Scale Formation on HSLA Steel during Continuous Casting Part II: The Effect of Surface Conditions
Abstract
1. Introduction
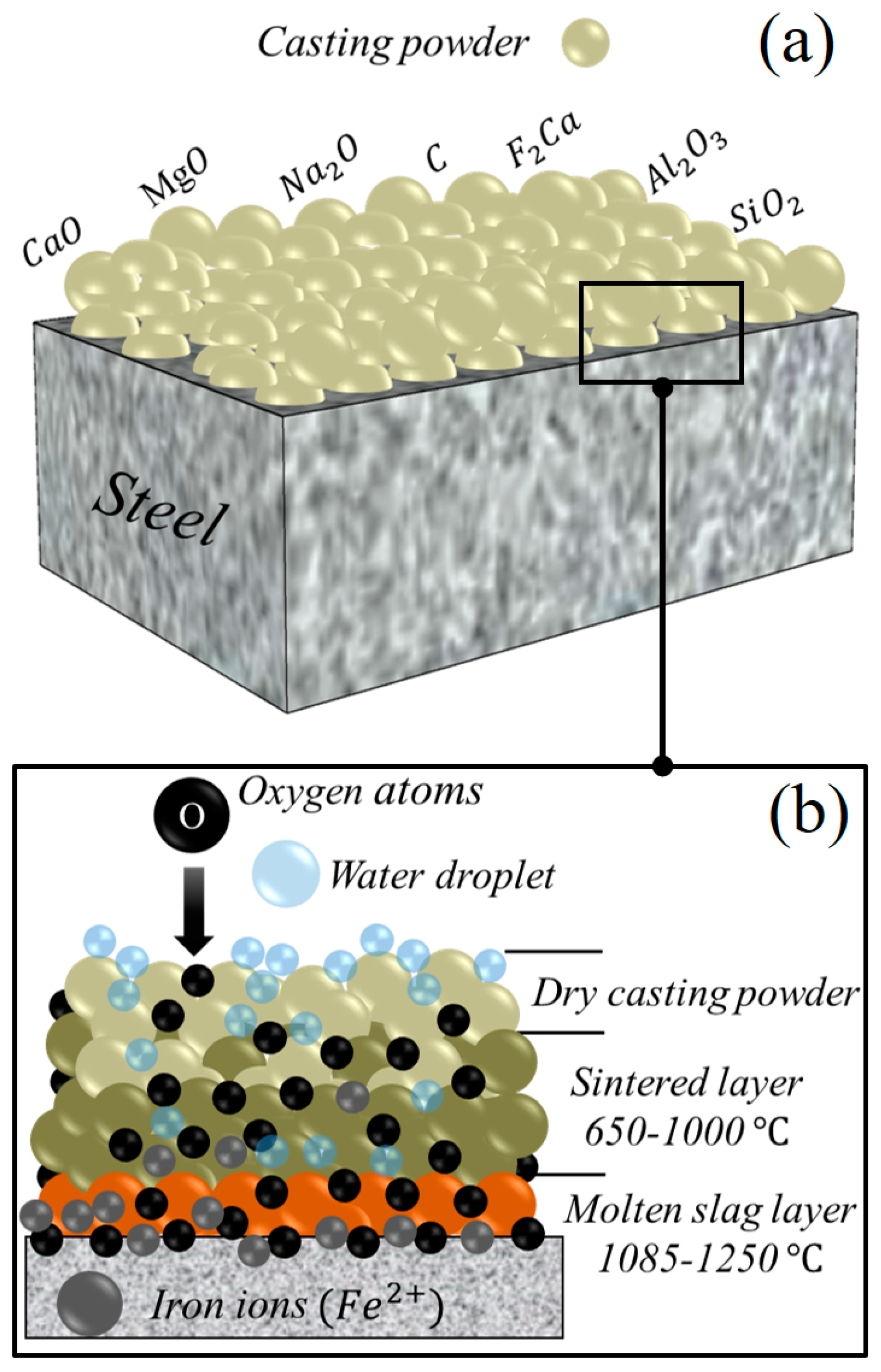
Surface Condition and Casting Powder Effects on Oxidation
2. Experiment Setup
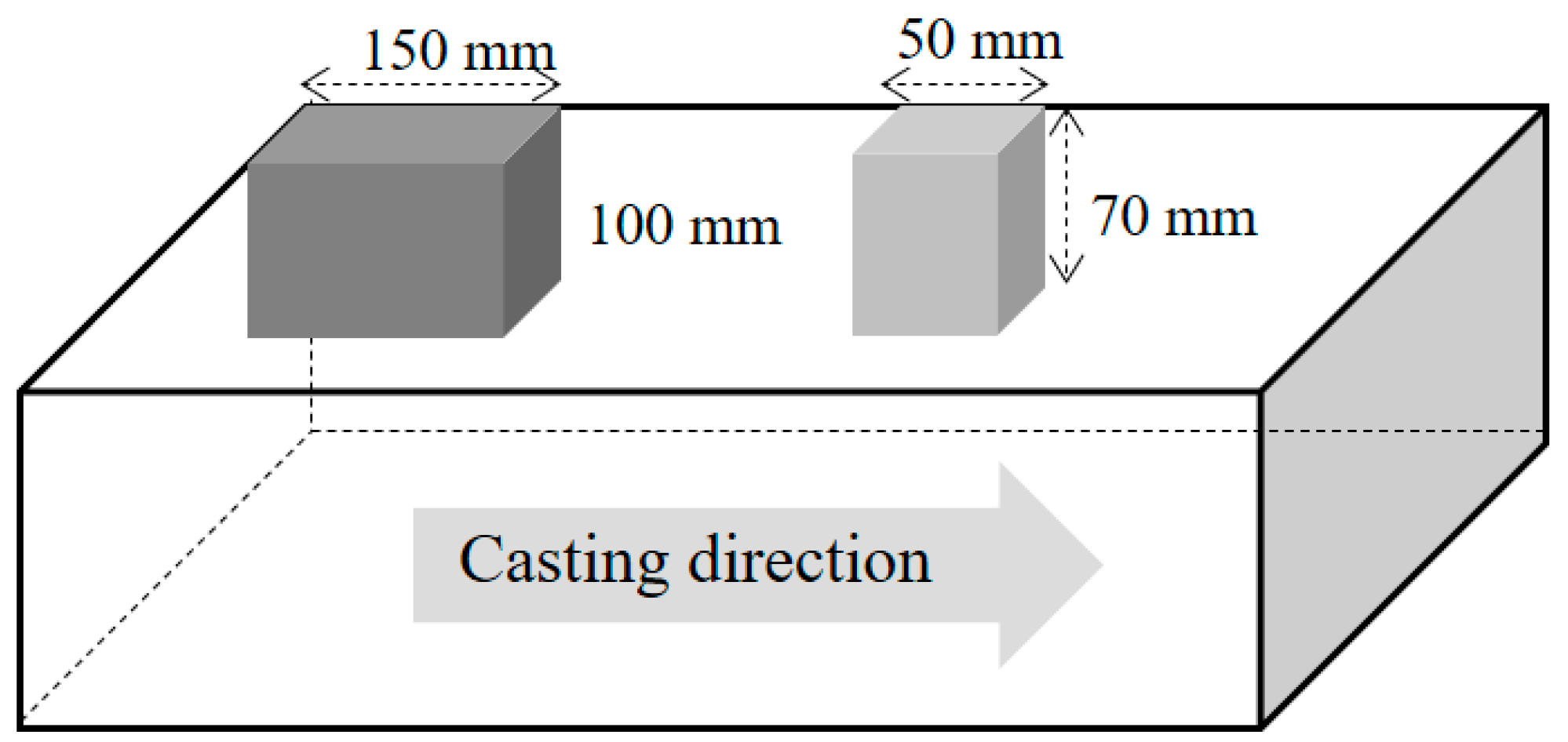
2.1. Sampling
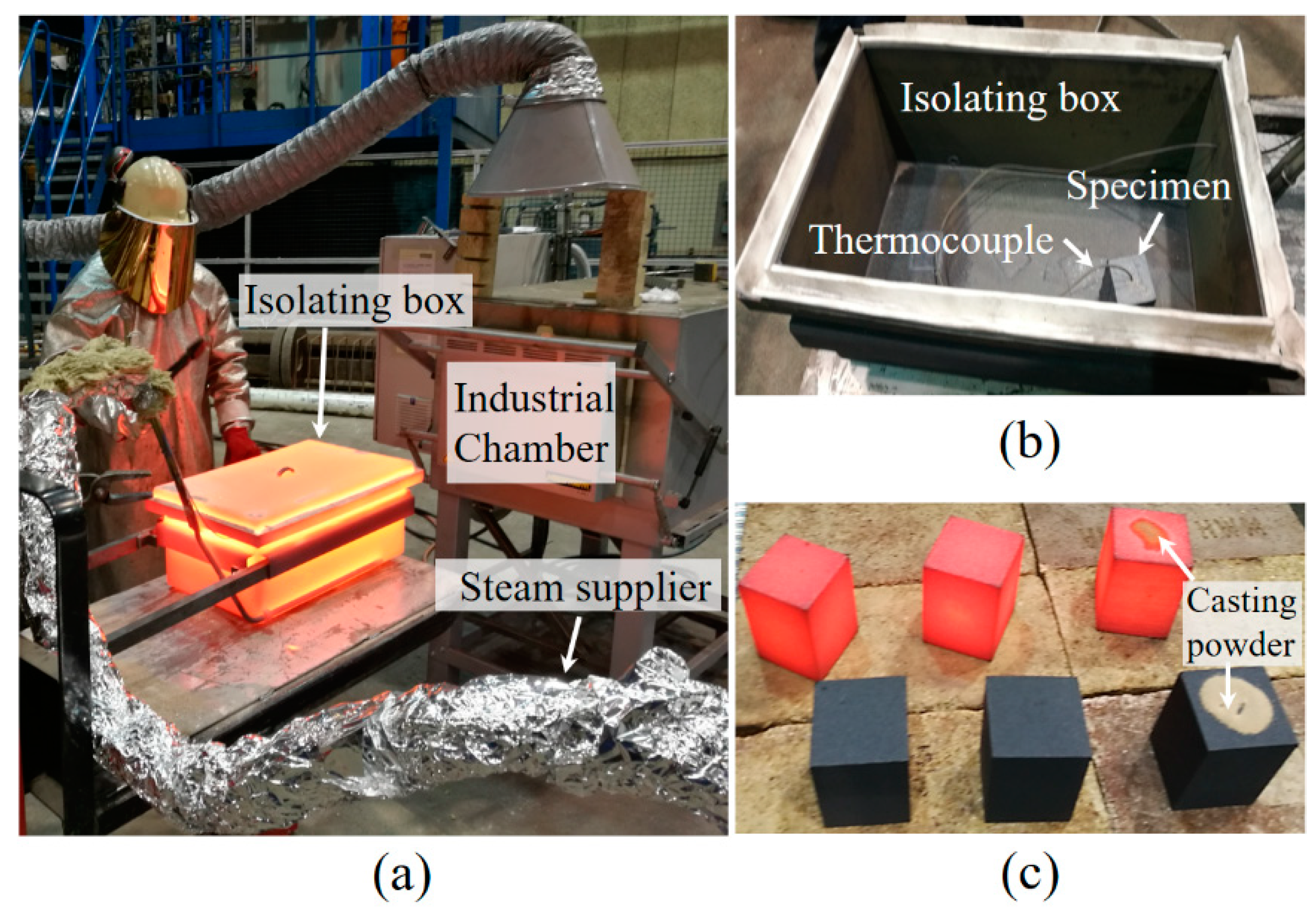
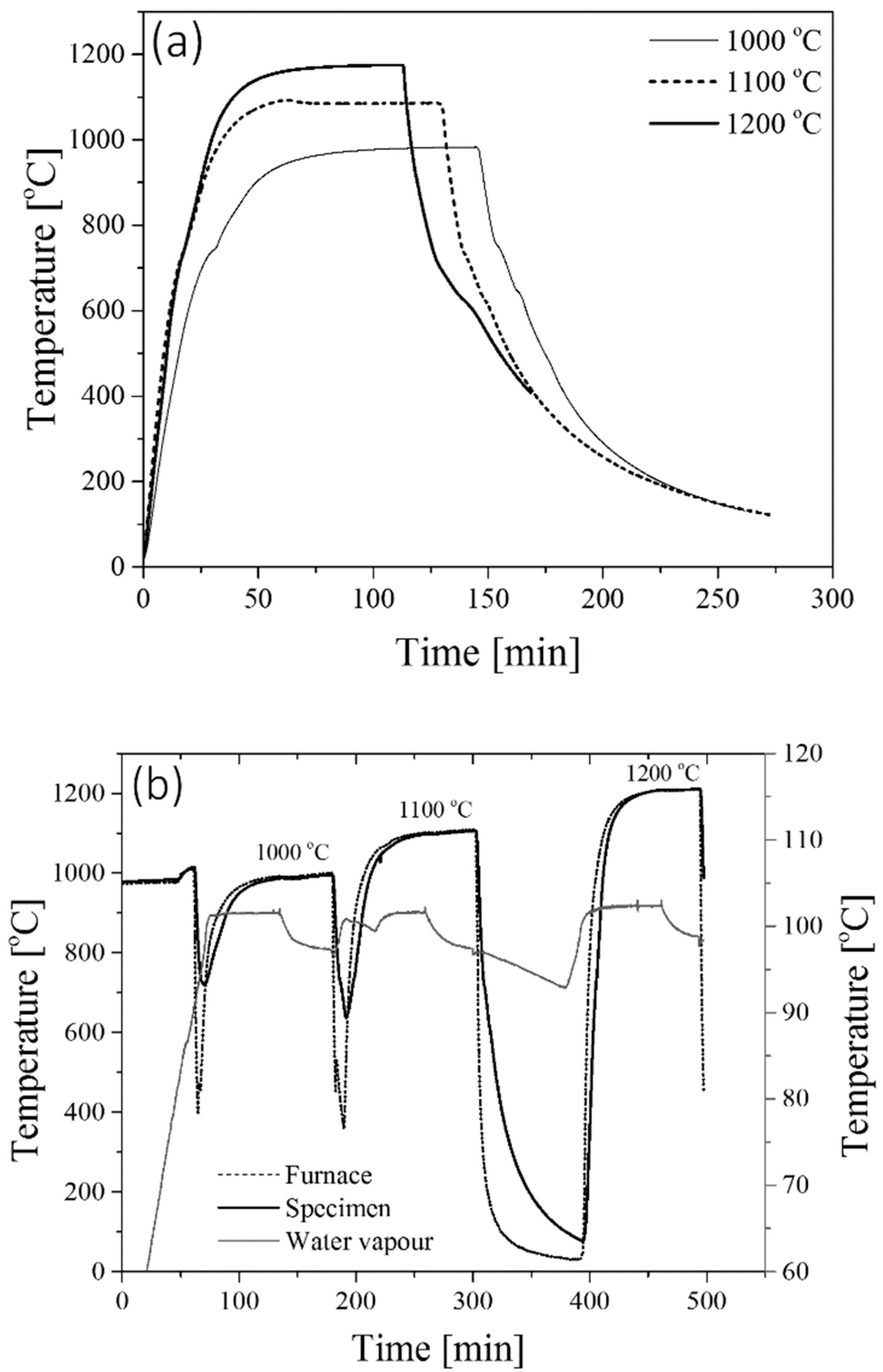
2.2. Oxidation Tests
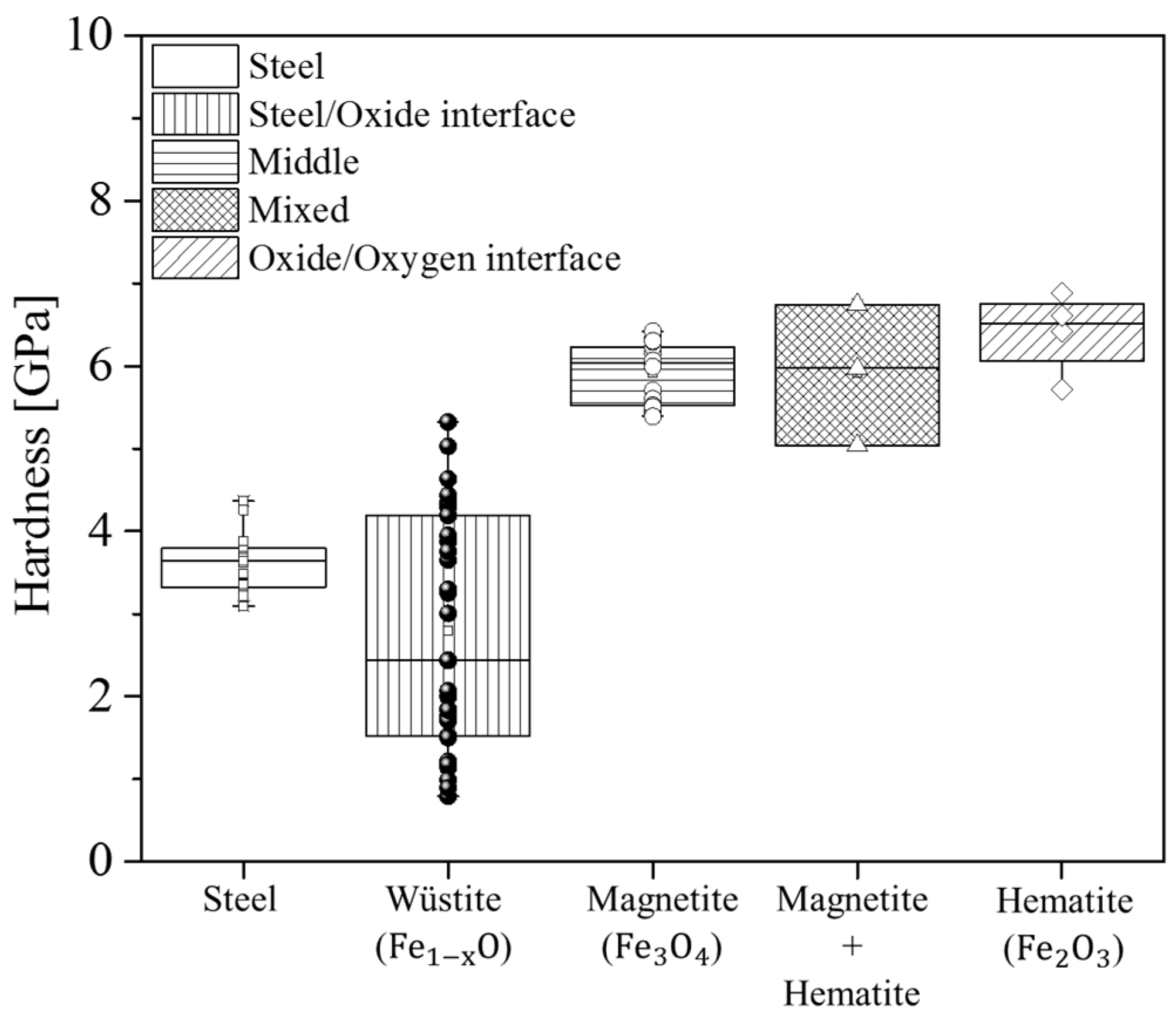
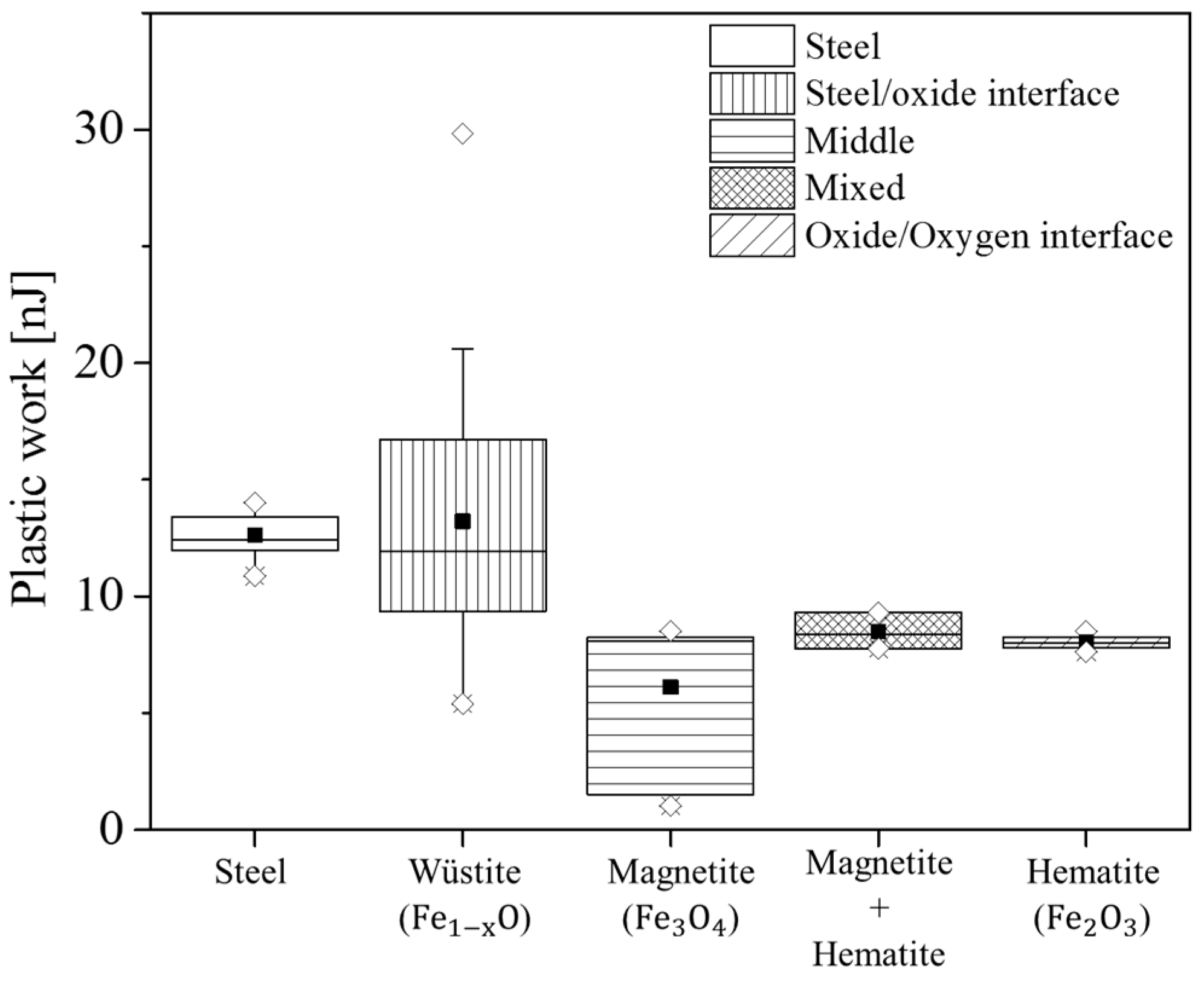
2.3. Nanoindentation
3. Results
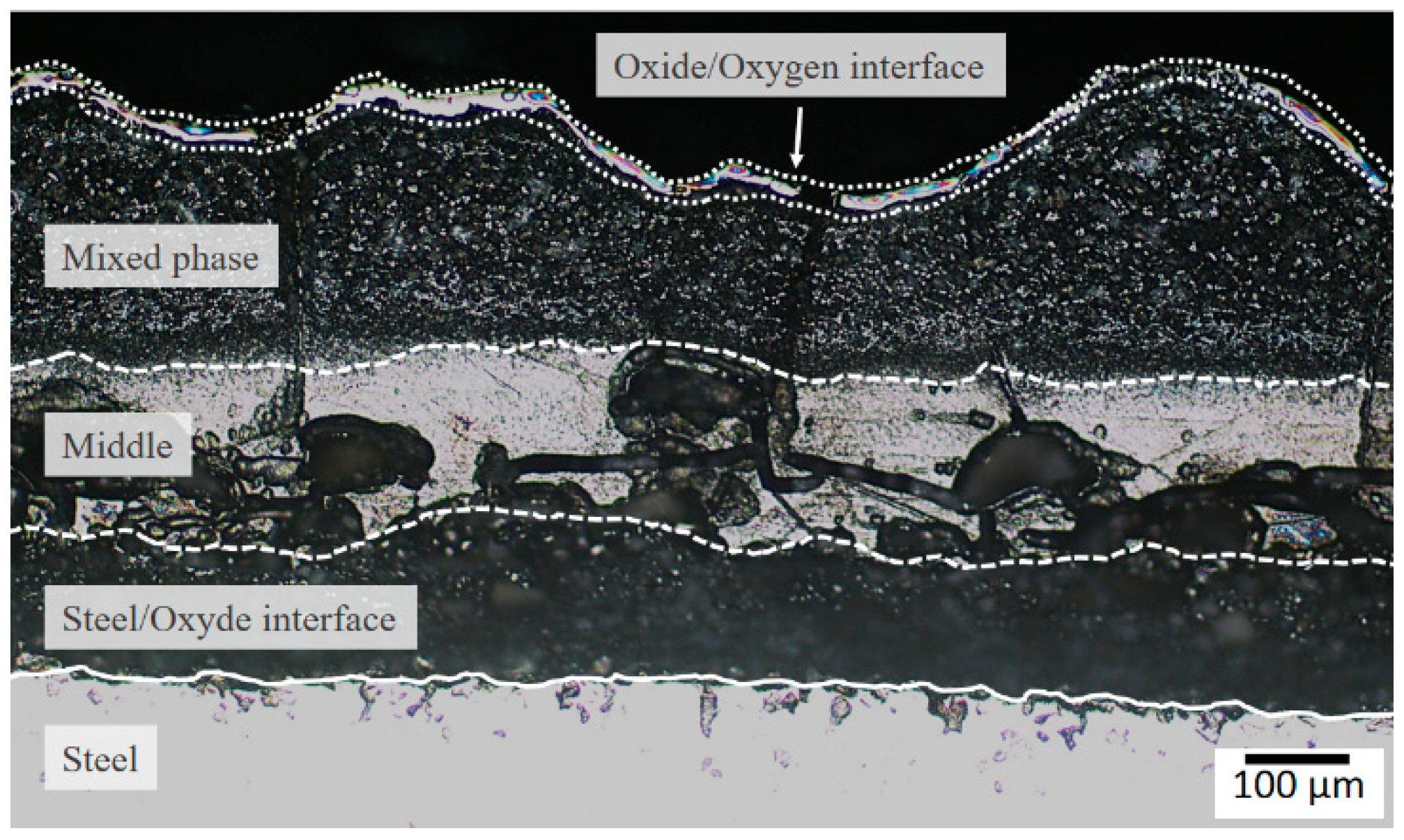
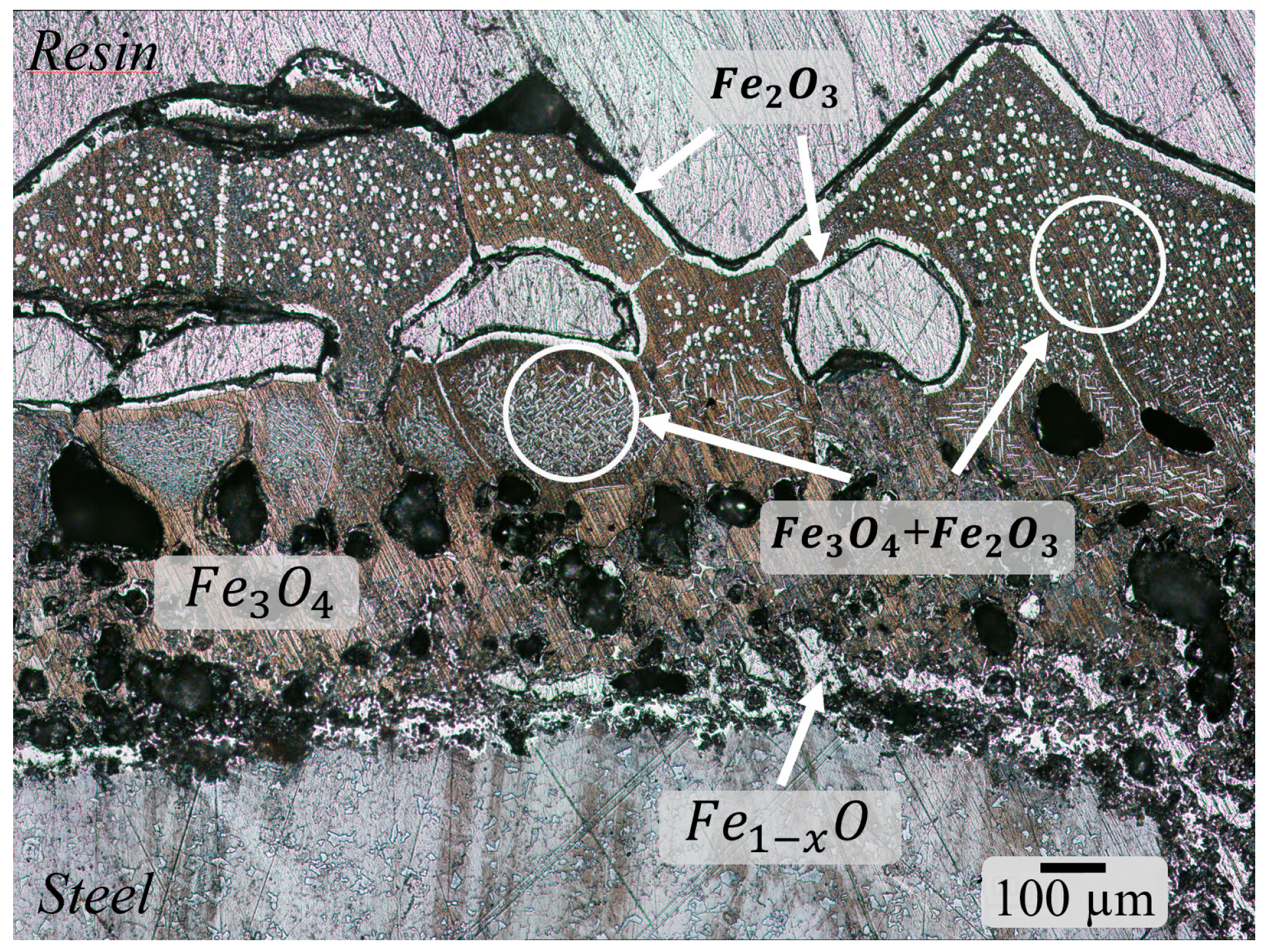
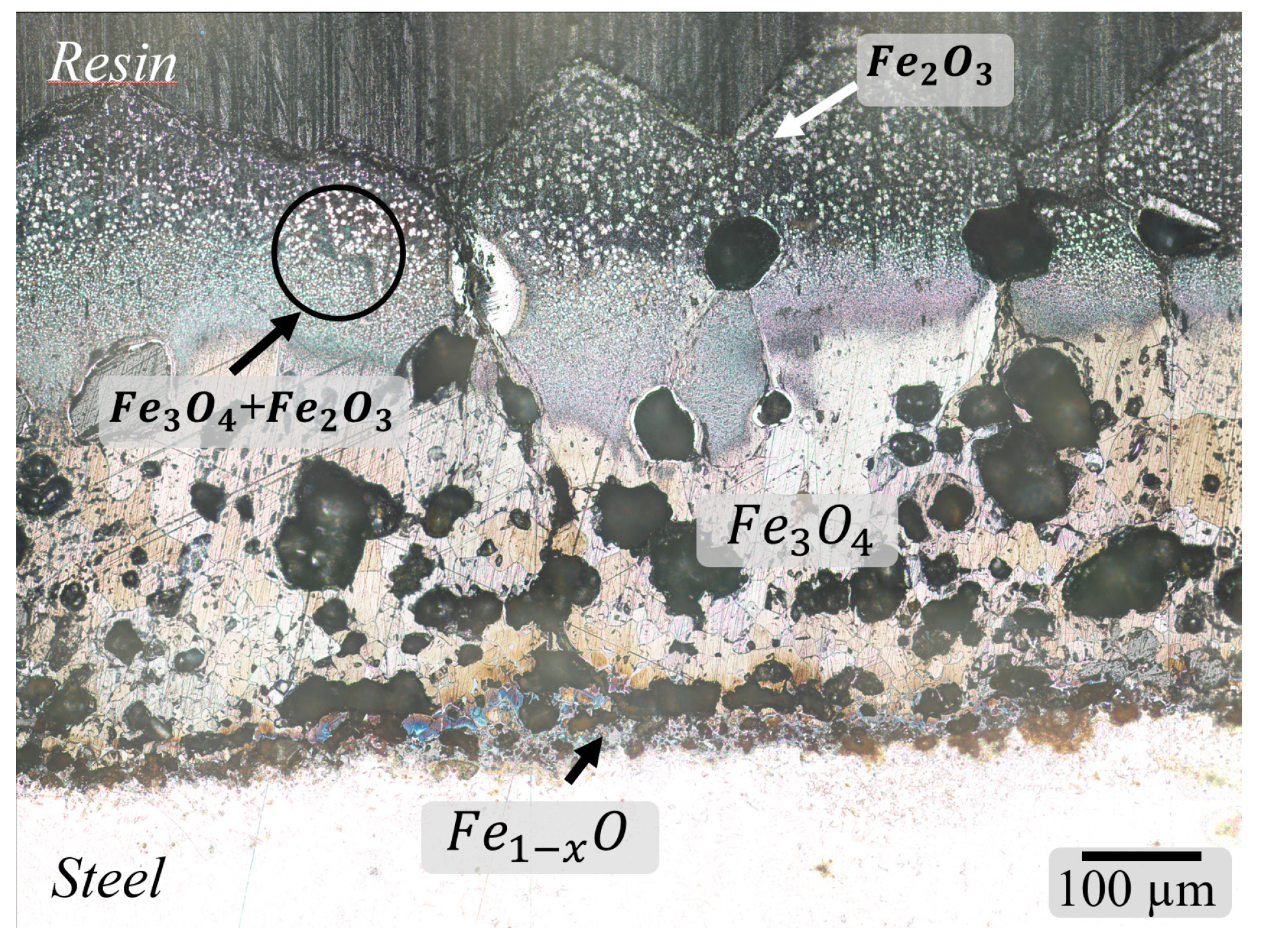
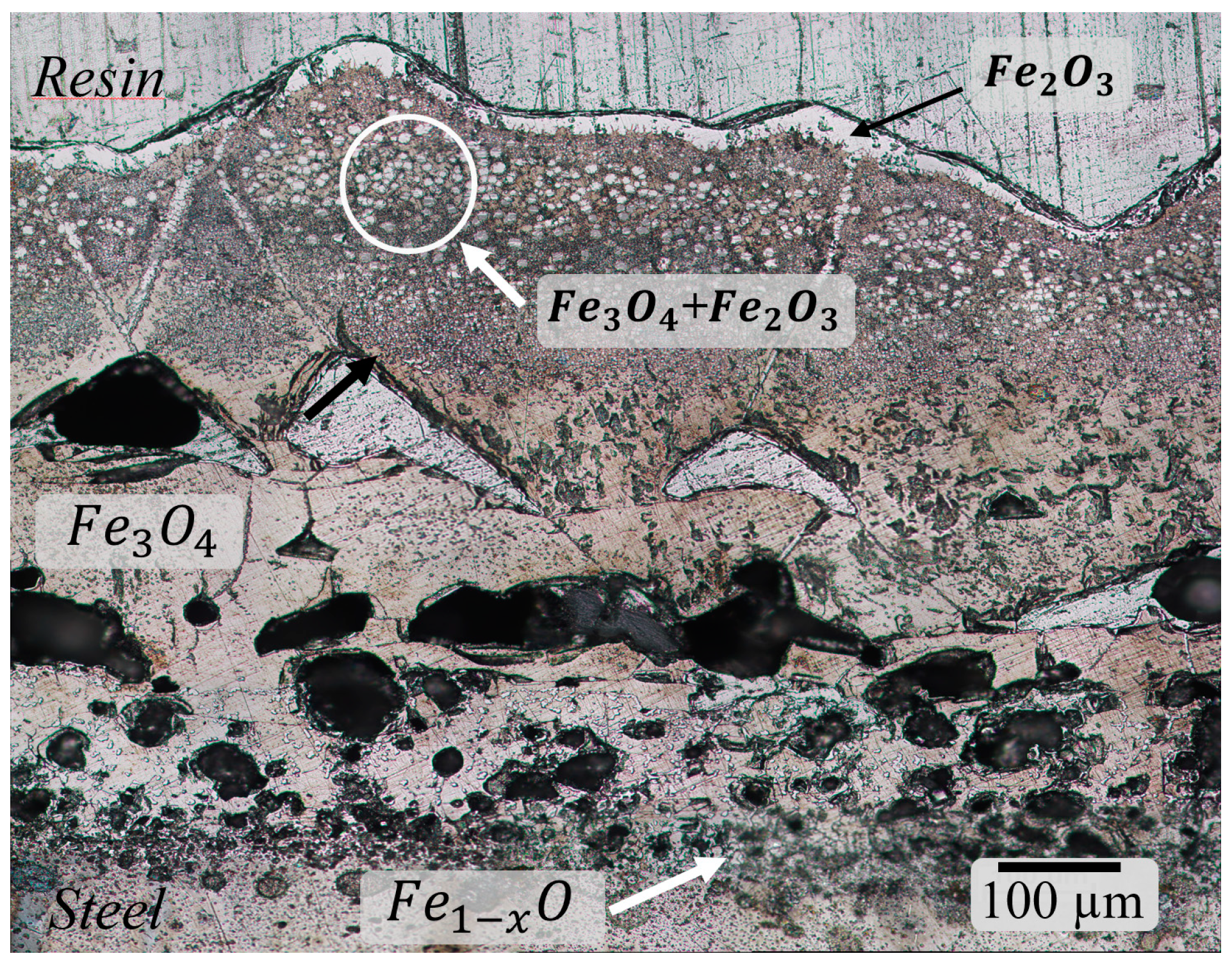
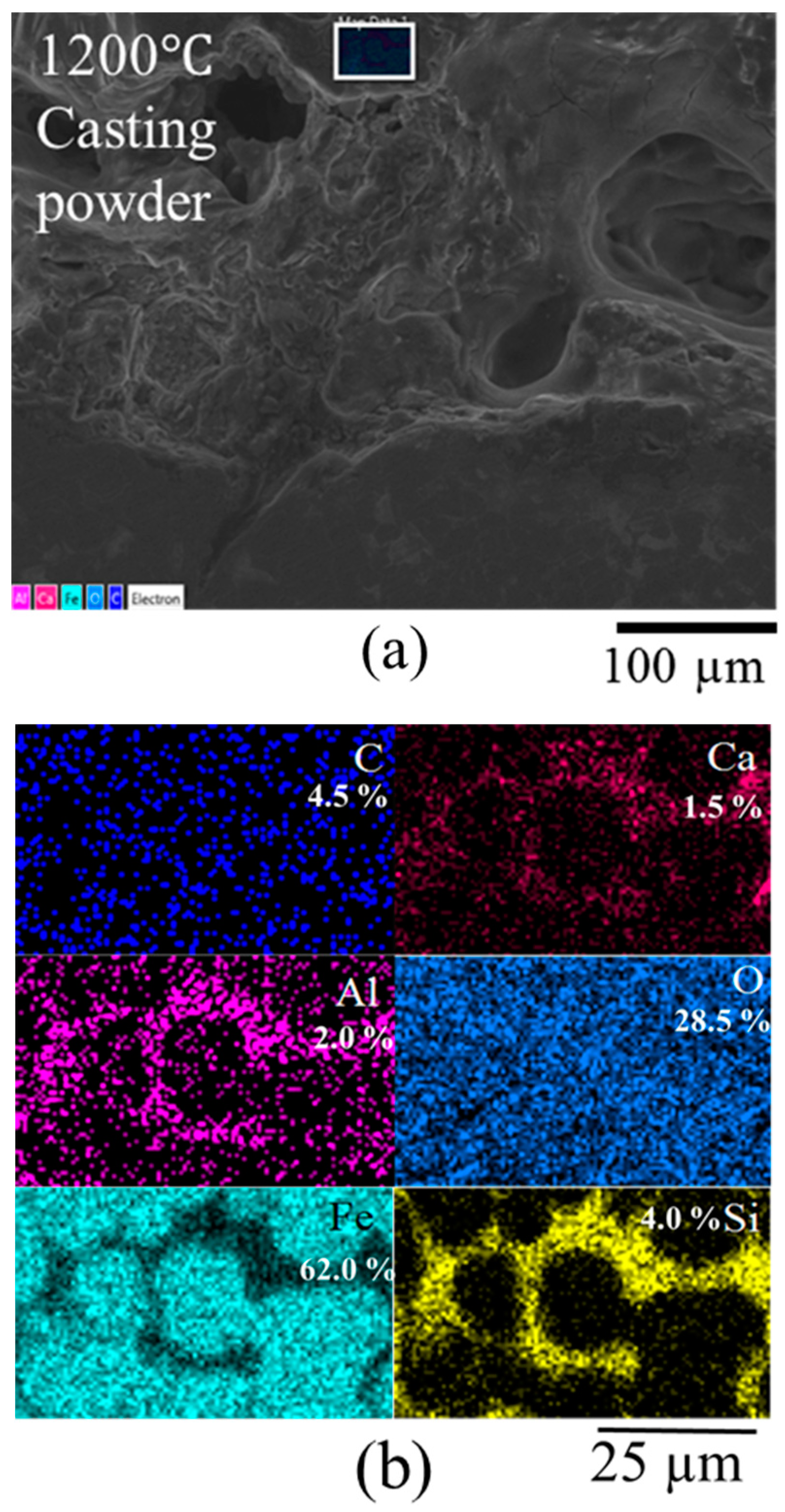
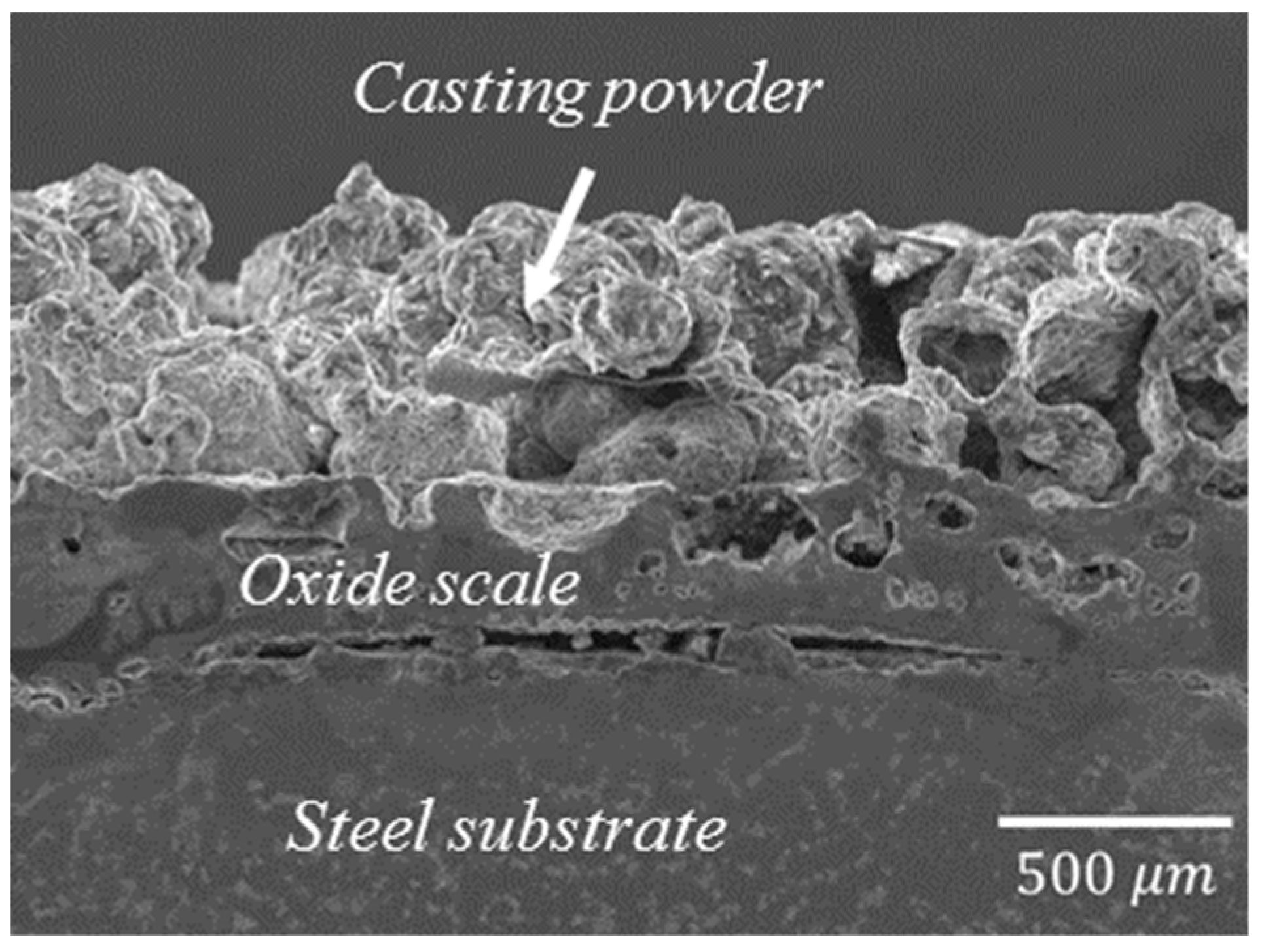
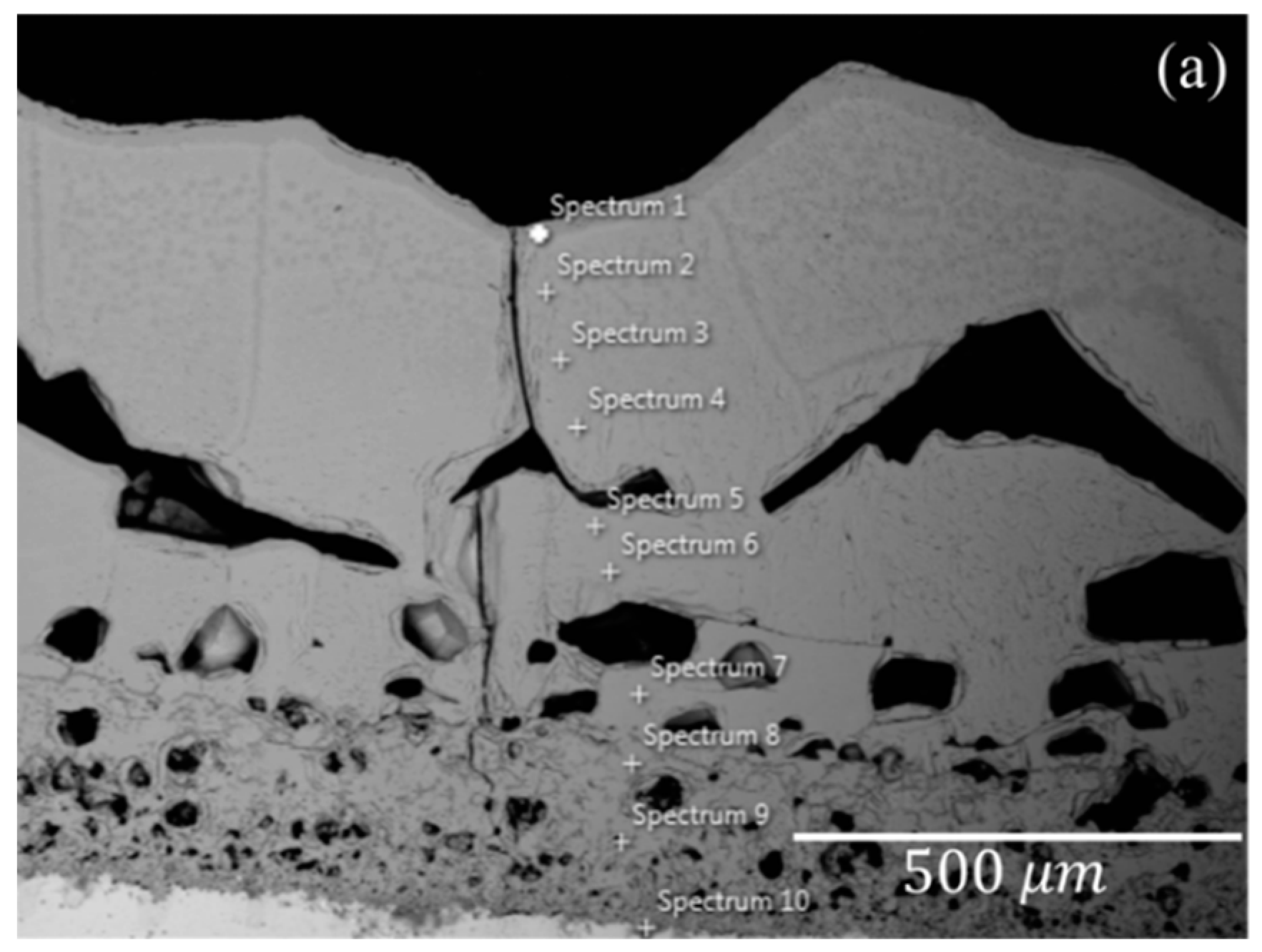
3.1. Characterization of Scale
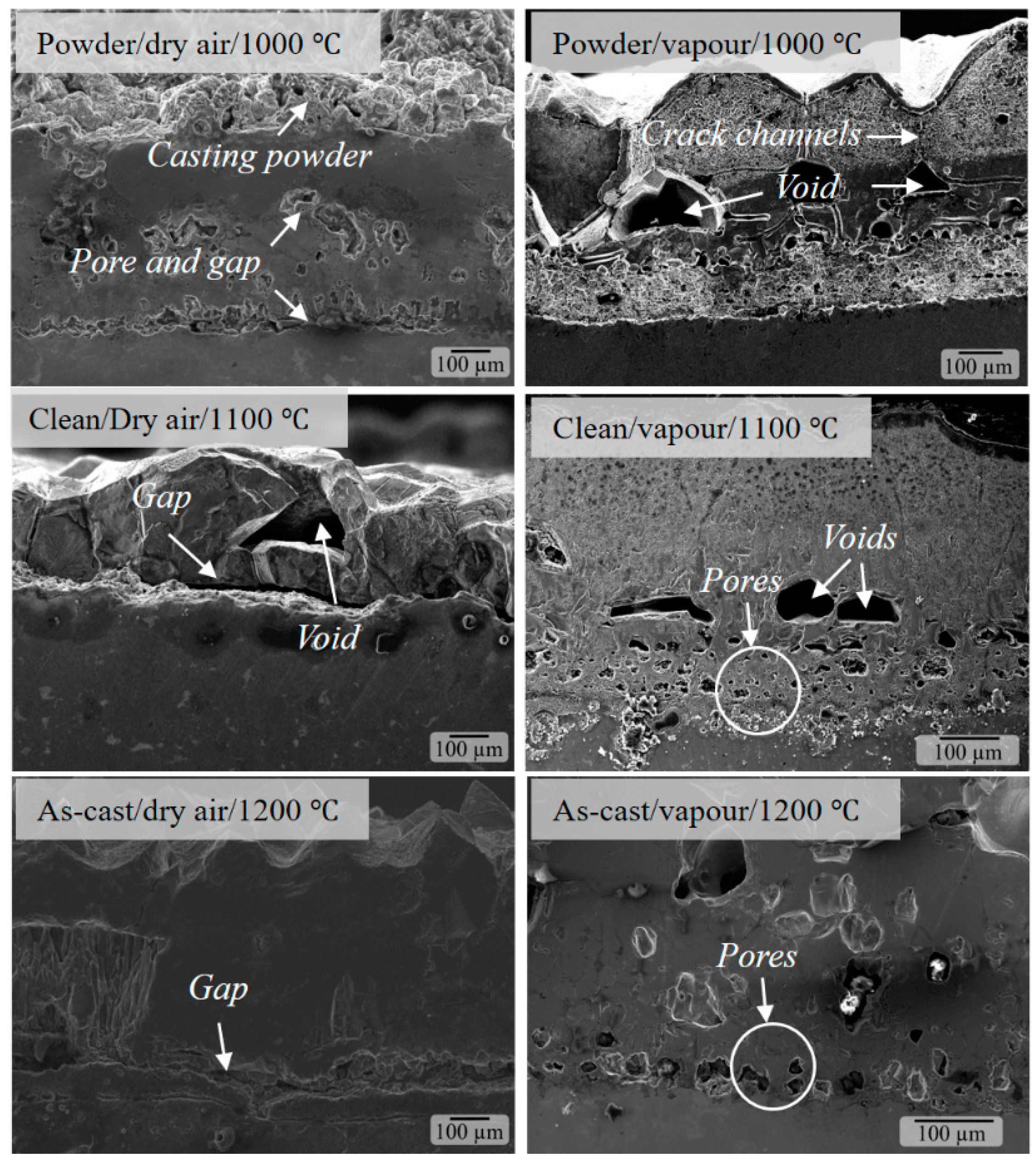
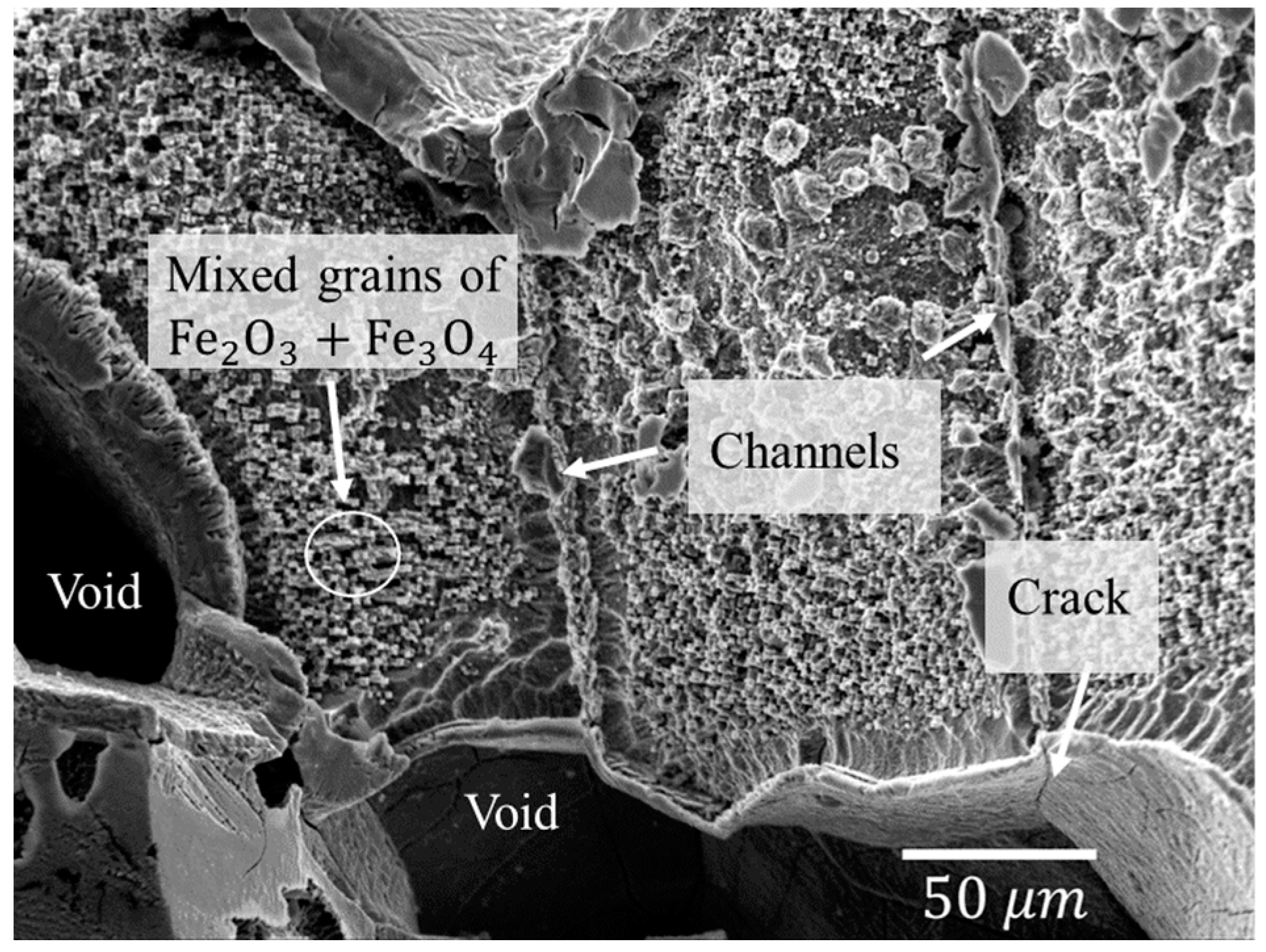
3.2. Defects in Oxide Scale
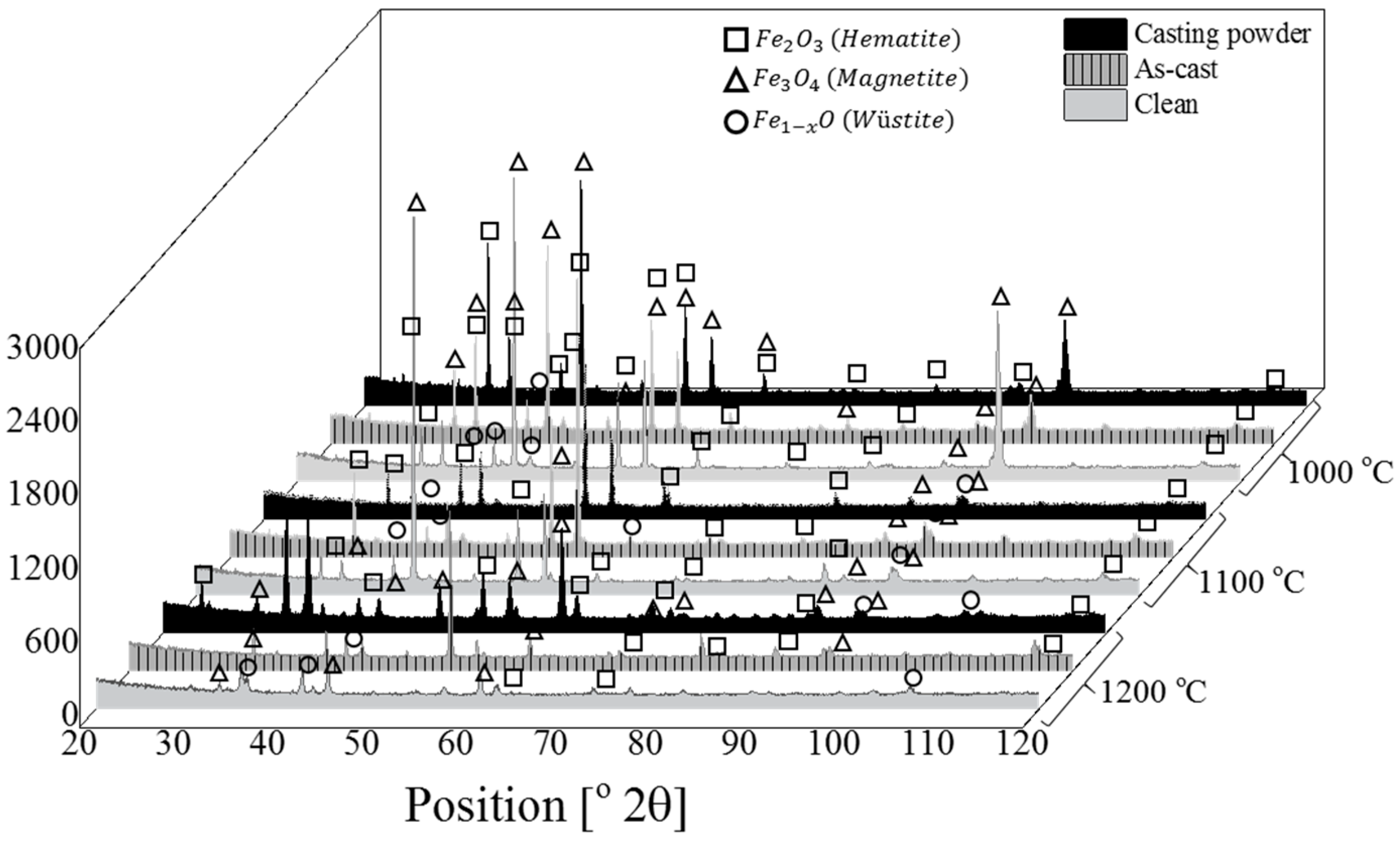
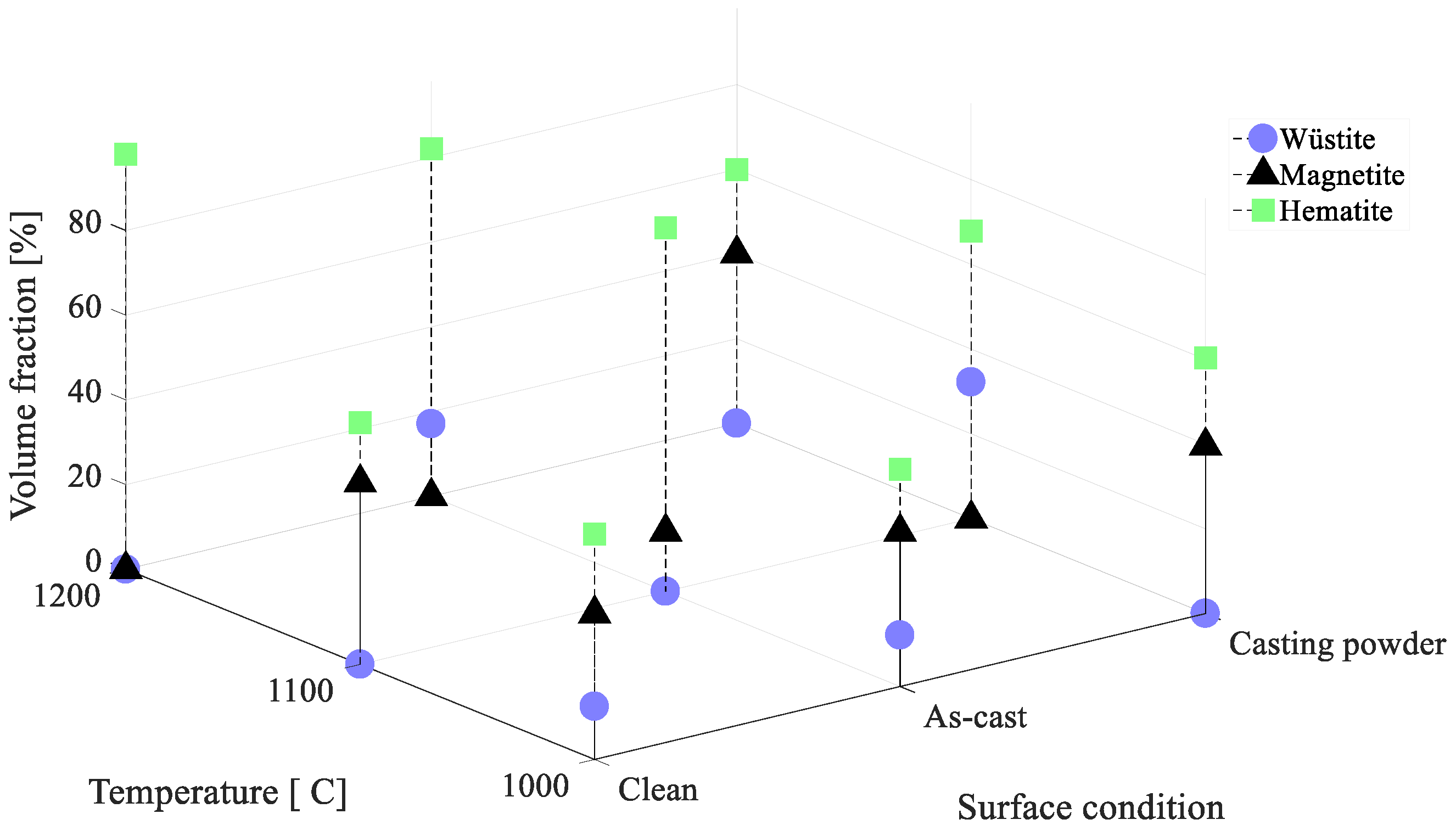
3.3. Phase Analysis
4. Discussion
4.1. Phase Analysis
4.2. Effect of Casting Powder on Oxidation Kinetics
4.3. Effect of Casting Powder on Micromechanics
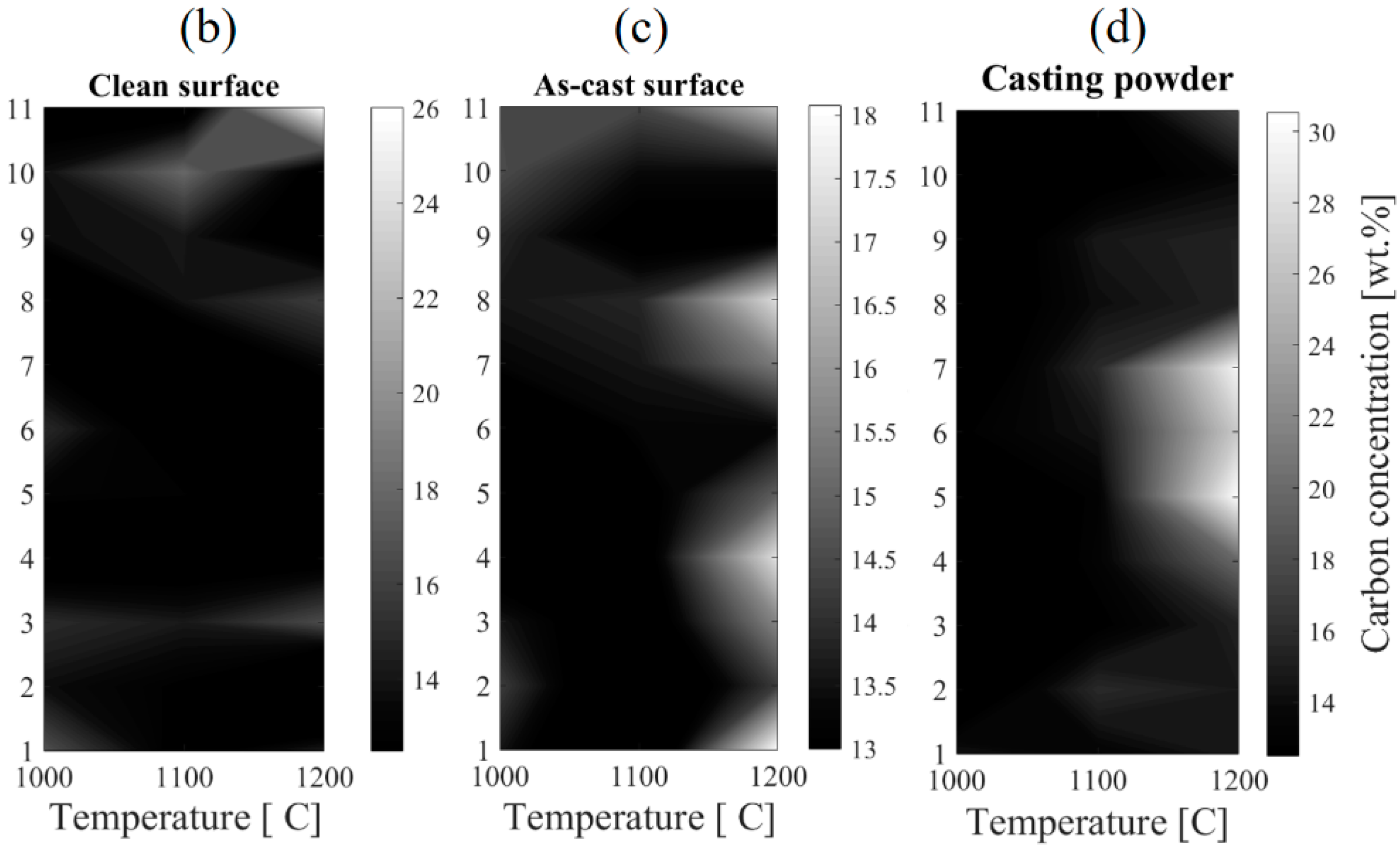
4.4. Effect of Carbon Content on Oxidation Behaviour
5. Conclusions
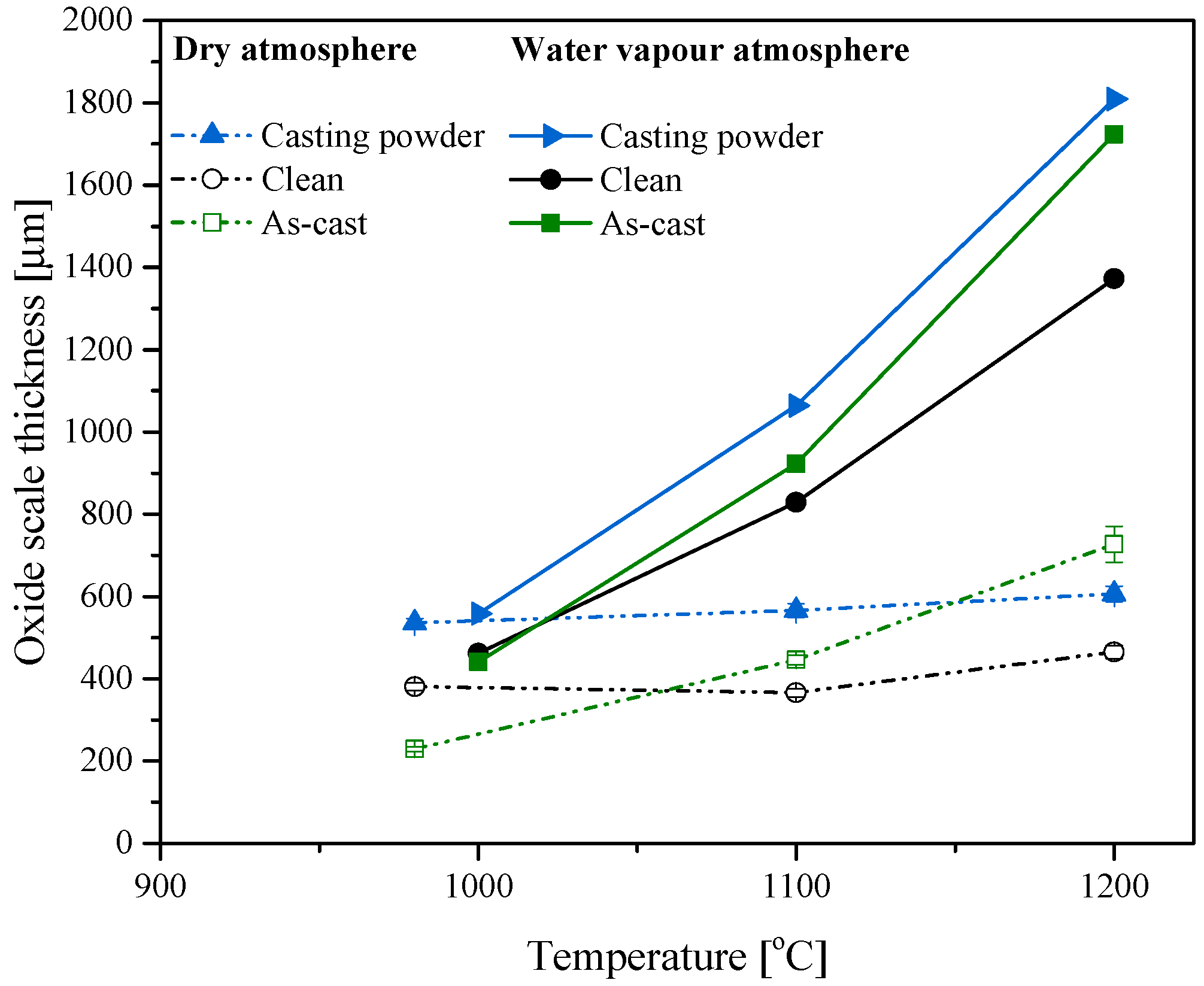
- Oxide scales are influenced not only by temperature and environmental conditions but also strongly by the status of the surface which increases rugosity or oxidation rate.
- Thick and dense scales are formed oxide under water vapour compared to dry air conditions.
- Scale thickness is more significant on as-cast and surface covered with casting powder than those formed on a clean surface.
- Fast oxidation and gases (CO and CO2) lead to stress accumulation, which make oxide scales prone to defects such as pores, voids and micro-cracks. The number of these defects increase with temperature, being more pronounced in the as-cast and surfaces covered with casting powder.
- Oxides and carbonates from the casting powder during melting and sintering accelerate the oxidation rate of oxide scale leading to thick and unstable scales.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, P.; Ying, L.; Li, Y.; Liao, Z. Effect of oxide scale on temperature-dependent interfacial heat transfer in hot stamping process. J. Mater. Process. Technol. 2013, 213, 1475–1483. [Google Scholar] [CrossRef]
- Li, Y.H.; Sellars, C.M. Comparative investigations of interfacial heat transfer behaviour during hot forging and rolling of steel with oxide scale formation. J. Mater. Process. Technol. 1998, 80–81, 282–286. [Google Scholar] [CrossRef]
- Marston, H.F.; Bolt, P.H.; Leprince, G.; Röder, M.; Klima, R.; Niska, J.; Jarl, M. Challenges in the modelling of scale formation and decarburisation of high carbon, special and general steels. Ironmak. Steelmak. 2004, 31, 1. [Google Scholar] [CrossRef]
- Sun, W.; Tieu, A.K.; Jiang, Z.; Lu, C. High temperature oxide scale characteristics of low carbon steel in hot rolling. J. Mater. Process. Technol. 2004, 155–156, 1307–1312. [Google Scholar] [CrossRef]
- Hidaka, Y.; Anraku, T.; Otsuka, N. Deformation of iron oxides upon tensile tests at 600–1250 °C. Oxid. Met. 2003, 59, 97–113. [Google Scholar] [CrossRef]
- Abuluwefa, H.; Guthrie, R.; Ajersch, F. The effect of oxygen concentration on the oxidation of low-carbon steel in the temperature range 1000 to 1250 °C. Oxid. Met. 1996, 46, 423–440. [Google Scholar] [CrossRef]
- Suarez, L.; Houbaert, Y.; Eynde, X.V.; Colás, R. High temperature deformation of oxide scale. Corros. Sci. 2009, 51, 309–315. [Google Scholar] [CrossRef]
- Chen, R.; Yeun, W. Review of the high-temperature oxidation of iron and carbon steels in air or oxygen. Oxid. Met. 2003, 59, 433–468. [Google Scholar] [CrossRef]
- Liu, S.; Tang, D.; Wu, H.; Wang, L. Oxide scales characterization of micro-alloyed steel at high temperature. J. Mater. Process. Technol. 2013, 213, 1068–1075. [Google Scholar] [CrossRef]
- Wang, N.; Wen, H.; Huang, W.; Dou, N.; Chen, M. Effect of Steel Composition on the Scale Layer Composition in Continuous Casting. In Proceedings of the 8th Pacific Rim International Congress on Advanced Materials and Processing, Waikoloa, HI, USA, 4–9 August 2013. [Google Scholar]
- Chabičovský, M.; Hnízdil, M.; Tseng, A.; Raudenský, M. Effects of oxide layer on Leidenfrost temperature during spray cooling of steel at high temperatures. Int. J. Heat Mass Transf. 2015, 88, 236–246. [Google Scholar] [CrossRef]
- Chabičovský, M.; Horský, J. Factors influencing spray cooling of hot steel surfaces. In Proceedings of the 26th International Conference on Metallurgy and Materials, Brno, Czech Republic, 24–26 May 2017. [Google Scholar]
- Brožová, T.; Chabičovský, M.; Horský, J. Influence of the surface roughness on the cooling intensity during spray cooling. In Proceedings of the 25th Anniversary International Conference on Metallurgy and Materials, Brno, Czech Republic, 25–27 May 2016. [Google Scholar]
- Scale Control. Control of Scale during Steel Processing; RFSR-CT-2005-00016; European Commission: London, UK, 2005. [Google Scholar]
- ODS-STEEL. Novel Oxide Dispersion Strengthened Steels Obtained by High Productivity Casting Process with Innovative Injection of Suitable Special Powder; RFSR-CT-2006-00020; European Commission: Roma, Italy, 2006. [Google Scholar]
- Wendelstorf, R.; Spitzer, K.; Wendelstorf, J. Effect of oxide layers on spray water cooling heat transfer at high surface temperatures. Int. J. Heat Mass Transf. 2008, 51, 4892–4901. [Google Scholar] [CrossRef]
- Yamauchi, A.; Sorimachi, K.; Sakuraya, T.; Fujii, T. Heat transfer between mold and strand through mold flux film in continuous casting of steel. ISIJ Int. 1993, 33, 140–147. [Google Scholar] [CrossRef]
- Bolender, T.; Cappel, J. Continuous casting without secondary spray water cooling. Steel Grips 2004, 2, 1–6. [Google Scholar]
- Sun, W.; Tieu, A.; Jiang, Z.; Zhu, H.; Lu, C. Oxide scales growth of low-carbon steel at high temperatures. J. Mater. Process. Technol. 2004, 155–156, 1300–1306. [Google Scholar] [CrossRef]
- Xie, Q.; Huang, Z.; Hou, Q.; Zhang, L.; Cai, J. Heat Transfer from a Hot Steel Plate Impinged by Air-atomized Water Jet and Impinging Water Jet. ISIJ Int. 2019, 59, 113–121. [Google Scholar] [CrossRef]
- Warzee, M.; Hennaut, J.; Maurice, M.; Sonnen, C.; Waty, J.; Berge, P. Effect of Surface Treatment on the Corrosion of Stainless Steels in High-Temperature Water and Steam. J. Electrochem. Soc. 1965, 112, 7. [Google Scholar] [CrossRef]
- Cissé, S.; Laffont, L.; Tanguy, B.; Lafont, M.; Andrieu, E. Effect of surface preparation on the corrosion of austenitic stainless steel 304L in high temperature steam and simulated PWR primary water. Corros. Sci. 2012, 56, 209–216. [Google Scholar] [CrossRef]
- Cooper, L.; Benhaddad, S.; Wood, A.; Ivey, D. The effect of surface treatment on the oxidation of ferritic stainless steels used for solid oxide fuel cell interconnects. J. Power Sources 2008, 184, 220–228. [Google Scholar] [CrossRef]
- Mills, K.C.; Däcker, C. The Casting Powders Book; Mills, K.C., Däcker, C., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Saleem, S. On the Surface Quality of Continuously Cast Steels and Phosphor Bronzes. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2016. [Google Scholar]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science Publishers: Essex, UK, 1988. [Google Scholar]
- Krzyzanowski, M.; Beynon, J.H.; Farrugia, D.C. Oxide Scale Behavior in High Temperature Metal Processing; Krzyzanowski, M., Beynon, J.H., Farrugia, D.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals; Young, D.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Srinivasan, M. Science and Technology of Casting Processes; Srinivasan, M., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Marschall, I.; Kölbl, N.; Harmuth, H.; Atzenhofer, C. Identification of secondary raw materials in mold powders and their melting behavior. J. Iron Steel Res. Int. 2019, 26, 403–411. [Google Scholar] [CrossRef]
- Kudlinski, Z.; Sosnowski, R.; Sikora, B.; Dankmeyer-Laczny, J. The influence of the molten casting powder on the steel surface phenomena in continuous casting mould. Acta Metall. Slovaca (Slovak Repub.) 2001, 7, 184–197. [Google Scholar]
- Yan, W.; Chen, W.; Carsten, L.; Zheng, H. Effect of Properties Change of Mold Flux and Slag Rim on Concasting of Non-Magnetic Steel 20Mn23AlV. Teshugang (Special Steel) 2013, 34, 45–48. [Google Scholar]
- Petzow, G. Metallographic Etching: Techniques for Metallography, Ceramography, Plastography; Petzow, G., Ed.; ASM International: Cleveland, OH, USA, 1999. [Google Scholar]
- Walker, P.; Tarn, W.H. CRC Handbook of Metal Etchants; Walker, P., Tarn, W.H., Eds.; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Zambrano, O.; Coronado, J.; Rodríguez, S. Mechanical properties and phases determination of low carbon steel oxide scales formed at 1200 C in air. Surf. Coat. Technol. 2015, 282, 155–162. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of hematite to wustite by hydrogen for chemical looping combustion. Energy Fuels 2014, 28, 5406–5414. [Google Scholar] [CrossRef]
- Pitschke, W.; Hermann, H.; Mattern, N. The influence of surface roughness on diffracted X-ray intensities in Bragg-Brentano geometry and its effect on the structure determination by means of Rietveld analysis. Powder Diffr. 1993, 8, 74–83. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Hallström, S.; Höglund, L.; Agren, J. Modeling of iron diffusion in the iron oxides magnetite and hematite with variable stoichiometry. Acta Mater. 2011, 59, 53–60. [Google Scholar] [CrossRef]
- Giletti, B.; Hess, K. Oxygen diffusion in magnetite. Earth Planet. Sci. Lett. 1988, 89, 115–122. [Google Scholar] [CrossRef]

| T (°C) | Surface Condition | Average Thickness | |
|---|---|---|---|
| (µm) | |||
| Dry Air | Water Vapour | ||
| 1000 | Clean | 381 | 462 |
| As-Cast | 229 | 441 | |
| Casting Powder | 536 | 558 | |
| 1100 | Clean | 366 | 829 |
| As-Cast | 446 | 923 | |
| Casting Powder | 566 | 1064 | |
| 1200 | Clean | 465 | 1373 |
| As-Cast | 727 | 1724 | |
| Casting Powder | 606 | 1809 | |
| T (°C) | Surface Condition | Wüstite | ||
|---|---|---|---|---|
| 1000 | Clean | 12.5 | 34.4 | 53.2 |
| As-Cast | 12.1 | 36.5 | 51.3 | |
| Casting Powder | - | 39.6 | 60.4 | |
| 1100 | Clean | - | 42.9 | 57.1 |
| As-Cast | - | 14.1 | 85.9 | |
| Casting Powder | 32.2 | - | 67.8 | |
| 1200 | Clean | - | - | 98 |
| As-Cast | 17.1 | - | 82.1 | |
| Casting Powder | - | 40.1 | 59.9 |
| Phase | HN (GPA) | E (GPa) | Plasticity Index | |||
|---|---|---|---|---|---|---|
| This Work | Part I | This Work | Part I | This Work | Part I | |
| Wüstite | 2.8 | 2.7 | 98 | 138 | 0.79 | 0.86 |
| Magnetite | 5.9 | 5 | 146 | 144 | 0.53 | 0.78 |
| Hematite | 6.4 | 5.5 | 141 | 150 | 0.71 | 0.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda Huitron, R.M.; Ramírez López, P.E.; Vuorinen, E.; Nazen Jalali, P.; Pelcastre, L.; Kärkkäinen, M. Scale Formation on HSLA Steel during Continuous Casting Part II: The Effect of Surface Conditions. Metals 2020, 10, 1245. https://doi.org/10.3390/met10091245
Pineda Huitron RM, Ramírez López PE, Vuorinen E, Nazen Jalali P, Pelcastre L, Kärkkäinen M. Scale Formation on HSLA Steel during Continuous Casting Part II: The Effect of Surface Conditions. Metals. 2020; 10(9):1245. https://doi.org/10.3390/met10091245
Chicago/Turabian StylePineda Huitron, Rosa Maria, Pavel Ernesto Ramírez López, Esa Vuorinen, Pooria Nazen Jalali, Leonardo Pelcastre, and Maija Kärkkäinen. 2020. "Scale Formation on HSLA Steel during Continuous Casting Part II: The Effect of Surface Conditions" Metals 10, no. 9: 1245. https://doi.org/10.3390/met10091245
APA StylePineda Huitron, R. M., Ramírez López, P. E., Vuorinen, E., Nazen Jalali, P., Pelcastre, L., & Kärkkäinen, M. (2020). Scale Formation on HSLA Steel during Continuous Casting Part II: The Effect of Surface Conditions. Metals, 10(9), 1245. https://doi.org/10.3390/met10091245