Effect of the Microsegregation on Martensitic and Bainitic Reactions in a High Carbon-High Silicon Cast Steel
Abstract
1. Introduction
2. Materials and Methods
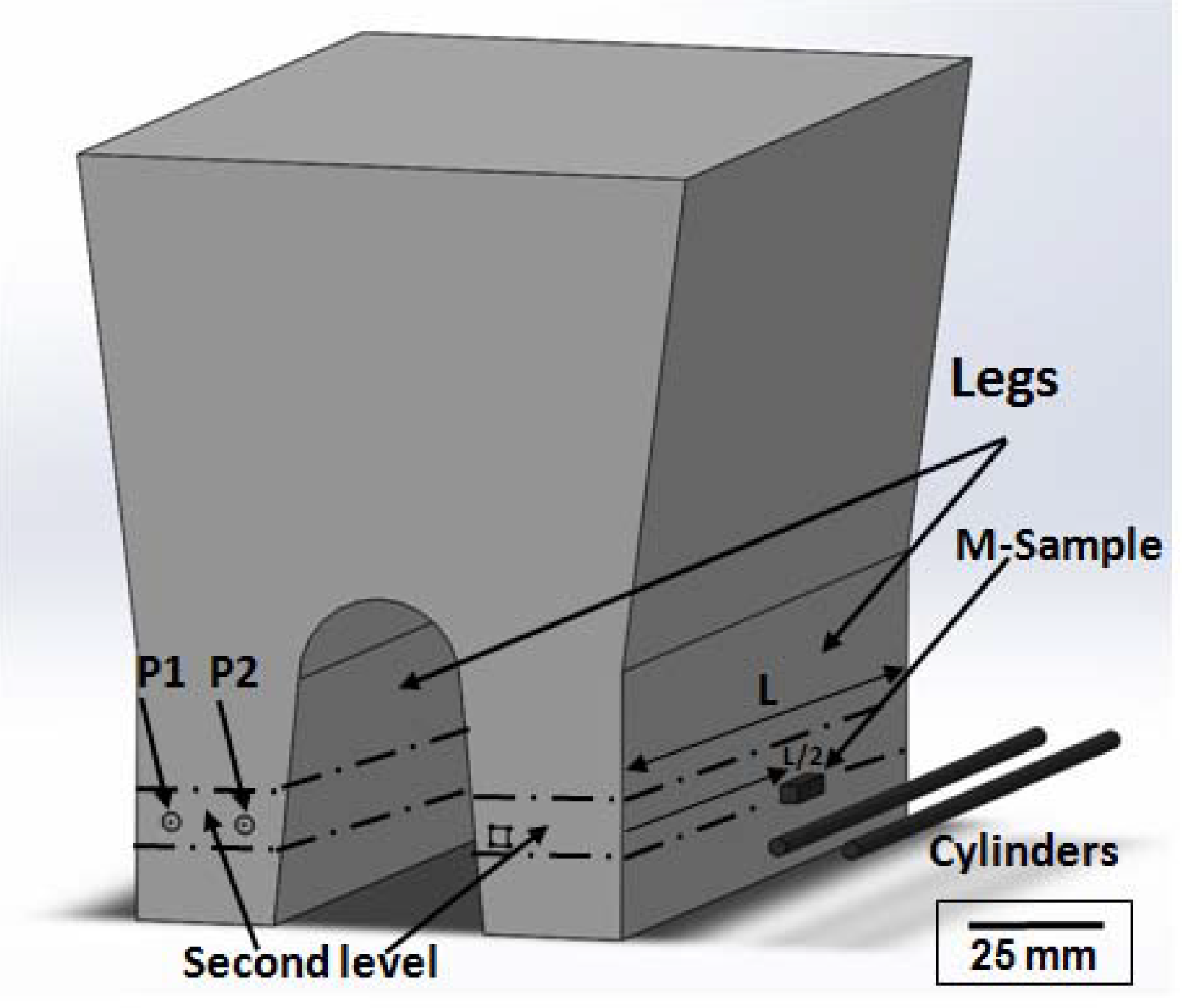
2.1. Material and Casting Procedure
2.2. As-Cast Structures: Matrix Phases and Microsegregation Characterization by Electron Probe Micro-Analysis (EPMA)
2.3. The Bainitic Transformation
2.3.1. Determination of the Prior Austenite Grain Size by Thermal Etching
2.3.2. High Resolution Dilatometry: Isothermal Austempering Heat Treatments
2.3.3. Microstructural Characterization by Light Optical and Electron Microscopy
2.3.4. X-Ray Diffraction
2.3.5. Hardness Tests
2.3.6. Electron Backscatter Diffraction (EBSD) Analysis
3. Results and Discussion
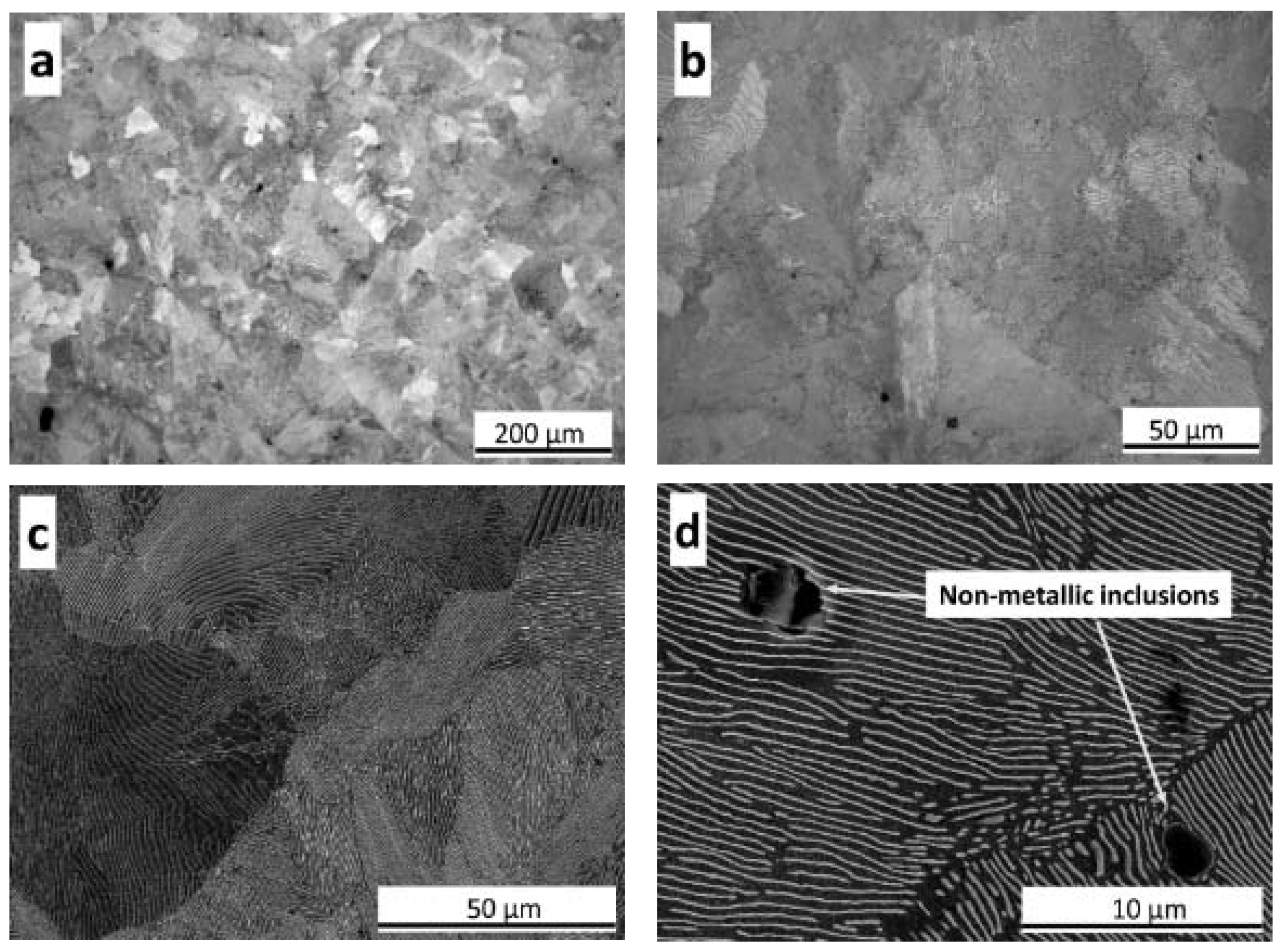
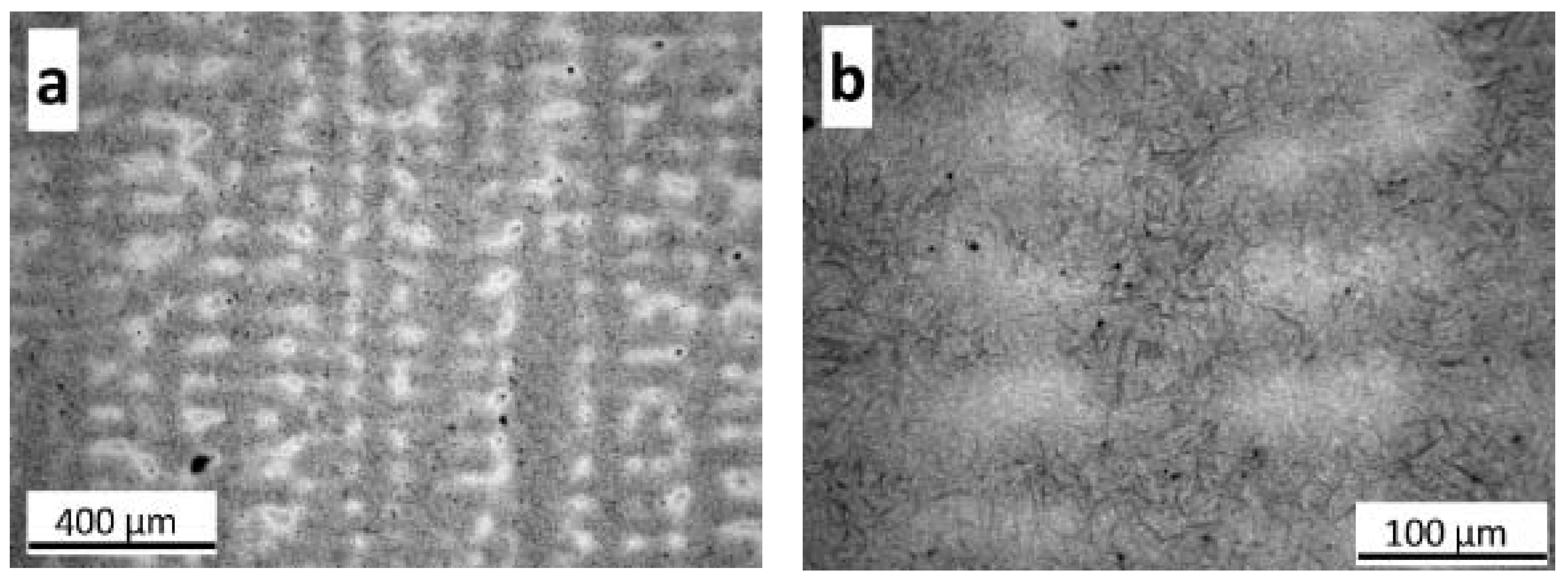
3.1. As-Cast Microstructure
3.1.1. Matrix Phases
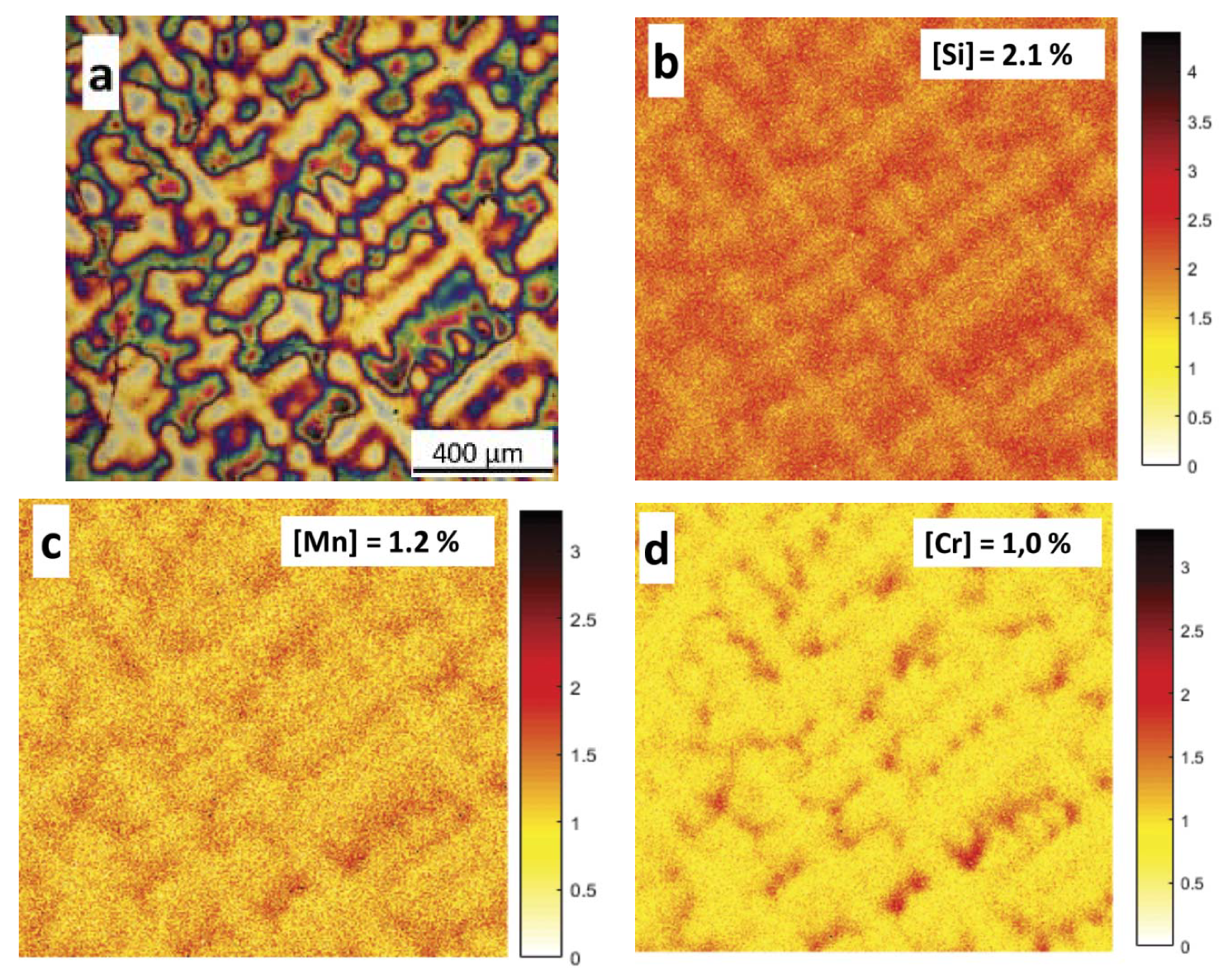
3.1.2. Microsegregation Analysis
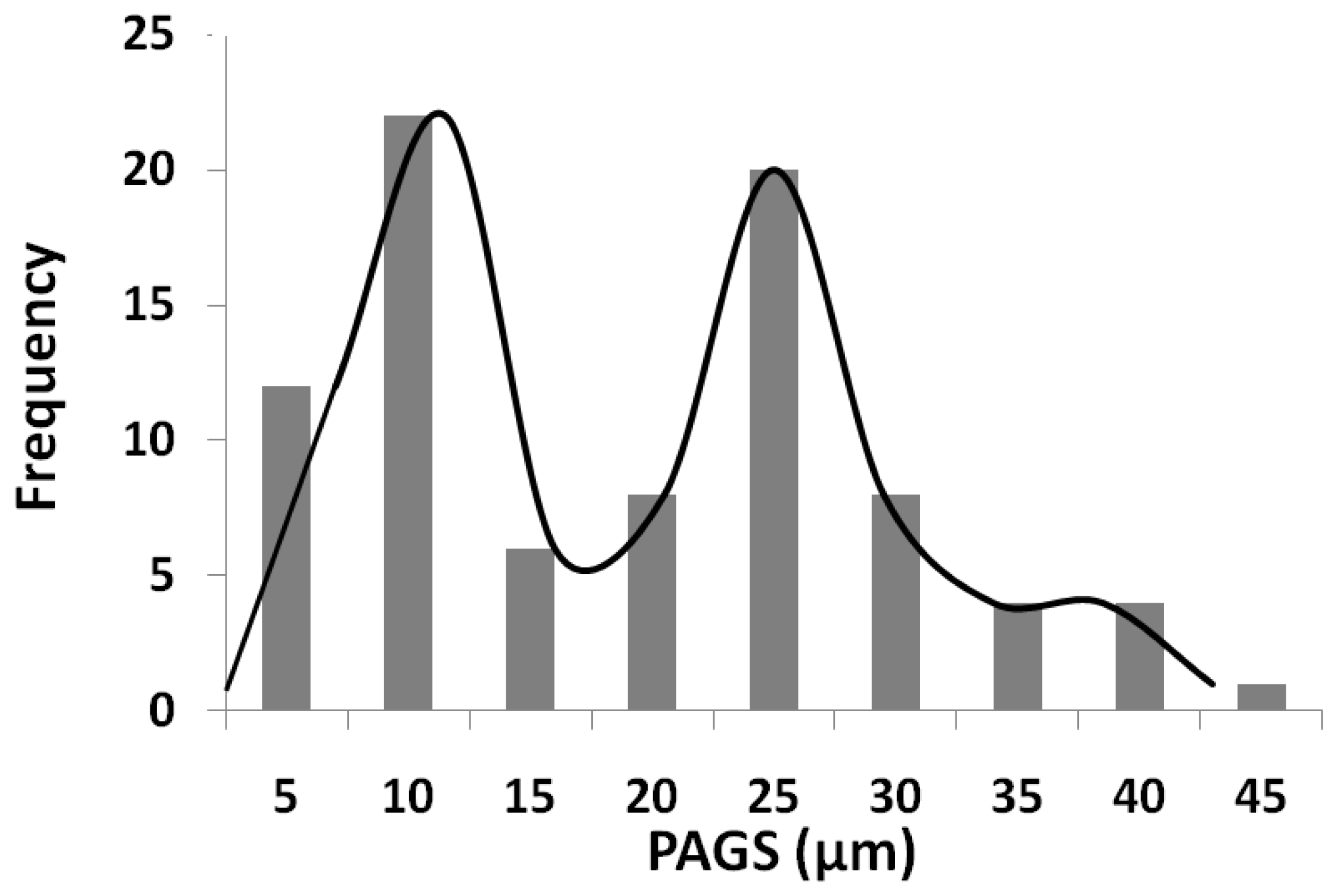
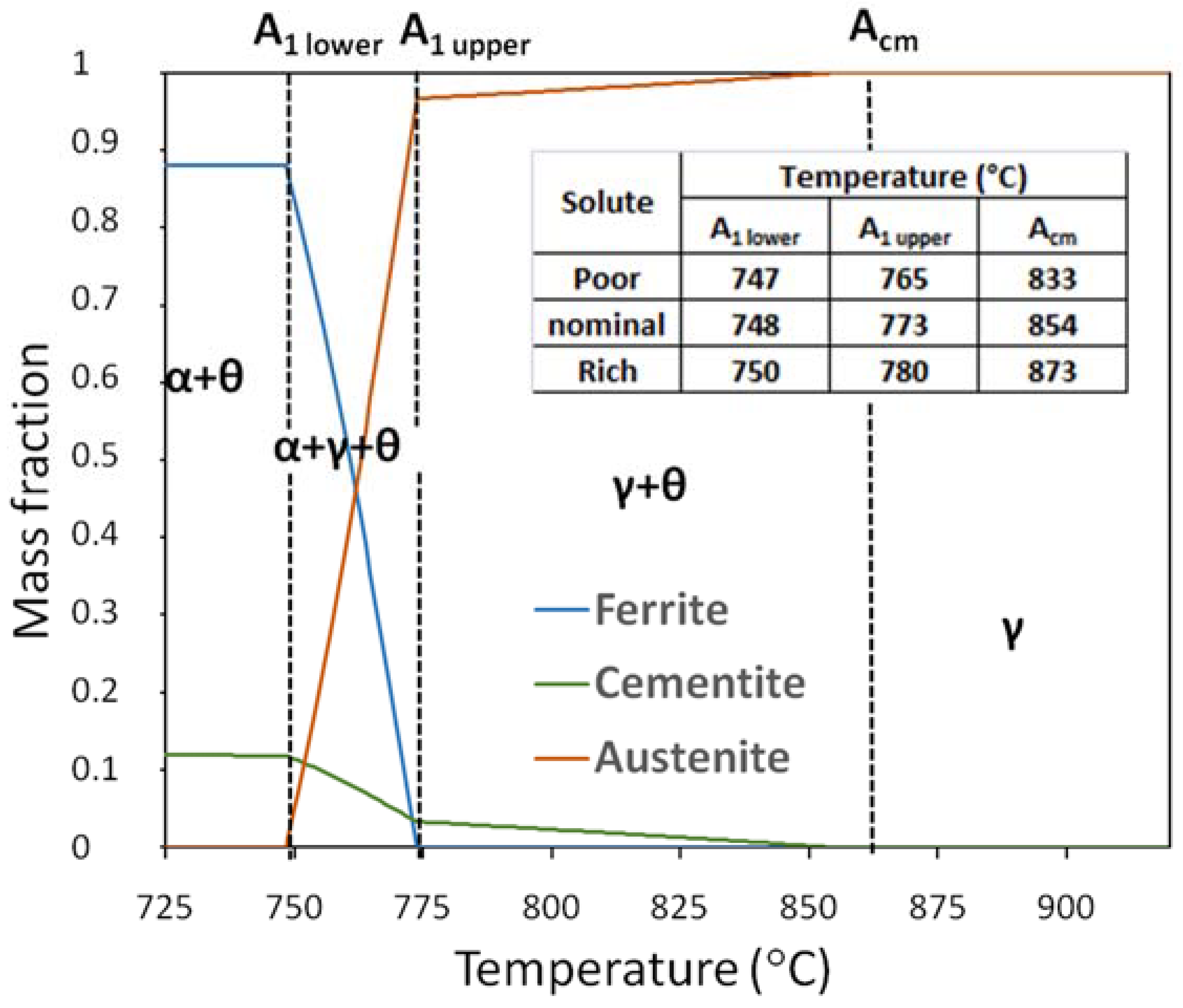
3.2. Austenitization of As-Cast Microstructures. Prior Austenitic Grain Size (PAGS)
3.3. Martensitic Transformation
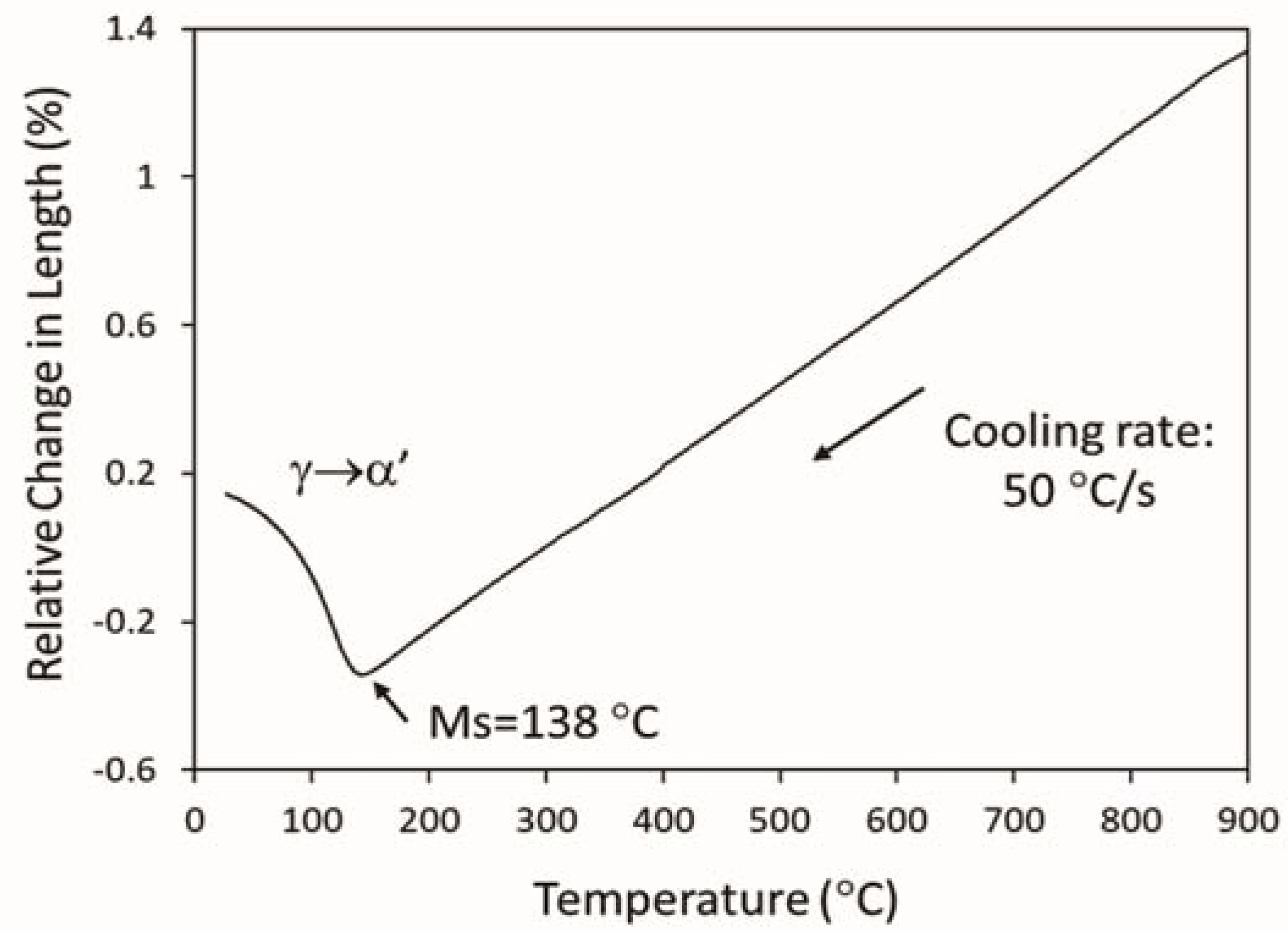
3.3.1. Determination of Ms Temperature
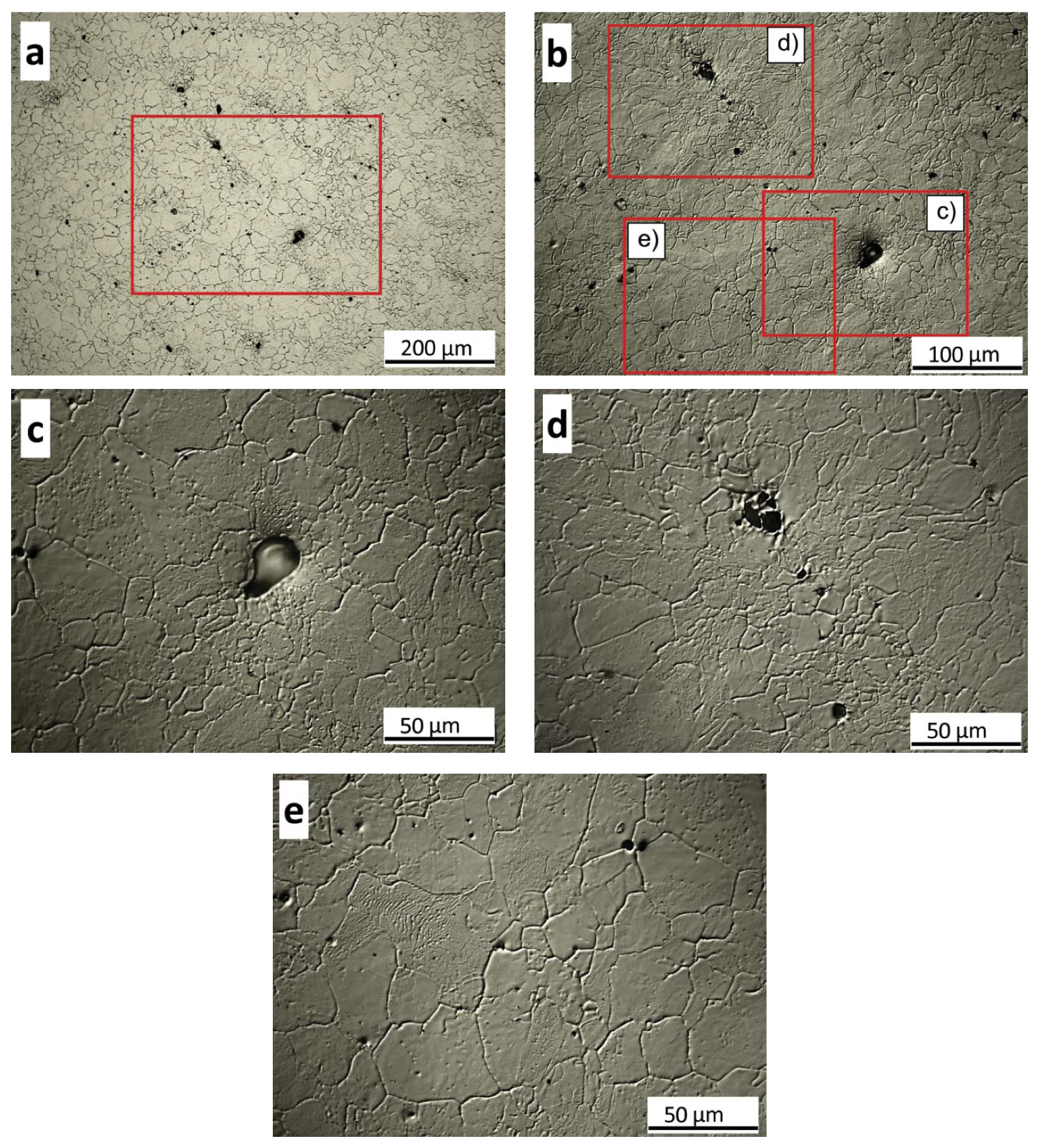
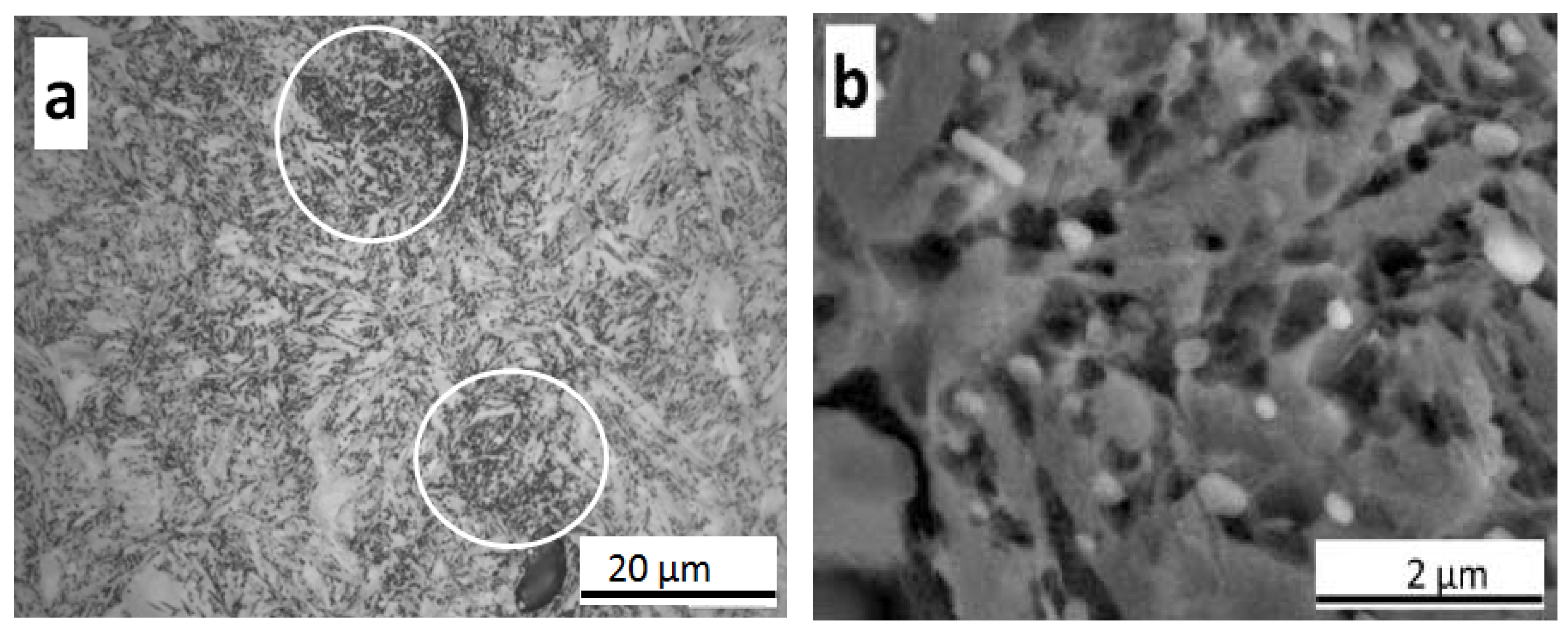
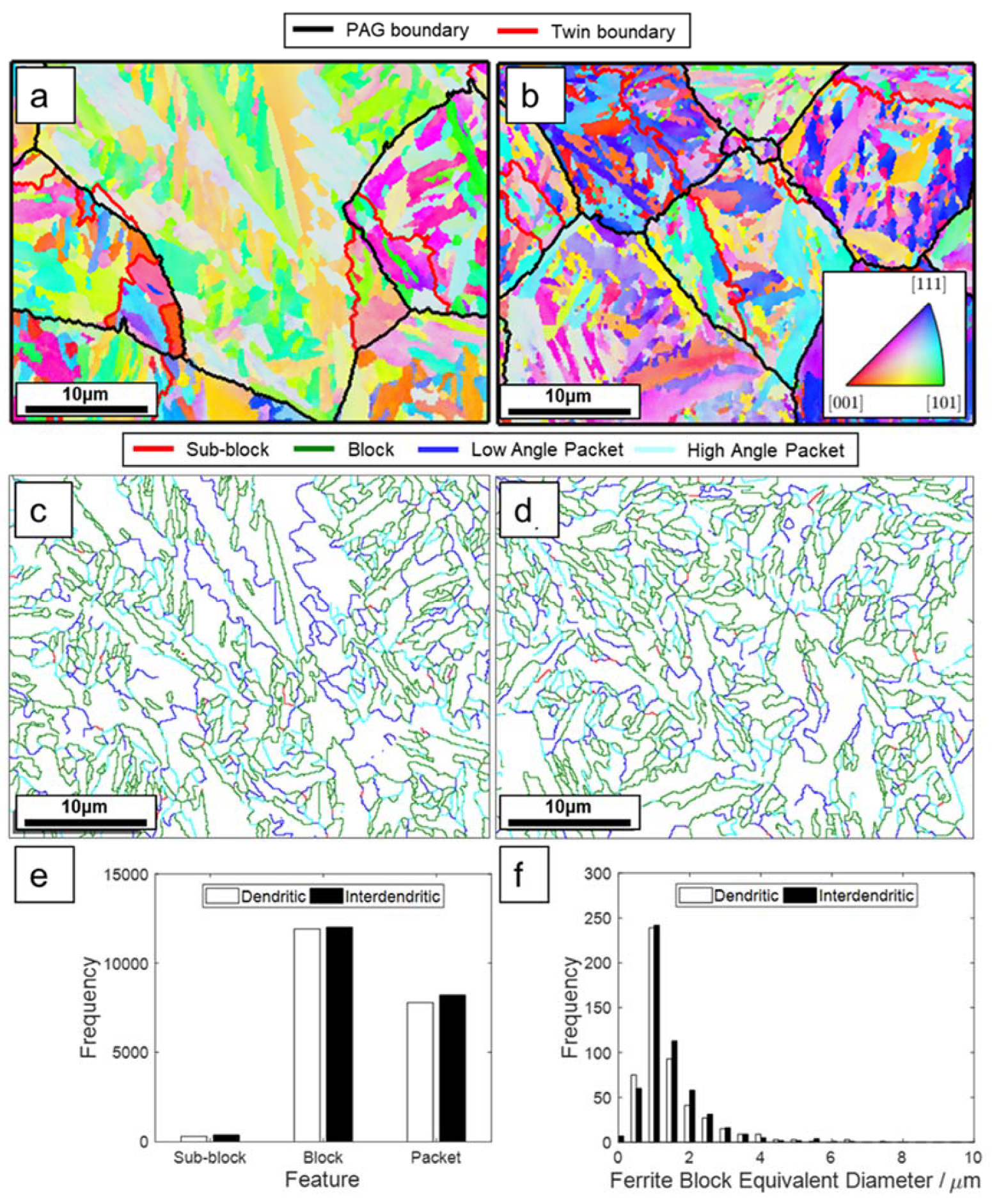
3.3.2. As-Quenched Microstructure
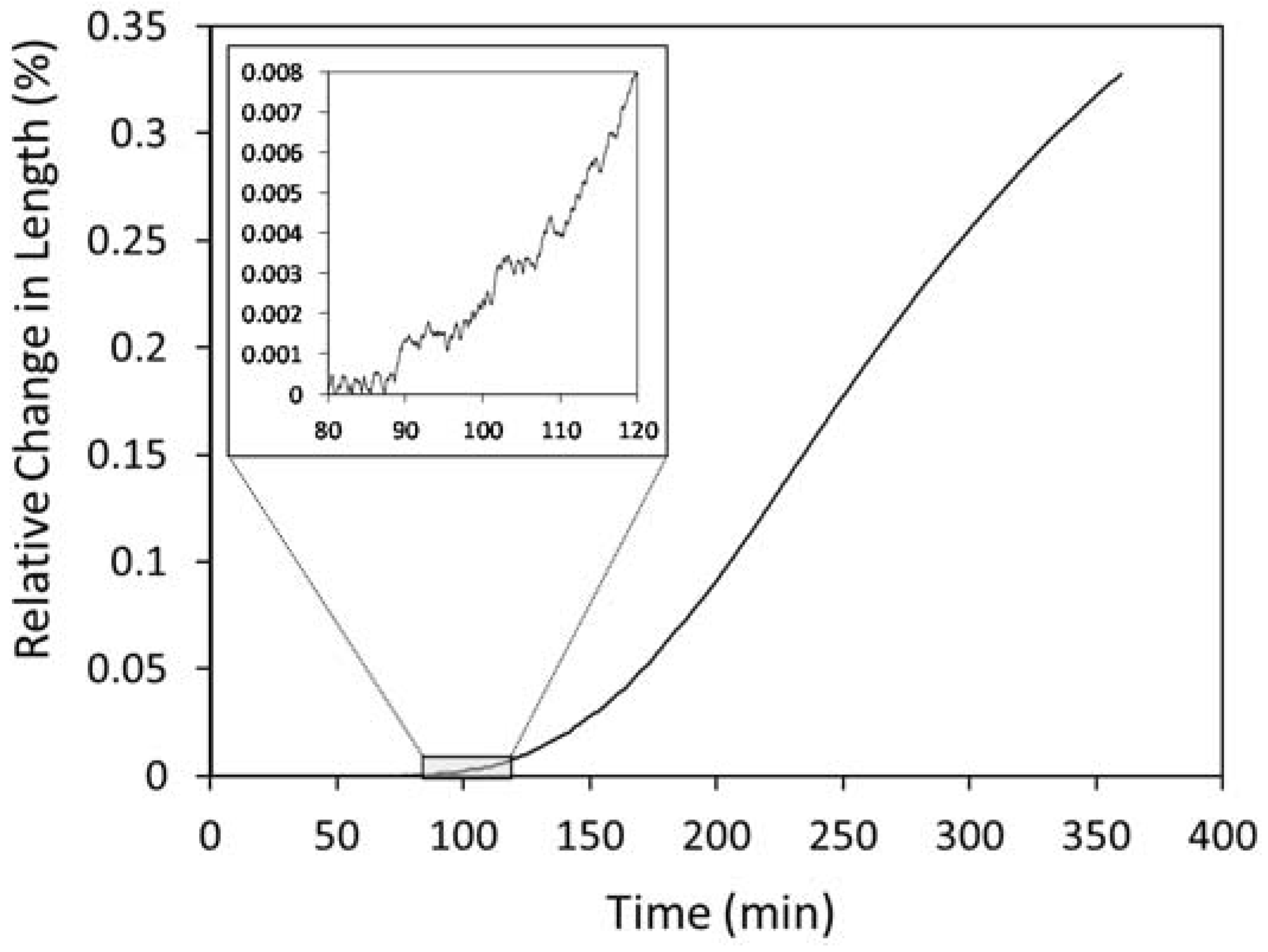
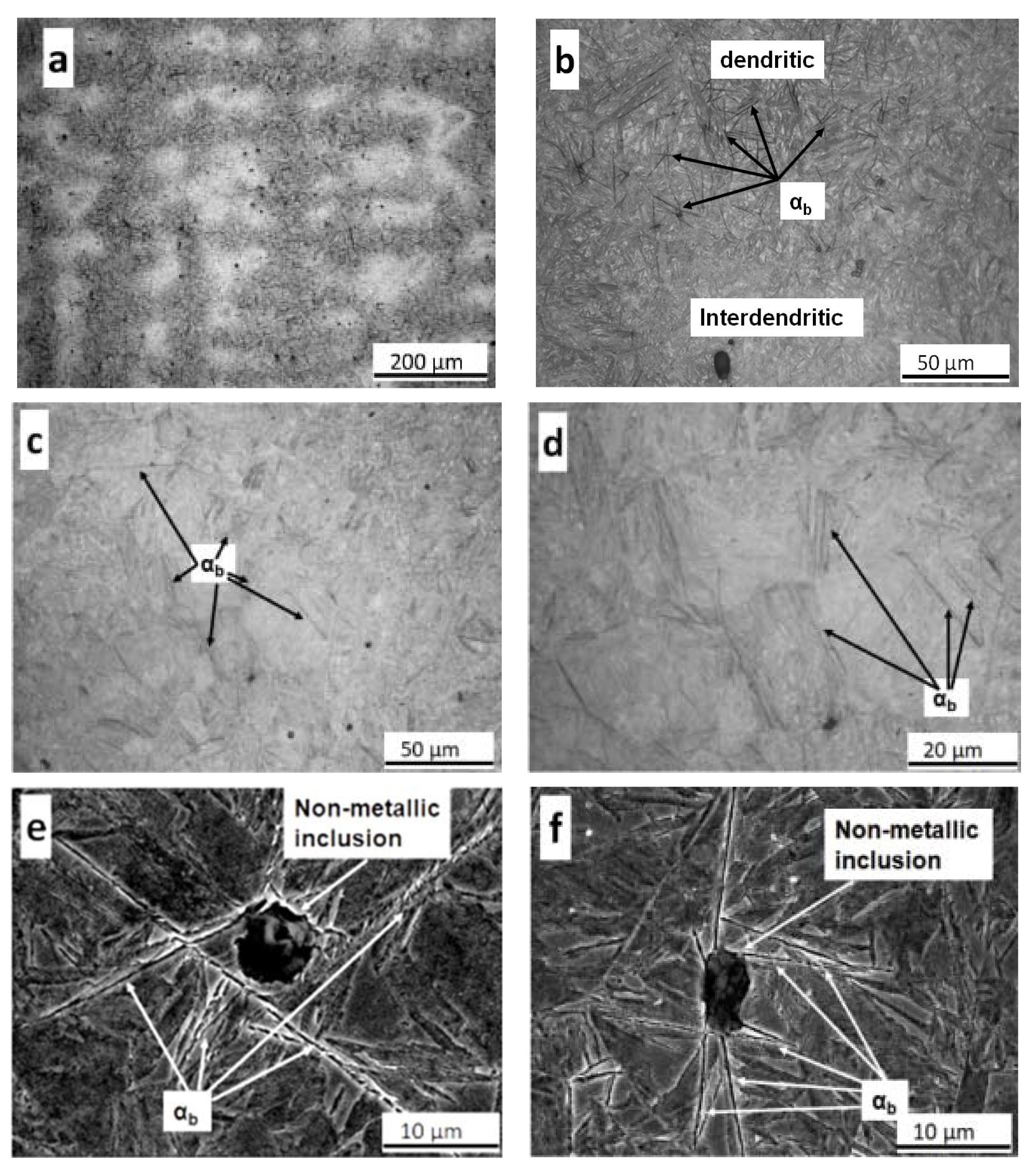
3.4. Bainitic Transformation
4. Conclusions
- Segregation of Cr, Si, and Mn during solidification causes the dendritic areas to have a lower concentration of alloying elements.
- Prior austenitic grain size shows a bimodal distribution associate to segregation of alloying elements. Dendritic regions, which have the lower concentration in solute, showed a larger average prior grain size than interdendritic areas (22.4 ± 8.1 µm and 6.4 ± 2.6 µm, respectively). This difference in grain size is attributed to the combined influence of the following. (1) The presence of undissolved cementite observed in segregated areas would pin the grain boundaries, inhibiting grain growth. (2) The Si, Mn, and Cr rich zones are the regions where the last austenite grains are formed during the heating cycle. This generates a smaller austenite grain size in the areas rich in solute with respect to areas poor in solute. (3) Moreover, in the enriched regions, solute drag could be lowering the mobility of the austenite grain boundaries, hampering their movement and refining the grain size.
- The microstructural inhomogeneities (microsegregation, bimodal grain size distribution) do not affect the nucleation and progress of the martensitic transformation as recorded by dilatometry contrary to other investigations. The EBSD analysis of the as-quenched microstructure shows a small difference in the PAGS measured in dendritic and interdendritic regions contrary to the thermal etching results. Further, no difference in the misorientation boundaries frequency and block size was observed between these two regions.
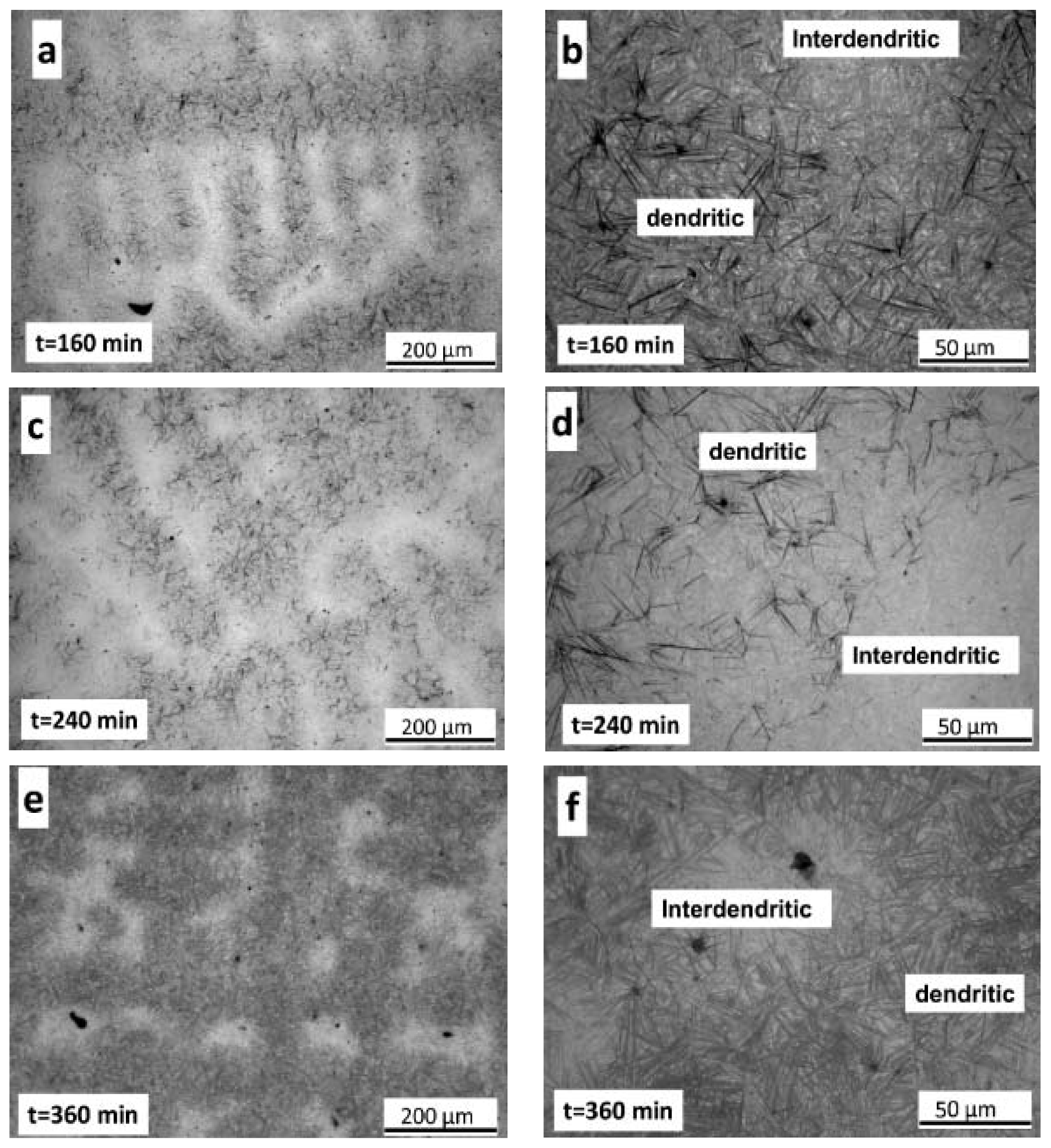
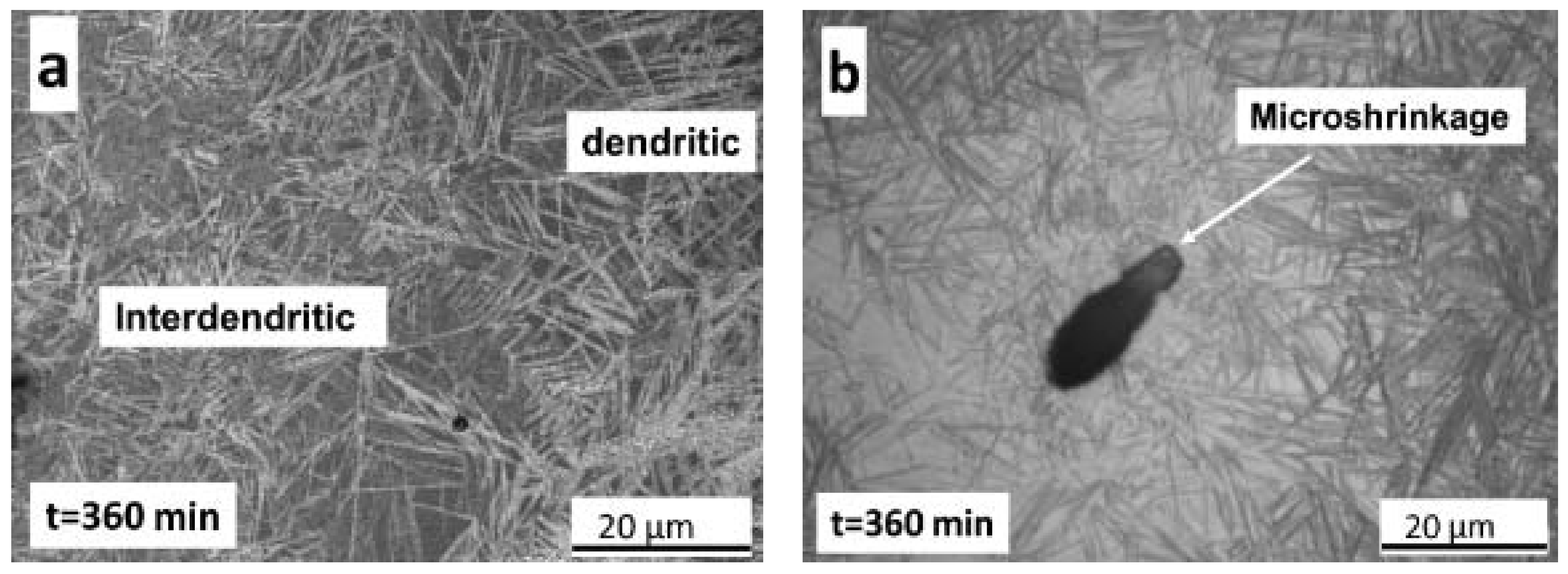
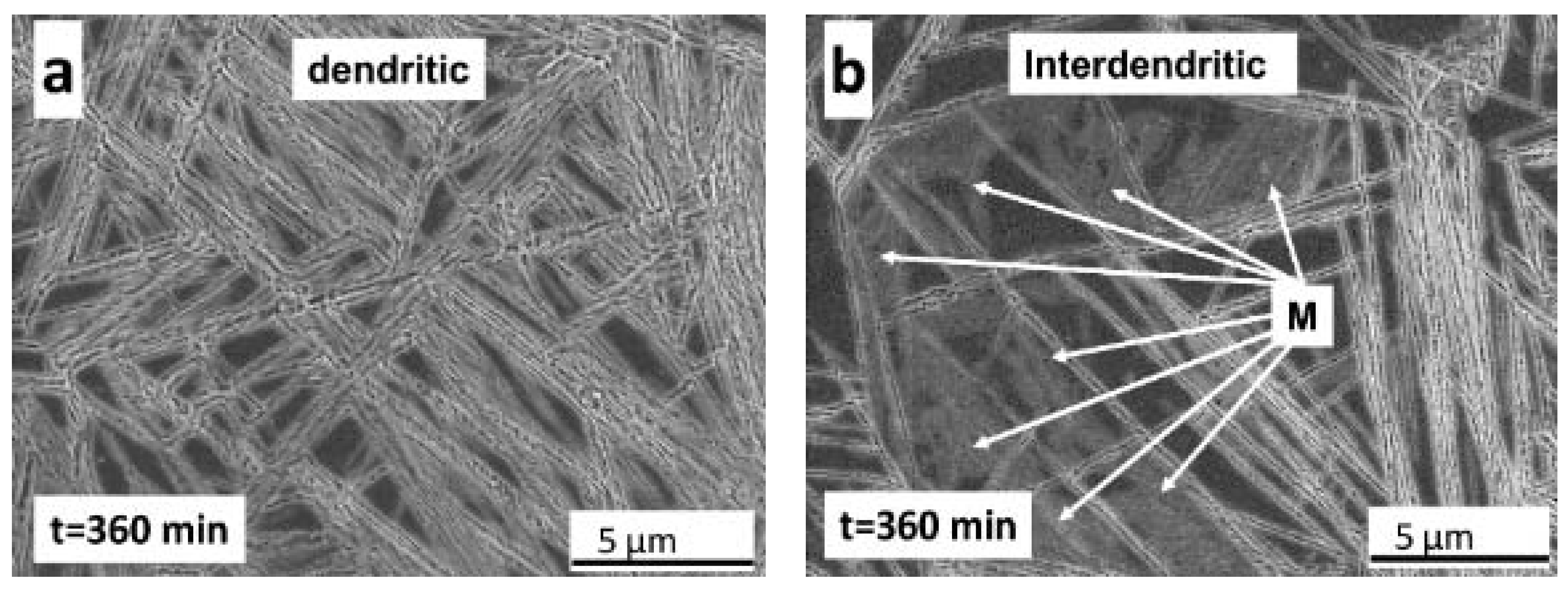
- Metallographic observations showed that the bainitic transformation from the austenite at 230°C starts after about 80 min, in reasonable correlation with dilatometric results. Differences in transformation rate between dendritic and interdendritic areas were related to the relationship between segregation and the influence of alloying elements on the transformation. In the interdendritic areas, the high Mn content reduced the transformation rate, and thus bainitic ferrite nucleates first, and grows faster in the dendritic zones. Further, it is possible to appreciate that the length of the bainitic ferrite plates is smaller in interdendritic regions, likely due to the presence of smaller austenite grains in these regions of the microstructure compared to dendritic ones.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Degarmo, E.P.; Black, J.T.; Kohser, R.A. Materials and Processes in Manufacturing, 9th ed.; Wiley: Hoboken, NJ, USA, 2003; p. 277. ISBN 0-471-65653-4. [Google Scholar]
- ASM Metals Handbook. Casting, Chapter: Casting Advantages. In Applications, and Market Size, 9th ed.; ASM International: Materials Park, OH, USA, 2005; Volume 15, pp. 54–71. [Google Scholar]
- The Metalcasting. Available online: http://www.themetalcasting.com/casting-process-advantages.html (accessed on 24 April 2020).
- Seppo, L. Treatise on Process Metallurgy. In Industrial Processes; Chapter 1.8—Continuous Casting of Steel; Elsevier Science: Edinburgh, UK, 2014; Volume 3, pp. 373–434. [Google Scholar]
- Brown, I. The role of microsegregation in centreline cold cracking of high strength low alloy steel weldments. Scr. Mater. 2006, 54, 489–492. [Google Scholar] [CrossRef]
- Won, Y.M.; Yeo, T.J.; Seol, D.J.; Oh, K.H. A new criterion for internal crack formation in continuously cast steels. Metall. Mater. Trans. B 2000, 31, 779–794. [Google Scholar] [CrossRef]
- Yansen, H.; Liu, W.; Li, J.; Nie, B.; Zhang, W.; Lui, Z. Microstructural bandings evolution behavior and their effects on microstructure and mechanical property of super-austenitic stainless steel. Mat. Sci. Eng. A 2018, 736, 258–268. [Google Scholar] [CrossRef]
- ÅStröm, H.; Loberg, B.; Bengtsson, B.; Easterling, K.E. Hot cracking and micro-segregation in 18–10 stainless steel welds. Met. Sci. 2013, 225–234. [Google Scholar] [CrossRef]
- Chojeckia, A.; Telejkoa, I.; Bogaczb, T. Influence of chemical composition on the hot tearing formation of cast steel. Theor. Appl. Frac. Mec. 1997, 27, 99–105. [Google Scholar] [CrossRef]
- Katayama, S.; Fujimoto, T.; Matsunawa, A. Correlation among solidification process, microstructure microsegregation and solidification cracking susceptibility in stainless steel weld metals. Trans. JWRI 1985, 14, 123–138. [Google Scholar]
- Lippold, J.; Savage, F. Solidification of austenitic stainless steel weldments: Part III-effect of solidification behaviour on hot cracking susceptibility. Weld Res. Suppl. 1982, 12, 288–296. [Google Scholar]
- Ennis, B.; Jimenez-Melero, E.; Mostert, R.; Santillana, B.; Lee, P. The role of aluminium in chemical and phase segregation in a TRIP assisted dual phase steel. Acta Mater. 2016, 115, 132–142. [Google Scholar] [CrossRef]
- Ahmed, M.; Salam, I.; Hashmi, F.; Khan, A. Influence of banded structure on the mechanical properties of a high-strength maraging steel. J. Mater. Eng. Perform. 1997, 6, 165–171. [Google Scholar] [CrossRef]
- Offerman, S.; Van Dijk, N.; Reckveldt, M.; Van der Swaag, S. Ferrite/pearlite band formation in hot rolled medium carbon steel. Mater. Sci. Technol. 2002, 18, 297–303. [Google Scholar] [CrossRef]
- Stauffer, A.; Koss, D.; Mc Kirgan, J. Microstructural banding and failure of a stainless steel. Metall. Mater. Trans. A 2004, 35, 1317–1324. [Google Scholar] [CrossRef]
- Caballero, F.; Capdevila, C.; García-Junqueda, A.; García de Andrés, C. Evolution of Microstructural Banding during the Manufacturing Process of Dual Phase Steels. Mater. Trans. 2006, 47, 2269–2276. [Google Scholar] [CrossRef]
- Ramanzani, A.; Queade, H.; Abbasi, M.; Prahl, H. The effect of martensite banding on the mechanical properties and formability of TRIP steels. Mater. Sci. Eng. A 2016, 651, 160–164. [Google Scholar] [CrossRef]
- Penha, R.; Vatavuk, J.; Couto, A.; Pereira, S.; Sousa, S.; Canale, L. Effect of chemical banding on the local hardenability in AISI 4340 steel bar. Eng. Fail. 2015, 53, 59–68. [Google Scholar] [CrossRef]
- Verhoeven, J. A Review of Microsegregation Induced Banding Phenomena in Steels. J. Mater. Eng. Perform. 2000, 9, 286–296. [Google Scholar] [CrossRef]
- Krauss, G. Solidification, Segregation, and Banding in Carbonand Alloy Steels. Metall. Mater. Trans. B 2003, 34, 781–792. [Google Scholar] [CrossRef]
- Tasan, C.; Hoefnagelsb, J.; Geersb, M. Microstructural banding effects clarified through micrographic digital image correlation. Scr. Mater. 2010, 62, 835–838. [Google Scholar] [CrossRef]
- Caballero, F.G.; Chao, J.; Cornide, J.; García-Mateo, C.; Santofimia, M.J.; Capdevila, C. Toughness of Advanced High Strength Bainitic Steels. Mater. Sci. Forum. 2010, 638, 118–123. [Google Scholar] [CrossRef]
- Edmonds, D.V. Advanced bainitic and martensitic steels with carbide-free microstructures containing retained austenite. Mater. Sci. Forum. 2010, 638, 110–117. [Google Scholar] [CrossRef]
- De Moor, E.; Speer, J.G. Bainitic and Quenching and Partitioning Steels. In Automotive Steels; Woodhead Publishing-Elsevier: Cambridge, UK, 2017; Chapter 10; pp. 298–316. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Modern Bainitic Alloys. In Bainite in Steels-Transformations, Microstructure and Properties, 2nd ed.; IOM Communications Ltd.: London, UK, 2001; Chapter 13; pp. 373–377. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Design of novel high strength bainiticsteels: Part 1. J. Mater. Sci. Technol. 2001, 17, 512–516. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Design of novel high strength bainiticsteels: Part 2. J. Mater. Sci. Technol. 2001, 17, 517–522. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Very strong low temperature bainite. J. Mater. Sci. Technol. 2001, 18, 279–284. [Google Scholar] [CrossRef]
- Carmo, D.J.; Días, J.; Santos, D. High cycle rotating bending fatigue property in high strength casting Steel with carbide free bainite. J. Mater. Sci. Technol. 2012, 28, 991–993. [Google Scholar] [CrossRef]
- Yoozbashi, M.; Wang, T. Design of a new nanostructures, high-Si bainitic steel with lower cost production. Mater. Des. 2011, 32, 3248–3253. [Google Scholar] [CrossRef]
- Basso, A.; Toda-Caraballo, I.; San-Martín, D.; Caballero, F.G. Influence of cast part size on macro- and microsegregation patterns in a high carbon high silicon steel. J. Mater. Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Tenaglia, N.; Boeri, R.; Massone, J.; Basso, A. Assessment of the austemperability of high-silicon cast steels through Jominy hardenability tests. J. Mater. Sci. Technol. 2018, 34, 1990–2000. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Acceleration of Low-temperature Bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H. Very strong bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251–257. [Google Scholar] [CrossRef]
- Singh, K.; Singh, A.; Kumar, A. Effect of Prior Austenite Grain Size on the Morphology of Nano-Bainitic Steels. Metall. Mater. Trans. A 2018, 49, 1348–1354. [Google Scholar] [CrossRef]
- Lan, L.Y.; Qiu, C.L.W.; Zhao, D.; Gao, X.H.; Du, L.X. Effect of austenite grain size on isothermal bainite transformation in low carbon microalloyed steel. J. Mater. Sci. Technol. 2011, 11, 1657–1663. [Google Scholar] [CrossRef]
- Yang, H.S.; Bhadeshia, H.K.D.H. Austenite grain size and the martensite-start temperature. Scr. Mater. 2009, 60, 493–495. [Google Scholar] [CrossRef]
- Sourmail, T.; Smanio, V. Determination of Mstemperature: Methods, meaning and influence of ‘slow start’ phenomenon. J. Mater. Sci. Technol. 2013, 29, 883–888. [Google Scholar] [CrossRef]
- Tenaglia, N.; Boeri, R.; Basso, A.; Massone, J. Macro and microstructural characterisation of high Si cast steels—Study of microsegregation patterns. Int. J. Cast Metals Res. 2017, 30, 103–111. [Google Scholar] [CrossRef]
- García de Andrés, C.; Bartolomé, M.J.; Capdevila, C.; San Martín, D.; Caballero, F.; López, V. Metallographic techniques for the determination of the austenite grain size in medium-carbon microalloyed steels. Mater. Charact. 2001, 46, 389–398. [Google Scholar] [CrossRef]
- San Martín, D.; Palizdar, Y.; Cochrane, R.; Brydson, R.; Scott, A. Application of Nomarski differential interference contrast microscopy to highlight the prior austenite grain boundaries revealed by thermal etching. Mater. Charact. 2010, 61, 584–588. [Google Scholar] [CrossRef]
- García de Andrés, C.F.; Capdevila, C.; San Martín, D. Revealing austenite grain boundaries by thermal etching: Advantages and disadvantages. Mater. Charact. 2003, 49, 121–127. [Google Scholar] [CrossRef]
- Palizdar, Y.; San Martin, D.; Ward, M.; Cochrane, R.C.; Brydson, R.; Scott, A.J. Observation of thermally etched grain boundarieswith the FIB/TEM technique. Mater. Charact. 2013, 83, 28–33. [Google Scholar] [CrossRef]
- Mullins, W. Theory of thermal grooving. Int. J. Appl. 1957, 28, 333–339. [Google Scholar] [CrossRef]
- Mullins, W. The effect of thermal grooving on grain boundary motion. Acta Metall. 1958, 6, 414–427. [Google Scholar] [CrossRef]
- Srinivasan, S.; Trivedi, R. Theory of grain boundary grooving under the combined action of the surface and volume diffusion mechanisms. Acta Metall. 1973, 21, 611–620. [Google Scholar] [CrossRef]
- Ohtani, H.; McMahon, J. Modes of fracture in temper embrittled steels. Acta Metall. 1975, 23, 377–386. [Google Scholar] [CrossRef]
- Han, J.; Da Silva, A.; Ponge, D.; Raabe, D.; Sang-Min, L.; Young-Kook, L.; Sang-In, L.; Hwang, B. The effects of prior austenite grain boundaries and microstructural morphology on the impact toughness of intercritically annealed medium Mn steel. Acta Mater. 2017, 122, 199–206. [Google Scholar] [CrossRef]
- Zhang, X.; Knott, J. Cleavage fracture in bainitic and martensitic microstructures. Acta Mater. 1999, 47, 3483–3495. [Google Scholar] [CrossRef]
- Archie, F.; Zaefferer, S. Micro-damage initiation in ferrite-martensite DP microstructures: Astatistical characterization of crystallographic and chemical parameters. J. Mater. Sci. Eng. A 2017, 701, 302–313. [Google Scholar] [CrossRef]
- Lianga, J.; Zhaoa, Z.; Di, T.; Nan, Y.; Yang, S.; Liu, W. Improved microstructural homogeneity and mechanical property of medium manganese steel with Mn segregation banding by alternating lath matrix. J. Mater. Sci. Eng. A 2018, 711, 175–181. [Google Scholar] [CrossRef]
- Brown, L.; Kirkaldy, J. Carbon diffusion in dilute ternary austenites. Trans. Metall. Soc. AIME 1964, 230, 223–230. [Google Scholar] [CrossRef]
- LEE, S.J.; Matlock, D.; Van, T. An Empirical Model for Carbon Diffusion in Austenite Incorporating Alloying Element Effects. ISIJ Int. 2011, 51, 1903–1911. [Google Scholar] [CrossRef]
- Zhukov, A.A.; Krishtal, M.A. Thermodynamic activity of alloy components. Met. Sci. Heat Treat. 1975, 17, 626–633. [Google Scholar] [CrossRef]
- Rowan, O.K.; Sisson, R.D. Effect of Alloy Composition on Carburizing Performance of Steel. J. Phase Equilib. Diffus. 2009, 30, 235–241. [Google Scholar] [CrossRef]
- Babu, S.S.; Bhadeshia, H.K.D.H. Diffusion of carbon in substitutionally alloyed austenite. J. Mater. Sci. Lett. 1995, 14, 314–316. [Google Scholar] [CrossRef]
- Bain, E.C. Alloying Elements in Unhardened Steels. In Alloying Elements in Steels; ASM: Cleveland, OH, USA, 1939; Chapter 3; pp. 59–99. [Google Scholar]
- Pace Technologies. Available online: https://www.metallographic.com/Metallographic-Etchants/Metallography-Etchants.htm (accessed on 26 April 2020).
- ASM Metals Handbook. Metallographic Techniques. In Metallography and Microstructures; Vander Voort, G.F., Ed.; ASM International: Novelty, OH, USA, 1992; Volume 9, p. 533. [Google Scholar]
- Cahn, J. The impurity-drag effect in grain boundary motion. Acta Metall. Mater. 1962, 10, 789–798. [Google Scholar] [CrossRef]
- Hillert, M. Solute drag, solute trapping and diffusional dissipation of Gibbs energy. Acta Mater. 1999, 47, 4481–4505. [Google Scholar] [CrossRef]
- Fujiyama, N.; Nishibata, T.; Seki, A.; Hirata, H.; Kojima, K.; Ogawa, K. Austenite grain growth simulation considering the solute-drag effect and pinning effect. Sci. Technol. Adv. Mater. 2017, 18, 88–95. [Google Scholar] [CrossRef]
- Babu, S.; Ivanov, D.; Porter, D. Influence of Microsegregation on the Onset of the Martensitic Transformation. ISIJ Int. 2019, 59, 169–175. [Google Scholar] [CrossRef]
- Van gen Hassend, F.; Weber, S. Influence of Chemical Inhomogeneities on Local Phase Stabilities and Material Properties in Cast Martensitic Stainless Steel. Steel Res. Int. 2019, 1900481. [Google Scholar] [CrossRef]
- Huang, Q.; Volkova, O.; Biermann, H.; Mola, J. Dilatometry Analysis of Dissolution of Cr-Rich Carbides in Martensitic Stainless Steels. Metall. Mater. Trans. A 2017, 48, 5771–5777. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C. The nonlinear lattice expansion of iron alloys in the range100–1600 K. Scr. Mater. 2013, 69, 315–318. [Google Scholar] [CrossRef]
- Andrews, K.W. Empirical formulae for calculation of some trans-formation temperatures. J. Iron Steel Inst. 1965, 203, 721–727. [Google Scholar]
- Kung, C.Y.; Rayment, J. An examination of the validity of existing empirical formulae for the calculation of Mstemperature. Metall. Mater. Trans. A 1982, 13, 328–331. [Google Scholar] [CrossRef]
- Sourmail, T.; Garcia-Mateo, C. Critical assessment of models for predicting the Mstemperature of steels. Comput. Mater. Sci. 2005, 34, 323–334. [Google Scholar] [CrossRef]
- Capdevilla, C.; Caballero, F.G.; Garcia de Andrés, C. Determination of ms temperature in steels: A bayesian neural network model. ISIJ Int. 2002, 42, 894–902. [Google Scholar] [CrossRef]
- Van Bohemen, S. Bainite and martensite start temperaturecalculated with exponential carbon dependence. J. Mater. Sci. Technol. 2012, 28, 487–495. [Google Scholar] [CrossRef]
- Caballero, F.G.; Álvarez, L.F.; Capdevila, C.; García de Andrés, C. The origin of splitting phenomena in the martensitic transformation of stainless steels. Scr. Mater. 2003, 49, 315–320. [Google Scholar] [CrossRef]
- Guimaraes, J.; Gomes, J. A metallographic study of the influence of the austenite grain size on martensite kinetics in Fe-31.9Ni-0.02C. Acta Metall. 1978, 26, 1571–1596. [Google Scholar] [CrossRef]
- García-Junceda, A.; Capdevila, C.; Caballero, F.G.; García de Andrés, C. Dependence of martensite start temperature on fine austenite grain size. Scr. Mater. 2008, 58, 134–137. [Google Scholar] [CrossRef]
- Nyyssönen, T.; Peura, P.; Kuokkala, V.T. Crystallography, morphology, and martensite transformation of prior austenite in intercritically annealed high-aluminum steel. Metall. Mater. Trans. A 2018, 49, 6426–6441. [Google Scholar] [CrossRef]
- Humphreys, F. Review grain and subgraincharacterization by electron backscatter diffraction. J. Mater. Sci. 2001, 36, 3833–3854. [Google Scholar] [CrossRef]
- Thomas, G.; Speer, J.; Matlock, D.; Michael, J. Application of electron backscatter diffraction techniques to quenched and partitioned steels. Microsc. Microanal. 2011, 17, 368–373. [Google Scholar] [CrossRef]
- Carpenter, H.C.H.; Tamura, S. Experiments on the production of large copper crystals. Proc. R. Soc. Lond. Ser. AContain. Papers Math.Phys. Charact. 1926, 13, 28–43. [Google Scholar] [CrossRef]
- Nishiyama, Z. Martensitic Transformation, 1st ed.; Academic Press: Cambridge, MA, USA, 1978; ISBN 9780323148818. [Google Scholar]
- Kurdjumow, G.; Sachs, G. Über den mechanismus der stahlhärtung. Z. Phys. 1930, 64, 325–343. [Google Scholar] [CrossRef]
- Pham, A.; Ohba, T.; Morito, S.; Hayashi, T. Effect of Chemical Composition on Average γ/α’Orientation Relationship in Carbon and Low Alloy Steels. Appl. Mater. Today 2015, 2, S663–S666. [Google Scholar] [CrossRef]
- Smallman, R.E.; Ngan, A.H.W. Steel Transformations. In Modern Physical Metallurgy; Smallman, R.E., Ngan, A.H.W., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; Chapter 12; pp. 473–498. ISBN 9780080982236. [Google Scholar]
- Du, C.; Hoefnagels, J.; Vaes, R.; Geers, M. Block and sub-block boundary strengthening in lath martensite. Scr. Mater. 2016, 116, 117–121. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Manmath, K.D.; Vijayalakshmi, M. Estimation of martensite feature size in a low-carbon alloy steel by microtexture analysis of boundaries. Micron 2015, 68, 77–90. [Google Scholar] [CrossRef]
- Hidalgo, J.; Santofimia, M.J. Effect of prior austenite grain size refinement by thermal cycling on the microstructural features of as-quenched lath martensite. Metall. Mater. Trans. A 2016, 47, 5288–5301. [Google Scholar] [CrossRef]
- Gyhlesten Back, J.; Engberg, G. Investigation of parent austenite grains from martensite structure using EBSD in a wear resistant steel. J. Mater. Sci. 2017, 10, 453. [Google Scholar] [CrossRef]
- Morito, S.; Saito, H.; Ogawa, T.; Furuhara, T.; Maki, T. Effect of austenite grain size on the morphology and crystallography of lath martensite in low carbon steels. ISIJ Int. 2005, 45, 91–94. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Thermodynamics. In Bainite in Steels-Transformations, Microstructure and Properties, 2nd ed.; IOM Communications Ltd.: London, UK, 2001; Chapter 5; pp. 122–128. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite: The Incomplete-Reaction phenomenon and the approach to equilibrium. In International Solid-Solid Phase Transformations Conference; The Metallurgical Society of the AIME: Warrendale, PA, USA, 1981; pp. 1041–1048. [Google Scholar]
- Garcia-Mateo, C.; Caballero, F.; Capdevila, C.; Garcia de Andres, C. Estimation of dislocation density in bainitic microstructures using high-resolution dilatometry. Scr. Mater. 2009, 61, 855–858. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; Santofimia, M.; Garcia de Andres, C. Time-Temperature-Transformation Diagram within the Bainitic Temperature Range in a Medium Carbon Steel. Mater. Trans. 2004, 12, 3272–3281. [Google Scholar] [CrossRef][Green Version]
- Eres-Castellanos, A.; Morales-Rivas, L.; Latz, A.; Caballero, F.; Garcia-Mateo, C. Effect of ausforming on the anisotropy of low temperature bainitic transformation. Mater. Charact. 2018, 145, 371–380. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels: Theory and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 117–131. [Google Scholar] [CrossRef]
- Rementeria, R.; Jimenez, J.A.; Allain, S.; Poplawasky, J.D.; Guo, W.; Urones-Garrote, E.; Garcia-Mateo, C.; Caballero, F.G. Quantitative assessment of carbon allocation anomalies in low temperature bainite. Acta Mater. 2017, 133, 333–345. [Google Scholar] [CrossRef]
- CeladaCasero, C.; San-Martin, D. Austenite Formation in a Cold-Rolled Semi-austenitic Stainless Steel. Metall. Mater. Trans. A 2014, 45, 1767–1777. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Introduction. In Bainite in Steels-Transformations, Microstructure and Properties, 2nd ed.; IOM Communications Ltd.: London, UK, 2001; Chapter 1; pp. 2–16. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.; Guo, J.; Liu, Y. Effect of austenite grain size on transformation of nanobainite and its mechanical properties. Mater. Sci. Eng. A 2016, 666, 207–213. [Google Scholar] [CrossRef]

| C | Si | Mn | Cr | S | P | Fe |
|---|---|---|---|---|---|---|
| 0.8 | 2.25 | 1.05 | 1.1 | 0.01 | 0.013 | Balance |
| Element | Si | Mn | Cr | |
|---|---|---|---|---|
| Concentration (wt. %) | ||||
| Minimum value (Dendritic/FTF region)-Poor zone | 1.6 | 0.8 | 0.7 | |
| Maximum value (Interdendritic/LTF region)-Rich zone | 2.6 | 1.6 | 1.3 | |
| [X] (average or nominal value-EPMA) | 2.1 | 1.2 | 1.0 | |
| Variation regarding average value | ±24% | ±33% | ±30% | |
| Overall value measured by X-Ray Fluorescence spectrometry | 2.2 | 1.2 | 1.0 | |
| EBSD Area Fractions | Non-Indexed | Austenite | Martensite | |
|---|---|---|---|---|
| Dendritic region | As-scanned | 0.22 | 0.08 | 0.70 |
| After post-processing | 0.22 | 0.02 | 0.76 | |
| Interdendritic region | As-scanned | 0.26 | 0.07 | 0.67 |
| After post-processing | 0.26 | 0.02 | 0.73 | |
| Austempering Time (min) | 80 | 160 | 240 | 360 |
|---|---|---|---|---|
| Retained austenite content | 0.29 ± 0.02 | 0.30 ± 0.02 | 0.29 ± 0.02 | 0.40 ± 0.02 |
| a lattice parameter for BCC/BCT (nm) | 0.286123 | 0.285755 | 0.286275 | 0.285096 |
| c lattice parameter for BCT (nm) | 0.289692 | 0.288465 | 0.288757 | 0.287541 |
| c/a ratio for BCC/BCT structure | 1.012473 | 1.009483 | 1.008669 | 1.008576 |
| a lattice parameter for FCC (nm) | 0.359859 | 0.359927 | 0.360173 | 0.360072 |
| Hardness (HV5) | 830 ± 8 | 815 ± 8 | 807 ± 8 | 627 ± 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, A.D.; Toda-Caraballo, I.; Eres-Castellanos, A.; San-Martin, D.; Jimenez, J.A.; Caballero, F.G. Effect of the Microsegregation on Martensitic and Bainitic Reactions in a High Carbon-High Silicon Cast Steel. Metals 2020, 10, 574. https://doi.org/10.3390/met10050574
Basso AD, Toda-Caraballo I, Eres-Castellanos A, San-Martin D, Jimenez JA, Caballero FG. Effect of the Microsegregation on Martensitic and Bainitic Reactions in a High Carbon-High Silicon Cast Steel. Metals. 2020; 10(5):574. https://doi.org/10.3390/met10050574
Chicago/Turabian StyleBasso, Alejandro Daniel, Isaac Toda-Caraballo, Adriana Eres-Castellanos, David San-Martin, José Antonio Jimenez, and Francisca G. Caballero. 2020. "Effect of the Microsegregation on Martensitic and Bainitic Reactions in a High Carbon-High Silicon Cast Steel" Metals 10, no. 5: 574. https://doi.org/10.3390/met10050574
APA StyleBasso, A. D., Toda-Caraballo, I., Eres-Castellanos, A., San-Martin, D., Jimenez, J. A., & Caballero, F. G. (2020). Effect of the Microsegregation on Martensitic and Bainitic Reactions in a High Carbon-High Silicon Cast Steel. Metals, 10(5), 574. https://doi.org/10.3390/met10050574








