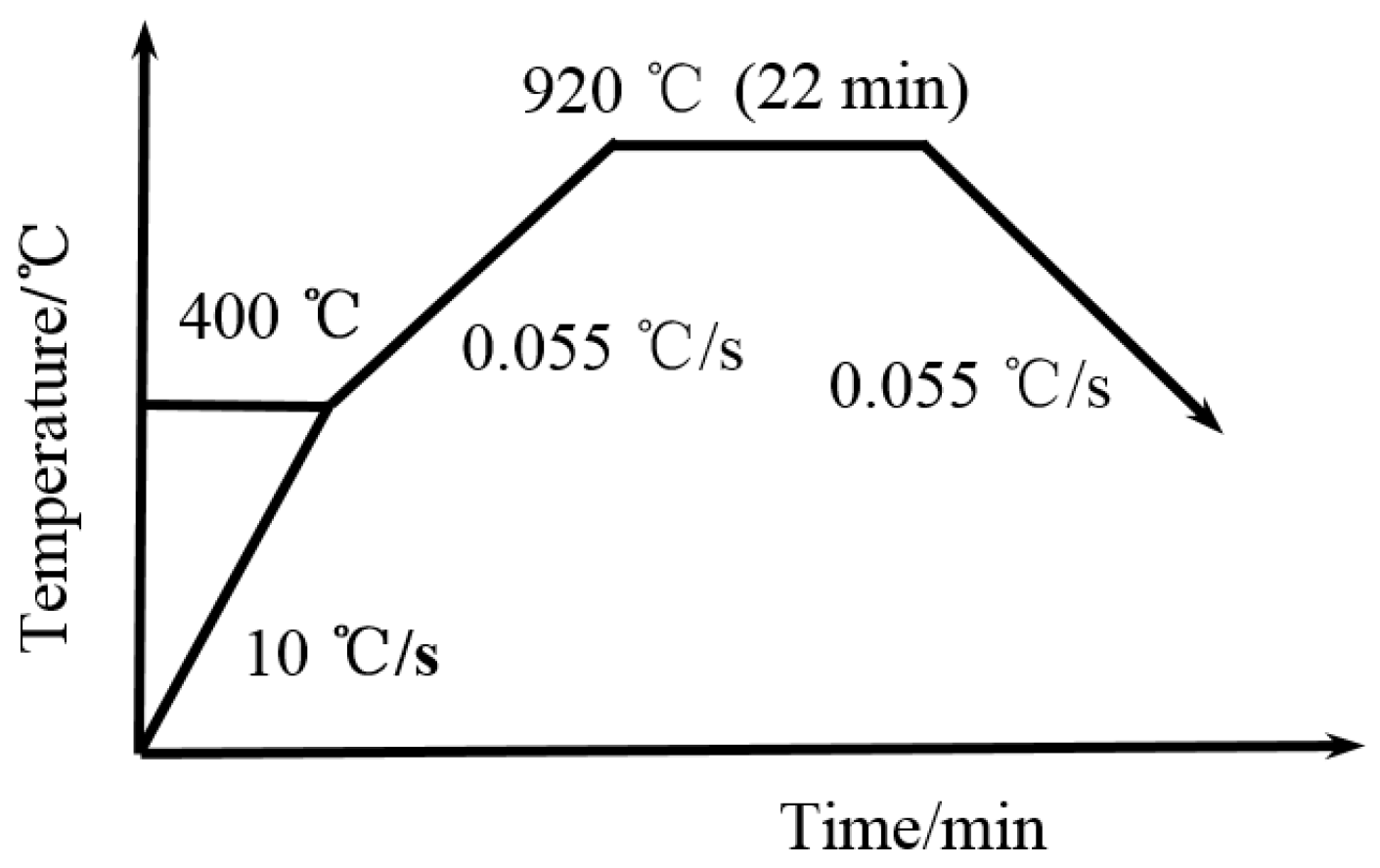
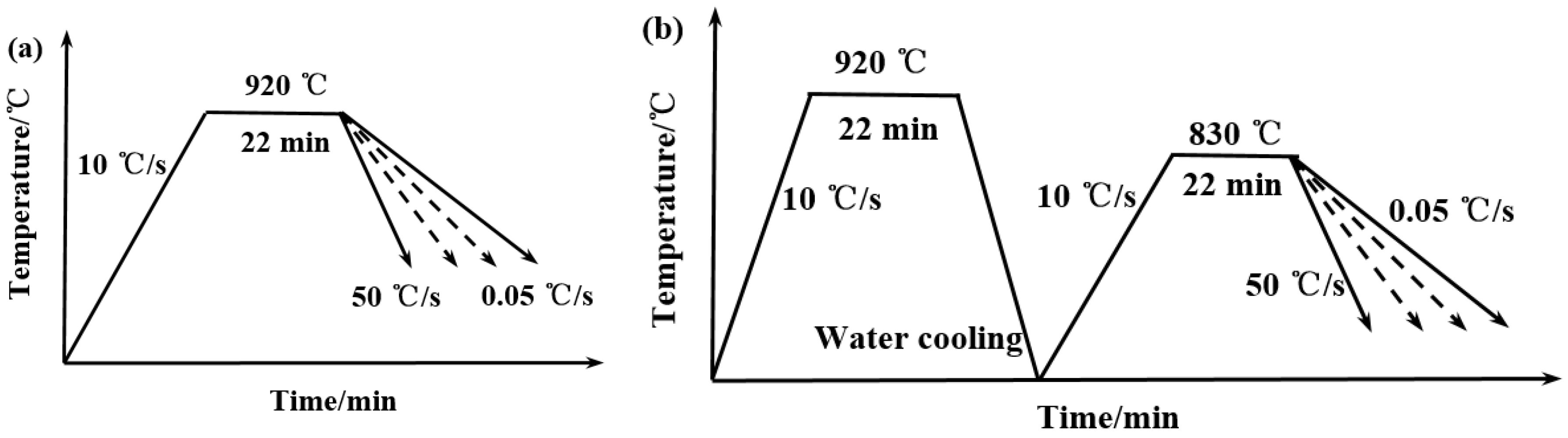
2.2. Experimental Method
3. Experimental Results and Discussion
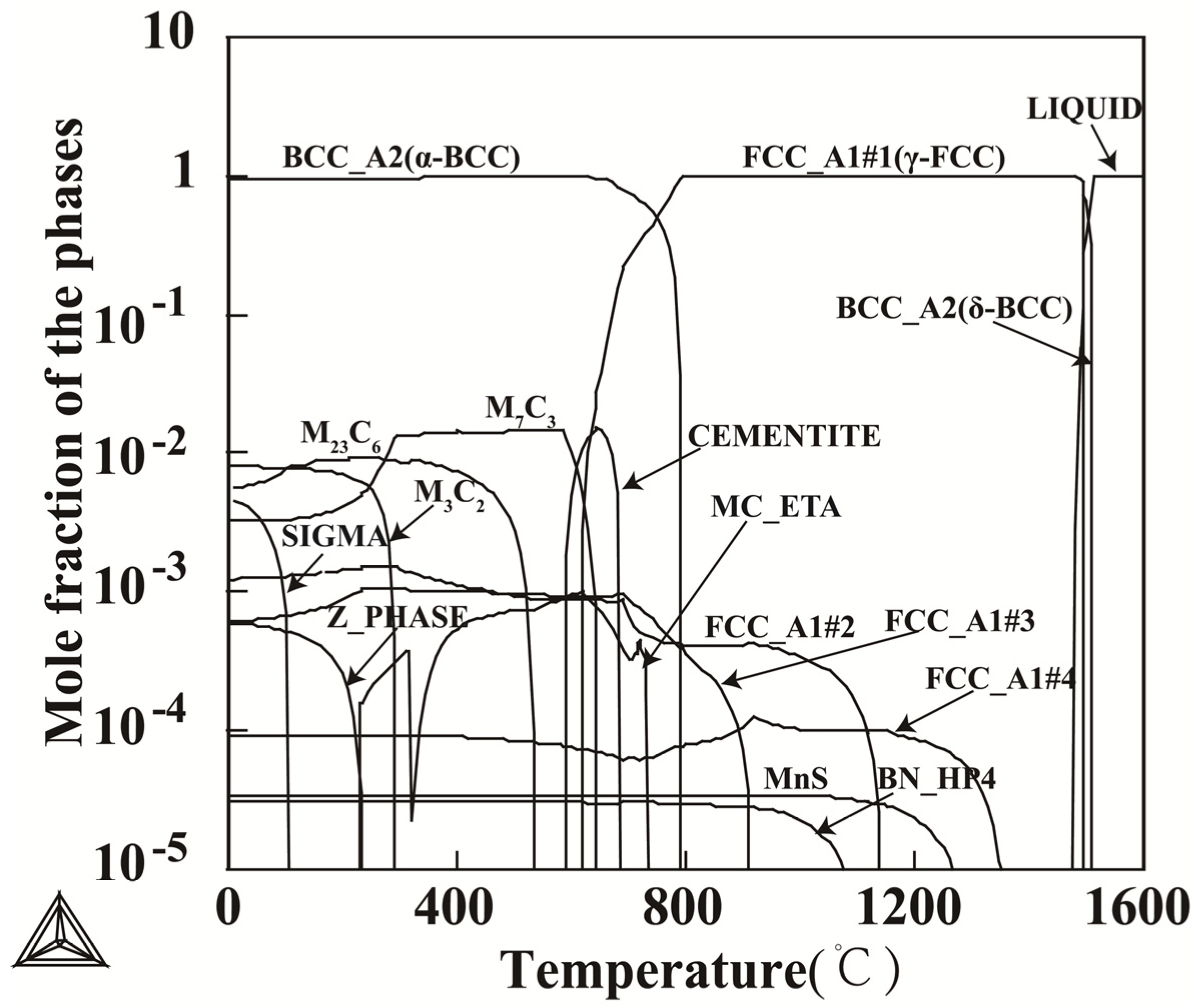
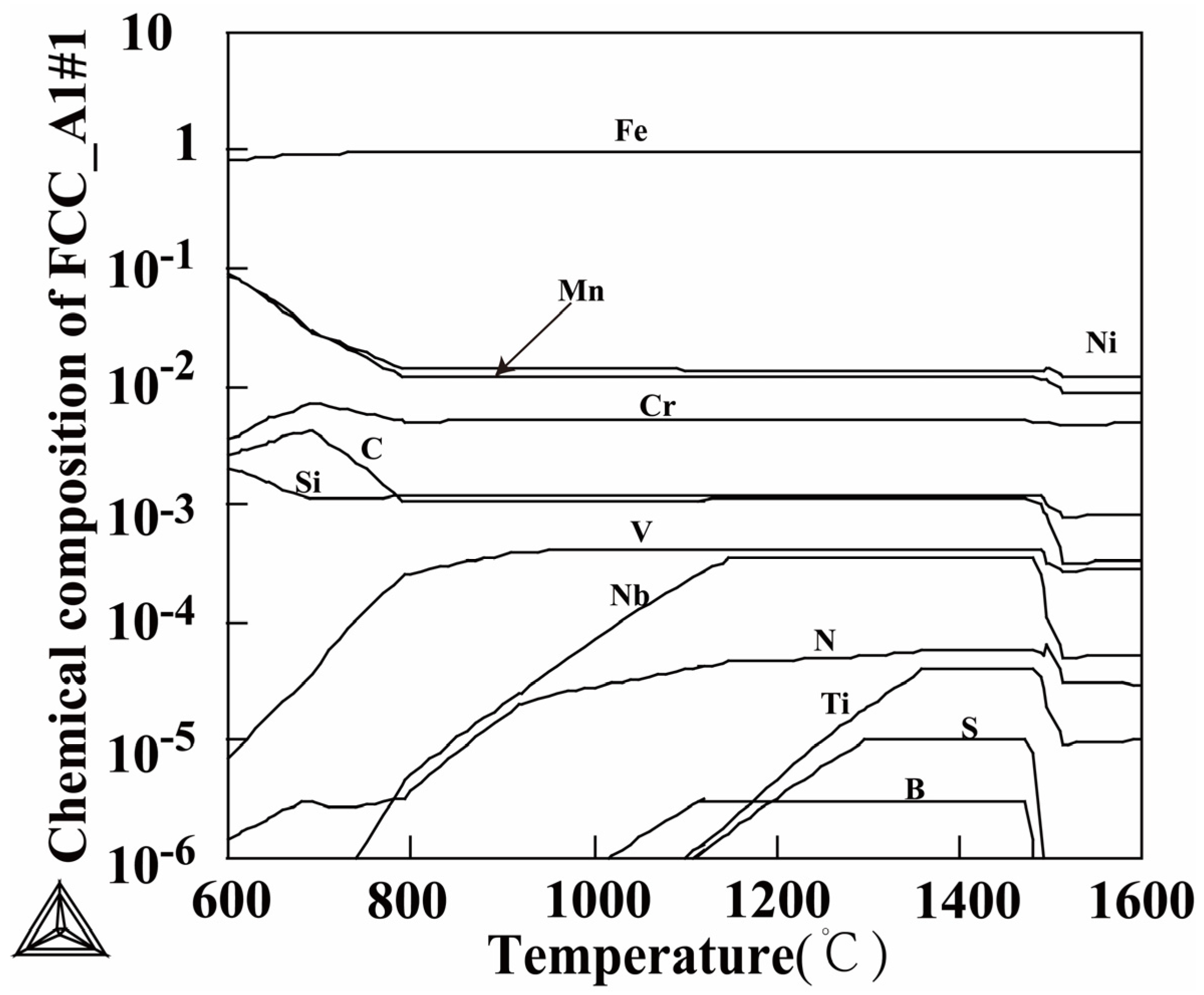
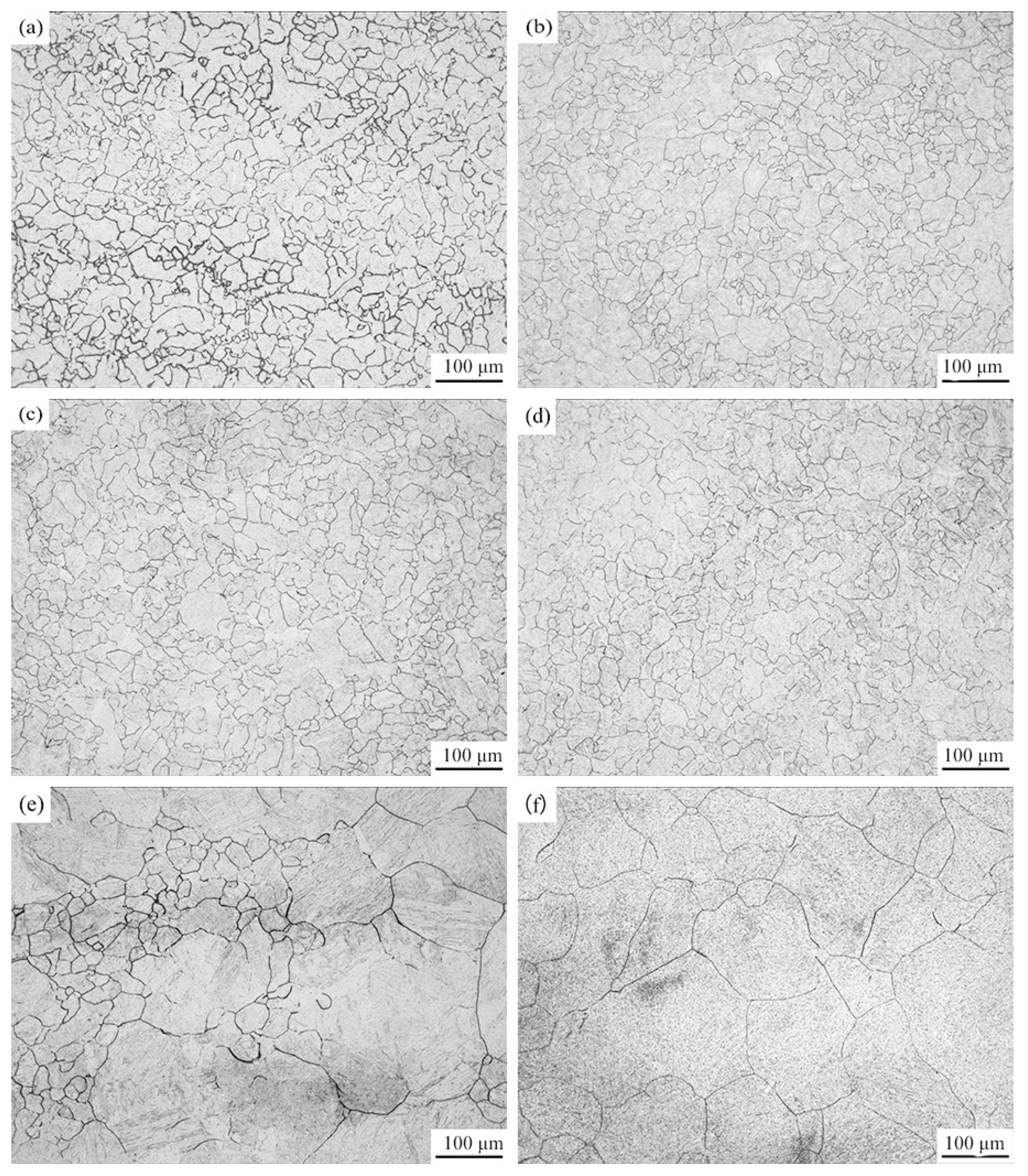
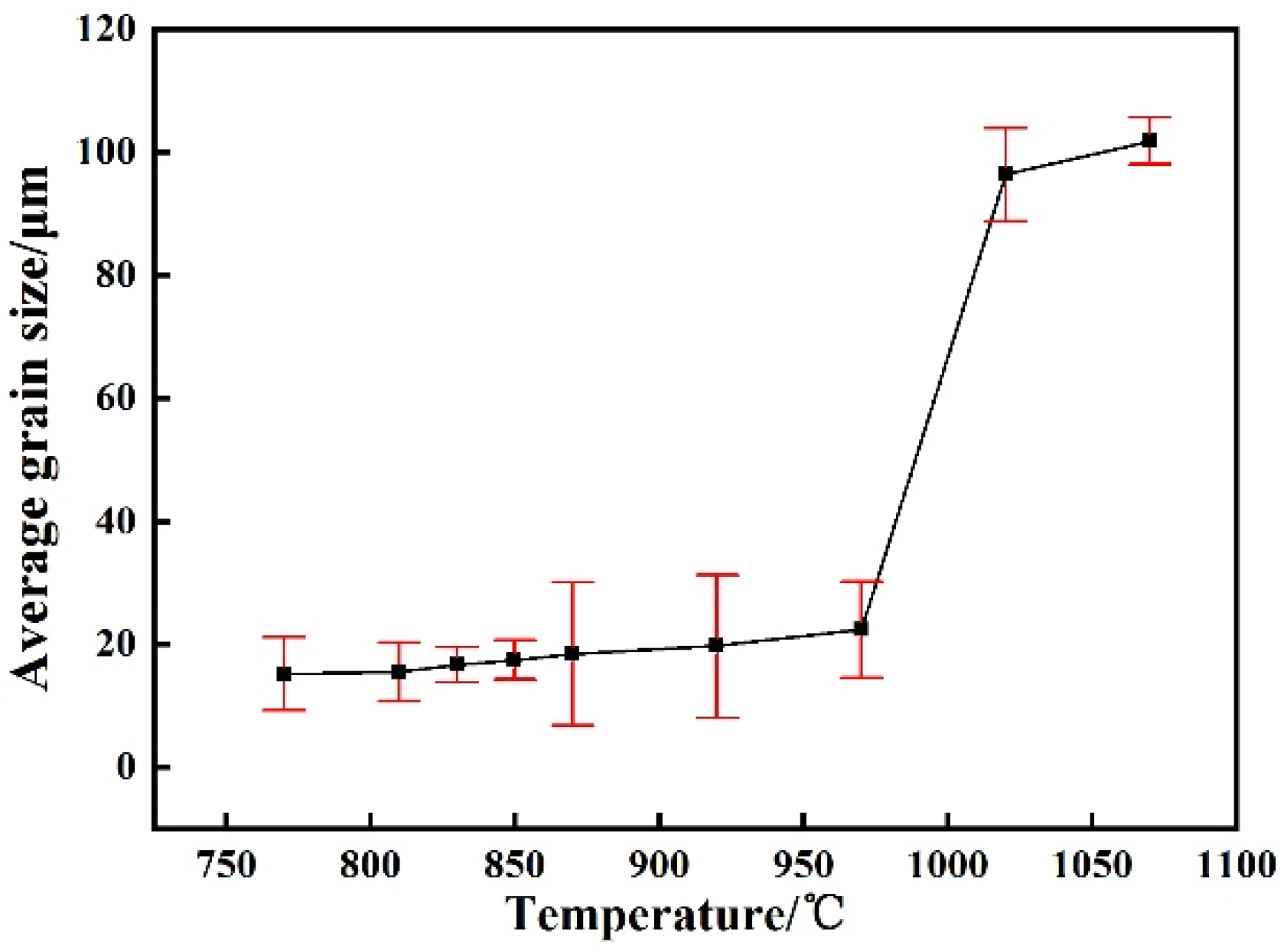
3.1. Determination of Austenitization Temperature
3.2. Determination of Critical Points
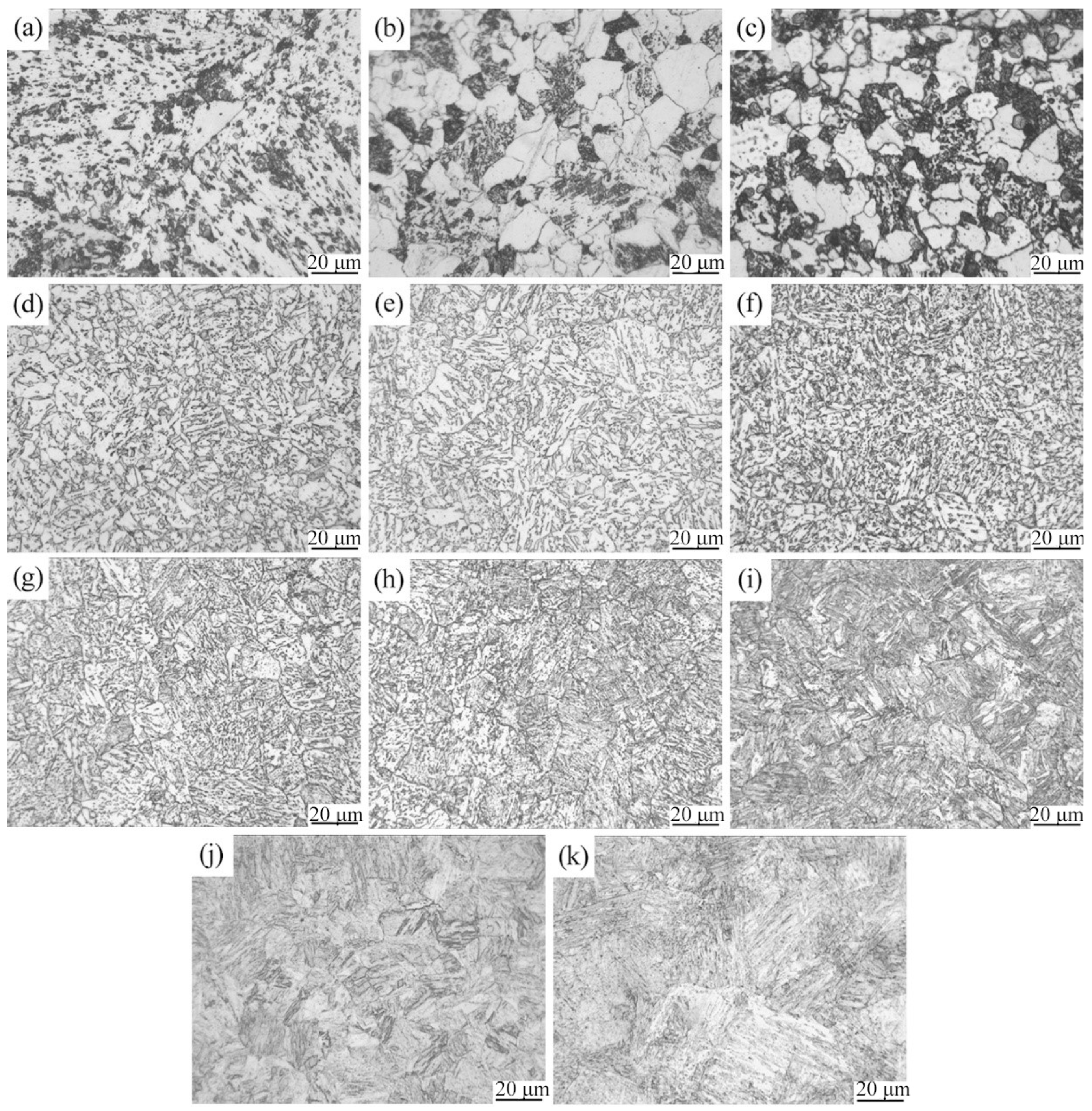
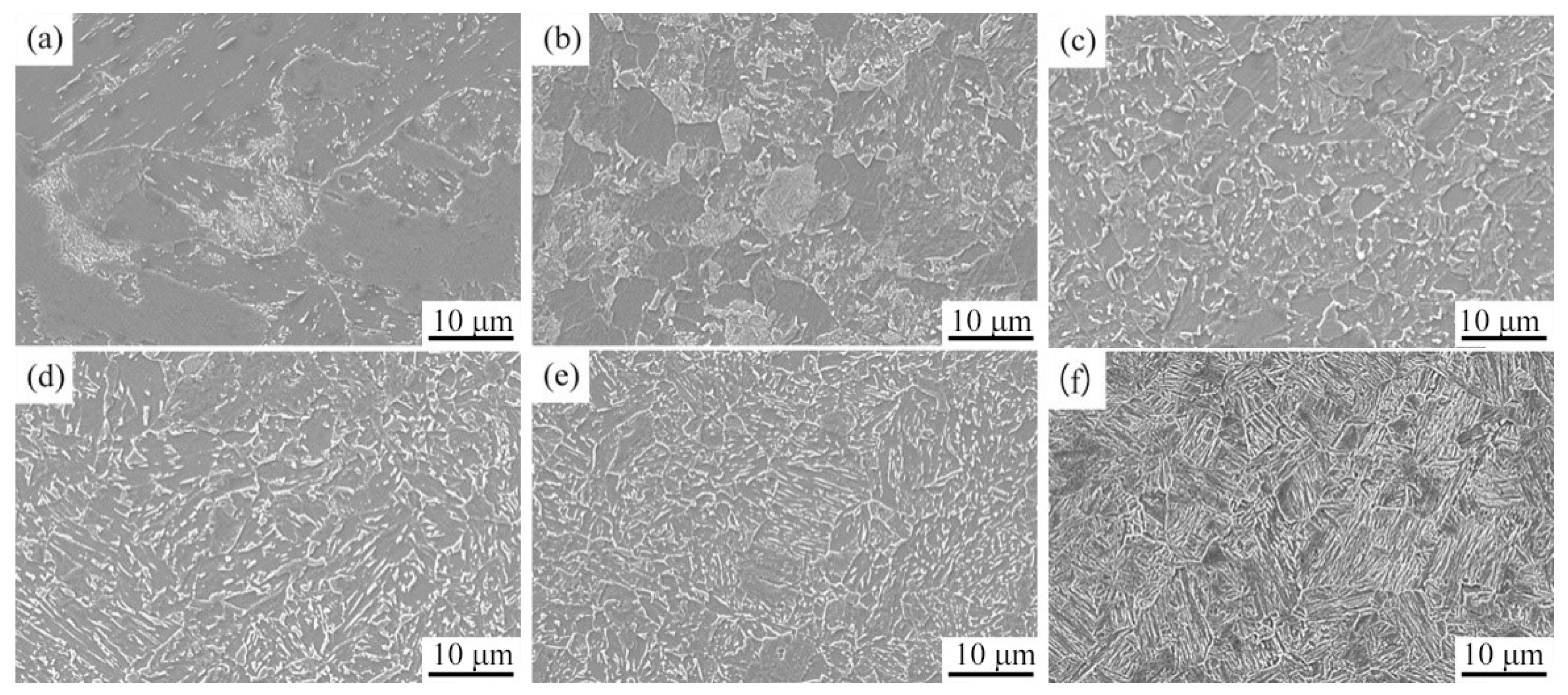
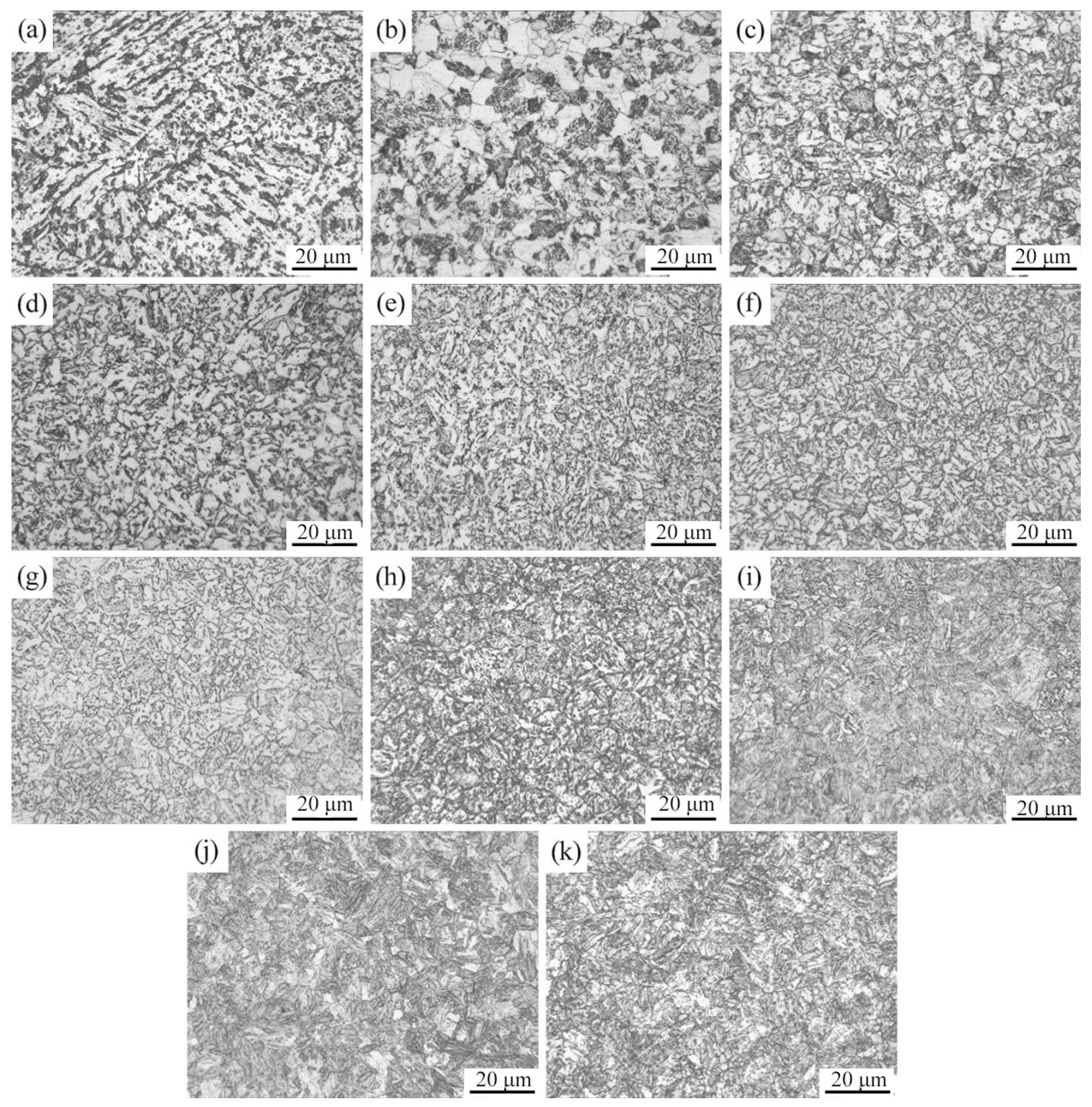
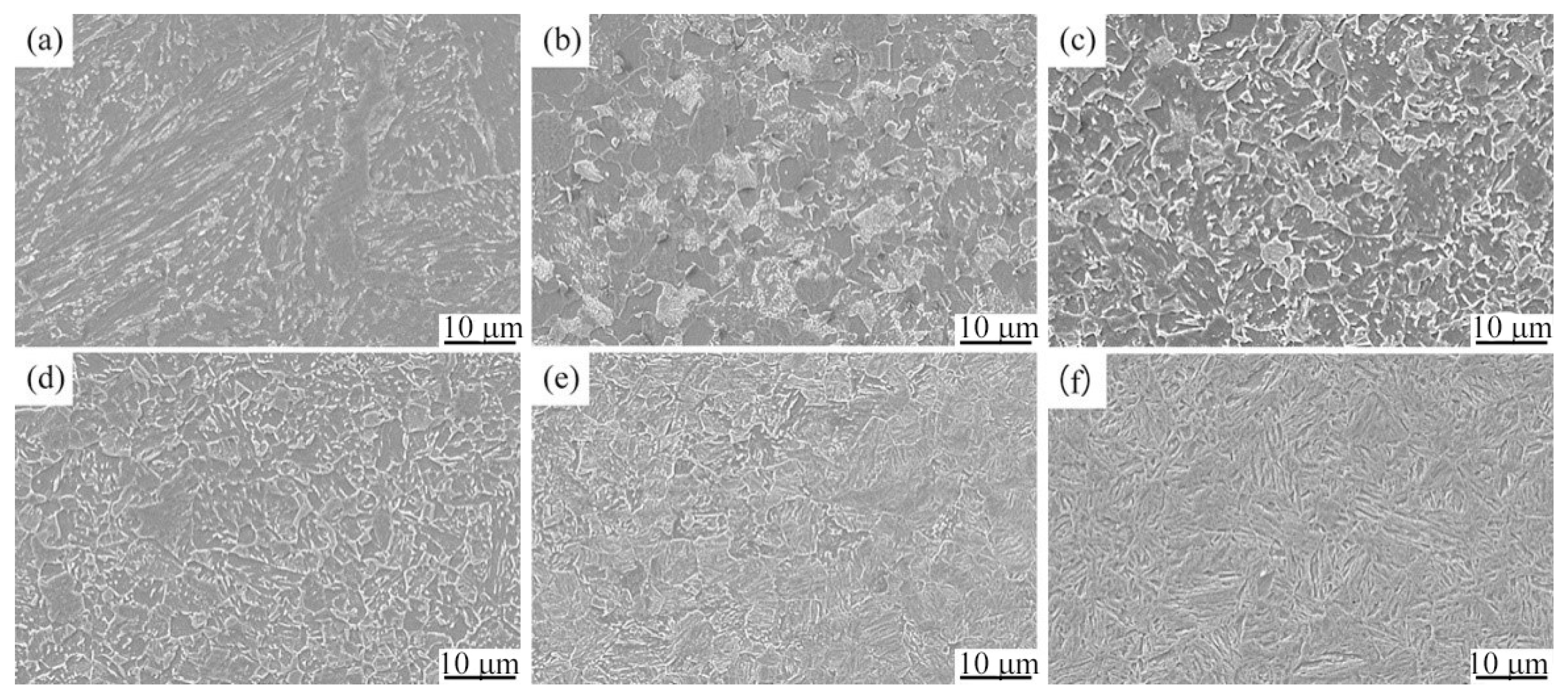
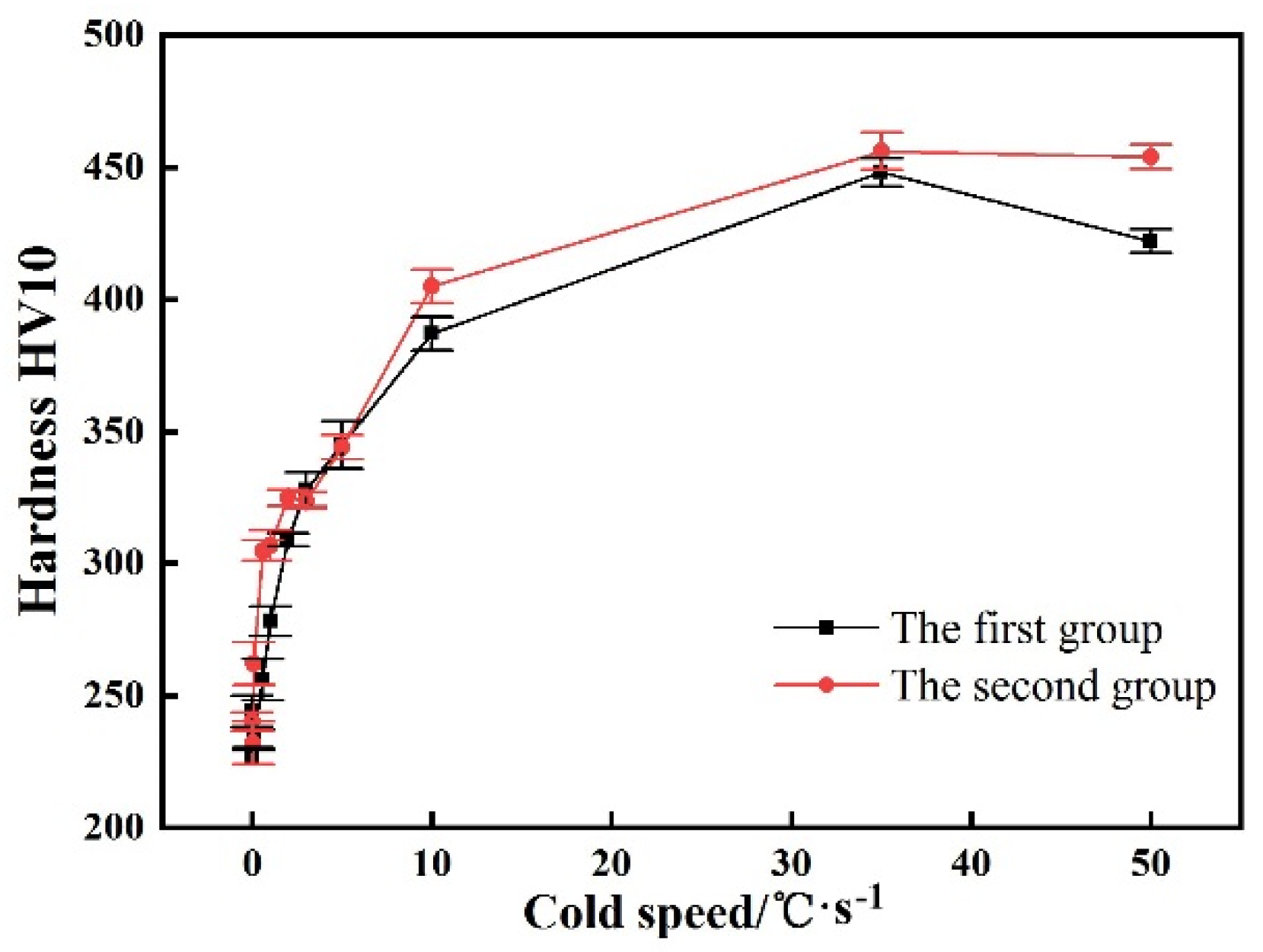
3.3. Microstructure and Hardness of the Experimental Steel at Different Cooling Rates
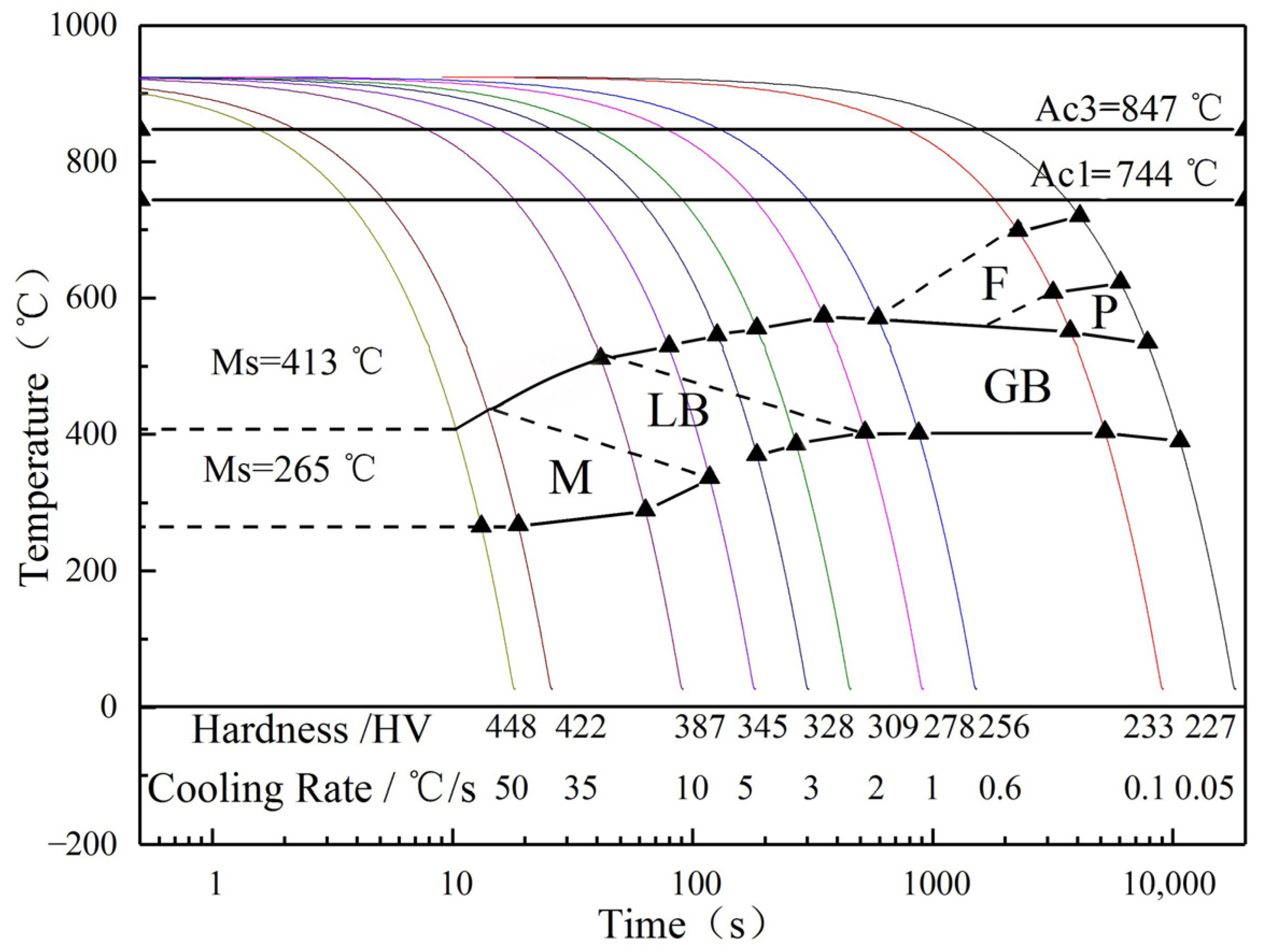
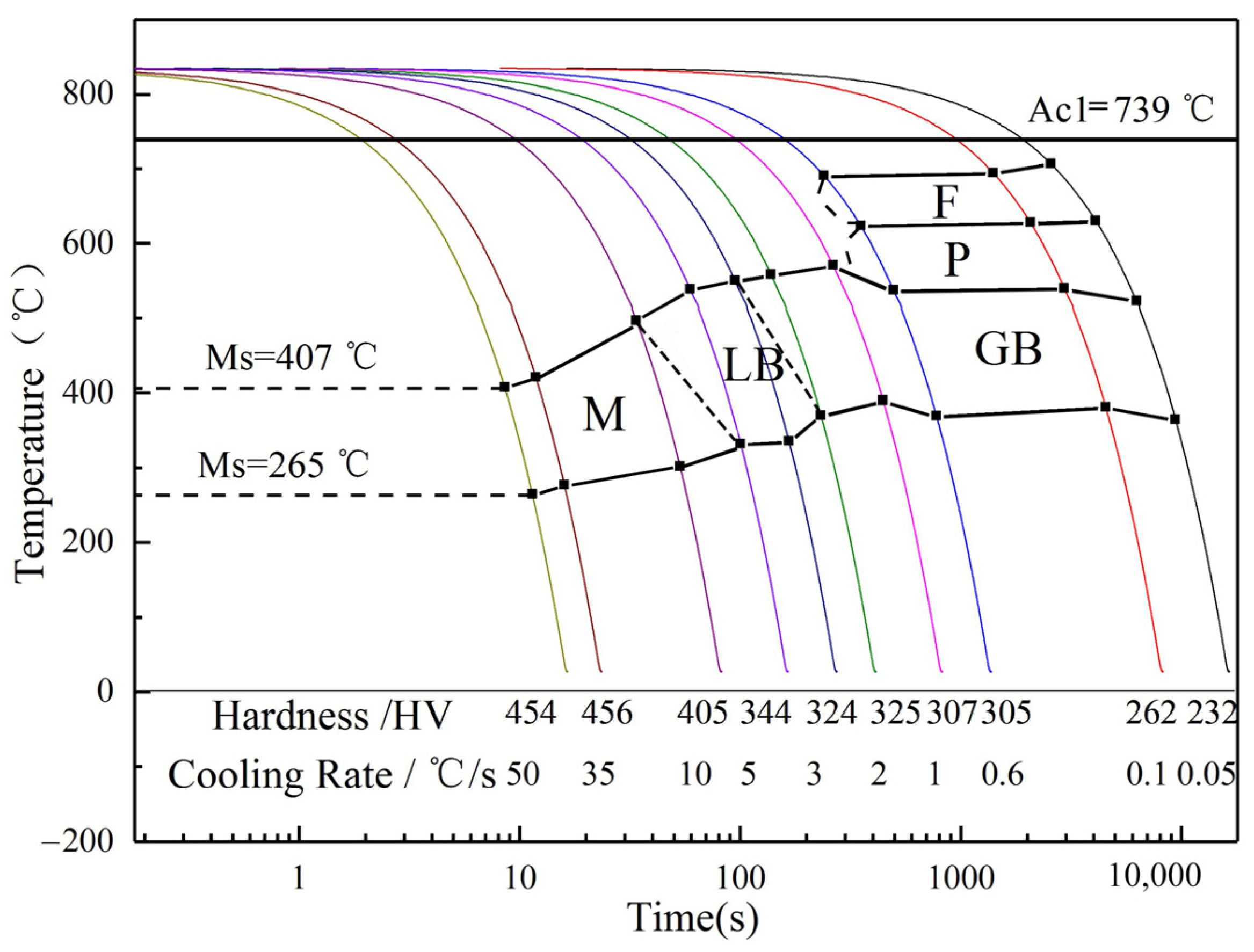
3.4. Drawing of CCT Curve of the Experimental Steel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.Y. Study on Microstructure Control and Low Temperature Toughness of 700 MPa Marine Steel. Ph.D. Thesis, Liaoning University of Science and Technology, Anshan, China, 2020. [Google Scholar]
- Sun, M.Y. Study on Multiphase Microstructure Control and Mechanical Behavior of Marine Engineering Steel for Low Temperature. Ph.D. Thesis, Beijing University of Science and Technology, Beijing, China, 2020. [Google Scholar]
- Ding, W.; Tang, D.; Jiang, H.T. Annealing process and microstructure evolution of 780 MPa grade TRIP steel for hot-dip galvanizing. J. Univ. Sci. Technol. Beijing 2010, 32, 753–758. [Google Scholar]
- Zhang, M. Study on Quenching and Tempering Treatment in Dual-Phase Zone of Q345 Structural Steel. Ph.D. Thesis, Shandong University, Jinan, China, 2019. [Google Scholar]
- Zhang, Y.; Wang, F.M.; Tang, Z.L.; Wang, L. Austenite grain growth behavior of SXQ500/550D steel and its influencing factors. Met. Heat Treat. 2019, 44, 110–118. [Google Scholar]
- Li, M.B.; Hu, P. Effect of TMCP process on microstructure and properties of Si-Mn bainitic steel. J. Eng. Sci. 2016, 38, 1115–1122. [Google Scholar]
- Huang, J.; Poole, W.J.; Militzer, M. Austenite formation during intercritical annealing. Metall. Mater. Trans. A 2004, 35, 3363–3375. [Google Scholar] [CrossRef]
- Lee, S.; De Cooman, B.C. Effect of the Intercritical Annealing Temperature on the Mechanical Properties of 10 Pct Mn Multi-Phase Steel. Metall. Mater. Trans. A 2014, 45, 5009–5016. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Cooman, B. Annealing Temperature Dependence of the Tensile Behavior of 10 pct Mn Multi-Phase TWIP-TRIP Steel. Metall. Mater. Trans. A 2014, 45, 6039–6052. [Google Scholar] [CrossRef] [Green Version]
- Wei, R.; Enomoto, M.; Hadian, R. Growth of austenite from as-quenched martensite during intercritical annealing in an Fe-0.1C-3Mn-1.5Si alloy. Acta Mater. 2013, 61, 697–707. [Google Scholar] [CrossRef]
- Mohanty, R.R.; Girina, O.A.; Fonstein, N.M. Effect of Heating Rate on the Austenite Formation in Low-Carbon High-Strength Steels Annealed in the Intercritical Region. Metall. Mater. Trans. A 2011, 42, 3680–3690. [Google Scholar] [CrossRef]
- Ding, R.; Tang, D.; Chen, Y.L.; Zhao, A.M.; Jiang, Y.H. Effect of annealing temperature on microstructure and mechanical properties of annealed martensitic TRIP steel. J. Univ. Sci. Technol. Beijing 2014, 36, 1476–1482. [Google Scholar]
- Yang, D.Z.; Sun, X.W.; Lei, T.C. Effect of intercritical quenching on microstructure and tensile properties of steels 15 and 15Mn2Nb. J. Mater. Sci. 1983, 18, 2727–2731. [Google Scholar] [CrossRef]
- Feng, L.L.; Hu, F.; Qiao, W.W. Study on Microstructure and Properties of Low Carbon Bainitic Steel 07MnCrMoVR for Hydropower Station. Iron Steel Res. 2020, 32, 1124–1131. [Google Scholar]
- Zhu, J.Q.; Tan, Z.L.; Zhang, M. Effect of Vanadium and Chromium on Tempering Microstructure and Properties of Bainitic Wheel Steel. Rare Met. 2020, 44, 957–966. [Google Scholar]
- Andersson, J.O. Thermo-Calc and DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Guidelines for preparation, execution and evaluation of dilatometric transformation tests on iron alloys. In Stahl-Eisen-Prüfblatt (SEP), 2nd ed.; Stahldaten: Wuppertal, Germany, 1681.
- ASTM A1033-10, Standard Practice for Quantitative Measurement and Reporting of Hypoeutectoid Carbon and Low-Alloy Steel Phase Transformations; Committee A01 on Steel, Stainless Steel and Related Alloys; ASTM International: West Conshohocken, PA, USA, 2010.
- YB/T 5127-2018, Steel-Determination of Critical Point-Dilatometric Method. CN-YB, 2018. Available online: https://www.chinesestandard.net/PDF/English.aspx/YBT5127-2018 (accessed on 25 August 2021).
- Huang, J.B.; He, I.; Huo, J. Continuous cooling phase transformation structure of 30NiCrMoV12 high speed rail axle steel. J. Mater. Heat Treat. 2021, 42, 132–139. [Google Scholar]
- Jang, J.; Ju, J.; Lee, B.; Kwon, D.; Kim, W.S. Effects of microstructural change on fracture characteristics in coarse-grained heat-affected zones of QLT-processed 9% Ni steel. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2003, 340, 68. [Google Scholar] [CrossRef]
- Wu, S.J.; Sun, G.J.; Ma, Q.S.; Shen, Q.Y.; Xu, L. Influence of QLT treatment on microstructure and mechanical properties of a high nickel steel. J. Mater. Process. Technol. 2013, 213, 120–128. [Google Scholar] [CrossRef]
- Liang, J.T.; Zhao, Z.Z.; Liu, K. Microstructure and properties of 1300 MPa Nb micro-alloyed DH steel. J. Eng. Sci. 2021, 43, 392–399. [Google Scholar]
- Yu, W.T.; Li, J.; Shi, C.B.; Zhu, Q.T.; Song, H.D.; Shi, C.M. As-cast microstructure and carbides of high carbon martensitic stainless steel 8Cr13MoV steel. J. Eng. Sci. 2016, 38, 1264–1269. [Google Scholar]
- Sugimoto, K.; Tanino, H. Influence of Cooling Process Routes after Intercritical Annealing on Impact Toughness of Duplex Type Medium Mn Steel. Metals 2021, 11, 1143. [Google Scholar] [CrossRef]
- Gonzalez-Pocino, A.; Asensio-Lozano, J.; Alvarez-Antolin, F.; García-Diez, A. Evaluation of Hardness, Sliding Wear and Strength of a Hypoeutectic White Iron with 25% Cr after Heat Treatments. Metals 2021, 11, 947. [Google Scholar] [CrossRef]
- Marshall, D.B.; Oliver, W.C. Measurement of Interfacial Mechanical Properties in Fiber-Reinforced Ceramic Composites. J. Am. Ceram. Soc. 2010, 70, 542–548. [Google Scholar] [CrossRef]
| C | Si | Mn | P | S | V | Ti | Als | O |
|---|---|---|---|---|---|---|---|---|
| 0.11 | 0.12 | 1.18 | 0.009 | 0.001 | 0.042 | 0.004 | 0.019 | 0.0015 |
| Cr | Mo | Nb | Ni | Cu | B | N | As | - |
| 0.506 | 0.386 | 0.035 | 1.380 | 0.020 | 0.0013 | 0.0058 | 0.017 | - |
| Cold Speed/°C·s−1 | First Group | Hardness | Second Group | Hardness | |
|---|---|---|---|---|---|
| a | original microstructure | F + GB + P | 244 | GB + LB | 240 |
| b | 0.05 | F + P + GB | 227 | F + P + GB | 232 |
| c | 0.1 | GB + F + P | 233 | GB + F + P | 262 |
| d | 0.6 | GB + F + LBless | 256 | GB + F | 305 |
| e | 1 | GB + LB | 278 | GB + F | 307 |
| f | 2 | GB + LB | 309 | GB + LB + F | 325 |
| g | 3 | GB + LB | 328 | GB + LB + F | 324 |
| h | 5 | LB + Mless | 345 | LB + Mless + F | 344 |
| i | 10 | M + LBless | 387 | M + LBless + F | 405 |
| j | 35 | M + A’ | 448 | M + A’ + F | 456 |
| k | 50 | M + A’ | 422 | M + A’ + F | 454 |
| Cold Speed/°C·s−1 | Fs/°C | Ff/°C | Ps/°C | Pf/°C | Bs/°C | Bf/°C | Ms/°C | Mf/°C |
| 0.05 | 721 | - | 623 | - | 535 | 390 | - | - |
| 0.1 | 699 | - | 608 | - | 552 | 404 | - | - |
| 0.6 | - | - | - | - | 571 | 402 | - | - |
| 1 | - | - | - | - | 574 | 403 | - | - |
| 2 | - | - | - | - | 556 | 386 | - | - |
| 3 | - | - | - | - | 546 | 370 | - | - |
| 5 | - | - | - | - | 530 | - | - | 337 |
| 10 | - | - | - | - | 512 | - | - | 289 |
| 35 | - | - | - | - | - | - | 435 | 267 |
| 50 | - | - | - | - | - | - | 413 | 265 |
| Cold Speed/°C·s−1 | Fs/°C | Ff/°C | Ps/°C | Pf/°C | Bs/°C | Bf/°C | Ms/°C | Mf/°C |
|---|---|---|---|---|---|---|---|---|
| 0.05 | 707 | - | 631 | - | 524 | 365 | - | - |
| 0.1 | 695 | - | 628 | - | 540 | 382 | - | - |
| 0.6 | 691 | - | 624 | - | 538 | 370 | - | - |
| 1 | - | - | - | - | 571 | 390 | - | - |
| 2 | - | - | - | - | 559 | 371 | - | - |
| 3 | - | - | - | - | 551 | 336 | - | - |
| 5 | - | - | - | - | 539 | 332 | - | - |
| 10 | - | - | - | - | 497 | - | - | 302 |
| 35 | - | - | - | - | - | - | 421 | 277 |
| 50 | - | - | - | - | - | - | 407 | 265 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Guo, R.; Zhang, Y.; Liu, Z.; Lu, Y.; Wang, F. Continuous Cooling Transformation of Under-Cooled Austenite of SXQ500/550DZ35 Hydropower Steel. Metals 2021, 11, 1562. https://doi.org/10.3390/met11101562
Tang Z, Guo R, Zhang Y, Liu Z, Lu Y, Wang F. Continuous Cooling Transformation of Under-Cooled Austenite of SXQ500/550DZ35 Hydropower Steel. Metals. 2021; 11(10):1562. https://doi.org/10.3390/met11101562
Chicago/Turabian StyleTang, Zhenglei, Ran Guo, Yang Zhang, Zhen Liu, Yuezhang Lu, and Fuming Wang. 2021. "Continuous Cooling Transformation of Under-Cooled Austenite of SXQ500/550DZ35 Hydropower Steel" Metals 11, no. 10: 1562. https://doi.org/10.3390/met11101562
APA StyleTang, Z., Guo, R., Zhang, Y., Liu, Z., Lu, Y., & Wang, F. (2021). Continuous Cooling Transformation of Under-Cooled Austenite of SXQ500/550DZ35 Hydropower Steel. Metals, 11(10), 1562. https://doi.org/10.3390/met11101562








