Alumina Reinforcement of Inconel 625 Coatings by Cold Gas Spraying
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Powder Characterization
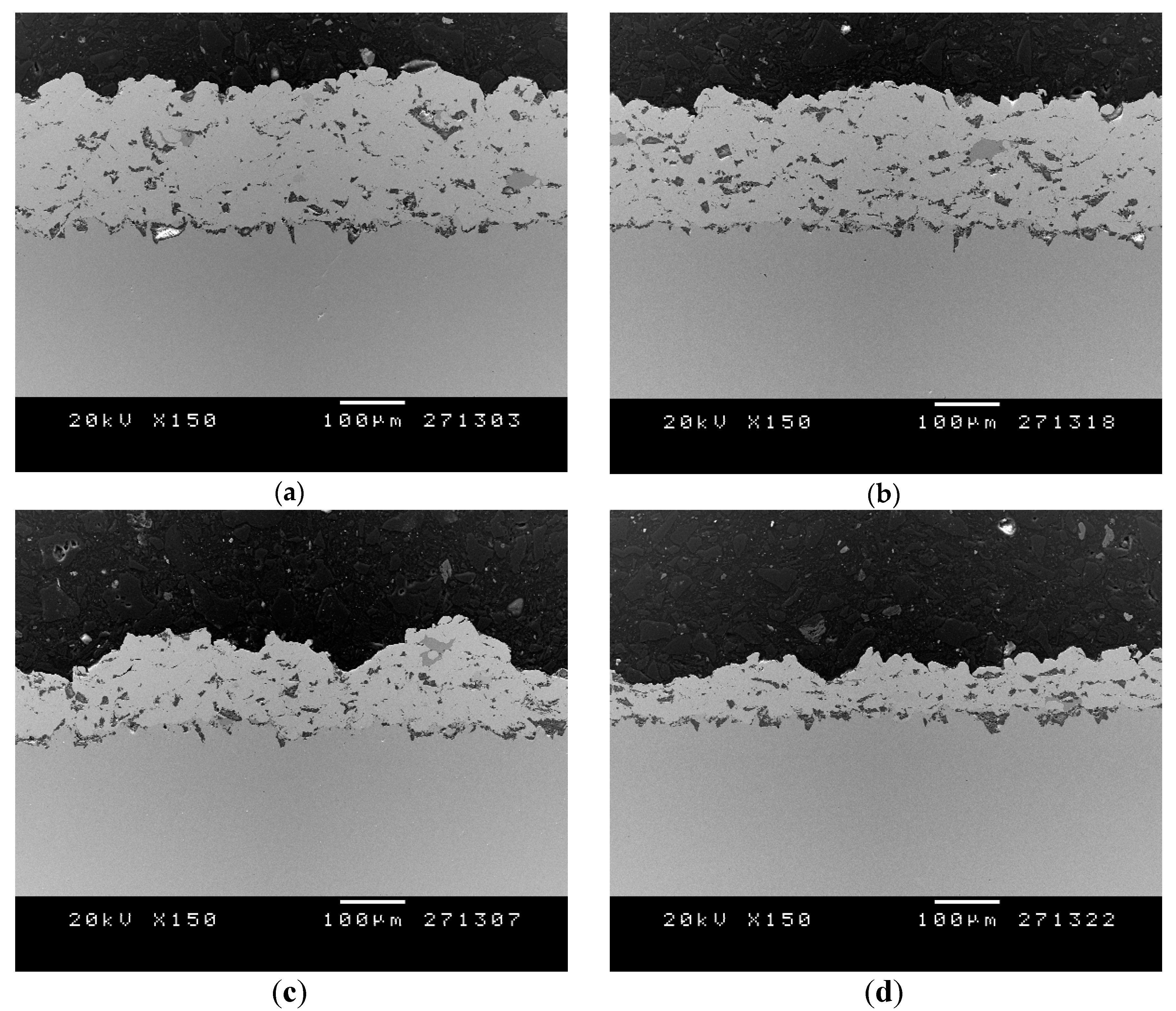
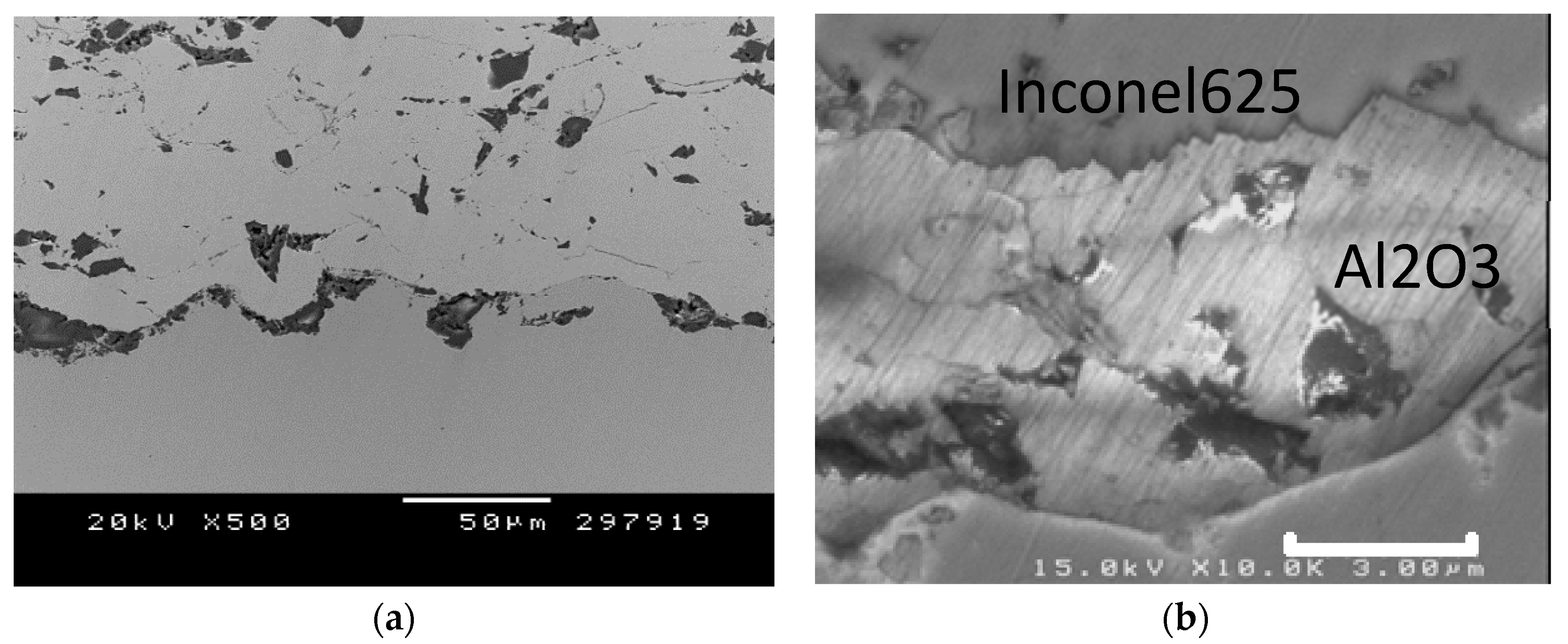
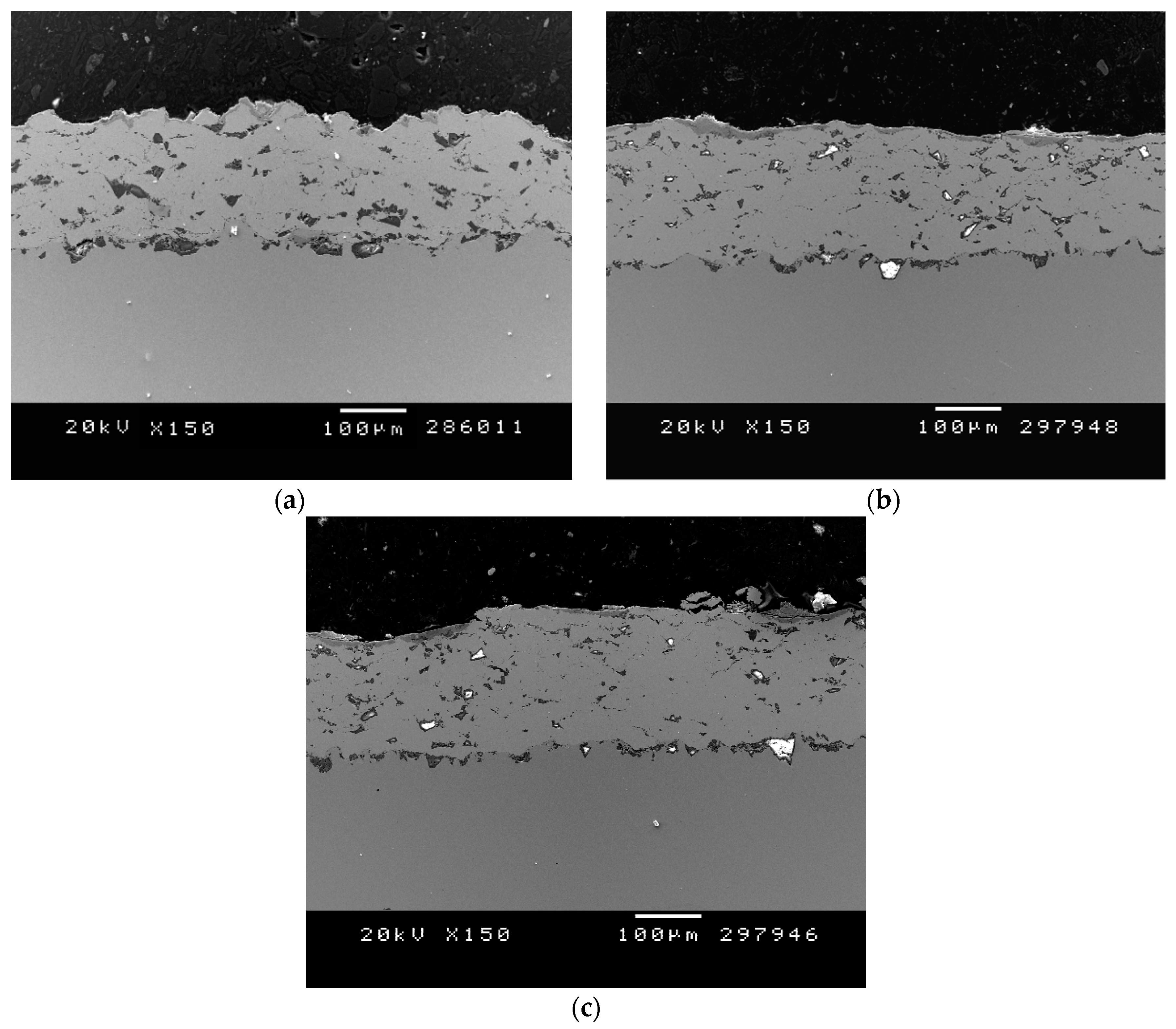
3.2. Structural Coating Characterization
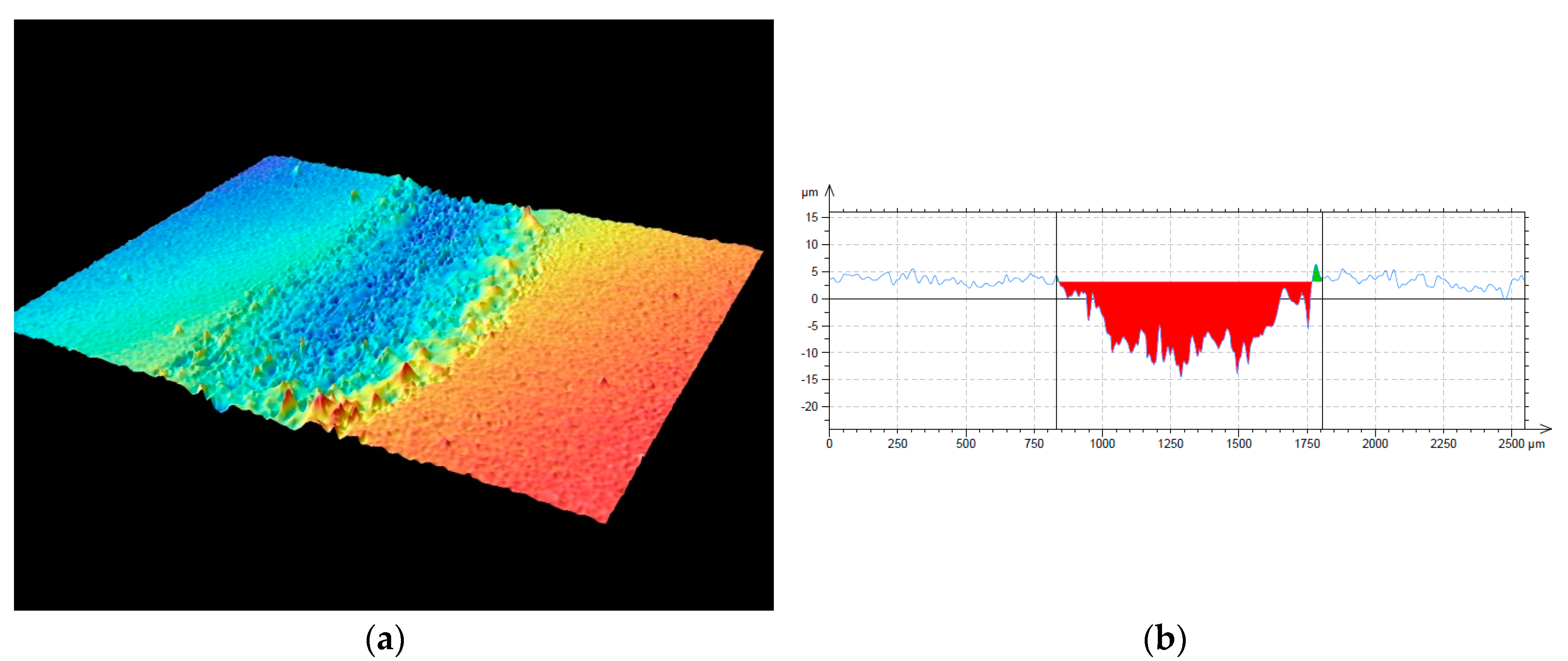
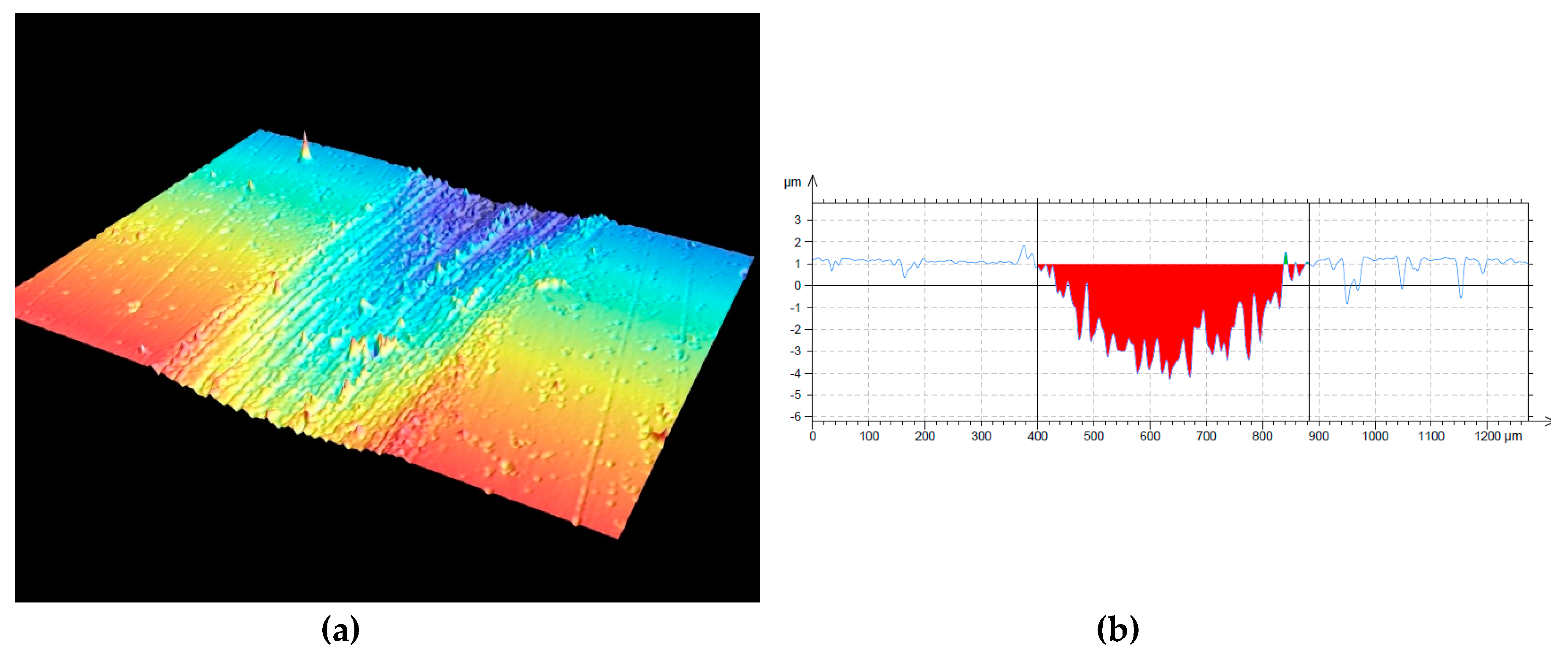
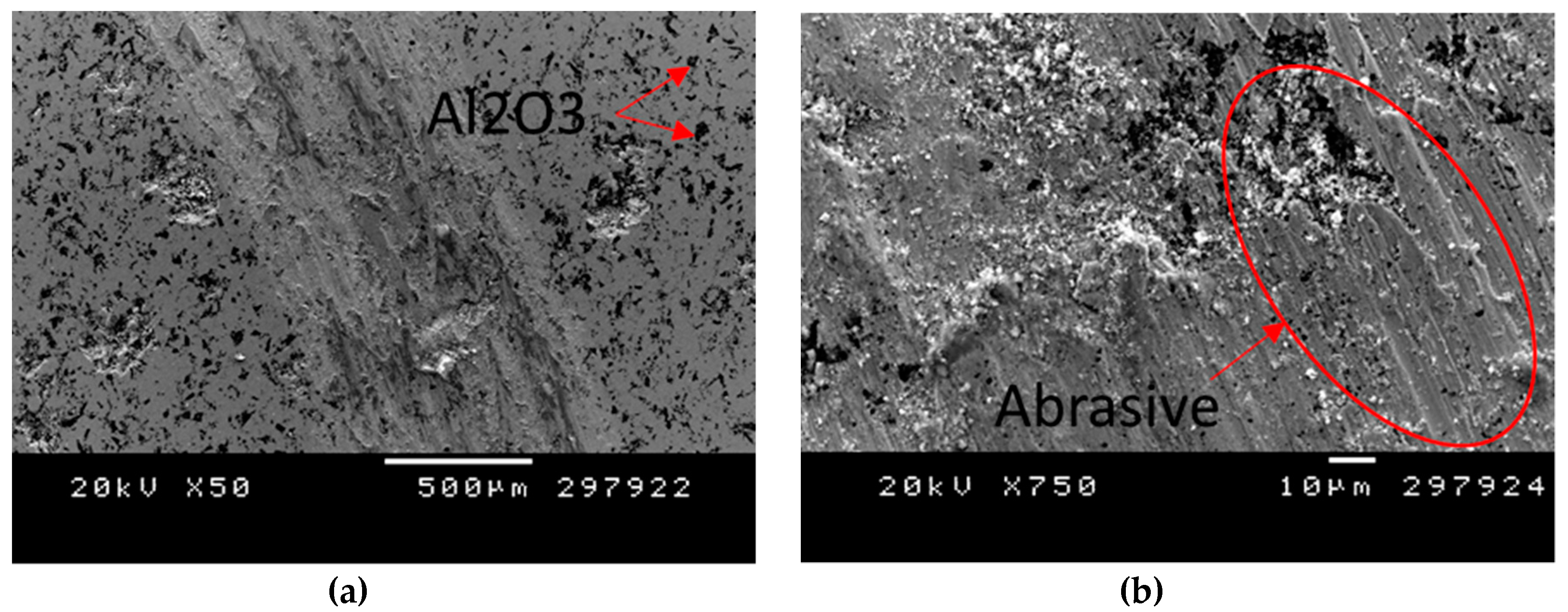
3.3. Mechanical and Tribological Properties
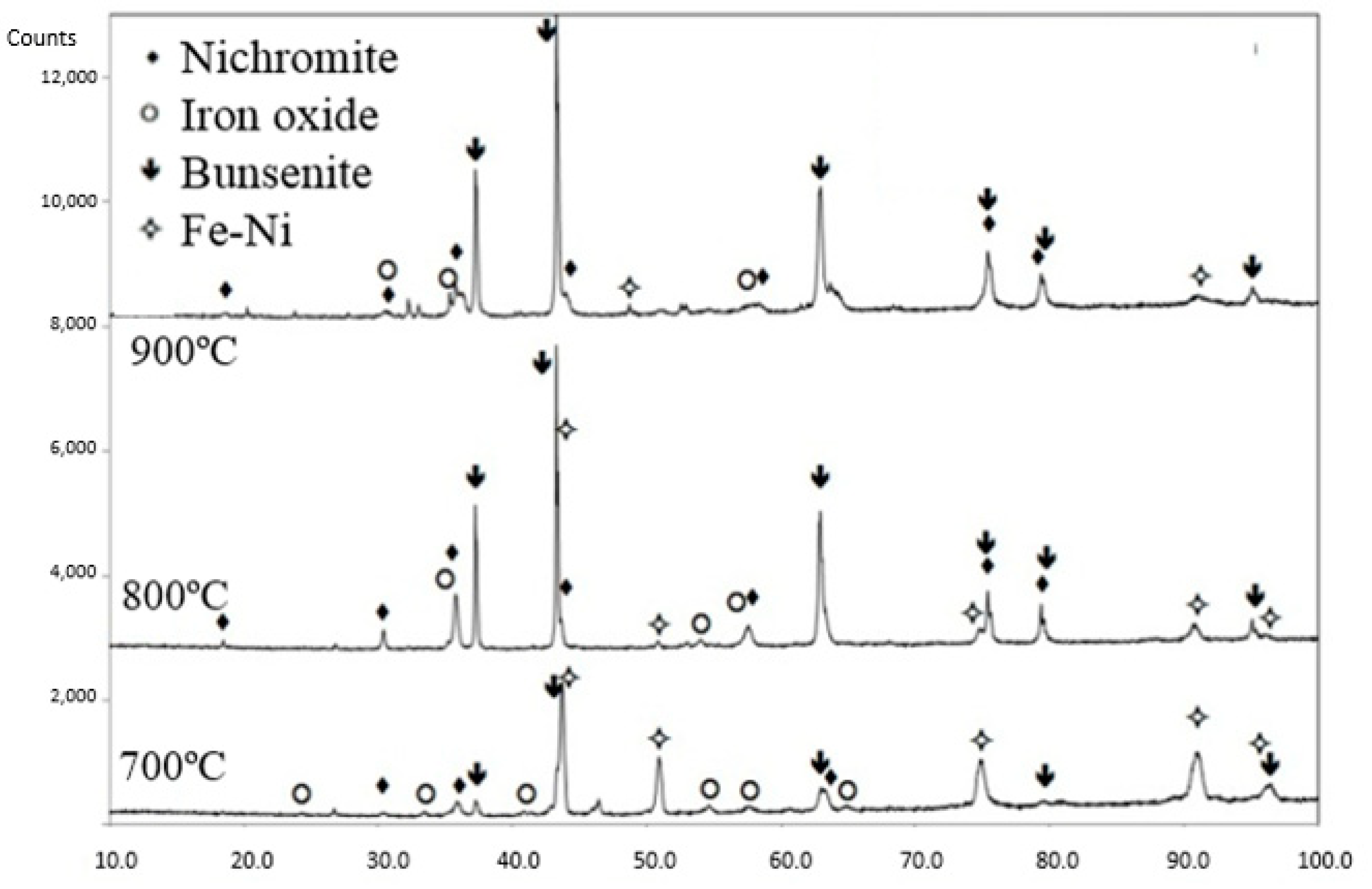
3.4. Oxidation Resistance
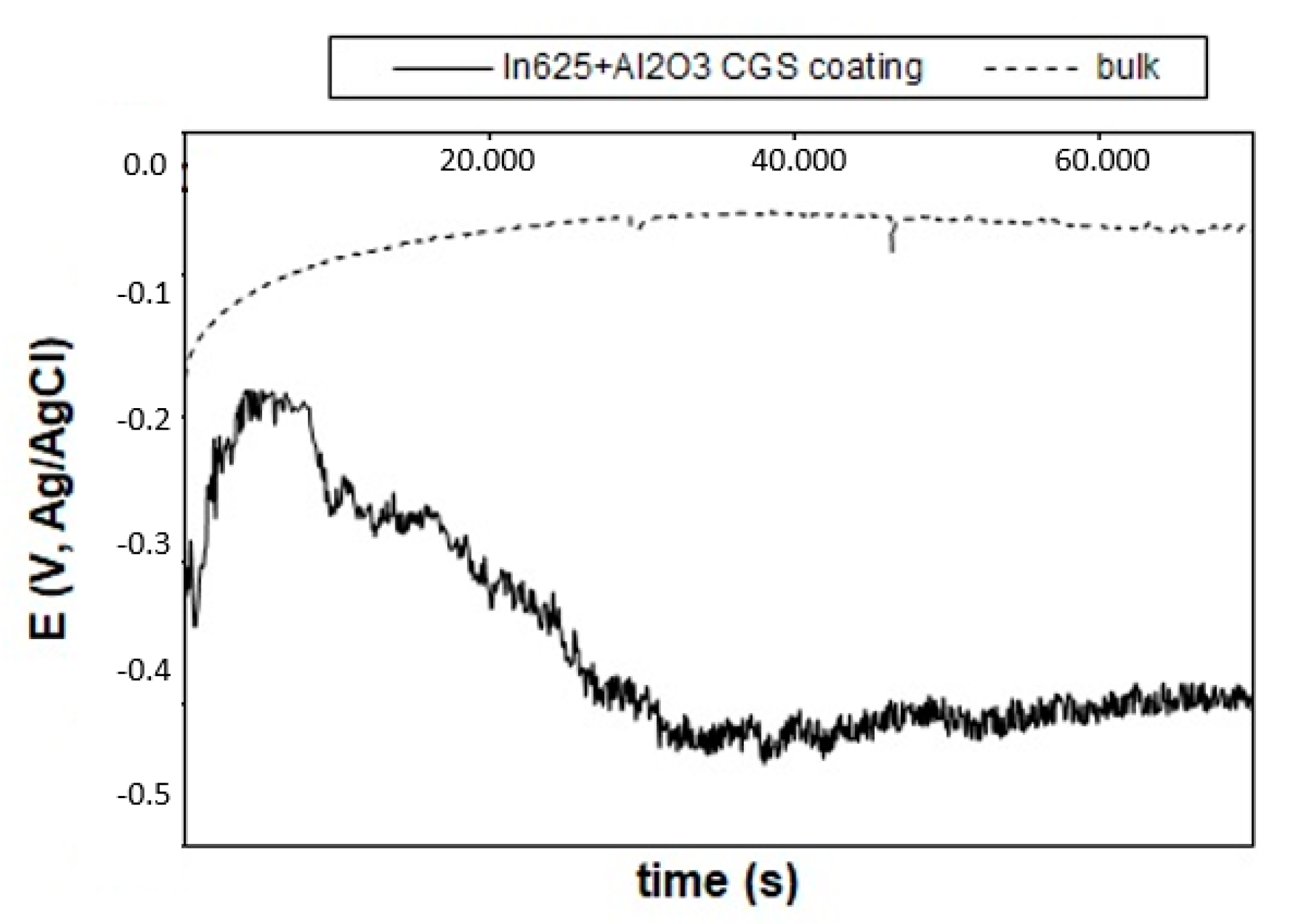
3.5. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ASM International. ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys; ASM International: Novelty, OH, USA, 2001; Volume 38. [Google Scholar] [CrossRef]
- Vander Voort, G.F. Atlas of T-T Diagrams of Nonferrous Alloys; ASM International: Novelty, OH, USA, 1991; Volume 1, pp. 2–5. ISBN 978-0-87170-428-3. [Google Scholar] [CrossRef]
- Special Metal Corporation, Inconel Alloy 625. 2013, Volume: 625, pp. 1–28. Available online: https://www.specialmetals.com (accessed on 26 May 2020).
- Scrivani, A.; Ianelli, S.; Rossi, A.; Groppetti, R.; Casadei, F.; Rizzi, G. A Contribution to the Surface Analysis and Characterisation of HVOF Coatings for Petrochemical Application. Wear 2001, 250, 107–113. [Google Scholar] [CrossRef]
- Boudi, A.A.; Hashmi, M.S.J.; Yilbas, B.S. HVOF Coating of Inconel 625 onto Stainless and Carbon Steel Surfaces: Corrosion and Bond Testing. J. Mater. Process. Technol. 2004, 155–156, 2051–2055. [Google Scholar] [CrossRef]
- Planche, M.P.; Normand, B.; Liao, H.; Rannou, G.; Coddet, C. Influence of HVOF Spraying Parameters on In-Flight Characteristics of Inconel 718 Particles and Correlation with the Electrochemical Behaviour of the Coating. Surf. Coat. Technol. 2002, 157, 247–256. [Google Scholar] [CrossRef]
- Torrell, M.; Dosta, S.; Miguel, J.R.; Guilemany, J.M. Optimisation of HVOF Thermal Spray Coatings for Their Implementation as MSWI Superheater Protectors. Corros. Eng. Sci. Technol. 2010, 45, 84–93. [Google Scholar] [CrossRef]
- Edris, H.; Mccartney, D.G.; Sturgeon, A.J. Microstructural Characterization of High Velocity Oxy-Fuel Sprayed Coatings of Inconel625. J. Mater. Sci. 1997, 32, 863–872. [Google Scholar] [CrossRef]
- Cinca, N.; Barbosa, M.; Dosta, S.; Guilemany, J.M. Study of Ti Deposition onto Al Alloy by Cold Gas Spraying. Surf. Coat. Technol. 2010, 205, 1096–1102. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold Spraying—A Materials Perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef] [Green Version]
- Cavaliere, P.; Silvello, A.; Cinca, N.; Canales, H.; Dosta, S.; Garcia Cano, I.; Guilemany, J.M. Microstructural and Fatigue Behavior of Cold Sprayed Ni-Based Superalloys Coatings. Surf. Coat. Technol. 2017. [Google Scholar] [CrossRef]
- Silvello, A.; Cavaliere, P.; Rizzo, A.; Valerini, D.; Dosta Parras, S.; Garcia Cano, I. Fatigue Bending Behavior of Cold-Sprayed Nickel-Based Superalloy Coatings. J. Therm. Spray Technol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Assadi, H.; Schmidt, T.; Richter, H.; Kliemann, J.O.; Binder, K.; Gärtner, F.; Klassen, T.; Kreye, H. On Parameter Selection in Cold Spraying. J. Therm. Spray Technol. 2011, 20, 1161–1176. [Google Scholar] [CrossRef]
- Rokni, M.R.; Nutt, S.R.; Widener, C.A.; Champagne, V.K.; Hrabe, R.H. Review of Relationship Between Particle Deformation, Coating Microstructure, and Properties in High-Pressure Cold Spray. J. Therm. Spray Technol. 2017, 26, 1308–1355. [Google Scholar] [CrossRef]
- Cavaliere, P.; Silvello, A. Processing Parameters Affecting Cold Spay Coatings Performances. Int. J. Adv. Manuf. Technol. 2014. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Raghupathy, Y.; Srinivasan, D.; Suwas, S.; Srivastava, C. Microstructural Evolution of Cold-Sprayed Inconel625 Superalloy Coatings on Low Alloy Steel Substrate. Acta Mater. 2017, 129, 11–25. [Google Scholar] [CrossRef]
- Fantozzi, D.; Matikainen, V.; Uusitalo, M.; Koivuluoto, H.; Vuoristo, P. Chlorine-Induced High Temperature Corrosion of Inconel 625 Sprayed Coatings Deposited with Different Thermal Spray Techniques. Surf. Coat. Technol. 2017, 318, 233–243. [Google Scholar] [CrossRef]
- Azarmi, F.; Sevostianov, I. Comparative Micromechanical Analysis of Alloy 625 Coatings Deposited by Air Plasma Spraying, Wire Arc Spraying, and Cold Spraying Technologies. Mech. Mater. 2020, 144, 103345. [Google Scholar] [CrossRef]
- Marinescu, I.; Huong, Y.A.N.; Liu, E. Additive Manufacturing of Inconel625 Superalloy parts via high pressure cold spray. In Proceedings of the 3rd International Conference on Progress in Additive Manufacturing (Pro-AM 2018), Singapore, 14–17 May 2018; pp. 433–438. [Google Scholar] [CrossRef]
- Sova, A.; Grigoriev, S.; Okunkova, A.; Smurov, I. Potential of Cold Gas Dynamic Spray as Additive Manufacturing Technology. Int. J. Adv. Manuf. Technol. 2013, 69, 2269–2278. [Google Scholar] [CrossRef]
- Levasseur, D.; Yue, S.; Brochu, M. Pressureless Sintering of Cold Sprayed Inconel 718 Deposit. Mater. Sci. Eng. A 2012, 556, 343–350. [Google Scholar] [CrossRef]
- Singh, R.; Schruefer, S.; Wilson, S.; Gibmeier, J.; Vassen, R. Influence of Coating Thickness on Residual Stress and Adhesion-Strength of Cold-Sprayed Inconel 718 Coatings. Surf. Coat. Technol. 2018, 350, 64–73. [Google Scholar] [CrossRef]
- Luo, X.T.; Yao, M.L.; Ma, N.; Takahashi, M.; Li, C.J. Deposition Behavior, Microstructure and Mechanical Properties of an in-Situ Micro-Forging Assisted Cold Spray Enabled Additively Manufactured Inconel 718 Alloy. Mater. Des. 2018, 155, 384–395. [Google Scholar] [CrossRef]
- Pérez-andrade, L.I.; Gärtner, F.; Villa-vidaller, M.; Klassen, T.; Muñoz-saldaña, J. Surface & Coatings Technology Optimization of Inconel718 Thick Deposits by Cold Spray Processing and Annealing. Surf. Coat. Technol. 2019, 378, 124997. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Vuoristo, P. Effect of Ceramic Particles on Properties of Cold-Sprayed Ni-20cr+ Al2O3 Coatings. J. Therm. Spray Technol. 2009, 18, 555–562. [Google Scholar] [CrossRef]
- Spencer, K.; Fabijanic, D.M.; Zhang, M.X. The Use of Al-Al2O3 cold Spray Coatings to Improve the Surface Properties of Magnesium Alloys. Surf. Coat. Technol. 2009. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Vuoristo, P. Effect of Powder Type and Composition on Structure and Mechanical Properties of Cu + Al2O3 Coatings Prepared by Using Low-Pressure Cold Spray Process. J. Therm. Spray Technol. 2010, 19, 1081–1092. [Google Scholar] [CrossRef]
- Irissou, E.; Legoux, J.G.; Arsenault, B.; Moreau, C. Investigation of Al-Al2O3 Cold Spray Coating Formation and Properties. J. Therm. Spray Technol. 2007, 16, 661–668. [Google Scholar] [CrossRef]
- Sansoucy, E.; Marcoux, P.; Ajdelsztajn, L.; Jodoin, B. Properties of SiC-Reinforced Aluminum Alloy Coatings Produced by the Cold Gas Dynamic Spraying Process. Surf. Coat. Technol. 2008, 202, 3988–3996. [Google Scholar] [CrossRef]
- Li, W.Y.; Zhang, C.; Liao, H.; Li, J.; Coddet, C. Characterizations of Cold-Sprayed Nickel-Alumina Composite Coating with Relatively Large Nickel-Coated Alumina Powder. Surf. Coat. Technol. 2008, 202, 4855–4860. [Google Scholar] [CrossRef]
- Al-Fadhli, H.Y.; Stokes, J.; Hashmi, M.S.J.; Yilbas, B.S. The Erosion-Corrosion Behaviour of High Velocity Oxy-Fuel (HVOF) Thermally Sprayed Inconel625 Coatings on Different Metallic Surfaces. Surf. Coat. Technol. 2006, 200, 5782–5788. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Torrell, M.; Miguel, J.R. Study of the HVOF Ni-Based Coatings’ Corrosion Resistance Applied on Municipal Solid-Waste Incinerators. J. Therm. Spray Technol. 2008, 17, 254–262. [Google Scholar] [CrossRef]
- Sova, A.; Kosarev, V.F.; Papyrin, A.; Smurov, I. Effect of Ceramic Particle Velocity on Cold Spray Deposition of Metal-Ceramic Coatings. J. Therm. Spray Technol. 2011, 20, 285–291. [Google Scholar] [CrossRef]
- Haines, R. Richard Haines. Notes Queries 1902, s9-IX, 341–342. [Google Scholar] [CrossRef]
- Conshohocken, W. Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus 1. Wear 2007, 5, 1–5. [Google Scholar]
- Koivuluoto, H. Microstructural Characteristics and Corrosion Properties of Cold-Sprayed Coatings; Tampere University of Technology: Tampere, Finland, 2010. [Google Scholar]
- Poza, P.; Múnez, C.J.; Garrido-Maneiro, M.A.; Vezzù, S.; Rech, S.; Trentin, A. Mechanical Properties of Inconel625 Cold-Sprayed Coatings after Laser Remelting. Depth Sensing Indentation Analysis. Surf. Coat. Technol. 2014, 243, 51–57. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Tribology: Friction and Wear of Engineering Materials, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 1–388. [Google Scholar]
- Zhang, D.; Harris, S.J.; McCartney, D.G. Microstructure Formation and Corrosion Behaviour in HVOF-Sprayed Inconel 625 Coatings. Mater. Sci. Eng. A 2003, 344, 45–56. [Google Scholar] [CrossRef]
- Liu, Z.; Cabrero, J.; Niang, S.; Al-Taha, Z.Y. Improving Corrosion and Wear Performance of HVOF-Sprayed Inconel 625 and WC-Inconel 625 Coatings by High Power Diode Laser Treatments. Surf. Coat. Technol. 2007, 201, 7149–7158. [Google Scholar] [CrossRef]




| (a) Inconel625 | (b) Alumina | ||||
|---|---|---|---|---|---|
| 0.375 μm to 2000 μm | 0.040 μm to 2000 μm | ||||
| Volume | 100% | μm | Volume | 100% | μm |
| Mean | 23.77 | μm | Mean | 60.31 | μm |
| Median | 24.18 | μm | Median | 71.54 | μm |
| SD | 1.230 | μm | SD | 2.660 | μm |
| d10 | 17.79 | μm | d10 | 36.07 | μm |
| d90 | 30.97 | μm | d90 | 118.0 | μm |
| Type | Al2O3 wt.% | Thickness (μm) |
|---|---|---|
| A | 23.1 | 191.3 ± 5.9 |
| B | 30.8 | 169.8 ± 22.7 |
| C | 34.7 | 132.7 ± 17.8 |
| D | 46.2 | 80.1 ±17.4 |
| Type | HV0.3 | Abrasive Wear Rate (mm3/Nm) | Friction Wear Rate (mm3/Nm) | Friction Coefficient |
|---|---|---|---|---|
| Inconel625 bulk | ~225 | 1.5 × 10−4 ± 1.2 × 10−5 | 1.13 × 10−4 ± 1.5 × 10−5 | 0.78 ± 0.02 |
| Inconel + Al2O3 CGS coating | 585 ± 56 | 8.3 × 10−5 ± 9.7 × 10−6 | 1.26 × 10−5 ± 6.5 × 10−7 | 0.79 ± 0.01 |
| Bulk Inconel | CGS Coating | |
|---|---|---|
| Ecorr (mV Ag/AgCl) | −70 | −404 |
| Icorr (µA/cm2) | 0.02 | 0.344 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosta, S.; Cinca, N.; Silvello, A.; Cano, I.G. Alumina Reinforcement of Inconel 625 Coatings by Cold Gas Spraying. Metals 2020, 10, 1263. https://doi.org/10.3390/met10091263
Dosta S, Cinca N, Silvello A, Cano IG. Alumina Reinforcement of Inconel 625 Coatings by Cold Gas Spraying. Metals. 2020; 10(9):1263. https://doi.org/10.3390/met10091263
Chicago/Turabian StyleDosta, Sergi, Nuria Cinca, Alessio Silvello, and Irene G. Cano. 2020. "Alumina Reinforcement of Inconel 625 Coatings by Cold Gas Spraying" Metals 10, no. 9: 1263. https://doi.org/10.3390/met10091263
APA StyleDosta, S., Cinca, N., Silvello, A., & Cano, I. G. (2020). Alumina Reinforcement of Inconel 625 Coatings by Cold Gas Spraying. Metals, 10(9), 1263. https://doi.org/10.3390/met10091263








