Effects of Ag-Rich Nano-Precipitates on the Antibacterial Properties of 2205 Duplex Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microstructure Characterization
2.3. Antibacterial Testing
2.4. Ag Ion Release
2.5. In Vitro Cytocompatibility
3. Results and Discussion
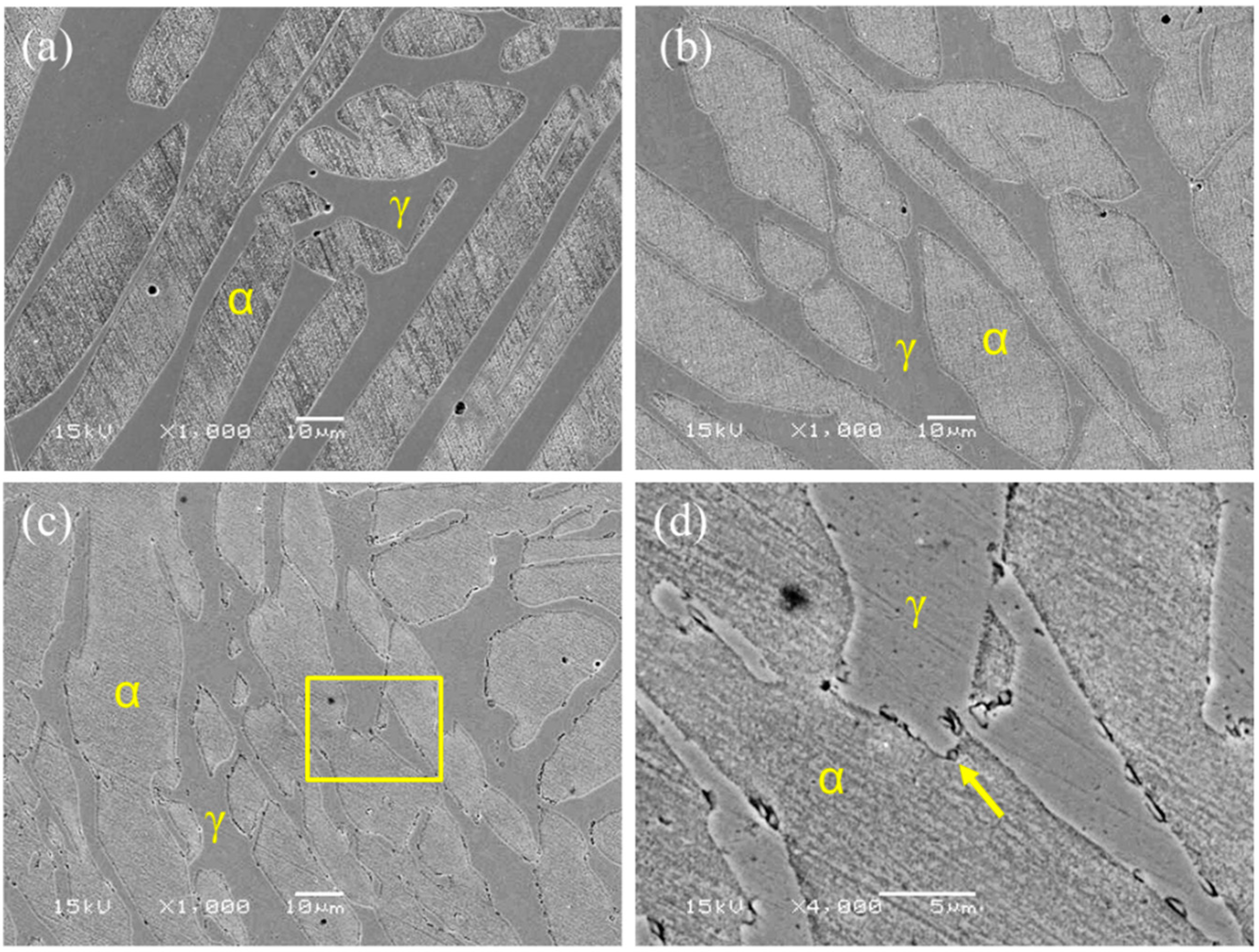
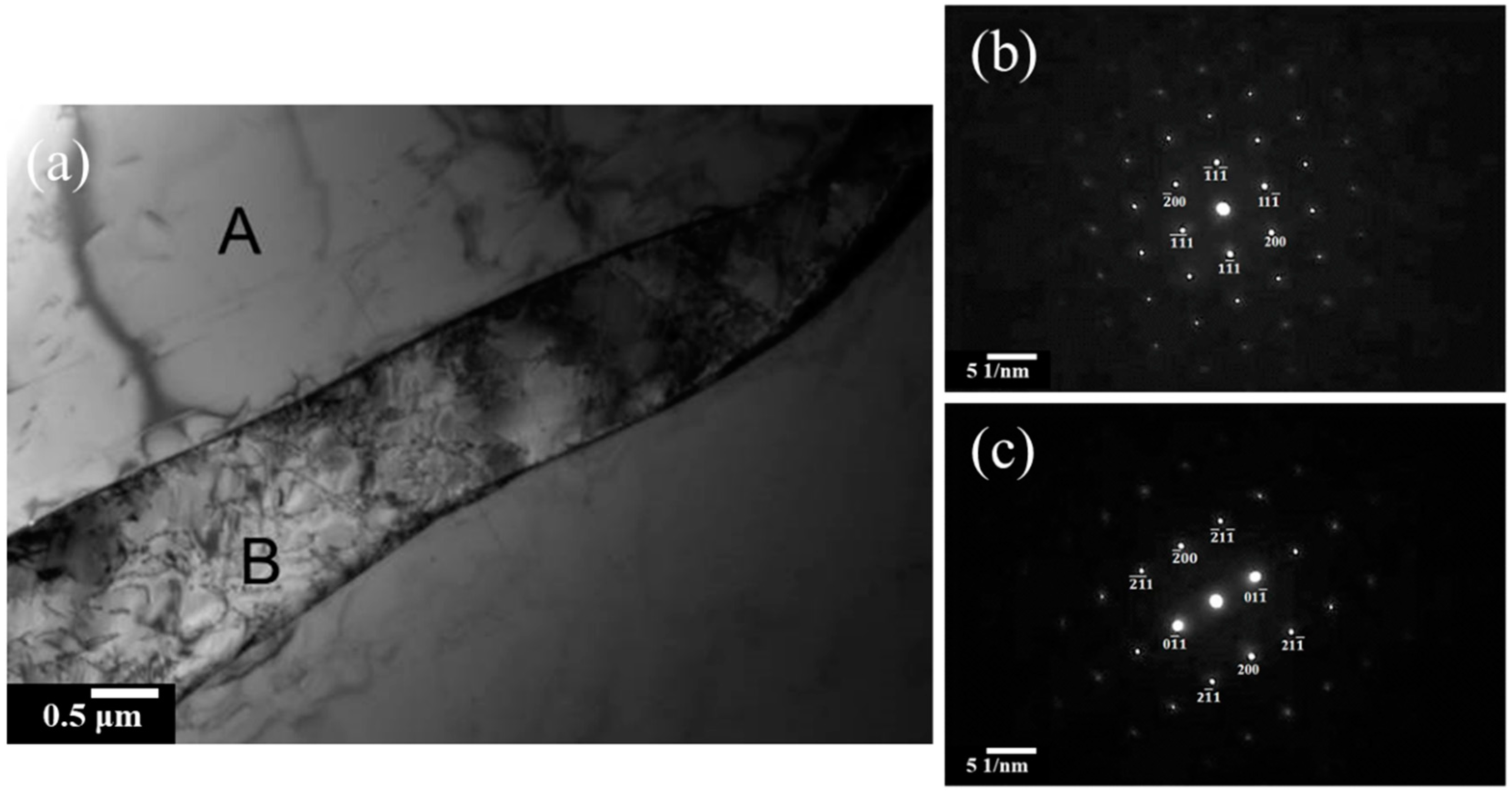
3.1. Microstructures
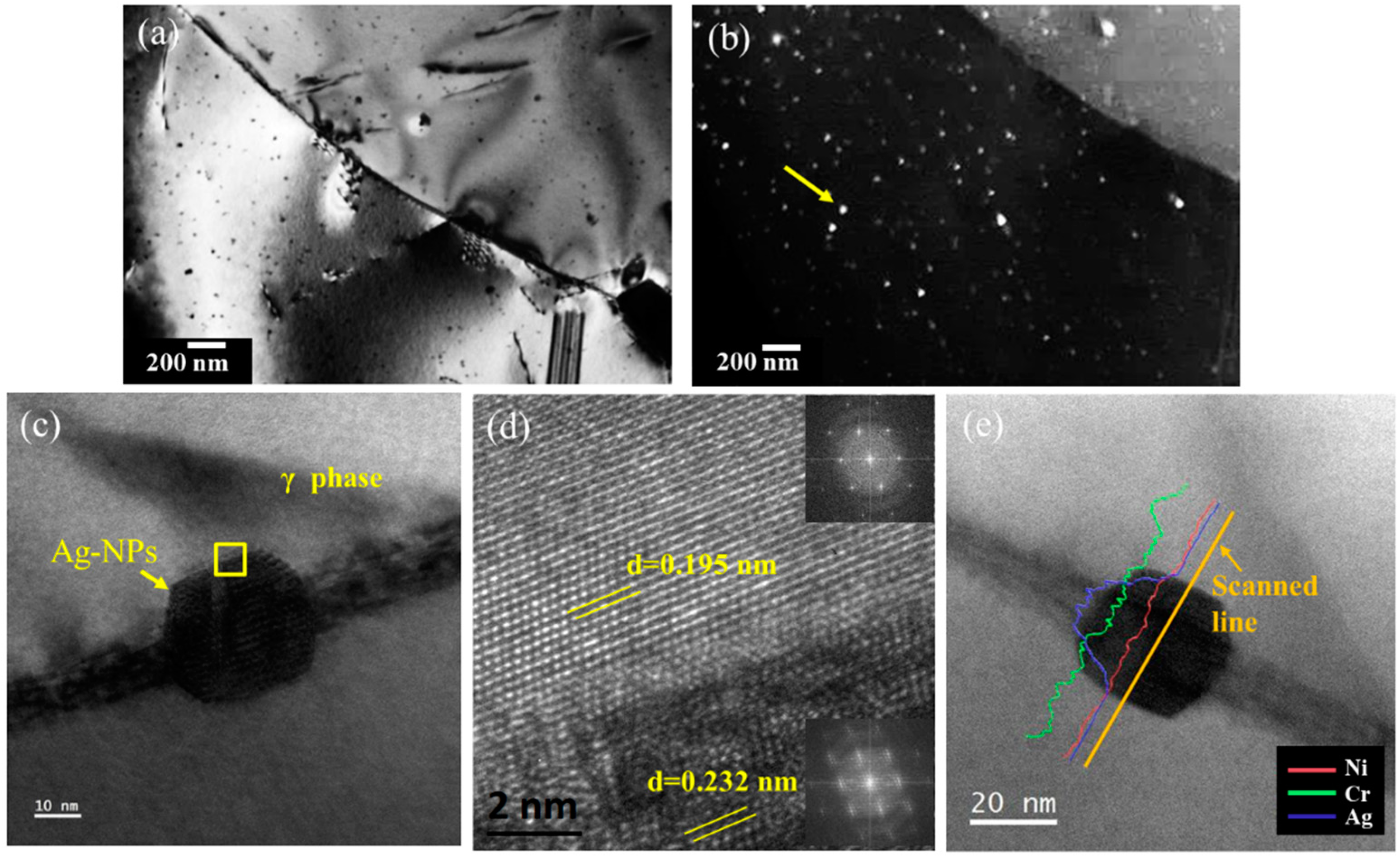
3.2. Effect of Ag-NPs on Antibacterial Properties
3.3. Cytocompatibility Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahmani-Oskooee, M.; Hossein Nedjad, M.; Samadi, A.; Kozeschnik, E. Cu-bearing, martensitic stainless steels for applications in biological environments. Mater. Des. 2017, 130, 442–451. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, L.; Zhang, B.; Cao, C.; Yang, K. In vitro study on infectious ureteral encrustation resistance of Cu-bearing stainless steel. J. Mater. Sci. Technol. 2017, 33, 1604–1609. [Google Scholar] [CrossRef]
- Zhao, J.; Zhai, Z.; Sun, D.; Yang, C.; Zhang, X.; Huang, N.; Jiang, X.; Yang, K. Antibacterial durability and biocompatibility of antibacterial-passivated 316L stainless steel in simulated physiological environment. Mater. Sci. Eng. C 2019, 100, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.K.; Brooks, R.P.; Ehrensberger, M.T. Effects of simulated inflammation on the corrosion of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Gervais, B.; Vadean, A.; Raison, M.; Brochu, M. Failure analysis of a 316L stainless steel femoral orthopedic implant. Case Stud. Eng. Fail. Anal. 2016, 5–6, 30–38. [Google Scholar] [CrossRef]
- AmaroVicente, T.; Oliveira, L.; Correa, E.; Barbosa, R.; Macanhan, V.; Alcântara, N. Stress Corrosion Cracking Behaviour of Dissimilar Welding of AISI 310S Austenitic Stainless Steel to 2304 Duplex Stainless Steel. Metals 2018, 8, 195. [Google Scholar] [CrossRef]
- Victoria, B.; George, G.; Kumar, K.A. Ultrasonic Characterization and Micro-Structural Studies On 2205 Duplex Stainless Steel in Thermal Variations. Int. J. Sci. Technol. Res. 2015, 4, 45–49. [Google Scholar]
- Conradi, M.; Schön, P.M.; Kocijan, A.; Jenko, M.; Vancso, G.J. Surface analysis of localized corrosion of austenitic 316L and duplex 2205 stainless steels in simulated body solutions. Mater. Chem. Phys. 2011, 130, 708–713. [Google Scholar] [CrossRef]
- Tranchida, G.; Clesi, M.; Di Franco, F.; Di Quarto, F.; Santamaria, M. Electronic properties and corrosion resistance of passive films on austenitic and duplex stainless steels. Electrochim. Acta 2018, 273, 412–423. [Google Scholar] [CrossRef]
- Du, J.K.; Wang, C.H.; Wang, K.C.; Chen, K.K. TEM analysis of 2205 duplex stainless steel to determine orientation relationship between M23C6 carbide and austenite matrix at 950 °C. Intermetallics 2014, 45, 80–83. [Google Scholar] [CrossRef]
- Lee, K.M.; Cho, H.S.; Choi, D.C. Effect of isothermal treatment of SAF 2205 duplex stainless steel on migration of δ/γ interface boundary and growth of austenite. J. Alloys Compd. 1999, 285, 156–161. [Google Scholar] [CrossRef]
- Horvath, W.; Prantl, W.; Stroißnigg, H.; Werner, E.A. Microhardness and microstructure of austenite and ferrite in nitrogen alloyed duplex steels between 20 and 500 °C. Mater. Sci. Eng. C 1998, 256, 227–236. [Google Scholar] [CrossRef]
- Michalska, J.; Sozańska, M. Qualitative and quantitative analysis of σ and χ phases in 2205 duplex stainless steel. Mater. Charact. 2006, 56, 355–362. [Google Scholar] [CrossRef]
- Palsson, N.; Pettersson, R.; Pan, J. Depletion effects at phase boundaries in 2205 duplex stainless steel characterized with SKPFM and TEM/EDS. Corros. Sci. 2009, 51, 1850–1860. [Google Scholar]
- Haghdadi, N.; Cizek, P.; Hodgson, P.D.; Beladi, H. Microstructure dependence of impact toughness in duplex stainless steels. Mater. Sci. Eng. A 2019, 745, 369–378. [Google Scholar] [CrossRef]
- de Lima, H.M.L.F.; Tavares, S.S.M.; Martins, M.; Araújo, W.S. The effect of copper addition on the corrosion resistance of cast duplex stainless steel. J. Mater. Res. Technol. 2019, 8, 2107–2119. [Google Scholar] [CrossRef]
- Yang, S.M.; Chen, Y.C.; Chen, C.H.; Huang, W.P.; Lin, D.Y. Microstructural characterization of δ/γ/σ/γ2/χ phases in silver-doped 2205 duplex stainless steel under 800 °C aging. J. Alloys Compd. 2015, 633, 48–53. [Google Scholar] [CrossRef]
- Liao, K.-H.; Ou, K.-L.; Cheng, H.-C.; Lin, C.-T.; Peng, P.-W. Effect of silver on antibacterial properties of stainless steel. Appl. Surf. Sci. 2010, 256, 3642–3646. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Y.; Li, X.; Dong, C. Interaction between austein-ferrite phases on passive performance of 2205 duplex stainless steel. J. Mater. Res. Technol. 2018, 34, 2140–2148. [Google Scholar] [CrossRef]
- Chen, T.H.; Yang, J.R. Effects of solution treatment and continuous cooling on σ-phase precipitation in a 2205 duplex stainless steel. Mater. Sci. Eng. A 2001, 311, 28–41. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandström, R. Sigma phase precipitation in duplex stainless steel 2205. Mater. Sci. Eng. A 2007, 44, 271–276. [Google Scholar] [CrossRef]
- Bettini, E.; Kivisäkk, U.; Leygraf, C.; Pan, J. Study of corrosion behavior of a 22% Cr duplex stainless steel: Influence of nano-sized chromium nitrides and exposure temperature. Electrochim. Acta 2013, 113, 280–289. [Google Scholar] [CrossRef]
- Chandra, K.; Singhal, R.; Kain, V.; Raja, V.S. Low temperature embrittlement of duplex stainless steel: Correlation between mechanical and electrochemical behavior. Mater. Sci. Eng. A 2010, 527, 3904–3912. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of intermetallic precipitations on the properties of duplex stainless steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar] [CrossRef]
- de Lacerda, J.C.; Cândido, L.C.; Godefroid, L.B. Effect of volume fraction of phases and precipitates on the mechanical behavior of UNS S31803 duplex stainless steel. Int. J. Fatigue 2015, 74, 81–87. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.W.; Kim, H.B.; Park, C.N.; Choi, Y.I.; Park, C.J. Effects of the precipitation of secondary phases on the erosion-corrosion of 25% Cr duplex stainless steel. Corros. Sci. 2019, 152, 202–210. [Google Scholar] [CrossRef]
- Reichman, D.E.; Greenberg, J.A. Reducing surgical site infections: A review. Rev. Obstet. Gynecol. 2009, 2, 212–221. [Google Scholar] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterial 2013, 34, 8018–8029. [Google Scholar] [CrossRef]
- Topouzelis, N.; Tsaousoglou, P. Clinical factors correlated with the success rate of miniscrews in orthodontic treatment. Int. J. Oral Sci. 2012, 4, 38–44. [Google Scholar] [CrossRef]
- Kravitz, N.D.; Kusnoto, B. Risks and complications of orthodontic miniscrews. Am. J. Orthod. Dentofac. Orthop. 2007, 131, S43–S51. [Google Scholar] [CrossRef]
- Patil, P.; Kharbanda, O.P.; Duggal, R.; Das, T.K.; Kalyanasundaram, D. Surface deterioration and elemental composition of retrieved orthodontic miniscrews. Am. J. Orthod. Dent. Orthoped. 2015, 14, S88–S100. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef] [PubMed]
- Ciacotich, N.; Din, R.U.; Sloth, J.J.; Møller, P.; Gram, L. An electroplated copper–silver alloy as antibacterial coating on stainless steel. Surf. Coat. Technol. 2018, 345, 96–104. [Google Scholar] [CrossRef]
- Devasconcellos, P.; Bose, S.; Beyenal, H.; Bandyopadhyay, A.; Zirkle, L.G. Antimicrobial Particulate Silver Coatings on Stainless Steel Implants for Fracture Management. Mater. Sci. Eng. C 2012, 32, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Sharifahmadian, O.; Salimijazi, H.R.; Fathi, M.H.; Mostaghimi, J.; Pershin, L. Relationship between surface properties and antibacterial behavior of wire arc spray copper coatings. Surf. Coat. Technol. 2013, 233, 74–79. [Google Scholar] [CrossRef]
- Dudognon, J.; Vayer, M.; Pineau, A.; Erre, R. Mo and Ag ion implantation in austenitic, ferritic and duplex stainless steels: A comparative study. Surf. Coat. Technol. 2008, 203, 180–185. [Google Scholar] [CrossRef]
- Osés, J.; Palacio, J.F.; Kulkarni, S.; Medrano, A.; García, J.A.; Rodríguez, R. Antibacterial PVD coatings doped with silver by ion implantation. Appl. Surf. Sci. 2014, 310, 56–61. [Google Scholar] [CrossRef]
- Chen, R.; Ni, H.; Zhang, H.; Yue, G.; Zhan, W.; Xiong, P. A preliminary study on antibacterial mechanisms of silver ions implanted stainless steel. Vacuum 2013, 89, 249–253. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Huang, X.-B.; Jiang, L.; Ma, Y.; Fan, A.I.; Tang, B. Antibacterial Property of Cu Modified Stainless Steel by Plasma Surface Alloying. Int. J. Iron Steel Res. 2012, 19, 75–79. [Google Scholar] [CrossRef]
- Alphonsa, J.; Raja, V.S.; Mukherjee, S. Study of plasma nitriding and nitrocarburizing for higher corrosion resistance and hardness of 2205 duplex stainless steel. Corros. Sci. 2015, 100, 121–132. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2020, 100735. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.-C. Simultaneous improvement of corrosion resistance and bioactivity of a titanium alloy via wet and dry plasma treatments. J. Alloys Compd. 2021, 851, 156840. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Shahzad, M.B.; Xu, D.; Sun, Z.; Zhao, J.; Yang, C.; Qi, M.; Yang, K. Effect of copper addition on mechanical properties, corrosion resistance and antibacterial property of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 1079–1085. [Google Scholar] [CrossRef]
- Nan, L.; Ren, G.; Wang, D.; Yang, K. Antibacterial Performance of Cu-Bearing Stainless Steel against Staphylococcus aureus and Pseudomonas aeruginosa in Whole Milk. J. Mater. Sci. Technol. 2016, 32, 445–451. [Google Scholar] [CrossRef]
- Yang, S.M.; Chen, Y.C.; Pan, Y.T.; Lin, D.Y. Effect of silver on microstructure and antibacterial property of 2205 duplex stainless steel. Mater. Sci. Eng. C 2016, 63, 376–383. [Google Scholar] [CrossRef]
- Arash, V.; Keikhaee, F.; Rabiee, S.M.; Rajabnia, R.; Khafri, S.; Tavanafar, S. Evaluation of Antibacterial Effects of Silver-Coated Stainless Steel Orthodontic Brackets. J. Dent. 2016, 13, 49–54. [Google Scholar]
- Moerman, F. Antimicrobial materials, coatings and biomimetic surfaces with modified microtography to control microbial fouling of product contact surfaces within food processing equipment: Legislation, requirements, effectiveness and challenges. J. Hyg. Eng. Des. 2014, 7, 8–29. [Google Scholar]
- Silvestry-Rodriguez, N.; Sicairos-Ruelas, E.E.; Gerba, C.P.; Bright, K.R. Silver as a Disinfectant, Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2007; pp. 23–45. [Google Scholar]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. In Vitro 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Qing, Y.A.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic Mechanism of Antimicrobial Activity of Ag+ in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. BioMetals 2013, 26, 609–621. [Google Scholar] [CrossRef]
- Shi, A.; Zhu, C.; Fu, S.; Wang, R.; Qin, G.; Chen, D.; Zhang, E. What controls the antibacterial activity of Ti-Ag alloy, Ag ion or Ti2Ag particles? Mater. Sci. Eng. C 2020, 109, 110548. [Google Scholar] [CrossRef]
- Chen, M.; Yang, L.; Zhang, L.; Han, Y.; Lu, Z.; Qin, G.; Zhang, E. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater. Sci. Eng. C 2017, 75, 906–917. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, E.; Zhang, L. Microstructure, mechanical properties, bio-corrosion properties and antibacterial properties of Ti–Ag sintered alloys. Mater. Sci. Eng. C 2016, 62, 350–360. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, H.; Zhang, E.; You, J.; Ma, X.; Bai, X. Antibacterial activities and biocompatibilities of Ti-Ag alloys prepared by spark plasma sintering and acid etching. Mater. Sci. Eng. C 2018, 92, 121–131. [Google Scholar] [CrossRef]
- Wang, E.; Huang, Y.; Du, Q.; Sun, Y. Silver nanoparticle induced toxicity to human sperm by increasing ROS (reactive oxygen species) production and DNA damage. Environ. Toxicol. Pharmacol. 2017, 52, 193–199. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-Eight-Day Oral Toxicity, Genotoxicity, and Gender-Related Tissue Distribution of Silver Nanoparticles in Sprague-Dawley Rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chao, C.Y.; Chang, Y.T.; Du, J.K. Effect of Phase Distribution on the Antibacterial Property and Cytotoxicity of Ti-5Al-2.5Cu Alloy after Heat Treatment at Various Temperatures. Metals 2020, 10, 858. [Google Scholar] [CrossRef]
- Swartzendruber, L.J. The Ag−Fe (Silver-Iron) system. Bull. Alloy Phase Diagr. 1984, 5, 560–564. [Google Scholar] [CrossRef]
- Yang, S.M.; Chen, Y.C.; Pan, Y.T.; Lin, D.Y. Effect of Ag doping and isothermal aging on phase transformation in 2205 duplex stainless steel. J. Alloys Compd. 2017, 704, 649–658. [Google Scholar] [CrossRef]
- Woehl, T.J.; Evans, J.E.; Arslan, I.; Ristenpart, W.D.; Browning, N.D. Direct in situ determination of the mechanisms controlling nanoparticle nucleation and growth. ACS Nano 2012, 6, 8599–8610. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Lu, W.; Yao, K.; Wang, J.; Yuan, J. Ionic liquids–water interfacial preparation of triangular Ag nanoplates and their shape-dependent antibacterial activity. J. Colloid Interface Sci. 2015, 437, 35–41. [Google Scholar] [CrossRef]
- Chiang, W.C.; Tseng, I.S.; Møller, P.; Hilbert, L.R.; Tolker-Nielsen, T.; Wu, J.-K. Influence of silver additions to type 316 stainless steels on bacterial inhibition, mechanical properties, and corrosion resistance. Mater. Chem. Phys. 2010, 119, 123–130. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Sato, M.; Amano, A.; Hara, A.; Tsuruta, S.; Sugie, A.; Nomiya, K. Light-stable and antimicrobial active silver(I) complexes composed of triphenylphosphine and amino acid ligands: Synthesis, crystal structure, and antimicrobial activity of silver(I) complexes constructed with hard and soft donor atoms (n∞{[Ag(L)(PPh3)]2} with L=α-ala− or asn− and n = 1 or 2). Inorg. Chim. Acta 2008, 361, 1267–1273. [Google Scholar]
- Zielińska-Jurek, A.; Wei, Z.; Wysocka, I.; Szweda, P.; Kowalska, E. The effect of nanoparticles size on photocatalytic and antimicrobial properties of Ag-Pt/TiO2 photocatalysts. Appl. Surf. Sci. 2015, 353, 317–325. [Google Scholar] [CrossRef]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef Univ. J. Bas. Appl. Sci. 2015, 4, 225–231. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, L.; Lv, Y.; Liu, H.; Wang, G.; Ren, N.; Liu, D.; Wang, J.; Boughton, R.I. Chemical assembly of silver nanoparticles on stainless steel for antimicrobial applications. Surf. Coat. Technol. 2010, 204, 3871–3875. [Google Scholar] [CrossRef]
- Polet, M.; Laloux, L.; Cambier, S.; Ziebel, J.; Gutleb, A.C.; Schneider, Y.-J. Soluble silver ions from silver nanoparticles induce a polarised secretion of interleukin-8 in differentiated Caco-2 cells. Toxicol. Lett. 2020, 325, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.R.; Kim, Y.S.; Kim, G.W.; Yang, H.W.; Ko, Y.G.; Shin, D.H. Effects of concentration of Ag nanoparticles on surface structure and in vitro biological responses of oxide layer on pure titanium via plasma electrolytic oxidation. Appl. Surf. Sci. 2015, 347, 574–582. [Google Scholar] [CrossRef]
- Soloviev, M.; Gedanken, A. Coating a stainless steel plate with silver nanoparticles by the sonochemical method. Ultrason. Sonochemistry 2011, 18, 356–362. [Google Scholar] [CrossRef]
- Jamuna-Thevi, K.; Abu Bakar, S.; Ibrahim, S.; Shahab, N.; Toff, M.R.M. Quantification of silver ion release, in vitro cytotoxicity and antibacterial properties of nanostuctured Ag doped TiO2 coatings on stainless steel deposited by RF magnetron sputtering. Vacuum 2011, 86, 235–241. [Google Scholar] [CrossRef]
- Wan, A.T.; Conyers, R.A.; Coombs, C.J.; Masterton, J.P. Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clin. Chem. 1991, 37, 1683–1687. [Google Scholar] [CrossRef]
- Hardes, J.; Ahrens, H.; Gebert, C.; Streitbuerger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Wedemeyer, C.; Saxler, G.; Winkelmann, W.; et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials 2007, 28, 2869–2875. [Google Scholar] [CrossRef]





| Elements | Fe | Cr | Ni | Mo | Mn | C | N | Si | Ag |
|---|---|---|---|---|---|---|---|---|---|
| 2205 | Bal. | 23.44 | 5.90 | 3.07 | 1.12 | 0.03 | 0.10 | 0.31 | <0.01 |
| 2205Ag | Bal. | 22.51 | 5.45 | 2.82 | 1.25 | 0.03 | 0.18 | 0.44 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.-K.; Chao, C.-Y.; Wei, L.-L.; Wang, C.-H.; Chen, J.-H.; Chen, K.-K.; Huang, R.-B. Effects of Ag-Rich Nano-Precipitates on the Antibacterial Properties of 2205 Duplex Stainless Steel. Metals 2021, 11, 23. https://doi.org/10.3390/met11010023
Du J-K, Chao C-Y, Wei L-L, Wang C-H, Chen J-H, Chen K-K, Huang R-B. Effects of Ag-Rich Nano-Precipitates on the Antibacterial Properties of 2205 Duplex Stainless Steel. Metals. 2021; 11(1):23. https://doi.org/10.3390/met11010023
Chicago/Turabian StyleDu, Je-Kang, Chih-Yeh Chao, Lin-Lung Wei, Chau-Hsiang Wang, Jeng-Huey Chen, Ker-Kong Chen, and Ruei-Bin Huang. 2021. "Effects of Ag-Rich Nano-Precipitates on the Antibacterial Properties of 2205 Duplex Stainless Steel" Metals 11, no. 1: 23. https://doi.org/10.3390/met11010023
APA StyleDu, J.-K., Chao, C.-Y., Wei, L.-L., Wang, C.-H., Chen, J.-H., Chen, K.-K., & Huang, R.-B. (2021). Effects of Ag-Rich Nano-Precipitates on the Antibacterial Properties of 2205 Duplex Stainless Steel. Metals, 11(1), 23. https://doi.org/10.3390/met11010023





