Research on the Mathematical Model of Local Equilibrium in the Top-Blown Smelting Process of Electronic Waste
Abstract
:1. Introduction
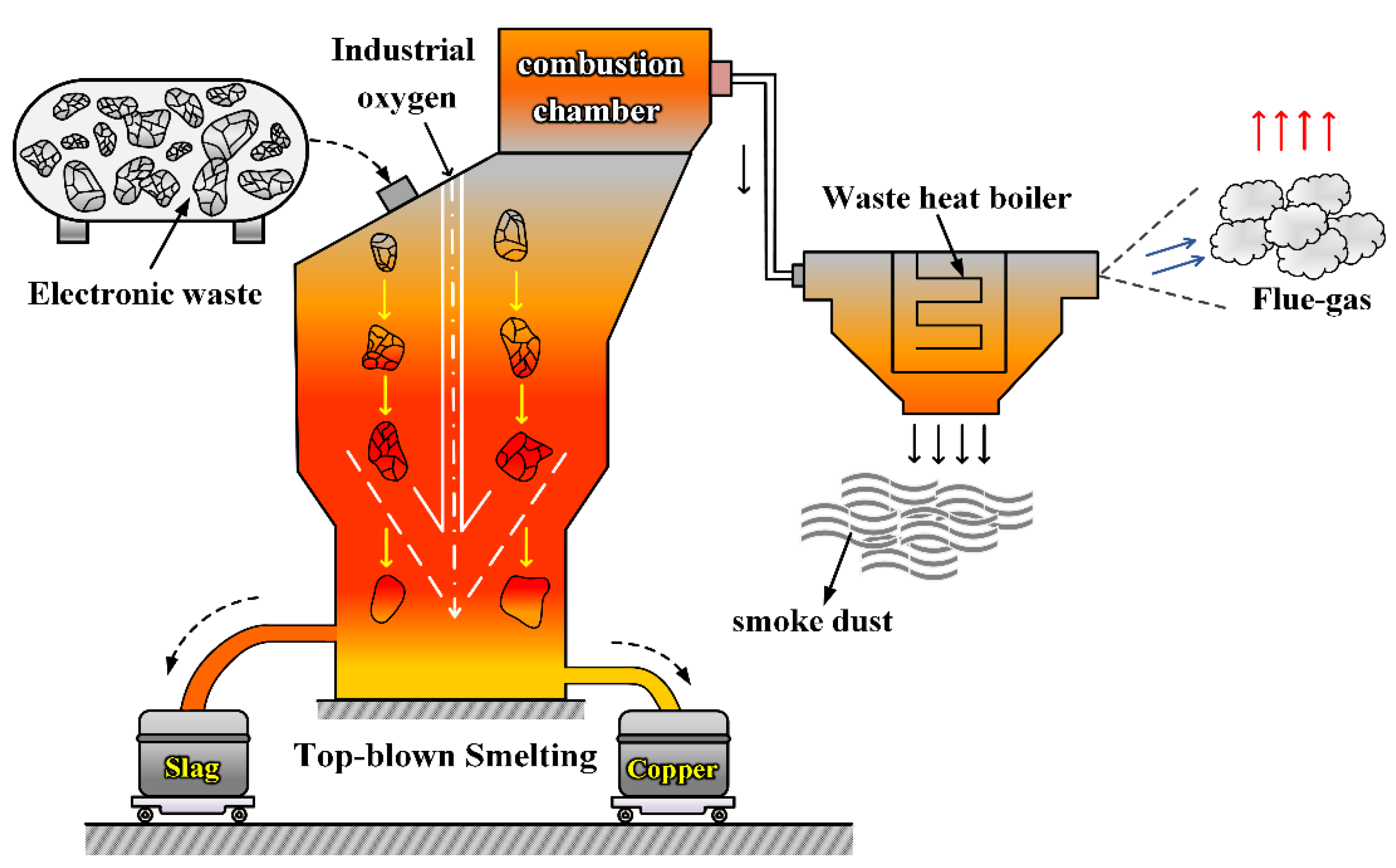
2. Top-Blowing Smelting System of Electronic Waste
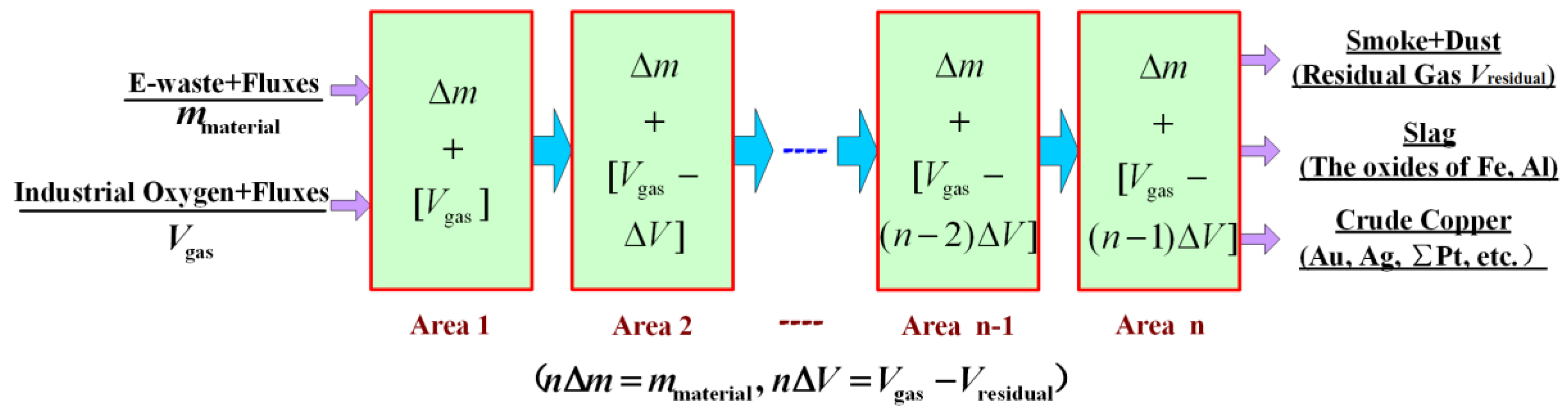
2.1. Modeling Method of Local Equilibrium
2.2. Physical Model Description
3. Mathematical Model and Algorithm
3.1. Mathematical Description
3.2. Solution Algorithm of the Model
3.3. The Calculation Process of the Mathematical Model
- Local iteration
- 2.
- Composition iteration
- 3.
- Activity coefficient iteration
4. Results and Discussion
4.1. Thermodynamic Parameters
4.2. Process Parameters
4.2.1. Material and Methods
4.2.2. Conditional Control
4.3. The Foundation of a Simulation System
4.4. Test Case
4.4.1. Material Balance of Input-Output
4.4.2. The Contents of Products
4.4.3. Heat Balance of Input–Output
4.4.4. Comparisons with Industrial Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cayumil, R.; Khanna, R.; Rajarao, R.; Mukherjee, P.S.; Sahajwalla, V. Concentration of precious metals during their recovery from electronic waste. Waste Manag. 2016, 57, 121–130. [Google Scholar] [CrossRef]
- Kumari, A.; Jha, M.K.; Lee, J.; Singh, R.P. Clean process for recovery of metals and recycling of acid from the leach liquor of PCBs. J. Clean Prod. 2016, 112, 4826–4834. [Google Scholar] [CrossRef]
- Bidini, G.; Fantozzi, F.; Bartocci, P.; D‘Alessandro, B.; D‘Amico, M.; Laranci, P.; Scozza, E.; Zagaroli, M. Recovery of precious metals from scrap printed circuit boards through pyrolysis. J. Anal. Appl. Pyrol. 2015, 111, 140–147. [Google Scholar] [CrossRef]
- Remeteiová, D.; Ružičková, S.; Mičková, V.; Laubertová, M.; Slezáková, R. Evaluation of US EPA Method 3052 Microwave Acid Digestion for Quantification of Majority Metals in Waste Printed Circuit Boards. Metals 2020, 10, 1511. [Google Scholar] [CrossRef]
- Burke, M. The gadget scrap heap. Chem. World 2007, 4, 45–48. [Google Scholar]
- Cucchiella, F.; D’Adamo, I.; Koh, S.C.L.; Rosa, P. Recycling of WEEEs: An economic assessment of present and future e-waste streams. Renew. Sustain. Energy Rev. 2015, 51, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Brodersen, K.; Tartler, D.; Danzer, B. Scrap of electronics a challenge to recycling activities. In Proceedings of the 1994 IEEE International Symposium on Electronics and the Environment, San Francisco, CA, USA, 2–4 May 1994; pp. 174–176. [Google Scholar]
- Duan, H.B.; Huang, Q.F.; Wang, Q.; Zhou, B.Y.; Li, J.H. Hazardous waste generation and management in China: A review. J. Hazard. Mater. 2008, 158, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kwapisiński, P.; Wołczyński, W.; Bydałek, A.W.; Schlafka, P.; Bydałek, A. Analysis of separation mechanism of the metallic phase of slag in the Direct-To-Blister Process. Arch. Metall. Mater. 2015, 60, 2347–2353. [Google Scholar]
- Meng, L.; Zhong, Y.W.; Guo, L.; Wang, Z.; Chen, K.Y.; Guo, Z.C. High-temperature centrifugal separation of Cu from waste printed circuit boards. J. Clean. Prod. 2018, 199, 831–839. [Google Scholar] [CrossRef]
- Meng, L.; Wang, Z.; Zhong, Y.W.; Guo, L.; Gao, J.T.; Chen, K.Y.; Cheng, H.J.; Guo, Z.C. Supergravity separation for recovering metals from waste printed circuit boards. Chem. Eng. J. 2017, 326, 540–550. [Google Scholar] [CrossRef]
- Tabassum, S.; Ghosh, A.; Meshram, P.; van Hullebusch, E.D. Microbial Processing of Waste Shredded PCBs for Copper Extraction Cum Separation—Comparing the Efficacy of Bacterial and Fungal Leaching Kinetics and Yields. Metals 2021, 11, 317. [Google Scholar]
- Argumedo-Delira, R.; Díaz-Martínez, M.E.; Gómez-Martínez, M.J. Microorganisms and plants in the recovery of metals from the printed circuit boards of computers and cell phones: A mini review. Metals 2020, 10, 1120. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; He, Y.; Xu, S.; Hu, B.; Cao, H.; Zhou, J.; Zheng, G. Efficient and safe disposition of arsenic by incorporation in smelting slag through copper flash smelting process. Miner. Eng. 2021, 160, 106661. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Yin, W.; Jiang, T.; Li, G. Thiosulfate leaching of Au, Ag and Pd from a high Sn, Pb and Sb bearing decopperized anode slime. Hydrometallurgy 2016, 164, 278–287. [Google Scholar] [CrossRef]
- Amer, A.M. Processing of copper anodic-slimes for extraction of valuable metals. Waste Manag. 2003, 23, 763–770. [Google Scholar] [CrossRef]
- Xia, Z. Simulation Research on Fluid Flow Behavior of Sirosmelt; Kunming University of Science and Technology: Kunming, China, 2015. [Google Scholar]
- Chen, Z.; Wang, Y.X.; Zhou, J.; Liu, A.M.; Mei, C. Simulation Study of Intensified Flash Smelting Process, Copper 2010; DGBM: Hamburg, Germany, 2010; pp. 1313–1323. [Google Scholar]
- Kang, H.Y.; Schoenung, J.M. Electronic waste recycling: A review of US infrastructure and technology options. Resour. Conserv. Recy. 2005, 45, 368–400. [Google Scholar] [CrossRef]
- Wołczyński, W.; Bydałek, A.W.; Tarasek, A.; Sypien, A. Copper droplets agglomeration/coagulation in the conditions similar to industrial ones. Arch. Metall. Mater. 2017, 62, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L. Metallurgical calculation and production practice of oxygen-enriched side-blown bath smelting process. China Nonferrous Metall. 2012, 41, 6–9. [Google Scholar]
- Xu, X.; Su, Y.; Huang, H. Simulation calculation of double side blow furnace’s process based on Metcal. Nonferrous Met. Extr. Metall. 2016, 6, 31–34. [Google Scholar]
- Zhang, H.L.; Zhou, C.Q.; Bing, W.U.; Chen, Y.M. Numerical simulation of multiphase flow in a Vanyukov furnace. J. S. Afr. I Min. Metall. 2015, 115, 457–463. [Google Scholar] [CrossRef]
- Li, M.Z.; Zhou, J.M.; Tong, C.R.; Zhang, W.H.; Chen, Z.; Wang, J.L. Thermodynamic Modeling and Optimization of the Copper Flash Converting Process Using the Equilibrium Constant Method. Metall. Mater. Trans. B. 2018, 49, 1794–1807. [Google Scholar] [CrossRef]
- Li, M.Z.; Tong, C.R.; Huang, J.; Wang, J.L. Simulated Calculation of Overall Process Flow of Copper Bottom Blowing Smelting. Chin. J. Process. Eng. 2016, 16, 1028–1037. [Google Scholar]
- Wołczyński, W. Pattern Selection in the Eutectic Growth—Thermodynamic Interpretation. Arch. Metall. Mater. 2020, 65, 653–666. [Google Scholar]
- Koshikawa, T.; Gandin, C.A.; Bellet, M.; Yamamura, H.; Bobadilla, M. Computation of phase transformation paths in steels by a combination of the partial- and para-equilibrium thermodynamic approximations. ISIJ Int. 2014, 54, 1274–1282. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.W.; Liu, Q.; Shibata, H.; Wang, Q.; Jönsson, P.; He, J.C.; Nakajima, K. Partial Equilibrium Prediction of Solidification and Carbide Precipitation in Ti-added High Cr Cast Irons. ISIJ Int. 2014, 54, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Zhang, C.F.; Zhang, W.H. Formation thermodynamic of Fe3O4 in reaction shaft of flash smelting furnace. J. Cent. South Univ. (Sci. Technol.) 2013, 44, 4787–4792. [Google Scholar]
- Lieb, E.H.; Yngvason, J. The physics and mathematics of the second law of thermodynamics. Phys. Rep. 1999, 310, 1–96. [Google Scholar] [CrossRef] [Green Version]
- Teh, Y.S.; Rangaiah, G.P. A study of equation-solving and Gibbs free energy minimization methods for phase equilibrium calculations. Chem. Eng. Res. Des. 2002, 80, 745–759. [Google Scholar] [CrossRef]
- Kambe, T. New perspectives on mass conservation law and waves in fluid mechanics. Fluid. Dyn. Res. 2020, 52, 031401. [Google Scholar] [CrossRef]
- Mohebujjaman, M.; Rebholz, L.G.; Xie, X.; Iliescu, T. Energy balance and mass conservation in reduced order models of fluid flows. J. Comput. Phys. 2017, 346, 262–277. [Google Scholar] [CrossRef]
- Chai, Z.H.; Sun, D.K.; Wang, H.L.; Shi, B.C. A comparative study of local and nonlocal Allen-Cahn equations with mass conservation. Int. J. Heat Mass. Tran. 2018, 122, 631–642. [Google Scholar] [CrossRef]
- Wang, J.L. Slag Activity Research and Numerical Simulation of Heavy Metal Smelting Process with Short Flow. Ph.D. Thesis, Central South University, Changsha, China, 2009. [Google Scholar]
- Ji, K.Y.; Wang, Z.; Zhou, Y.; Liang, Y.B. Improved zeroth-order variance reduced algorithms and analysis for nonconvex optimization. In Proceedings of the International Conference on Machine Learning (PMLR), Long Beach, CA, USA, 9–15 June 2019; pp. 3100–3109. [Google Scholar]
- Bloom, S.L.; Ésik, Z. Iteration Theories; Springer: Berlin/Heidelberg, Germany, 1993; pp. 159–213. [Google Scholar]
- Ye, D.; Hu, J. Handbook of Inorganic Thermodynamics; Metallurgical Industry Press: Beijing, China, 2002. [Google Scholar]
- Nagamori, M.; Chaubal, P.C. Thermodynamics of copper matte converting: Part III. Steady-state volatilization of Au, Ag, Pb, Zn, Ni, Se, Te, Bi, Sb, and As from slag, matte, and metallic copper. J. Electron. Mater. 1991, 20, 319–329. [Google Scholar] [CrossRef]
- Tan, P.; Neuschütz, D. A thermodynamic model of nickel smelting and direct high-grade nickel matte smelting processes: Part I. Model development and validation. Metall. Mater. Trans. B. 2001, 32, 341–351. [Google Scholar] [CrossRef]
- Tan, P.F.; Zhang, C.F. Computer model of copper smelting process and distribution behaviors of accessory elements. J. Cent. South Univ. 1997, 4, 36–41. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L. Research of melting temperature of the smelting slag of copper clad laminate scrap. Non-Ferr. Met. Sci. Eng. 2020, 11, 46–50. [Google Scholar]
- Li, M.Z.; Tong, C.R.; Huang, J.D.; Li, J.; Wang, J.L. Simulated calculation of overall process flow of copper flash smelting and converting based on Metcal. Non-Ferr. Met. Sci. Eng. 2016, 6, 31–34. [Google Scholar]



| Components | Phase | Activity Coefficient |
|---|---|---|
| Cu | Crude copper | 1 |
| Au | Crude copper | |
| Ag | Crude copper | |
| Pb | Crude copper | |
| Pd | Crude copper | 1 |
| FeO | Slag | |
| Fe3O4 | Slag | |
| SiO2 | Slag | 2.1 |
| CaO | Slag | 1 |
| PbO | Slag | 1 |
| Cu2O | Slag | |
| Ag2O | Slag | |
| Al2O3 | Slag | 1 |
| B2O3 | Slag | 1 |
| Au | Slag | 50 |
| Ag | Slag | 255 |
| Pd | Slag | 200 |
| Component | State | ||||||
|---|---|---|---|---|---|---|---|
| a | b | c | d | ||||
| Cu | l | 8.028 | 34.236 | 32.845 | 0.000 | 0.000 | 0.000 |
| Au | l | 0.000 | 47.489 | 23.326 | 6.053 | 0.237 | −1.181 |
| Ag | l | 0.000 | 42.678 | 24.347 | 2.544 | 0.025 | 2.895 |
| Pb | l | 3.873 | 70.506 | 32.490 | −3.088 | 0.000 | 0.000 |
| Pd | l | 0.000 | 37.824 | 32.192 | −14.188 | −3.368 | 11.480 |
| FeO | l | −256.323 | 52.148 | 52.148 | 0.000 | 0.000 | 0.000 |
| Fe3O4 | l | −993.310 | 198.380 | 213.384 | 0.000 | 0.000 | 0.000 |
| SiO2 | l | −927.526 | 9.309 | 85.772 | 0.000 | 0.000 | 0.000 |
| CaO | l | −572.895 | 40.979 | 62.760 | 0.000 | 0.000 | 0.000 |
| PbO | l | −202.244 | 73.377 | 73.377 | 0.000 | 0.000 | 0.000 |
| Cu2O | l | −130.221 | 96.399 | 99.914 | 0.000 | 0.000 | 0.000 |
| Ag2O | l | −31.131 | 121.003 | 59.433 | 40.341 | −4.812 | −2.390 |
| Al2O3 | l | −1675.732 | 50.950 | 9.777 | 294.732 | −2.485 | −198.179 |
| B2O3 | l | −1273.530 | 59.971 | 64.160 | 64.598 | −18.366 | 0.033 |
| CO2 | g | −393.515 | 213.774 | 22.227 | 56.202 | 0.105 | −22.519 |
| O2 | g | 0.000 | 205.154 | 22.060 | 20.887 | 1.621 | −8.207 |
| N2 | g | 0.000 | 191.615 | 23.529 | 12.116 | 1.210 | −3.076 |
| H2O | g | −285.837 | 69.952 | 186.889 | −464.258 | −19.565 | 548.644 |
| Pb | g | 0.000 | 64.802 | 26.354 | −6.154 | −0.157 | 25.977 |
| PbO | g | 70.300 | 240.042 | 33.413 | 6.852 | −2.395 | −2.940 |
| HCl | g | −92.314 | 186.900 | 29.151 | −0.365 | 0.000 | 1.099 |
| Cl2 | g | 0.000 | 223.084 | 23.274 | 51.224 | 0.144 | −53.560 |
| Br2 | g | 0.000 | 152.214 | −39.004 | 368.864 | 28.711 | −310.331 |
| HBr | g | −36.291 | 196.702 | 29.102 | 0.053 | 0.000 | 0.231 |
| Contents | Cu | Au(g/t) | Ag(g/t) | Pd | Pb | Fe | SiO2 | CaO | Al2O3 | B2O3 | H2O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt %) | 19.43 | 0.20 | 0.50 | 0.48 | 0.038 | 0.19 | 16.91 | 9.59 | 6.84 | 16.89 | 5.00 |
| Organic | C2H3Cl | C2H4 | CH3NO2 | C7H8O2 | C12H6Cl4 | C12H8Br2 | C15H16O2 | ||||
| (wt %) | 5.00 | 10.00 | 3.00 | 7.00 | 3.00 | 12.00 | 60.00 | ||||
| No. | Input | Output | ||||||
|---|---|---|---|---|---|---|---|---|
| Phase | °C | kg/h | Nm3/h | Phase | °C | kg/h | Nm3/h | |
| 1 | Scrap of copper-clad laminate | 25 | 3130.00 | Crude copper | 1250 | 515.60 | ||
| 2 | Iron powder | 25 | 67.51 | Slag | 1280 | 2058.51 | ||
| 3 | Limestone | 25 | 740.30 | Off-gas | 1317 | 8926.77 | 6730.56 | |
| 4 | Arenaceous quartz | 25 | 0.00 | Dust | 1317 | 159.88 | ||
| 5 | Industrial oxygen | 25 | 0.00 | 0.00 | ||||
| 6 | Air | 25 | 7722.94 | 6000.00 | ||||
| Total | 11,660.75 | 6000.00 | Total | 11,660.75 | 6730.56 | |||
| No. | Crude Copper | Slag | Off-Gas | Dust | |||||
|---|---|---|---|---|---|---|---|---|---|
| Phase | wt% | Phase | wt% | Phase | wt% | v% | Phase | wt% | |
| 1 | Cu | 97.18 | FeO | 3.00 | CO2 | 25.49 | 17.22 | Cu | 16.98 |
| 2 | Au | 1.14 × 10−4 | SiO2 | 25.46 | O2 | 0.05 | 0.05 | Pb | 0.05 |
| 3 | Ag | 2.73 × 10−4 | Cu2O | 4.37 | N2 | 66.42 | 70.49 | Fe | 13.21 |
| 4 | Pb | 4.51 × 10−5 | CaO | 31.00 | H2O | 7.11 | 11.74 | SiO2 | 23.49 |
| 5 | Pd | 2.82 | Fe3O4 | 0.16 | Pb | 0.00 | 0.00 | CaO | 13.32 |
| 6 | - | - | PbO | 0.06 | PbO | 0.00 | 0.00 | Al2O3 | 9.49 |
| 7 | - | - | Au | 0.00 | HCl | 0.38 | 0.31 | B2O3 | 23.46 |
| 8 | - | - | Ag | 0.00 | Cl2 | 0.00 | 0.00 | - | - |
| 9 | - | - | Pd | 0.02 | Br2 | 0.01 | 0.00 | - | - |
| 10 | - | - | Ag2O | 0.00 | HBr | 0.53 | 0.19 | - | - |
| 11 | - | - | Al2O3 | 9.66 | - | - | - | - | - |
| 12 | - | - | B2O3 | 23.87 | - | - | - | - | - |
| 13 | - | - | Other | 2.40 | - | - | - | - | - |
| Total | 100.00 | Total | 100.00 | Total | 100.00 | 100.00 | Total | 100.00 | |
| No. | Heat Income | Heat Output | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Materials | T (°C) | MJ/h | % | Materials | T (°C) | MJ/h | % | |
| 1 | Physical heat | Scrap of copper-clad laminate | 25 | 0.00 | 0.00 | Copper | 1250 | 322.09 | 1.49 |
| Iron powder | 25 | 0.00 | 0.00 | Slag | 1280 | 3605.05 | 16.62 | ||
| Limestone | 25 | 0.00 | 0.00 | Off-gas | 1317 | 14,183.16 | 65.39 | ||
| Arenaceous quartz | 25 | 0.00 | 0.00 | Dust | 1317 | 238.04 | 1.10 | ||
| Industrial oxygen | 25 | 0.00 | 0.00 | ||||||
| Air | 25 | 0.00 | 0.00 | ||||||
| Total | 0.00 | 0.00 | Total | 18,348.34 | 84.60 | ||||
| 2 | Chemical heat | 25 | 21,689.46 | 100.00 | 25 | ||||
| 3 | Exchanged heat | Top cooling water | 25 | Top cooling water | 30 | 1044.34 | 4.81 | ||
| 4 | Natural Cooling | 208 | 2296.78 | 10.59 | |||||
| Total | 21,689.46 | 100.00 | 21,689.46 | 100.00 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Wang, J.; Wang, H. Research on the Mathematical Model of Local Equilibrium in the Top-Blown Smelting Process of Electronic Waste. Metals 2021, 11, 1500. https://doi.org/10.3390/met11101500
Wen X, Wang J, Wang H. Research on the Mathematical Model of Local Equilibrium in the Top-Blown Smelting Process of Electronic Waste. Metals. 2021; 11(10):1500. https://doi.org/10.3390/met11101500
Chicago/Turabian StyleWen, Xiaochun, Jinliang Wang, and Houqing Wang. 2021. "Research on the Mathematical Model of Local Equilibrium in the Top-Blown Smelting Process of Electronic Waste" Metals 11, no. 10: 1500. https://doi.org/10.3390/met11101500
APA StyleWen, X., Wang, J., & Wang, H. (2021). Research on the Mathematical Model of Local Equilibrium in the Top-Blown Smelting Process of Electronic Waste. Metals, 11(10), 1500. https://doi.org/10.3390/met11101500







