Effect of Al Concentration on the Microstructural Evolution of Fe-Cr-Al Systems: A Phase-Field Approach
Abstract
1. Introduction
2. CALPHAD-Based Phase-Field Method
2.1. Phase-Field Modeling Applied Using the Cahn–Hilliard Equation
2.2. Semi-Implicit Fourier Spectral Method with Variable Mobility
2.3. CALPHAD-Type Free Energy
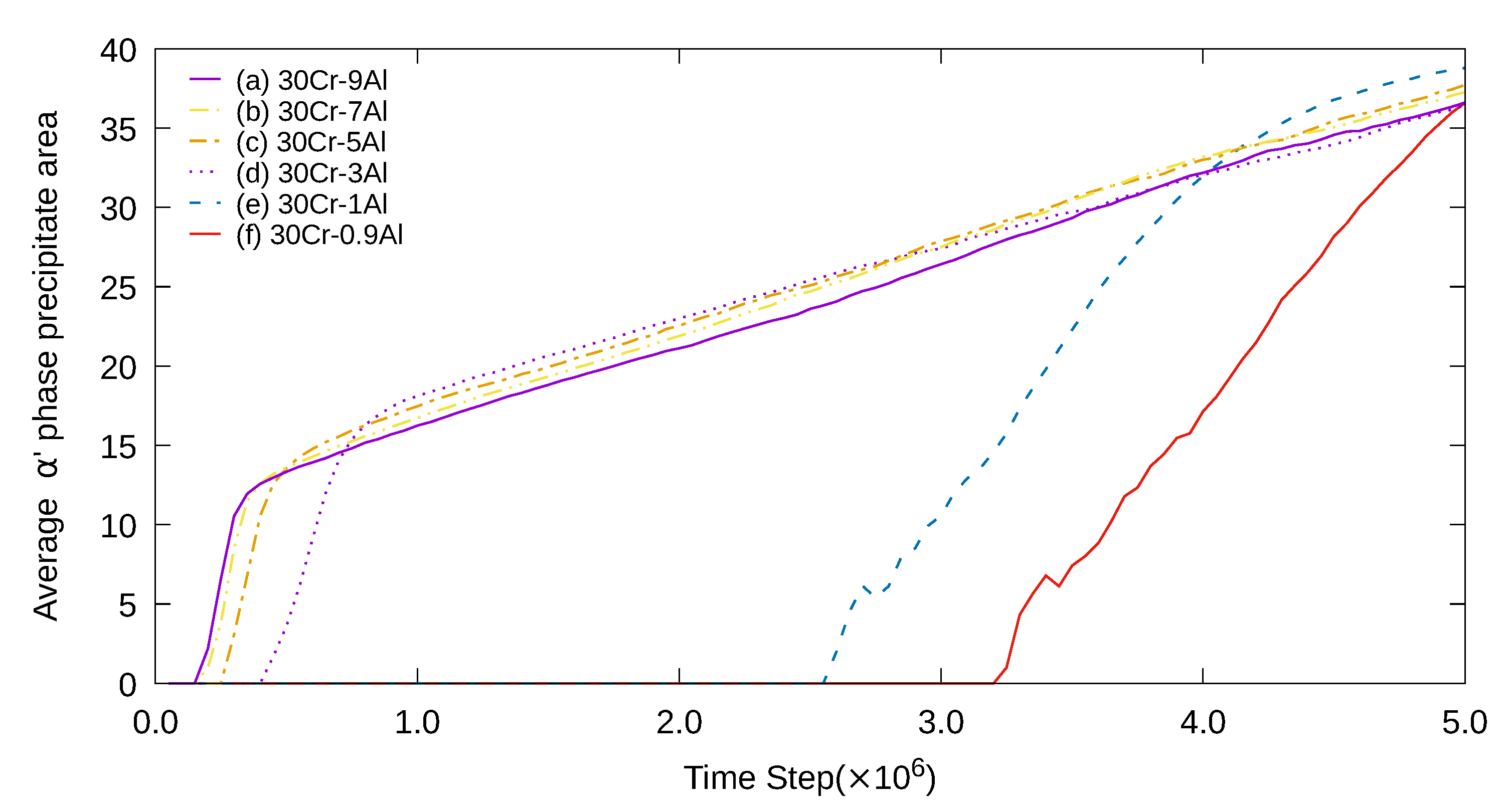
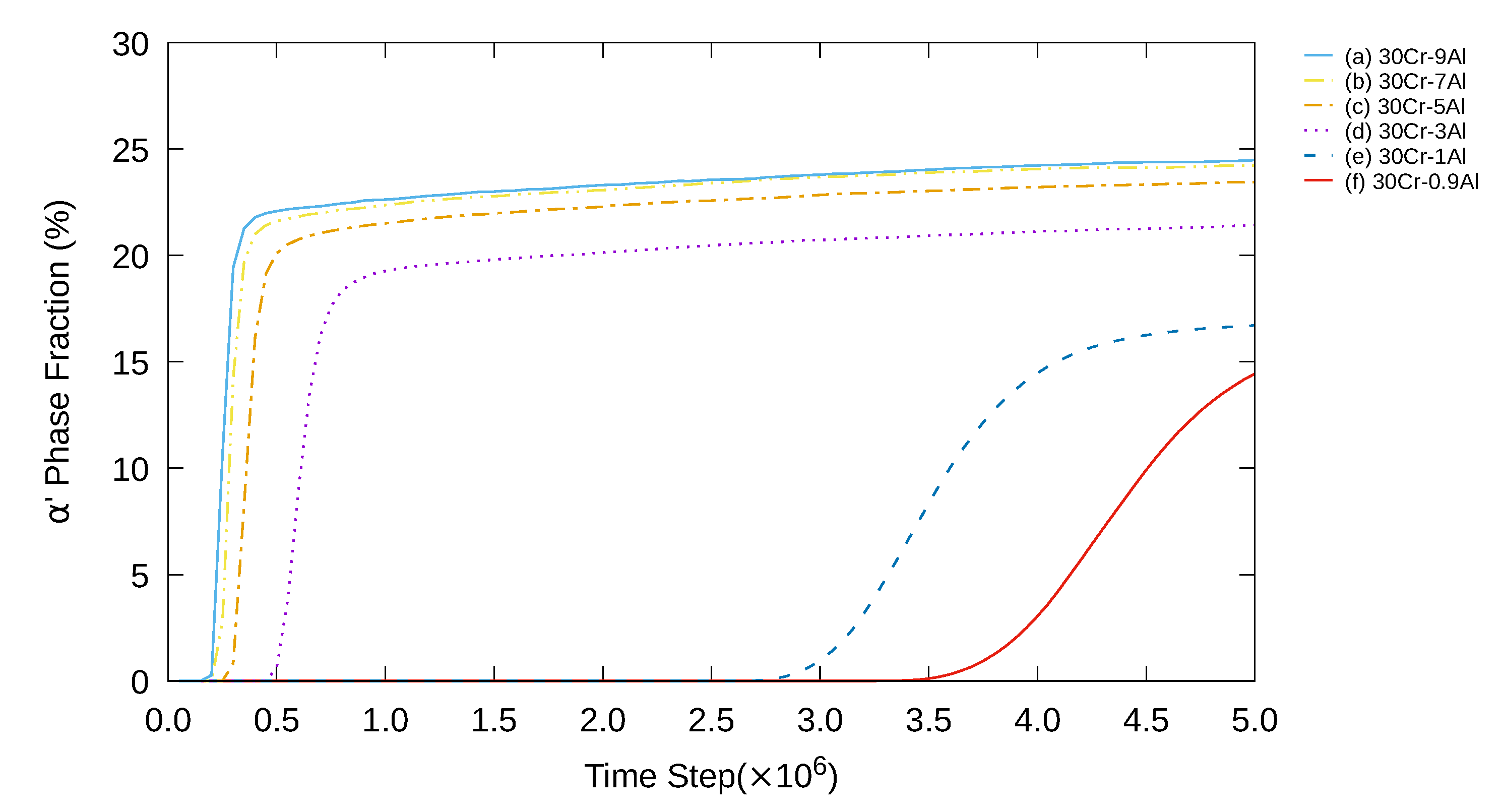
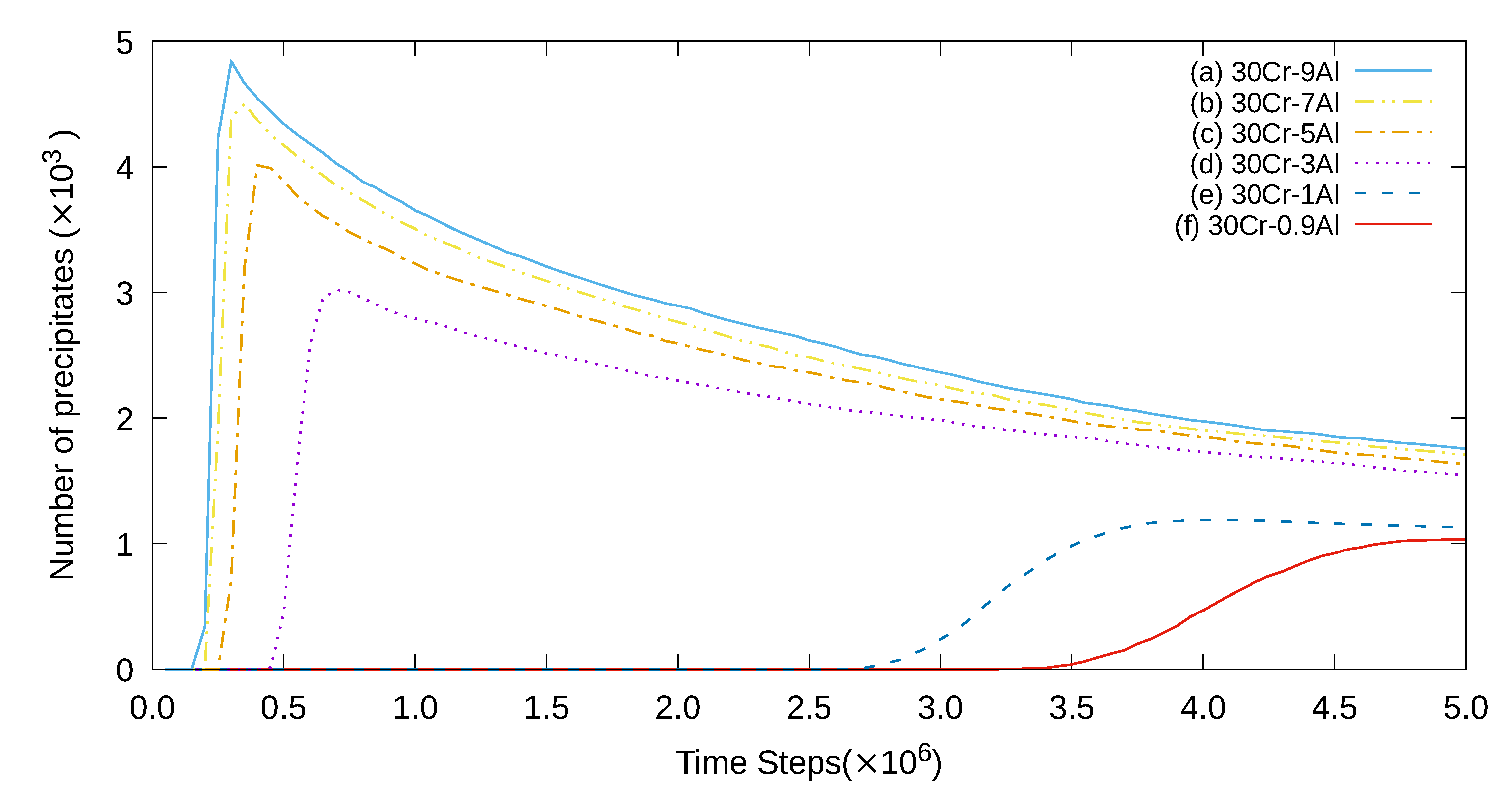
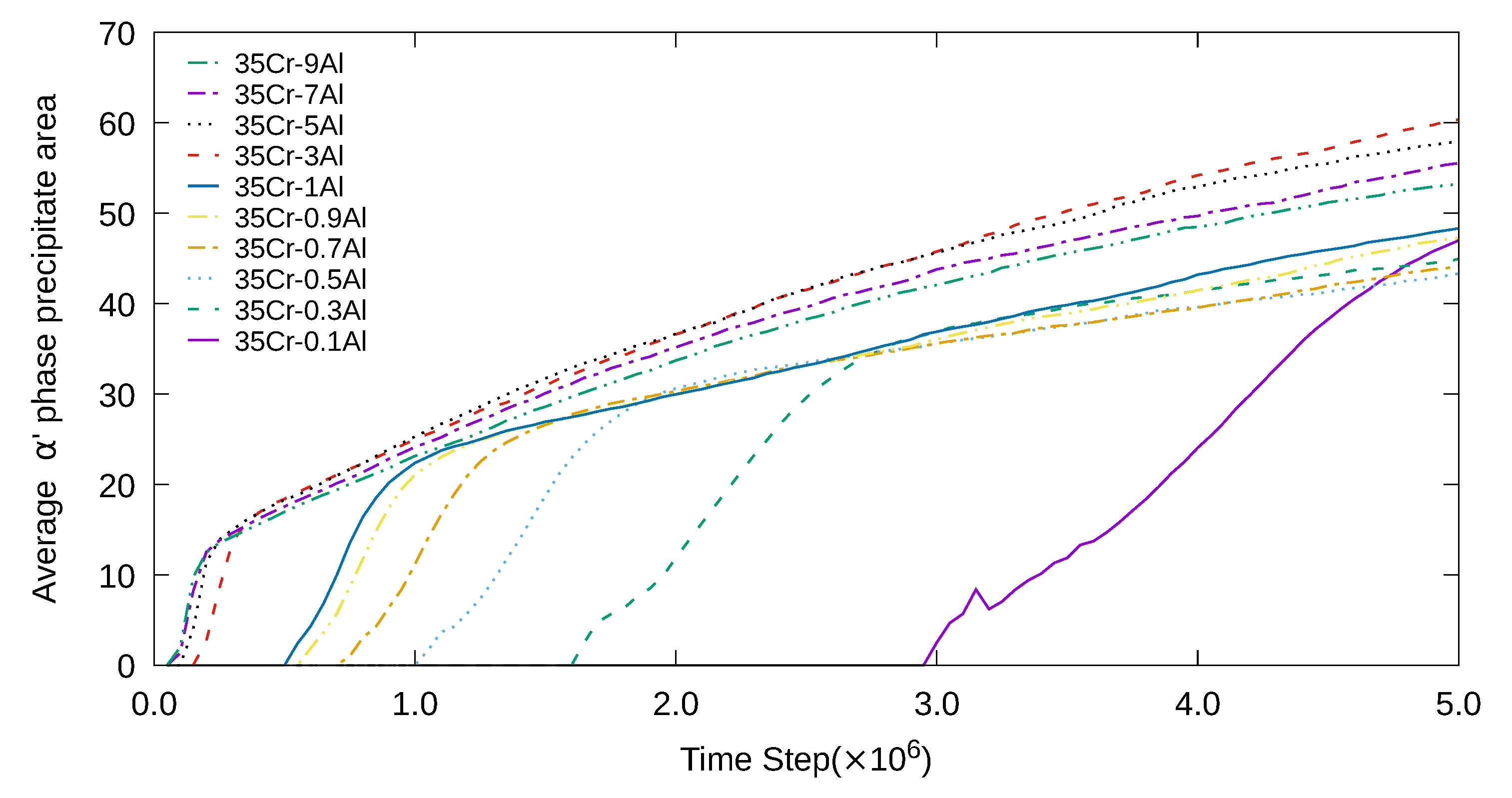
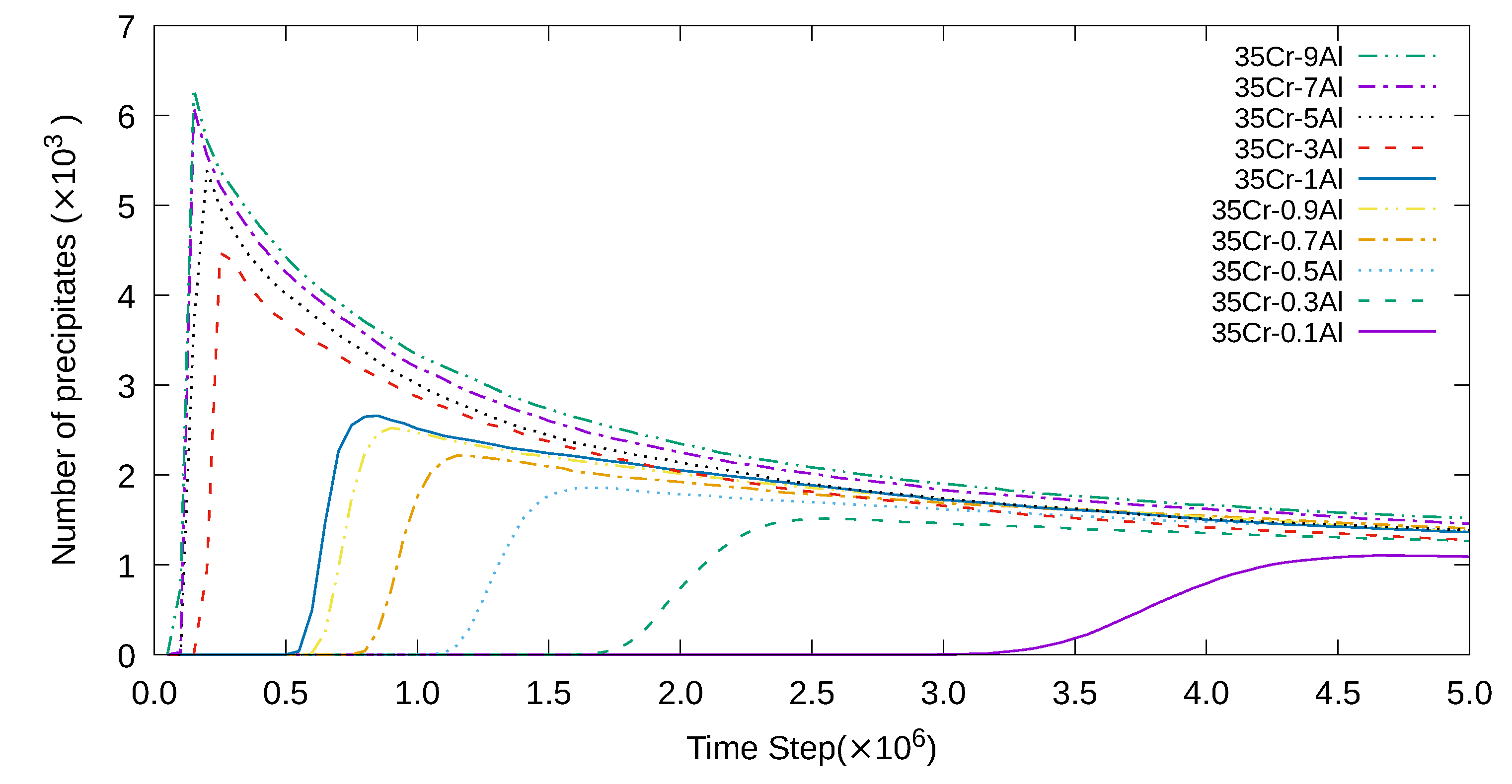
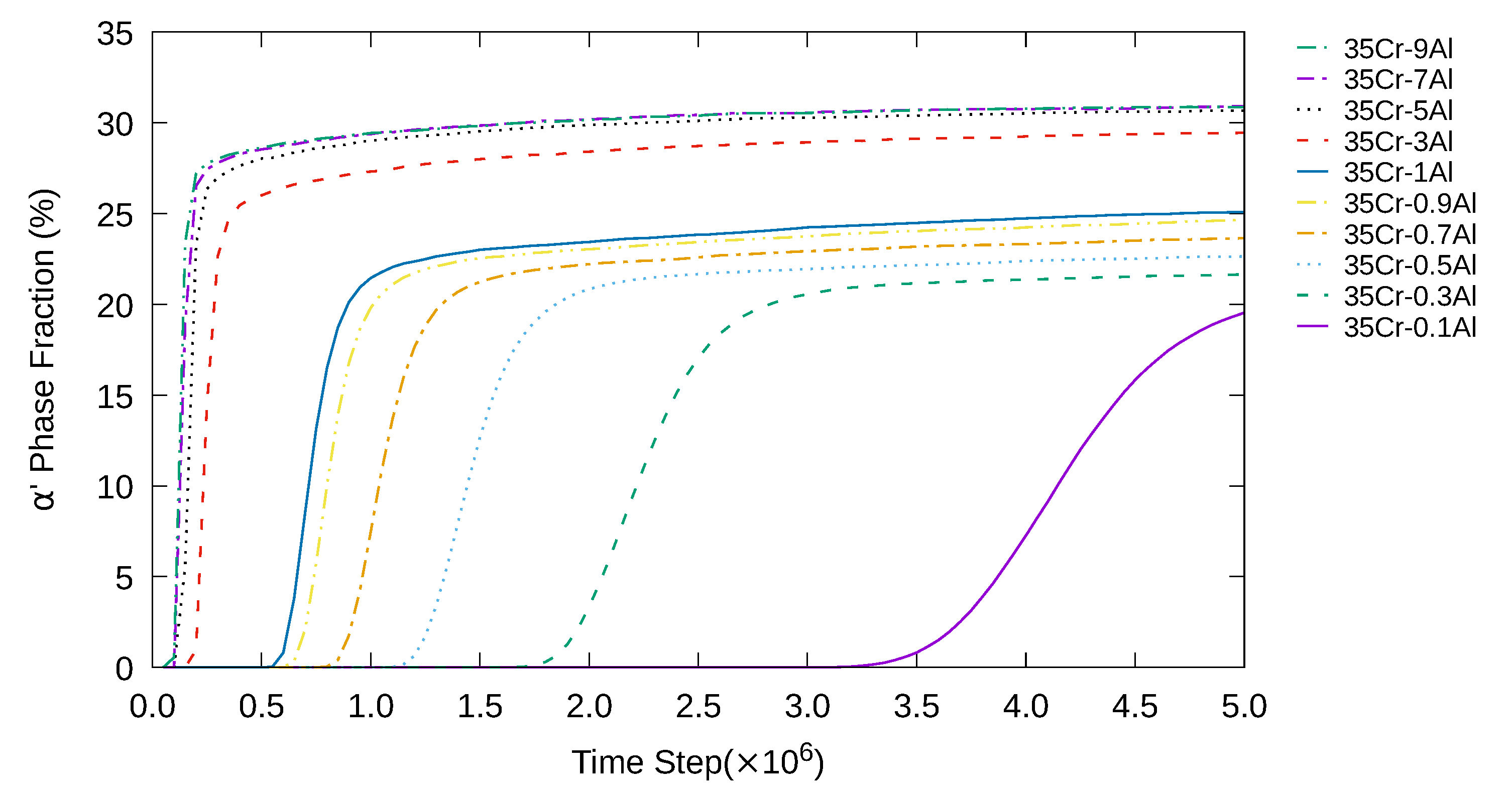
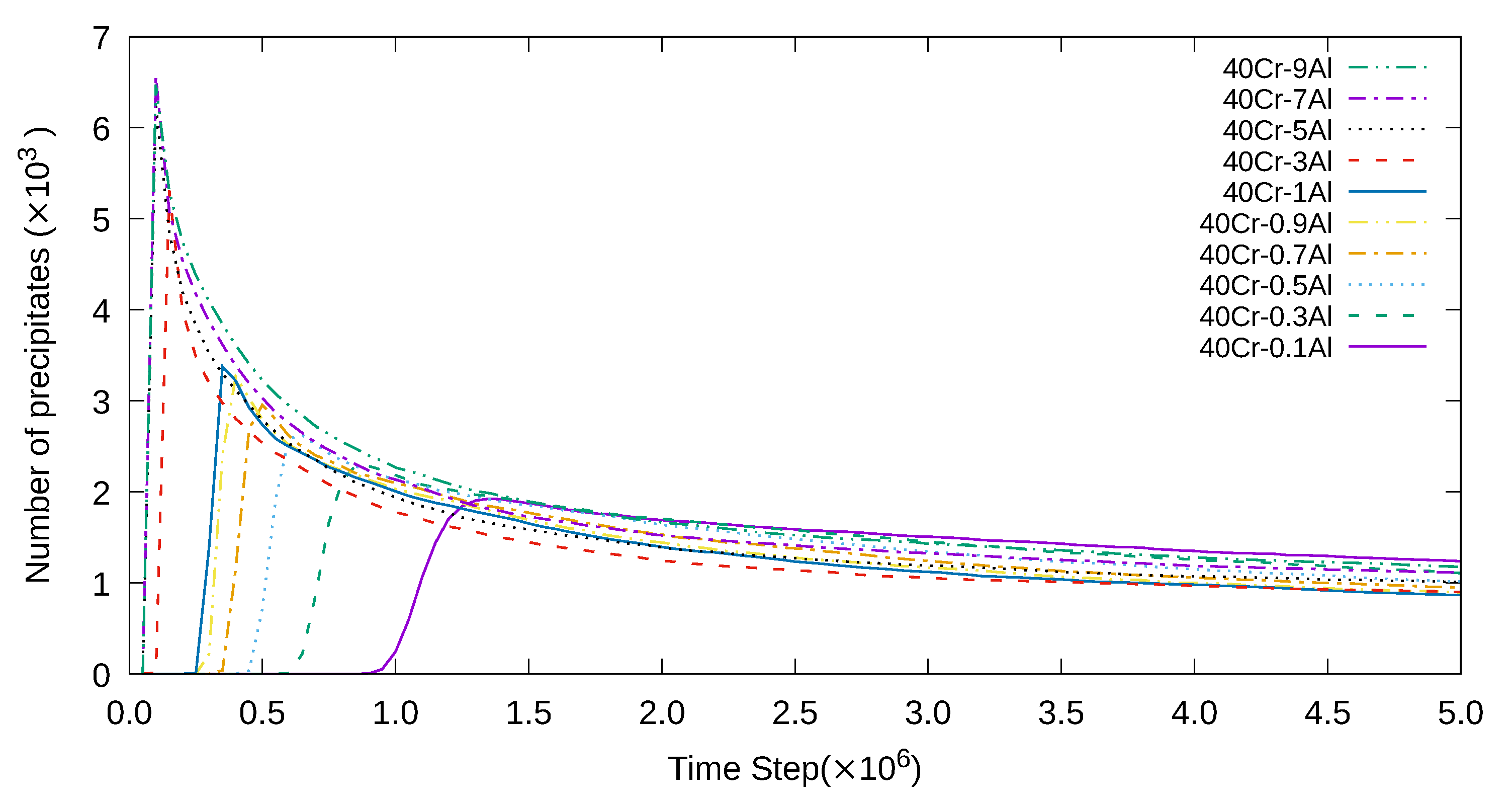
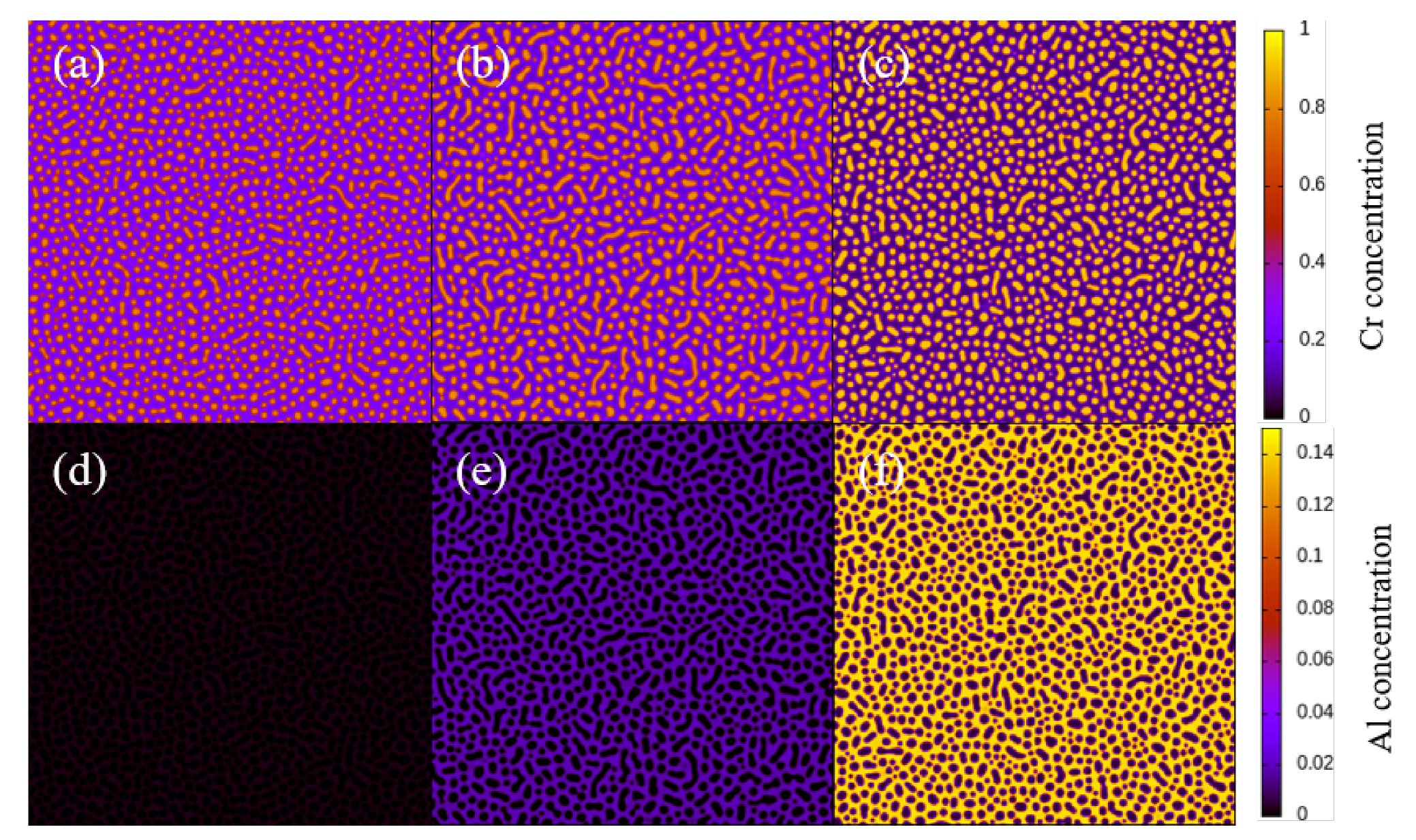
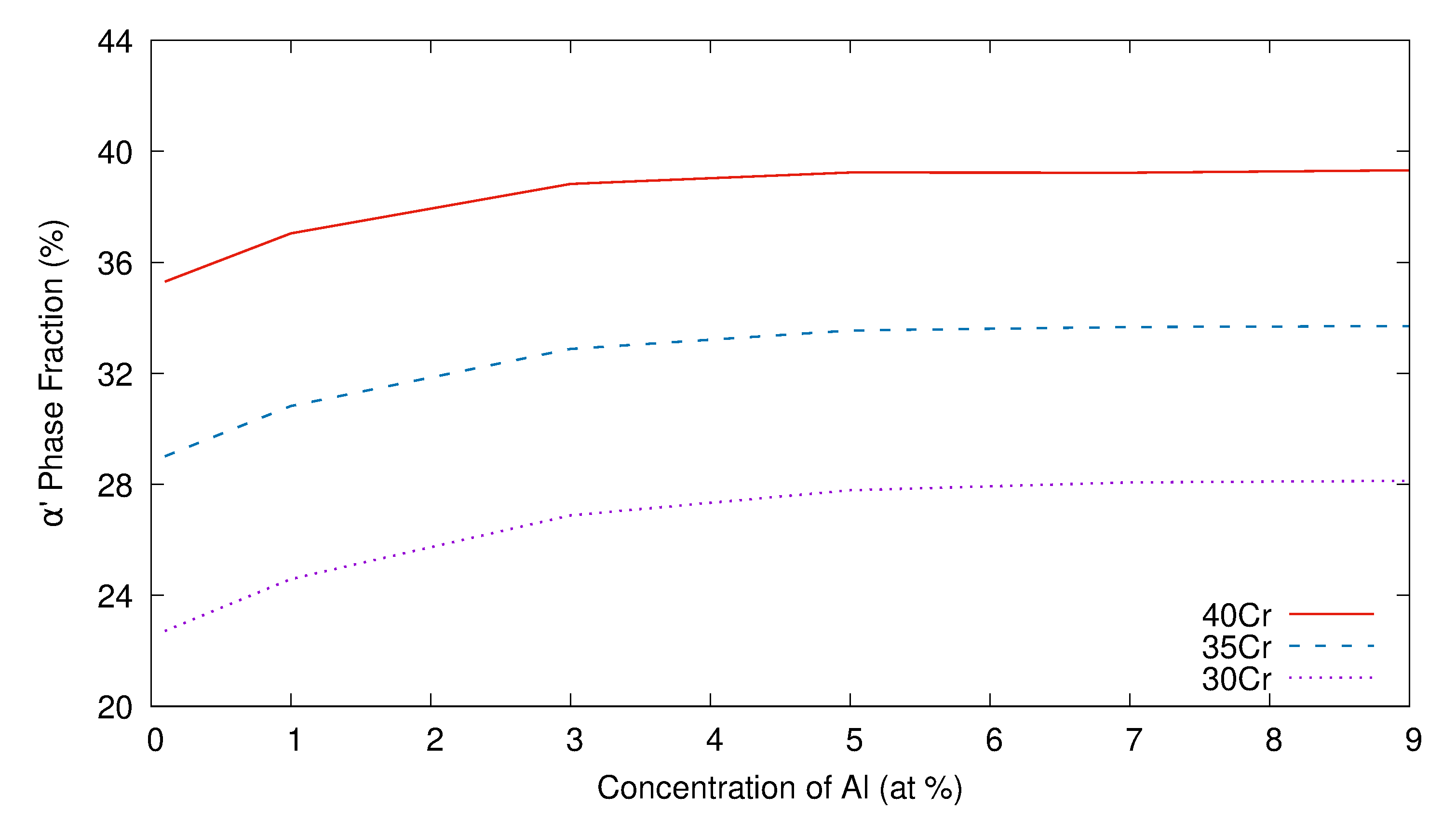
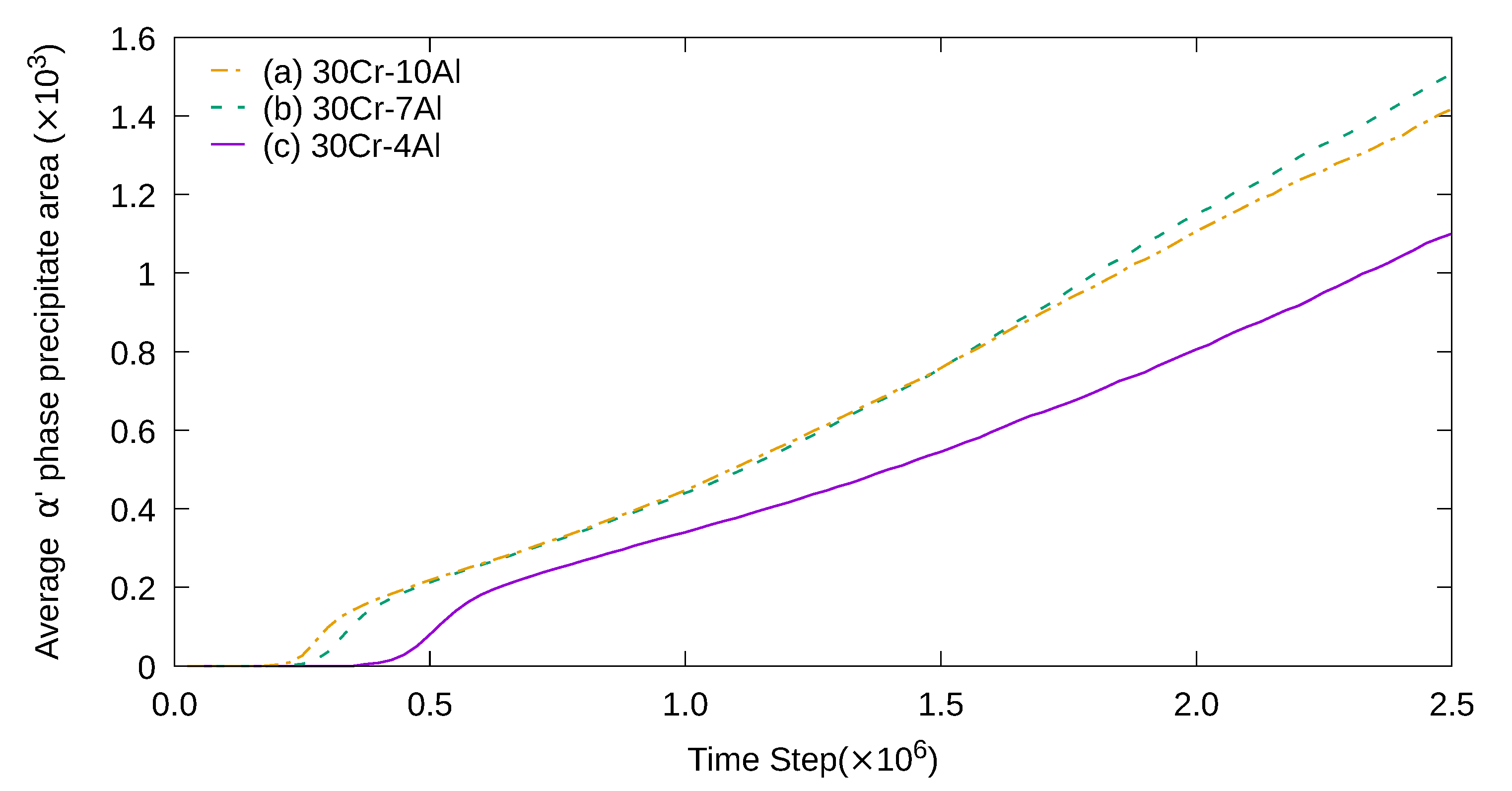
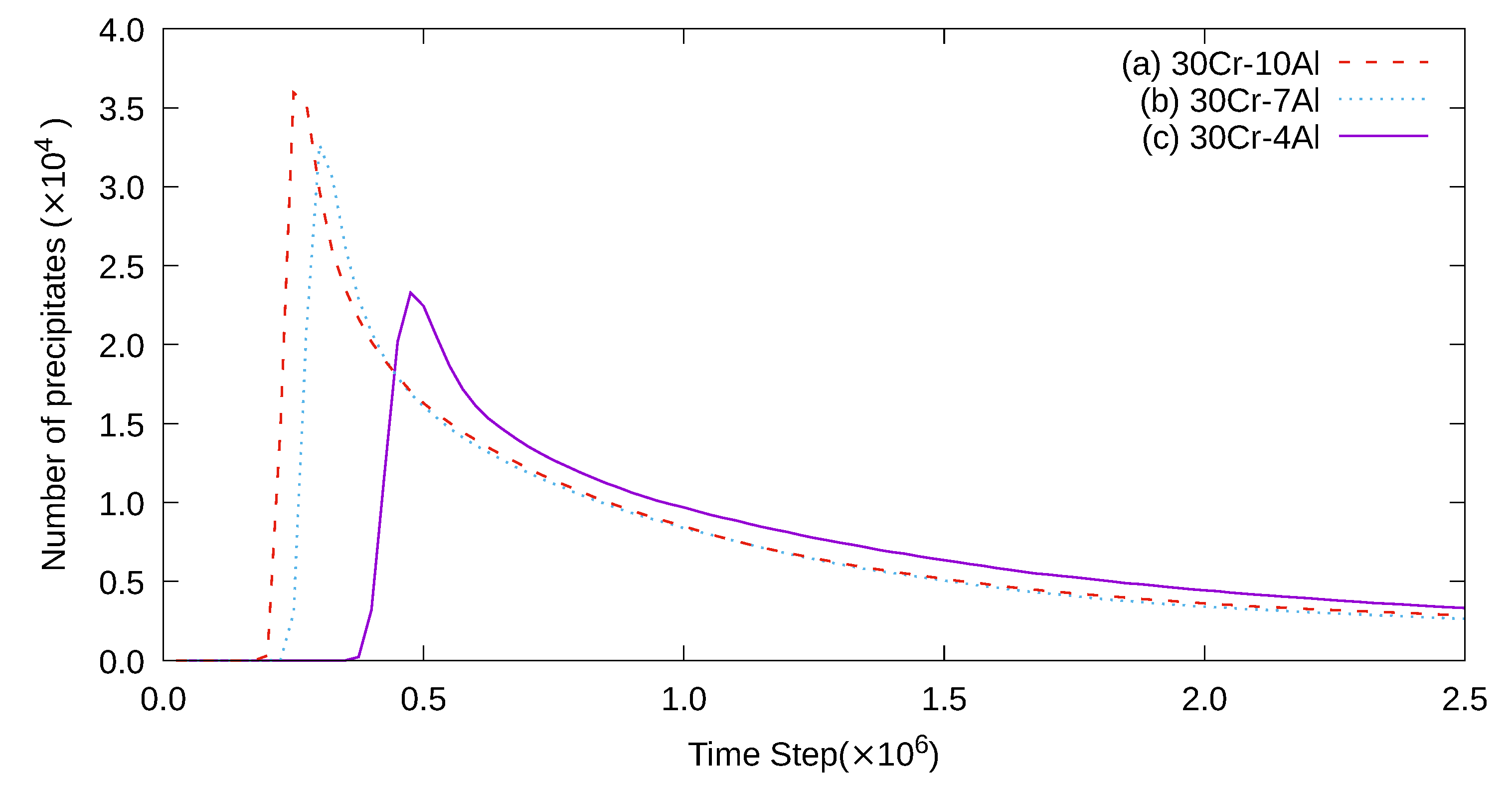
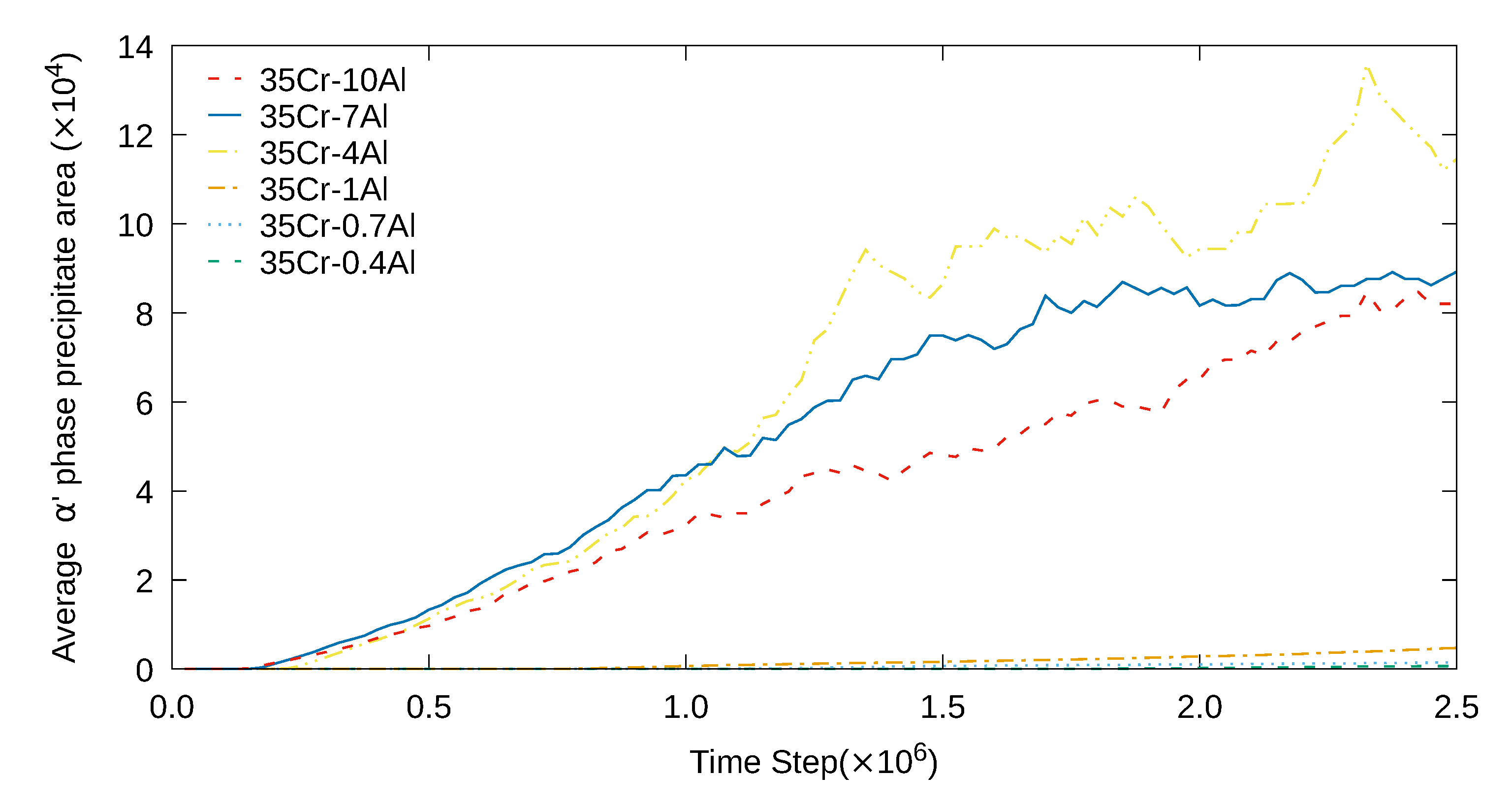
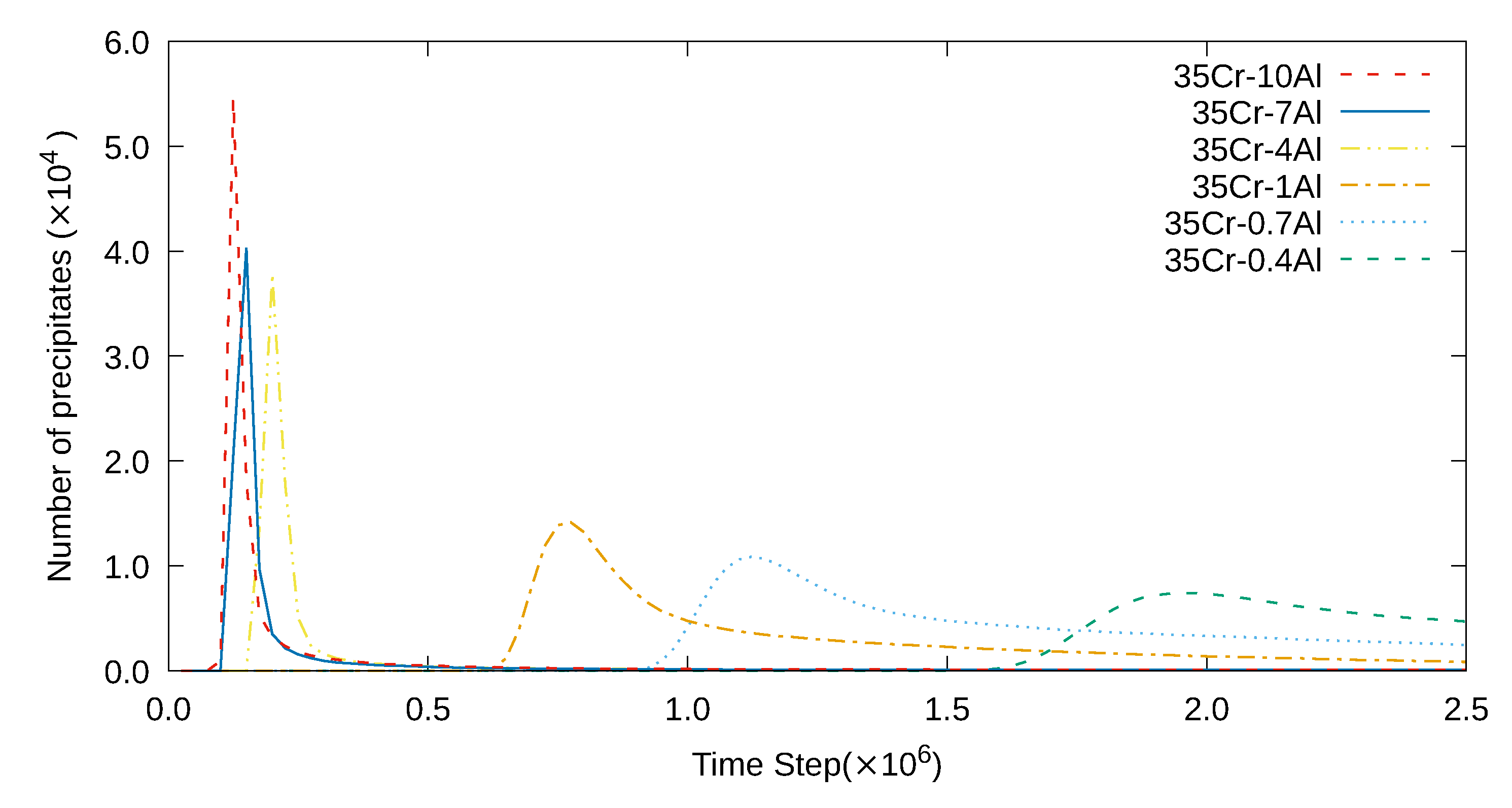
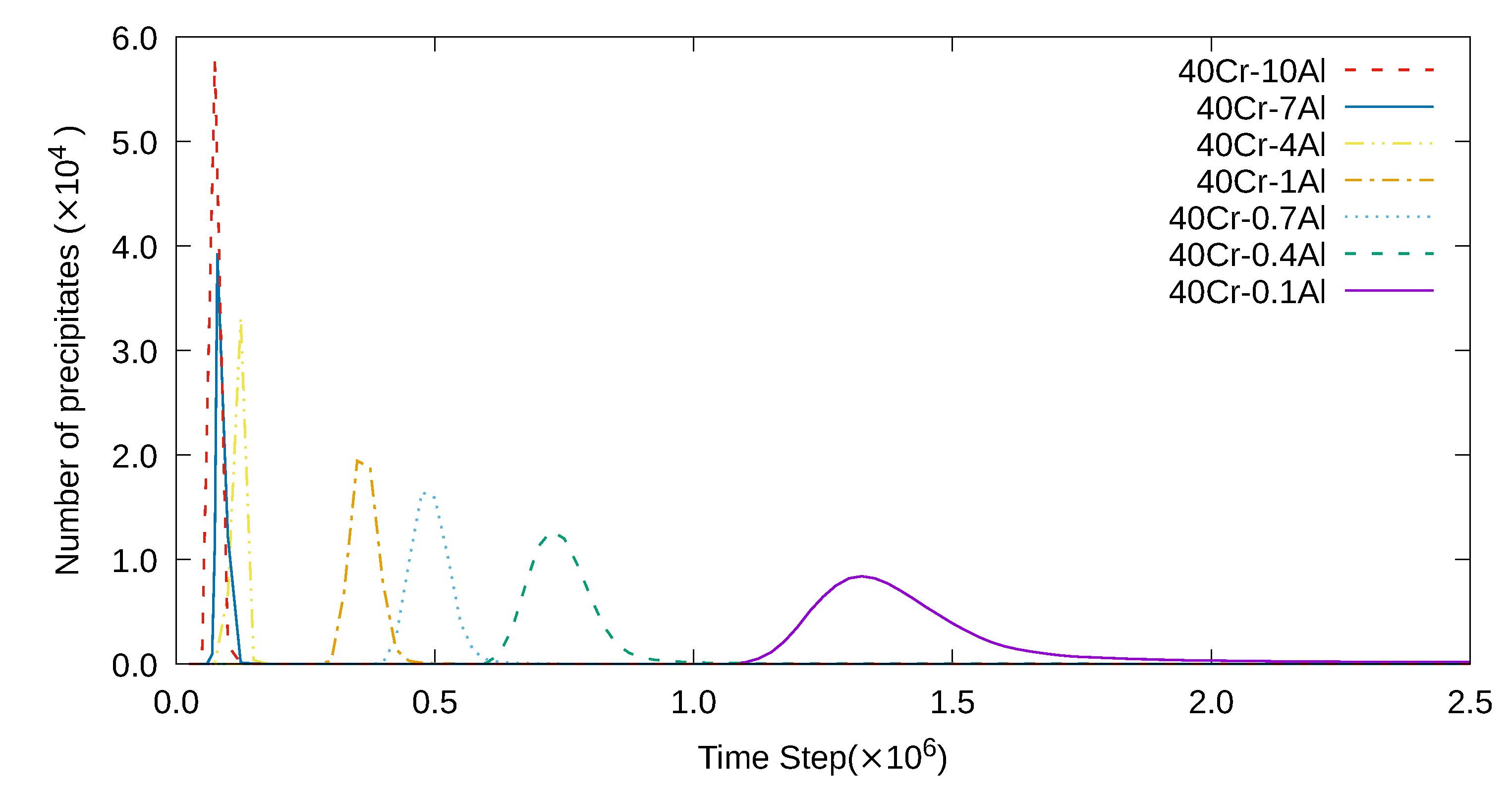
3. Simulation Results and Analyses
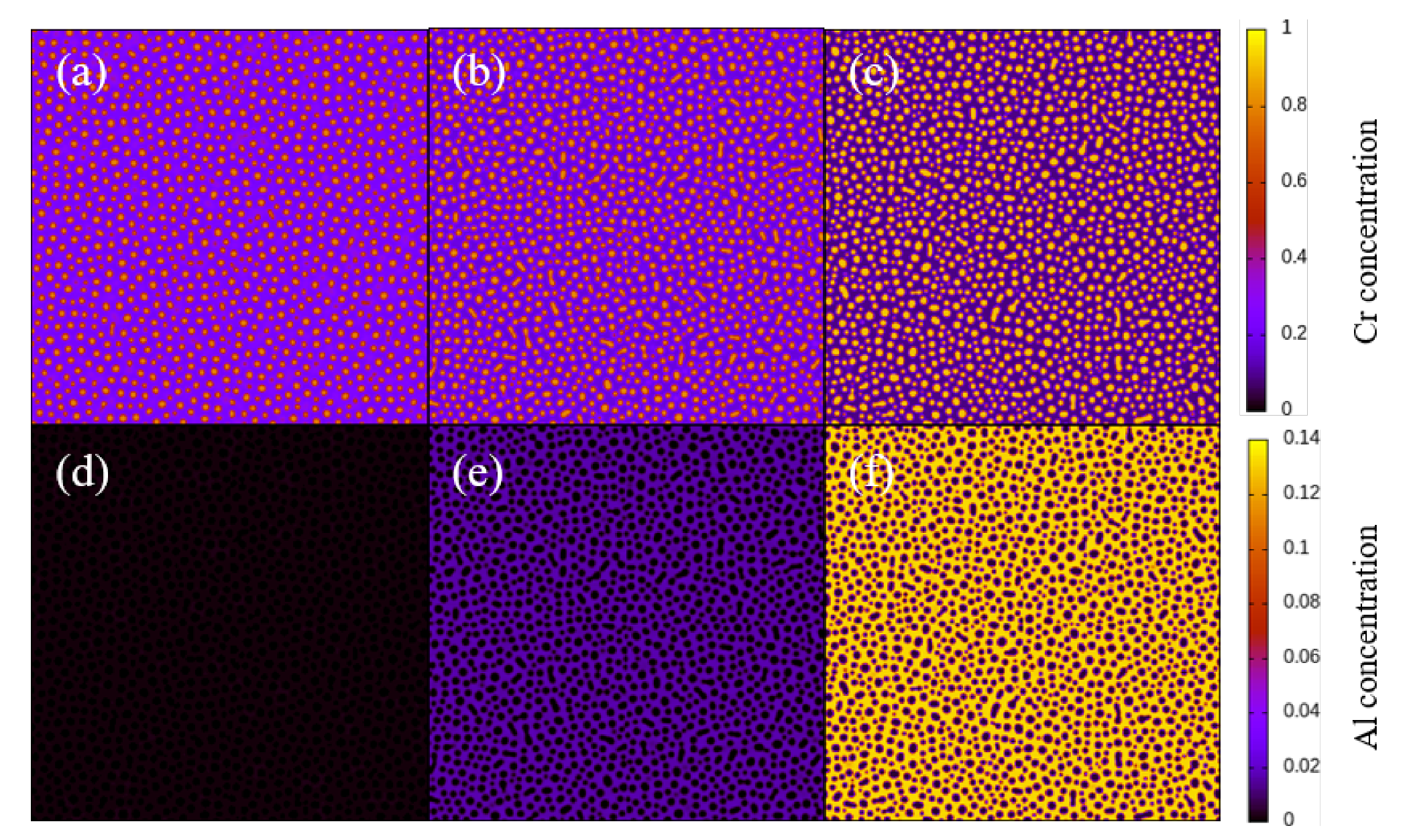
3.1. Two-Dimensional Simulation of the Microstructural Evolution
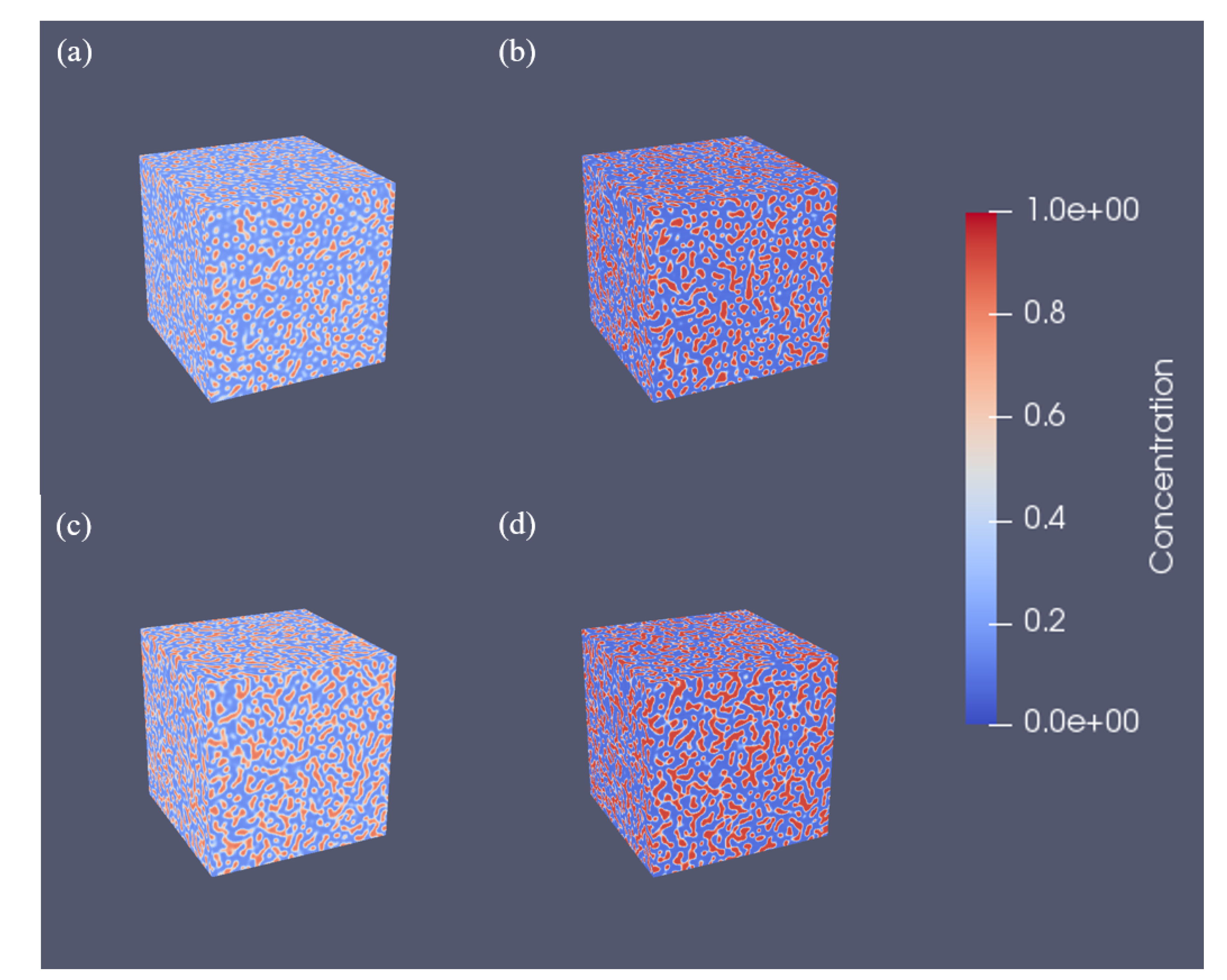
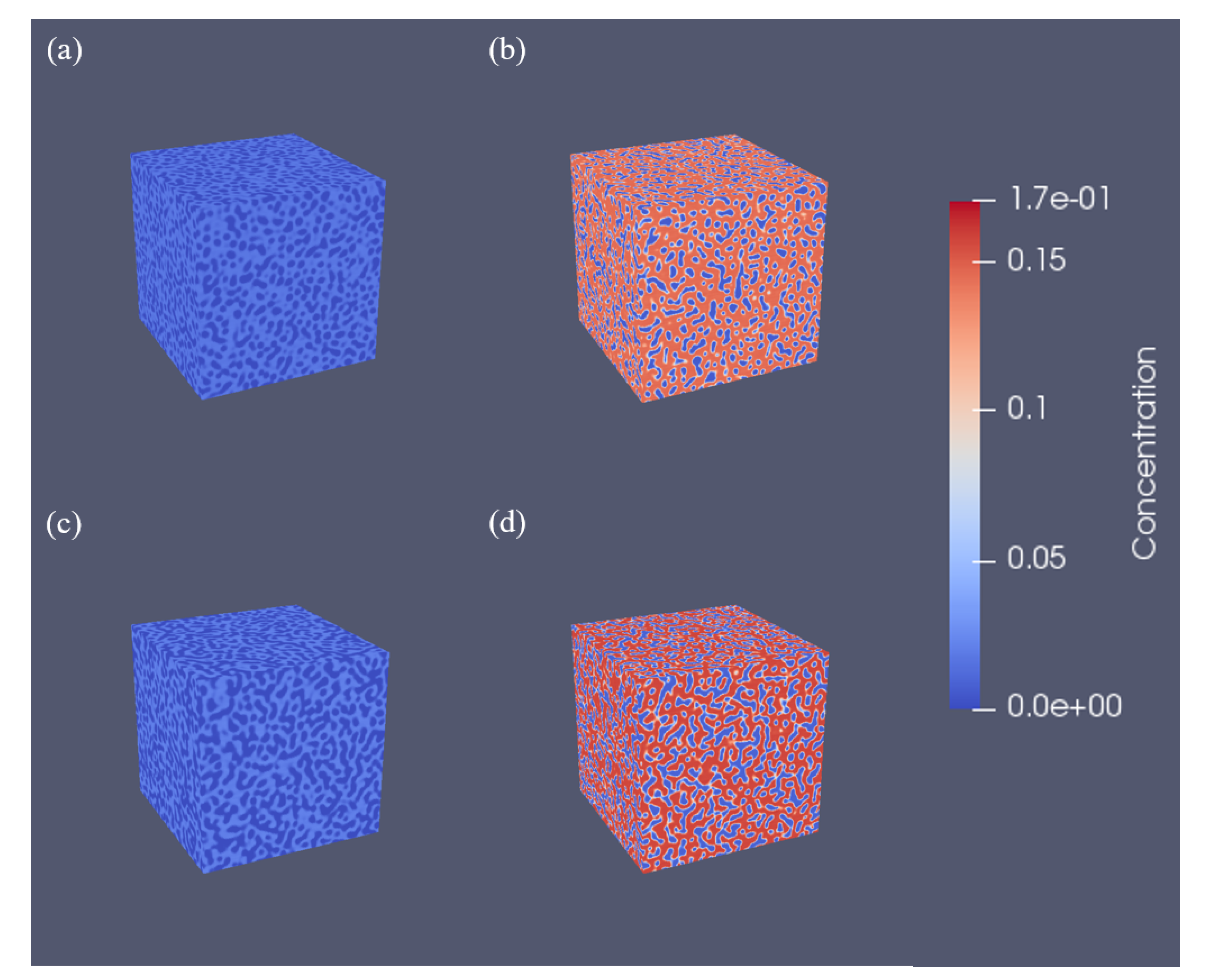
3.2. Three-Dimensional Simulation of Microstructural Evolution
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klueh, R.; Nelson, A.T. Ferritic/martensitic steels for next-generation reactors. J. Nucl. Mater. 2007, 371, 37–52. [Google Scholar] [CrossRef]
- Tedmon, C., Jr. The effect of oxide volatilization on the oxidation kinetics of Cr and Fe-Cr alloys. J. Electrochem. Soc. 1966, 113, 766. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Pint, B.A.; Terrani, K.A.; Brady, M.P.; Cheng, T.; Keiser, J.R. High temperature oxidation of fuel cladding candidate materials in steam–hydrogen environments. J. Nucl. Mater. 2013, 440, 420–427. [Google Scholar] [CrossRef]
- Terrani, K.A.; Zinkle, S.J.; Snead, L.L. Advanced oxidation-resistant iron-based alloys for LWR fuel cladding. J. Nucl. Mater. 2014, 448, 420–435. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Pint, B.A.; Terrani, K.A.; Field, K.G.; Yang, Y.; Snead, L.L. Development and property evaluation of nuclear grade wrought FeCrAl fuel cladding for light water reactors. J. Nucl. Mater. 2015, 467, 703–716. [Google Scholar] [CrossRef]
- Gussev, M.N.; Cakmak, E.; Field, K.G. Impact of neutron irradiation on mechanical performance of FeCrAl alloy laser-beam weldments. J. Nucl. Mater. 2018, 504, 221–233. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Dai, C.; Wang, J.; Chen, X.; Xie, J.; Yang, W.; Chen, D. Effect of aluminum on microstructure and property of Cu–Ni–Si alloys. Mater. Sci. Eng. A 2013, 572, 65–74. [Google Scholar] [CrossRef]
- Field, K.G.; Hu, X.; Littrell, K.C.; Yamamoto, Y.; Snead, L.L. Radiation tolerance of neutron-irradiated model Fe–Cr–Al alloys. J. Nucl. Mater. 2015, 465, 746–755. [Google Scholar] [CrossRef]
- Hsiung, L.L.; Fluss, M.J.; Tumey, S.J.; Choi, B.W.; Serruys, Y.; Willaime, F.; Kimura, A. Formation mechanism and the role of nanoparticles in Fe-Cr ODS steels developed for radiation tolerance. Phys. Rev. B 2010, 82, 184103. [Google Scholar] [CrossRef]
- Parish, C.M.; Field, K.G.; Certain, A.G.; Wharry, J.P. Application of STEM characterization for investigating radiation effects in BCC Fe-based alloys. J. Mater. Res. 2015, 30, 1275–1289. [Google Scholar] [CrossRef]
- Airiskallio, E.; Nurmi, E.; Heinonen, M.; Väyrynen, I.; Kokko, K.; Ropo, M.; Punkkinen, M.; Pitkänen, H.; Alatalo, M.; Kollár, J.; et al. High temperature oxidation of Fe–Al and Fe–Cr–Al alloys: The role of Cr as a chemically active element. Corros. Sci. 2010, 52, 3394–3404. [Google Scholar] [CrossRef]
- Wolff, I.; Iorio, L.; Rumpf, T.; Scheers, P.; Potgieter, J. Oxidation and corrosion behaviour of Fe–Cr and Fe–Cr–Al alloys with minor alloying additions. Mater. Sci. Eng. A 1998, 241, 264–276. [Google Scholar] [CrossRef]
- Mrowec, S.; Wedrychowska, M. Kinetics and mechanism of high-temperature sulfur corrosion of Fe-Cr-Al alloys. Oxid. Met. 1979, 13, 481–504. [Google Scholar] [CrossRef]
- Field, K.G.; Briggs, S.A.; Sridharan, K.; Howard, R.H.; Yamamoto, Y. Mechanical properties of neutron-irradiated model and commercial FeCrAl alloys. J. Nucl. Mater. 2017, 489, 118–128. [Google Scholar] [CrossRef]
- Grobner, P. The 885 F (475 C) embrittlement of ferritic stainless steels. Metall. Trans. 1973, 4, 251–260. [Google Scholar] [CrossRef]
- Chandra, D.; Schwartz, L. Mössbauer effect study of the 475‡ C decomposition of Fe-Cr. Metall. Trans. 1971, 2, 511–519. [Google Scholar] [CrossRef]
- Solomon, H.; Levinson, L.M. Mössbauer effect study of ‘475 C embrittlement’of duplex and ferritic stainless steels. Acta Metall. 1978, 26, 429–442. [Google Scholar] [CrossRef]
- Miller, M.; Hyde, J.; Hetherington, M.; Cerezo, A.; Smith, G.; Elliott, C. Spinodal decomposition in Fe-Cr alloys: Experimental study at the atomic level and comparison with computer models—I. Introduction and methodology. Acta Metall. Mater. 1995, 43, 3385–3401. [Google Scholar] [CrossRef]
- Lee, J.; Chang, K. Effect of magnetic ordering on the spinodal decomposition of the Fe-Cr system: A GPU-accelerated phase-field study. Comput. Mater. Sci. 2019, 169, 109088. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Liu, C.; Chen, S.; Shi, S.; Jin, S. Effect of applied strain on phase separation of Fe–28 at.% Cr alloy: 3D phase-field simulation. Model. Simul. Mater. Sci. Eng. 2018, 26, 035015. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takasugi, T. Mapping of 475 C embrittlement in ferritic Fe–Cr–Al alloys. Scr. Mater. 2010, 63, 1104–1107. [Google Scholar] [CrossRef]
- Li, W.; Lu, S.; Hu, Q.M.; Mao, H.; Johansson, B.; Vitos, L. The effect of Al on the 475 C embrittlement of Fe–Cr alloys. Comput. Mater. Sci. 2013, 74, 101–106. [Google Scholar] [CrossRef]
- Han, W.; Yabuuchi, K.; Kimura, A.; Ukai, S.; Oono, N.; Kaito, T.; Torimaru, T.; Hayashi, S. Effect of Cr/Al contents on the 475 ºC age-hardening in oxide dispersion strengthened ferritic steels. Nucl. Mater. Energy 2016, 9, 610–615. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Shi, S.; Jin, S. Quantitative Phase-Field Simulation of Composition Partition and Separation Kinetics of Nanoscale Phase in Fe-Cr-Al Alloy. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Lukas, H.; Fries, S.G.; Sundman, B. Computational Thermodynamics: The Calphad Method; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Saunders, N.; Miodownik, A.P. CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Dinsdale, A. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Chang, K.; Meng, F.; Ge, F.; Zhao, G.; Du, S.; Huang, F. Theory-guided bottom-up design of the FeCrAl alloys as accident tolerant fuel cladding materials. J. Nucl. Mater. 2019, 516, 63–72. [Google Scholar] [CrossRef]
- Capdevila, C.; Miller, M.K.; Chao, J. Phase separation kinetics in a Fe–Cr–Al alloy. Acta Mater. 2012, 60, 4673–4684. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.Q.; Shen, J.; Tikare, V. Coarsening kinetics from a variable-mobility Cahn-Hilliard equation: Application of a semi-implicit Fourier spectral method. Phys. Rev. E 1999, 60, 3564. [Google Scholar] [CrossRef]
- Chen, L.Q.; Shen, J. Applications of semi-implicit Fourier-spectral method to phase field equations. Comput. Phys. Commun. 1998, 108, 147–158. [Google Scholar] [CrossRef]
- Hu, S.; Chen, L. Solute segregation and coherent nucleation and growth near a dislocation—Phase-field model integrating defect and phase microstructures. Acta Mater. 2001, 49, 463–472. [Google Scholar] [CrossRef]
- Jodra, J.L.; Gurrutxaga, I.; Muguerza, J. A study of memory consumption and execution performance of the cufft library. In Proceedings of the 2015 10th International Conference on P2P, Parallel, Grid, Cloud and Internet Computing (3PGCIC), Krakow, Poland, 4–6 November 2015; pp. 323–327. [Google Scholar]
- Stoer, J.; Bulirsch, R. Introduction to Numerical Analysis; Springer Science & Business Media: Berlin, Germany, 2013; Volume 12. [Google Scholar]
- Cahn, J.W. On spinodal decomposition. Acta Metall. 1961, 9, 795–801. [Google Scholar] [CrossRef]
- Huang, C.; de La Cruz, M.O.; Swift, B. Phase separation of ternary mixtures: Symmetric polymer blends. Macromolecules 1995, 28, 7996–8005. [Google Scholar] [CrossRef]
- Wu, K.; Morral, J.; Wang, Y. A phase field study of microstructural changes due to the Kirkendall effect in two-phase diffusion couples. Acta Mater. 2001, 49, 3401–3408. [Google Scholar] [CrossRef]
- Metals Data Book; Japan Institute of Metals: Maruzen, Tokyo, 2004; Volume 21.
- Cahn, J.W. Phase separation by spinodal decomposition in isotropic systems. J. Chem. Phys. 1965, 42, 93–99. [Google Scholar] [CrossRef]
- Mohanty, R.; Sohn, Y. Phase-field investigation of multicomponent diffusion in single-phase and two-phase diffusion couples. J. Phase Equilibria Diffus. 2006, 27, 676–683. [Google Scholar] [CrossRef]
- Hillert, M.; Kjellqvist, L.; Mao, H.; Selleby, M.; Sundman, B. Parameters in the compound energy formalism for ionic systems. Calphad 2009, 33, 227–232. [Google Scholar] [CrossRef]
- Luo, Z.; Du, Y.; Liu, Y.; Tang, S.; Pan, Y.; Mao, H.; Peng, Y.; Liu, W.; Liu, Z. Phase field simulation of the phase separation in the TiC-ZrC-WC system. Calphad 2018, 63, 190–195. [Google Scholar] [CrossRef]
- Jacobs, M.; Schmid-Fetzer, R.; Markus, T.; Motalov, V.; Borchardt, G.; Spitzer, K.H. Thermodynamics and diffusion in ternary Fe–Al–Cr alloys, Part I: Thermodynamic modeling. Intermetallics 2008, 16, 995–1005. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Chang, K.; Krill III, C.E.; Du, Q.; Chen, L.Q. Evaluating microstructural parameters of three-dimensional grains generated by phase-field simulation or other voxel-based techniques. Model. Simul. Mater. Sci. Eng. 2012, 20, 075009. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, K.; Chang, K. Effect of Al Concentration on the Microstructural Evolution of Fe-Cr-Al Systems: A Phase-Field Approach. Metals 2021, 11, 4. https://doi.org/10.3390/met11010004
Lee J, Park K, Chang K. Effect of Al Concentration on the Microstructural Evolution of Fe-Cr-Al Systems: A Phase-Field Approach. Metals. 2021; 11(1):4. https://doi.org/10.3390/met11010004
Chicago/Turabian StyleLee, Jeonghwan, Kwangheon Park, and Kunok Chang. 2021. "Effect of Al Concentration on the Microstructural Evolution of Fe-Cr-Al Systems: A Phase-Field Approach" Metals 11, no. 1: 4. https://doi.org/10.3390/met11010004
APA StyleLee, J., Park, K., & Chang, K. (2021). Effect of Al Concentration on the Microstructural Evolution of Fe-Cr-Al Systems: A Phase-Field Approach. Metals, 11(1), 4. https://doi.org/10.3390/met11010004




