Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructural Characterization and Hardness Test
2.3. Electrochemical Technique
3. Results
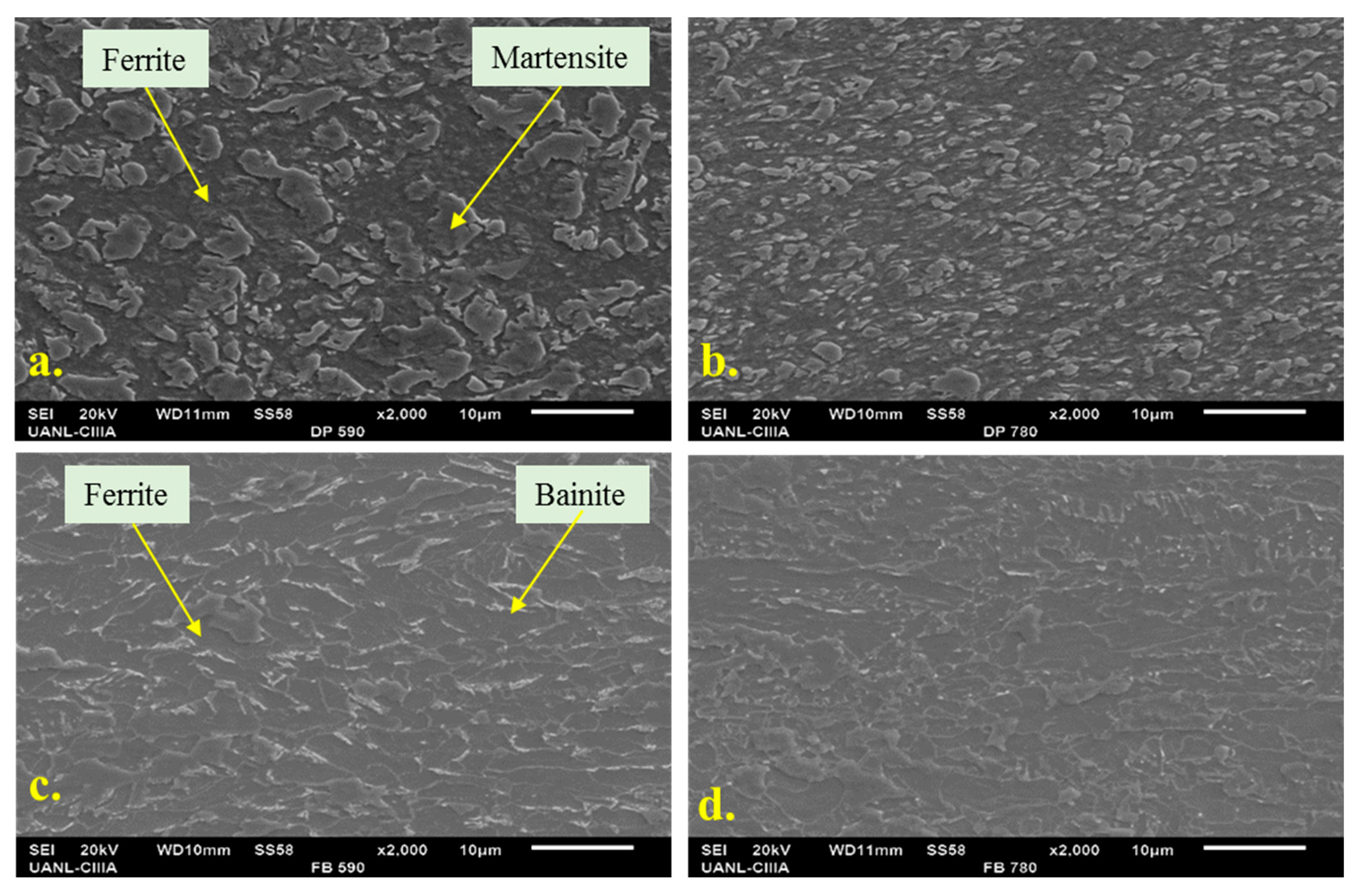
3.1. Microstructure
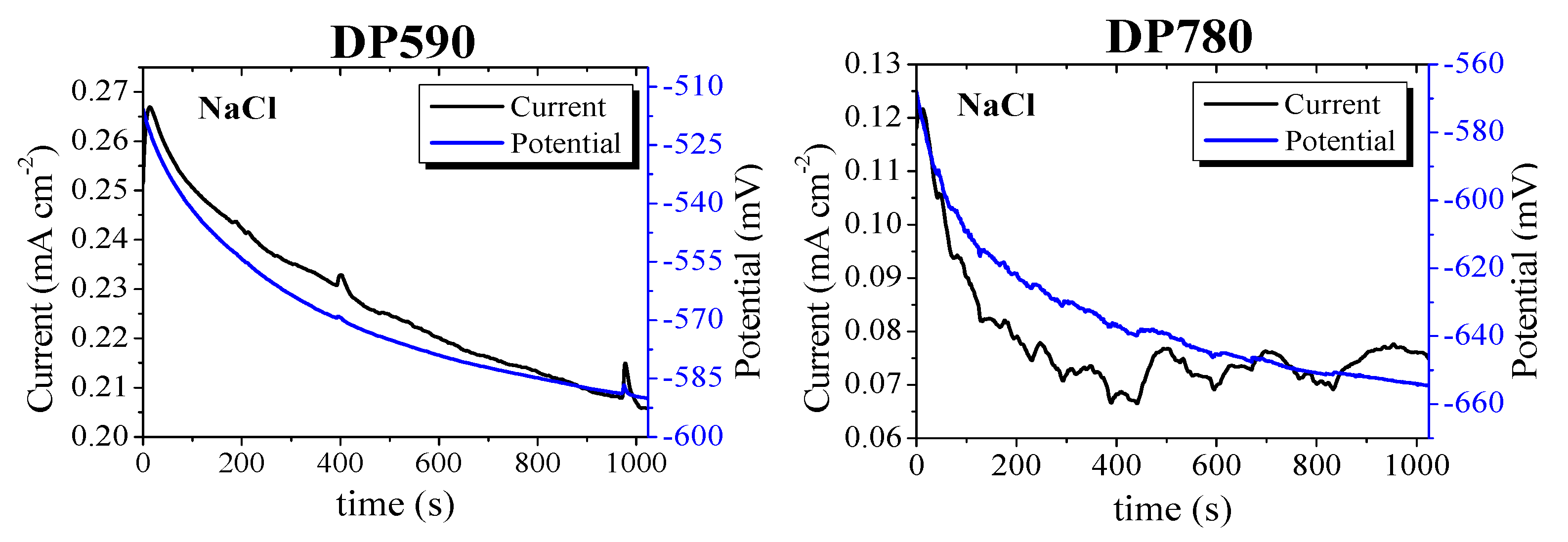
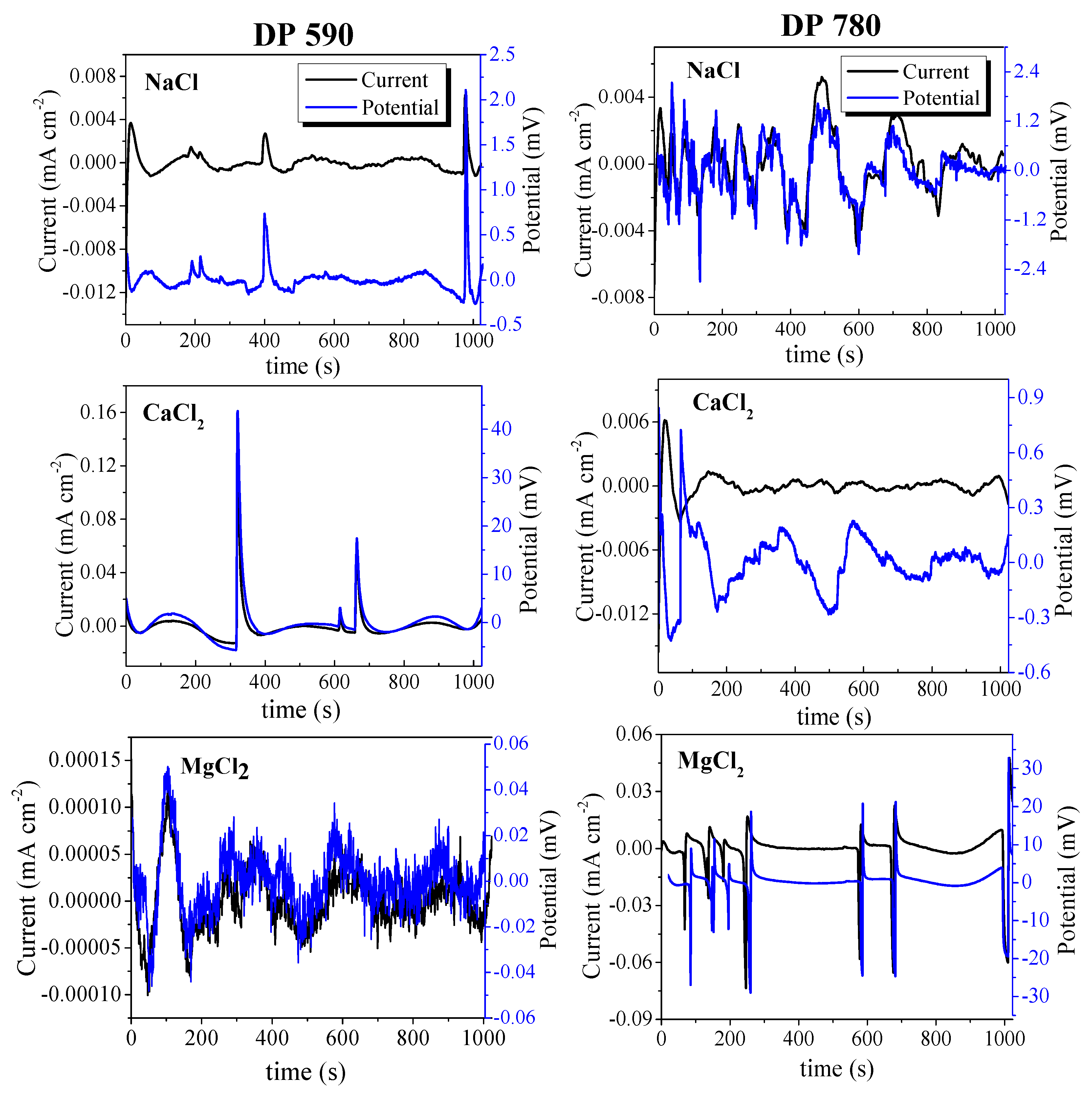
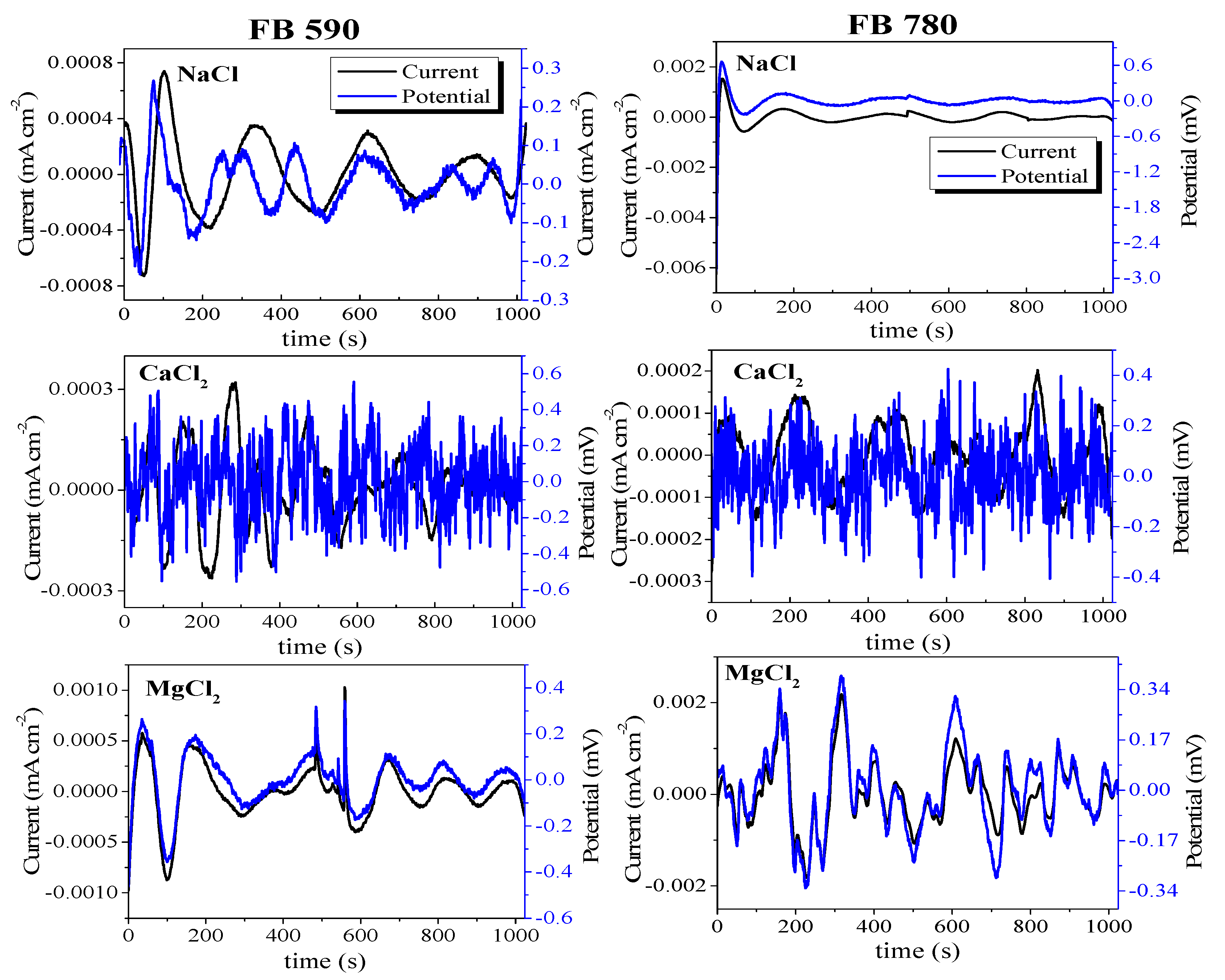
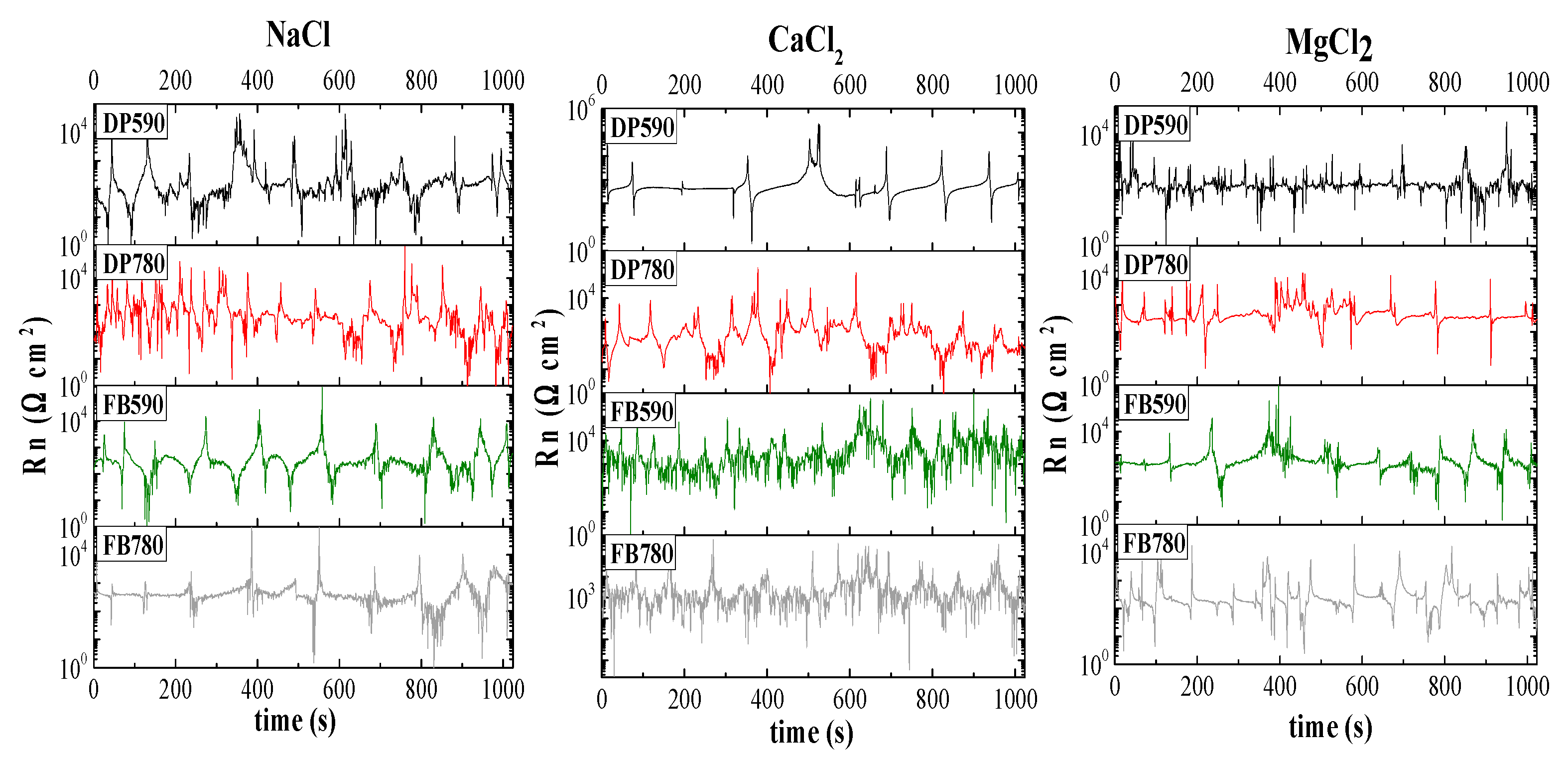
3.2. Electrochemical Noise
4. Discussion
4.1. Microstructure
4.2. Corrosion Analysis
4.2.1. Noise Resistance
4.2.2. Localization Index
4.2.3. Skewness and Kurtosis Measurements
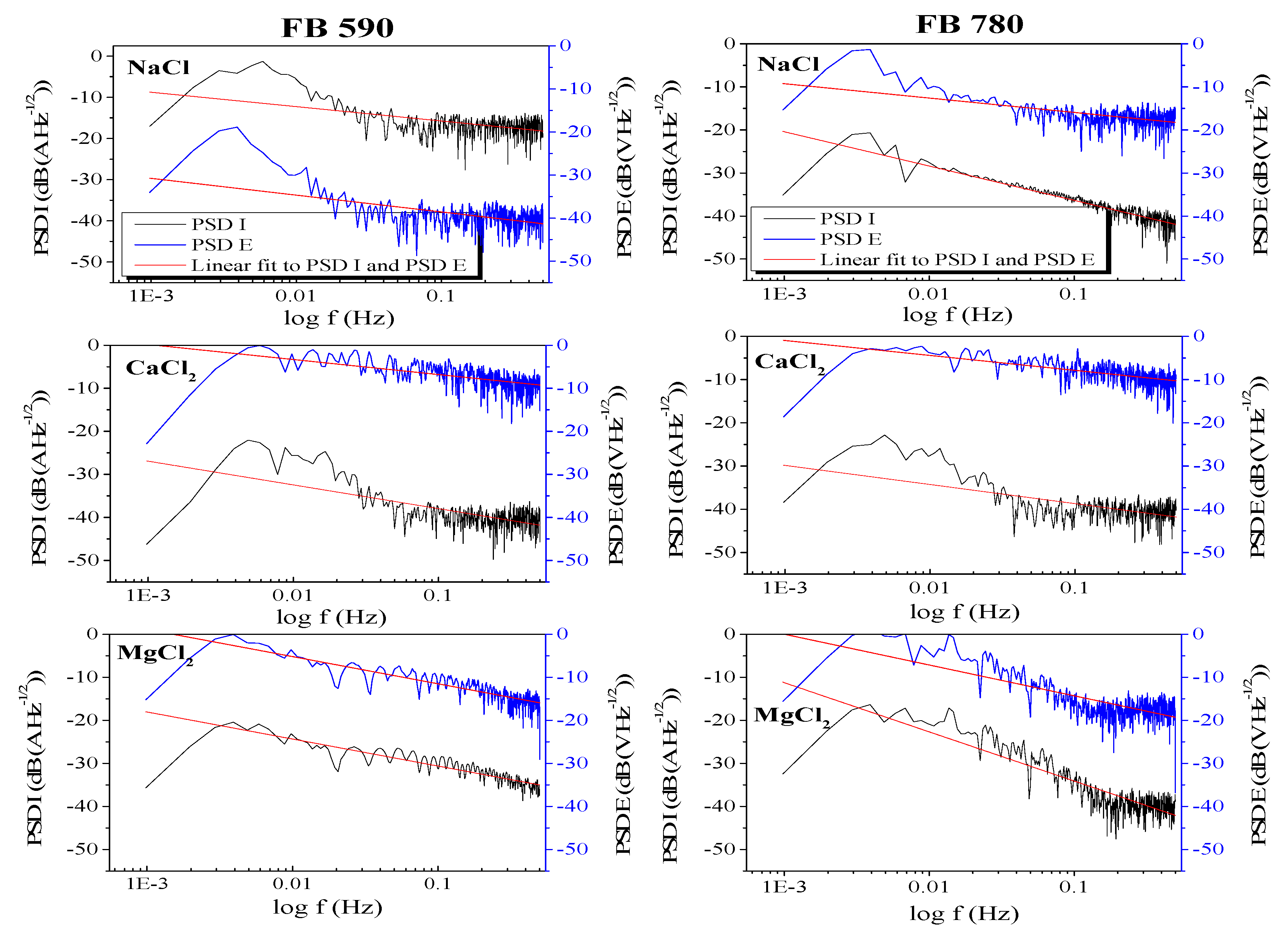
4.2.4. Power Spectral Density (PSD) Analysis
5. Conclusions
- This work presents a study on the corrosion behavior of four important types of AHSS steels i.e., DP (590 and 7890 MPa) and FB (590 and 780 MPa) steels exposed to 3.5 wt.% NaCl, 2 wt.% CaCl2 and 2wt.% MgCl2 solutions. These chemical compounds are the basis of de-icing salts commonly used in highways particularly in winter time. As a first approach, electrochemical noise technique was used.
- The current and potential time series show different behavior for each electrolyte. High-frequency fluctuations for short periods of time were observed for DP590 steel in MgCl2 solution and DP780 steel in NaCl solution. In the case of FB steels, the same behavior occurred in FB590 and FB780 in CaCl2 solution. In general, the current tan potential fluctuations can be associated with a general a corrosion process.
- From the Rn data obtained, an inverse relationship between the reciprocal of noise resistance value, 1/Rn, and mechanical strength of both DP and FB steels was found in NaCl solution. By contrast, the behavior in CaCl2 solution did not show any relationship from Rn and mechanical strength. Here, a higher 1/Rn value for DP780 (0.00271 Ω·cm−2) and FB780 (0.00509 Ω·cm−2) steels compared to that for DP590 (0.00286 Ω·cm−2) and FB590 (0.000592 Ω·cm−2) steels was noted. Immersion in MgCl2 solution gave very similar 1/Rn values for DP90 (0.00237 Ω·cm−2), DP780 (0.00237 Ω·cm−2) and FB590 (0.00252 Ω·cm−2) steels, with the 1/Rn values being somewhat higher for the FB780 (0.00509 Ω·cm−2) steel.
- Current and potential transients can be related to the corrosion type occurring according to statistical parameters such as the localization index [(L.I.) from 0.00054 to 0.15431 were calculated], skewness (values from −6.18 to 7.35 were calculated) and kurtosis (high values 37.15, 74.84 and 106.52). In general, the results indicated that the main corrosion process is related to one of uniform corrosion.
- The PSD current and potential slopes obtained were in the range from −2.85 to −5.42 in the entire frequency range for the FB590 steel in NaCl, CaCl2 and MgCl2 solutions, whereas somewhat higher PSD current and potential slopes were obtained for DP590, DP780 and FB780 steels exposed in all electrolytes.
- Corrosion behavior of AHSS steels exposed in NaCl solution could be related to the morphology of the phase constituents. However, under exposure in CaCl2 and MgCl2 solutions, an increase in martensite/bainite content or an increased refinement of phase constituents controls the corrosion behavior.
- Given the enormous industrial importance of this type of AHSS steels, and in order to obtain a better understanding of their corrosion behavior, it is recognized that the use of powerful techniques such as electrochemical impedance spectroscopy (EIS) would be of great benefit.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tamarelli, C.M. AHSS 101: The evolving use of advanced high-strength steel for automotive applications. Steel Mark. Dev. Inst. 2011, 1, 42. [Google Scholar]
- Keeler, S.; Kimchi, M. Advanced High-Strength Steels Application Guidelines Version 5.0. WorldAutoSteel 2014, 511. [Google Scholar] [CrossRef]
- Galán, J.; Samek, L.; Verleysen, P.; Verbeken, K.; Houbaert, Y. Advanced high strength steels for automotive industry. Rev. Metal. 2012, 48, 118–131. [Google Scholar] [CrossRef]
- Maffei, B.; Salvatore, W.; Valentini, R. Dual-phase steel rebars for high-ductile r.c. structures, Part 1: Microstructural and mechanical characterization of steel rebars. Eng. Struct. 2007, 29, 3325–3332. [Google Scholar] [CrossRef]
- Khan, A.S.; Baig, M.; Choi, S.H.; Yang, H.S.; Sun, X. Quasi-static and dynamic responses of advanced high strength steels: Experiments and modeling. Int. J. Plast. 2012, 30–31, 1–17. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.B.; Ray, K.K. Influence of bainite/martensite-content on the tensile properties of low carbon dual-phase steels. Mater. Sci. Eng. A 2008, 474, 270–282. [Google Scholar] [CrossRef]
- Qu, S.; Zhang, Y.; Pang, X.; Gao, K. Influence of temperature field on the microstructure of low carbon microalloyed ferrite-bainite dual-phase steel during heat treatment. Mater. Sci. Eng. A 2012, 536, 136–142. [Google Scholar] [CrossRef]
- Lesch, C.; Kwiaton, N.; Klose, F.B. Advanced High Strength Steels (AHSS) for Automotive Applications −Tailored Properties by Smart Microstructural Adjustments. Steel Res. Int. 2017, 88, 1–21. [Google Scholar] [CrossRef]
- Mintz, B. Hot dip galvanising of transformation induced plasticity and other intercritically annealed steels. Int. Mater. Rev. 2001, 46, 169–197. [Google Scholar] [CrossRef]
- Uzun, H.; Önal, E. Mechanical Properties and Corrosion Behaviors in 3.5% NaCl Solution of Grade-A and Dual-Phase Steels Welded by FCAW. Period. Eng. Nat. Sci. 2013, 1. [Google Scholar] [CrossRef]
- Abedini, O.; Behroozi, M.; Marashi, P.; Ranjbarnodeh, E.; Pouranvari, M. Intercritical heat treatment temperature dependence of mechanical properties and corrosion resistance of dua-phase steel. Mater. Res. 2019, 22, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Keleştemur, O.; Yildiz, S. Effect of various dual-phase heat treatments on the corrosion behavior of reinforcing steel used in the reinforced concrete structures. Constr. Build. Mater. 2009, 23, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zuo, X.; Li, J. Corrosion Resistance of the Welded Joint of Submarine Pipeline Steel with Ferrite Plus Bainite Dual-Phase Microstructure. Steel Res. Int. 2015, 86, 1260–1270. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, P.H.; Guan, Y.; Chen, Q.F.; Pu, S.K. The corrosion resistance of ultra-low carbon bainitic steel. Corros. Sci. 2009, 51, 954–961. [Google Scholar] [CrossRef]
- Mehdipour, M.; Naderi, R.; Markhali, B.P. Electrochemical study of effect of the concentration of azole derivatives on corrosion behavior of stainless steel in H2SO4. Prog. Org. Coat. 2014, 77, 1761–1767. [Google Scholar] [CrossRef]
- Xia, D.; Song, S.; Wang, J.; Shi, J.; Bi, H.; Gao, Z. Determination of corrosion types from electrochemical noise by phase space reconstruction theory. Electrochem. Commun. 2012, 15, 88–92. [Google Scholar] [CrossRef]
- Monticelli, C. Evaluation of Corrosion Inhibitors by Electrochemical Noise Analysis. J. Electrochem. Soc. 1992, 139, 706. [Google Scholar] [CrossRef]
- Park, C.J.; Kwon, H.S. Electrochemical noise analysis of localized corrosion of duplex stainless steel aged at 475 °C. Mater. Chem. Phys. 2005, 91, 355–360. [Google Scholar] [CrossRef]
- Suresh, G.U.; Kamachi, M.S. Electrochemical Noise Analysis of Pitting Corrosion of Type 304L Stainless Steel. Corrosion 2014, 70, 283–293. [Google Scholar] [CrossRef]
- Homborg, A.M.; Cottis, R.A.; Mol, J.M.C. An integrated approach in the time, frequency and time-frequency domain for the identification of corrosion using electrochemical noise. Electrochim. Acta 2016, 222, 627–640. [Google Scholar] [CrossRef]
- Nagiub, A.M. Electrochemical Noise Analysis for Different Green Corrosion Inhibitors for Copper Exposed to Chloride Media. Corros. Sci. 2017, 35, 201–210. [Google Scholar] [CrossRef]
- Ikpeseni, S.C.; Ameh, E.S. Effect of Temperature and Microstructure on the Corrosion Behaviour of a low Carbon dua-phase Steel. Am J Eng. Res. 2017, 6, 1–7. [Google Scholar]
- Si, Y. Investigation of Galvanic Corrosion Behavior of dua-phase Steel. ECS Trans. 2016, 72, 13. [Google Scholar] [CrossRef]
- Fushimi, K.; Yanagisawa, K.; Nakanishi, T.; Hasegawa, Y.; Kawano, T.; Kimura, M. Microelectrochemistry of dual-phase steel corroding in 0.1 M sulfuric acid. Electrochim. Acta 2013, 114, 83–87. [Google Scholar] [CrossRef]
- Gerengi, H.; Sen, N.; Uygur, I.; Kaya, E. Corrosion behavior of dua-phase 600 and 800 steels in 3.5 wt.% NaCl environment. J. Adhes. Sci. Technol. 2020, 34, 903–915. [Google Scholar] [CrossRef]
- Park, I.J.; Kim, S.T.; Lee, I.S.; Park, Y.S.; Moon, M.B. A study on corrosion behavior of DP-type and TRIP-type cold rolled steel sheet. Mater. Trans. 2009, 50, 1440–1447. [Google Scholar] [CrossRef] [Green Version]
- Park, I.J.; Lee, S.M.; Kang, M.; Lee, S.; Lee, Y.K. Pitting corrosion behavior in advanced high strength steels. J. Alloys Compd. 2015, 619, 205–210. [Google Scholar] [CrossRef]
- ASTM G199-09. Standard Guide for Electrochemical Noise Measurement; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Thewlis, G. Classification and quantification of microstructures in steels. J. Mater. Sci. Technol. 2004, 20, 143–160. [Google Scholar] [CrossRef]
- Kang, Y.; Han, Q.; Zhao, X.; Cai, M. Influence of nanoparticle reinforcements on the strengthening mechanisms of an ultrafine-grained dua-phase steel containing titanium. Mater. Des. 2013, 44, 331–339. [Google Scholar] [CrossRef]
- Schmitta, J.-H.; Iungb, T. New developments of advanced high-strength steels for automotive applications. Comptes Rendus Phys. 2018, 19, 641–656. [Google Scholar] [CrossRef]
- Hilditch, T.B.; de Souza, T.; Hodgson, P.D. Properties and automotive applications of advanced high-strength steels (AHSS). In Welding and Joining of Advanced High Strength Steels (AHSS), 1st ed.; Shome, M., Tumuluru, M., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2015; pp. 9–28. [Google Scholar]
- Kwon, O.; Lee, K.; Kim, G.; Chin, K.-G. New Trends in Advanced High Strength Steel Developments For Automotive Application. Mat. Sci. Forum. 2010, 638–642, 136–141. [Google Scholar] [CrossRef]
- Aydin, K.; Essadiqi, E.; Yue, S. Development of 3rd generation AHSS with medium Mn content alloying compositions. Mater. Sci. Eng. A 2013, 564, 501–508. [Google Scholar] [CrossRef]
- Saeidi, N.; Ekrami, A. Comparison of mechanical properties of martensite/ferrite and bainite/ferrite dua-phase 4340 steels. Mater. Sci. Eng. A 2009, 523, 125–129. [Google Scholar] [CrossRef]
- Brockwell, P.J.; Davis, R.A. Introduction to Time Series and Forecasting; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-29852-8. [Google Scholar]
- Bertocci, U.; Frydman, J.; Gabrielli, C.; Huet, F.; Keddam, M. Analysis of Electrochemical Noise by Power Spectral Density Applied to Corrosion Studies. J. Electrochem. Soc. 1998, 145, 2780–2786. [Google Scholar] [CrossRef]
- Tan, Y.; Bailey, S.; Kinsella, B. Studying the formation process of chromate conversion coatings on aluminium using continuous electrochemical noise resistance measurements. Corros. Sci. 2002, 44, 1277–1286. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Shao, Y.; Meng, G.; Wang, F. In-situ study of the formation process of stannate conversion coatings on AZ91D magnesium alloy using electrochemical noise. Corros. Sci. 2010, 52, 892–900. [Google Scholar] [CrossRef]
- Mansfeld, F.; Sun, Z.; Hsu, C.H.; Nagiub, A. Concerning trend removal in electrochemical noise measurements. Corros. Sci. 2001, 43, 341–352. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Basharnavaz, H.; Bezaatpour, A. A Schiff base compound as effective corrosion inhibitor for magnesium in acidic media. Mater. Chem. Phys. 2013, 138, 794–802. [Google Scholar] [CrossRef]
- Lentka, Ł.; Smulko, J. Methods of trend removal in electrochemical noise data - overview. Measurement 2018, 131, 569–581. [Google Scholar] [CrossRef]
- Tan, Y.J.; Bailey, S.; Kinsella, B. The monitoring of the formation and destruction of corrosion inhibitor films using electrochemical noise analysis (ENA). Corros. Sci. 1996, 38, 1681–1695. [Google Scholar] [CrossRef]
- Bhattacharya, D. Formable steels. In Advanced Steels; Springer: Berlin, Germany, 2011; pp. 163–175. ISBN 978-3-642-17665-4. [Google Scholar]
- Bakhtiari, R.; Ekrami, A. The effect of bainite morphology on the mechanical properties of a high bainite dua-phase (HBDP) steel. Adv. Mater. Sci. Eng. 2009, 525, 159–165. [Google Scholar] [CrossRef]
- Davies, R.G. The Deformation Behavior of a Vanadium-Strengthened dua-phase Steel. Metall. Trans. 1978, 9A, 41–52. [Google Scholar] [CrossRef]
- Ramazani, A.; Mukherjee, K.; Prahl, U.; Bleck, W. Modelling the effect of microstructural banding on the flow curve behaviour of dual-phase (DP) steels. Comput. Mater. Sci. 2012, 52, 46–54. [Google Scholar] [CrossRef]
- Davis, J.R. Engineering Data for Metals and Alloys. In Metals Handbook, Desk Edition, 2nd ed.; Davis, J.R., Ed.; ASM International: Cleveland, OH, USA, 1998; pp. 64–84. ISBN 978-1-62708-199-3. [Google Scholar]
- Calcagnotto, M.; Ponge, D.; Demir, E.; Raabe, D. Orientation gradients and geometrically necessary dislocations in ultrafine grained dual-phase steels studied by 2D and 3D EBSD. Mater. Sci. Eng. A 2010, 527, 2738–2746. [Google Scholar] [CrossRef]
- Ramazani, A.; Pinard, P.T.; Richter, S.; Schwedt, A.; Prahl, U. Characterisation of microstructure and modelling of flow behaviour of bainite-aided dual-phase steel. Comput. Mater. Sci. 2013, 80, 134–141. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, B.K.; Modi, O.P.; Das, S.; Yegneswaran, A.H. Correlating microstructural features and mechanical properties with abrasion resistance of a high strength low alloy steel. Wear 2003, 254, 120–128. [Google Scholar] [CrossRef]
- Puget, Y.; Trethewey, K.; Wood, R.J.K. Electrochemical noise analysis of polyurethane-coated steel subjected to erosion—Corrosion. Wear 1999, 233, 552–567. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Zhang, Z.; Cao, F.H.; Zhang, J.Q. Dimensional analysis applied to pitting corrosion measurements. Electrochim. Acta 2008, 53, 2688–2698. [Google Scholar] [CrossRef]
- Hashimoto, M.; Miyajime, S.; Murata, T. An experimental study of potential fluctuation during passive film breakdown and repair on iron. Corros. Sci. 1992, 33, 905–915. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. Passivity and pitting of carbon steel in chromate solutions. Electrochim. Acta 1999, 44, 4795–4804. [Google Scholar] [CrossRef]
- Almeraya, C.F.; Estupiñán, F.; Zambrano, R.P.; Martínez Villafañe, A.; Borunda, T.A.; Colás, R.; Gaona-Tiburcio, C. Análisis de los transitorios de ruido electroquímico para aceros inoxidables 316 Y – DUPLEX 2205 en NaCl Y FeCl (·) Electrochemical noise transient analysis for 316 and Duplex 2205 stainless steels in NaCl and FeCl. Rev. Metal. 2012, 48, 147–156. [Google Scholar] [CrossRef]
- Cuevas, C.; Brito, M.L.; Ocampo, A.M.-; Serna, S.; Torres, A. Analysis of Electrochemical Impedance and Noise Data for AISI-310 Exposed to Lithium Bromide Solution. Int. J. Electrochem. Sci. 2013, 8, 9593–9606. [Google Scholar]
- Burstein, G.T.; Souto, R.M. Observations of localised instability of passive titanium in chloride solution. Electrochem. Commun. 1995, 40, 1881–1888. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Guo, X.P. Initiation and repassivation of pitting corrosion of carbon steel in carbonated concrete pore solution. Corros. Sci. 2011, 53, 1322–1330. [Google Scholar] [CrossRef]
- Ramirez, A.; Gonzalez, J.; Campillo, B.; Gaona, T.; Dominguez, P.; Lezama, L.; Chacón, N.; Neri, F.; Martinez, V. An Electrochemical Study of the Corrosion Behavior of a dua-phase Steel in 0.5m H2SO4. Int. J. Electrochem. Sci. 2010, 5, 1786–1798. [Google Scholar]
- Bogaerts, J.F.; Chen, W.F. The physical meaning of noise resistance. Corros. Sci. 1995, 37, 1839–1842. [Google Scholar] [CrossRef]
- Cottis, R.A.; Turgoose, S.; Neuman, R. Corrosion Testing Made Easy: Impedance and Noise Analysis; Syrett, B.C., Ed.; NACE International: Houston, TX, USA, 1999. [Google Scholar]
- Sanchez, J.M.; Cottis, R.A. Shot noise and statistical parameters for the estimation of corrosion mechanisms. Corros. Sci. 2005, 47, 3280–3299. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, P.; Shi, X.; Nguyen, T.A.; Xiao, Z.; Wu, J. Electroless Synthesis of Ni-P and Ni-P-Zn Alloy Coatings for Protecting Steel Rebar from Chloride-Induced Corrosion. Int. J. Electrochem. Sci. 2012, 7, 8151–8169. [Google Scholar]
- Salamci, E.; Candan, S.; Kabakci, F. Effect of microstructure on corrosion behavior of dual-phase steels. Kov. Mater. 2017, 55, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.A.; Antonissen, J.; Palacio, C.A.; Grave, E. De Effects of Si as alloying element on corrosion resistance of weathering steel. Corros. Sci. 2012, 59, 198–203. [Google Scholar] [CrossRef]
- Yoo, Y.H.; Hong, J.H.; Kim, J.G.; Lee, H.Y.; Han, J.G. Effect of Si addition to CrN coatings on the corrosion resistance of CrN/stainless steel coating/substrate system in a deaerated 3.5 wt.% NaCl solution. Surf. Coat. Technol. 2007, 201, 9518–9523. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Xu, Y.; Liu, Z. Effects of Cr, Ni and Cu on the Corrosion Behavior of Low Carbon Microalloying Steel in a Cl L Containing Environment. J. Mater. Sci. Technol. 2013, 29, 168–174. [Google Scholar] [CrossRef]
- Sarkar, P.P.; Kumar, P.; Manna, M.K.; Chakraborti, P.C. Microstructural influence on the electrochemical corrosion behaviour of dual-phase steels in 3.5% NaCl solution. Mater. Lett. 2005, 59, 2488–2491. [Google Scholar] [CrossRef]
- Shahzad, M.; Tayyaba, Q.; Manzoor, T.; Ud-din, R.; Subhani, T.; Qureshi, A. The effects of martensite morphology on mechanical properties, corrosion behavior and hydrogen assisted cracking in A516 grade steel. Mater. Res. Express 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Wafaa, G.; Hussein, W.A.; Aboushahba, R.A.; Morad, M.M. The Corrosion Behavior of Ferrite Bainite dua-phase Steel in 3.5% NaCl Solution. Middle East J. Appl. Sci. 2016, 6, 627–635. [Google Scholar]
- Qu, S.; Pang, X.; Wang, Y.; Gao, K. Corrosion behavior of each phase in low carbon microalloyed ferrite – bainite dual-phase steel: Experiments and modeling. Corros. Sci. 2013, 75, 67–77. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Yang, F.; Chen, H.; Liang, N.; Wu, Y.; Zhang, J.; Ma, H.; Eyram, E.; Gao, B.; et al. Corrosion behavior and mechanism of Cr e Mo alloyed steel: Role of ferrite/bainite duplex microstructure. J. Alloys Compd. 2019, 809, 151787. [Google Scholar] [CrossRef]
- Jamali, S.S.; Mills, D.J. Progress in Organic Coatings A critical review of electrochemical noise measurement as a tool for evaluation of organic coatings. Prog. Org. Coat. 2016, 95, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento, E.; Uruchurtu, J.; Menchaca, C.; Sarmiento, O. Electrochemical Noise Analysis of Type 316L Stainless Steel in a LiBr + Ethylene Glycol + H2O Solution. Corrosion 2011, 67, 1–8. [Google Scholar] [CrossRef]
- Cottis, R.A. A practical evaluation of electrochemical noise parameters as indicators of corrosion type. Electrochim. Acta 2004, 49, 2787–2793. [Google Scholar] [CrossRef]
- Mansfeld, F.; Sun, Z. Localization index obtained from electrochemical noise analysis. Corrosion 1999, 55, 915–918. [Google Scholar] [CrossRef]
- Mansfeld, F.; Sun, Z.; Hsu, C.H. Electrochemical noise analysis (ENA) for active and passive systems in chloride media. Electrochim. Acta 2001, 46, 3651–3664. [Google Scholar] [CrossRef]
- Lara Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Cabral, M.J.A.; Estupinan, F.; Baltazar-Zamora, M.A.; Almeraya-Calderon, F. Corrosion Behaviour of 304 Austenitic, 15-5PH and 17-4PH Passive Stainless Steels in acid solutions. Int. J. Electrochem. Sci. 2018, 13, 10314–10324. [Google Scholar] [CrossRef]
- Eden, D.A.; Rothwell, A.N. Electrochemical Noise Data: Analysis Interpretation and Presentation. In Proceedings of Corrosion # 92; NACE: Huoston, TX, USA, 1992; p. 292. [Google Scholar]
- Lara, M.; Gaona, C.; Zambrano, P.; Delgado, M.; Cabral, J.; Nieves, D.; Maldonado, E.; Estupiñan, F.; Chacón, J.; Almeraya, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using. Materials 2020, 12, 2836. [Google Scholar] [CrossRef] [PubMed]
- Nagiub, A.M. Comparative Electrochemical Noise Study of the Corrosion of Different Alloys Exposed to Chloride Media. Engineering 2014, 6, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Legat, A.; Dolecek, V. Corrosion Monitoring System Based on Measurement and Analysis of Electrochemical Noise. Corrosion 1995, 51, 295–300. [Google Scholar] [CrossRef]
- Legat, A. Chaotic Analysis of Electrochemical Noise Measured on Stainless Steel. Electrochim. Acta 1995, 142, 1851–1858. [Google Scholar] [CrossRef]
- Fukuda, T. The evaluation of pitting corrosion from the spectrum slope of noise fluctuation 304 stainless steel electrodes. Corros. Sci. 1996, 38, 1085–1091. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Montoya, -R.M.; Garza Montes de Oca, N.; Castorena, G.H.J.; Estupiñan, L.F.; Cabral, M.J.; Maldonado, B.E.; Gaona-Tiburcio, C. Corrosion behavior of multilayer coatings deposited by PVD on Inconel 718 in Chloride and Sulphuric Acid solutions. Int. J. Electrochem. Sci. 2019, 14, 9596–9609. [Google Scholar] [CrossRef]
- He, L.; Jiang, Y.; Guo, Y.; Wu, X.; Li, J.; He, L.; Jiang, Y.; Guo, Y.; Wu, X.; Li, J. Electrochemical noise analysis of duplex stainless steel 2101 exposed to different corrosive solutions. Corros. Eng. Sci. Technol. 2016, 51, 187–194. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, M.; Xiong, Z.; Du, Y.; Qiao, Z.; Zhang, H. Development of Low Carbon Niobium Bearing High Strength F-B dua-phase Steel with High Hole Expansion Property. In Proceedings of the HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015, Hangzhou, China, 11–13 November 2015; pp. 677–681. [Google Scholar] [CrossRef]
- Basantia, S.K.; Bhattacharya, A.; Khutia, N.; Das, D. Plastic Behavior of Ferrite–Pearlite, Ferrite–Bainite and Ferrite–Martensite Steels: Experiments and Micromechanical Modelling. Met. Mater. Int. 2019, 1–19. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Ponge, D.; Raabe, D. Effect of grain refinement to 1μm on strength and toughness of dual-phase steels. Mater. Sci. Eng. A 2010, 527, 7832–7840. [Google Scholar] [CrossRef]


| AHSS | Fe | C | Mn | Cr | Ni | Mo | Si | P | Ti | Nb |
|---|---|---|---|---|---|---|---|---|---|---|
| DP590 | Bal. | 0.09 | 1.20 | 0.440 | 0.069 | 0.039 | 0.330 | 0.022 | - | - |
| DP780 | Bal. | 0.10 | 2.61 | 0.420 | - | - | 0.510 | - | 0.080 | - |
| FB590 | Bal. | 0.09 | 1.51 | 0.022 | - | - | 0.290 | - | - | 0.043 |
| FB780 | Bal. | 0.09 | 1.73 | 0.640 | - | 0.006 | 0.300 | - | 0.021 | - |
| AHSS | Solution | Rn Ω·cm2 | Localization Index | Type Corrosion | Skewness (i) | Kurtosis (i) | |||
|---|---|---|---|---|---|---|---|---|---|
| DP | 590 | NaCl | 156 | 0.00472 | uniform | −1.14 | 37.15 | −10.79 | −7.18 |
| CaCl2 | 349 | 0.15431 | Localized | 7.35 | 74.84 | −9.73 | −8.80 | ||
| MgCl2 | 421 | 0.00054 | uniform | 0.22 | 3.71 | −2.49 | −0.82 | ||
| 780 | NaCl | 353 | 0.02445 | Mixed | 0.13 | 3.47 | −9.34 | −4.56 | |
| CaCl2 | 120 | 0.00853 | uniform | −2.23 | 42.23 | −8.94 | −5.72 | ||
| MgCl2 | 422 | 0.14547 | Localized | −2.99 | 19.62 | −7.25 | −2.41 | ||
| FB | 590 | NaCl | 291 | 0.00355 | uniform | 0.16 | 3.58 | −3.37 | −2.85 |
| CaCl2 | 1686 | 0.00201 | uniform | 0.14 | 3.22 | −4.62 | −2.32 | ||
| MgCl2 | 425 | 0.00383 | uniform | −0.5 | 4.98 | −5.42 | −5.49 | ||
| FB | 780 | NaCl | 426 | 0.00698 | uniform | −6.18 | 106.52 | −7.17 | −2.74 |
| CaCl2 | 1622 | 0.00159 | uniform | −0.07 | 2.28 | −3.77 | −2.59 | ||
| MgCl2 | 196 | 0.00837 | uniform | 0.42 | 3.65 | −10.47 | −6.33 | ||
| Property | DP590 | DP780 | FB590 | FB780 |
|---|---|---|---|---|
| Vickers hardness number, HVN | 182 | 303 | 184 | 258 |
| Ultimate tensile strength, UTS (MPa) | 589 | 961 | 594 | 816 |
| Type of Corrosion | Slope | |||
|---|---|---|---|---|
| (dB[V]/decade) | (dB[A]/decade) | |||
| Max | Min | Max | Min | |
| Passivation | −15 | −25 | 1 | −1 |
| Pitting | −20 | −25 | −7 | −14 |
| Uniform | 0 | −7 | 0 | −7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Rangel, M.; Garza-Montes de Oca, N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions. Metals 2020, 10, 1232. https://doi.org/10.3390/met10091232
Montoya-Rangel M, Garza-Montes de Oca N, Gaona-Tiburcio C, Colás R, Cabral-Miramontes J, Nieves-Mendoza D, Maldonado-Bandala E, Chacón-Nava J, Almeraya-Calderón F. Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions. Metals. 2020; 10(9):1232. https://doi.org/10.3390/met10091232
Chicago/Turabian StyleMontoya-Rangel, Marvin, Nelson Garza-Montes de Oca, Citlalli Gaona-Tiburcio, Rafael Colás, José Cabral-Miramontes, Demetrio Nieves-Mendoza, Erick Maldonado-Bandala, José Chacón-Nava, and Facundo Almeraya-Calderón. 2020. "Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions" Metals 10, no. 9: 1232. https://doi.org/10.3390/met10091232
APA StyleMontoya-Rangel, M., Garza-Montes de Oca, N., Gaona-Tiburcio, C., Colás, R., Cabral-Miramontes, J., Nieves-Mendoza, D., Maldonado-Bandala, E., Chacón-Nava, J., & Almeraya-Calderón, F. (2020). Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions. Metals, 10(9), 1232. https://doi.org/10.3390/met10091232









