Bio-Oxidation of a Double Refractory Gold Ore and Investigation of Preg-Robbing of Gold from Thiourea Solution
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Microorganisms
2.3. Bio-Oxidation Experiments
2.4. Preg-Robbing Experiments
2.5. Thiourea Leaching Experiments
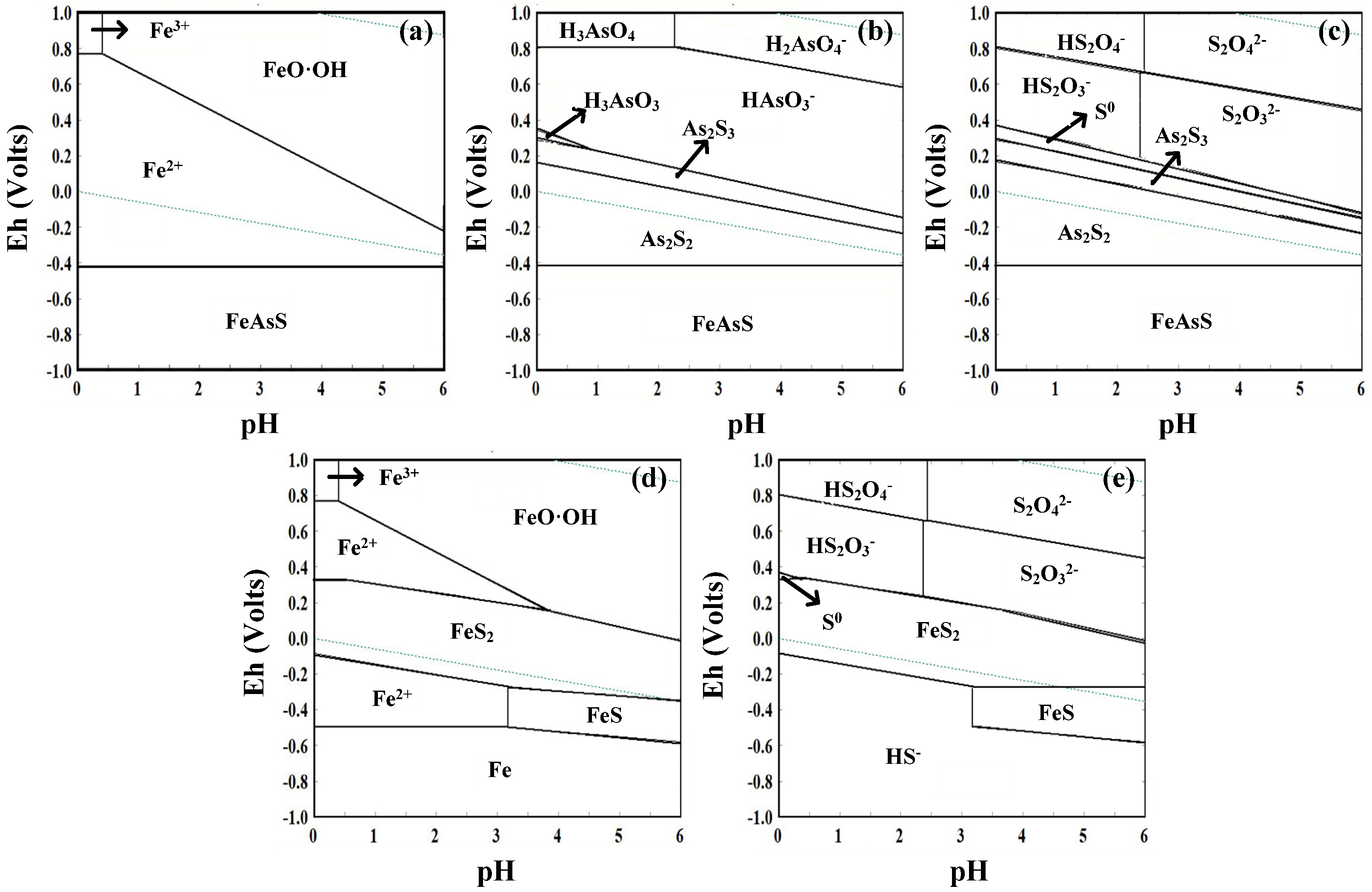
2.6. Thermodynamic Calculation
2.7. Analysis Methods
3. Results and Discussion
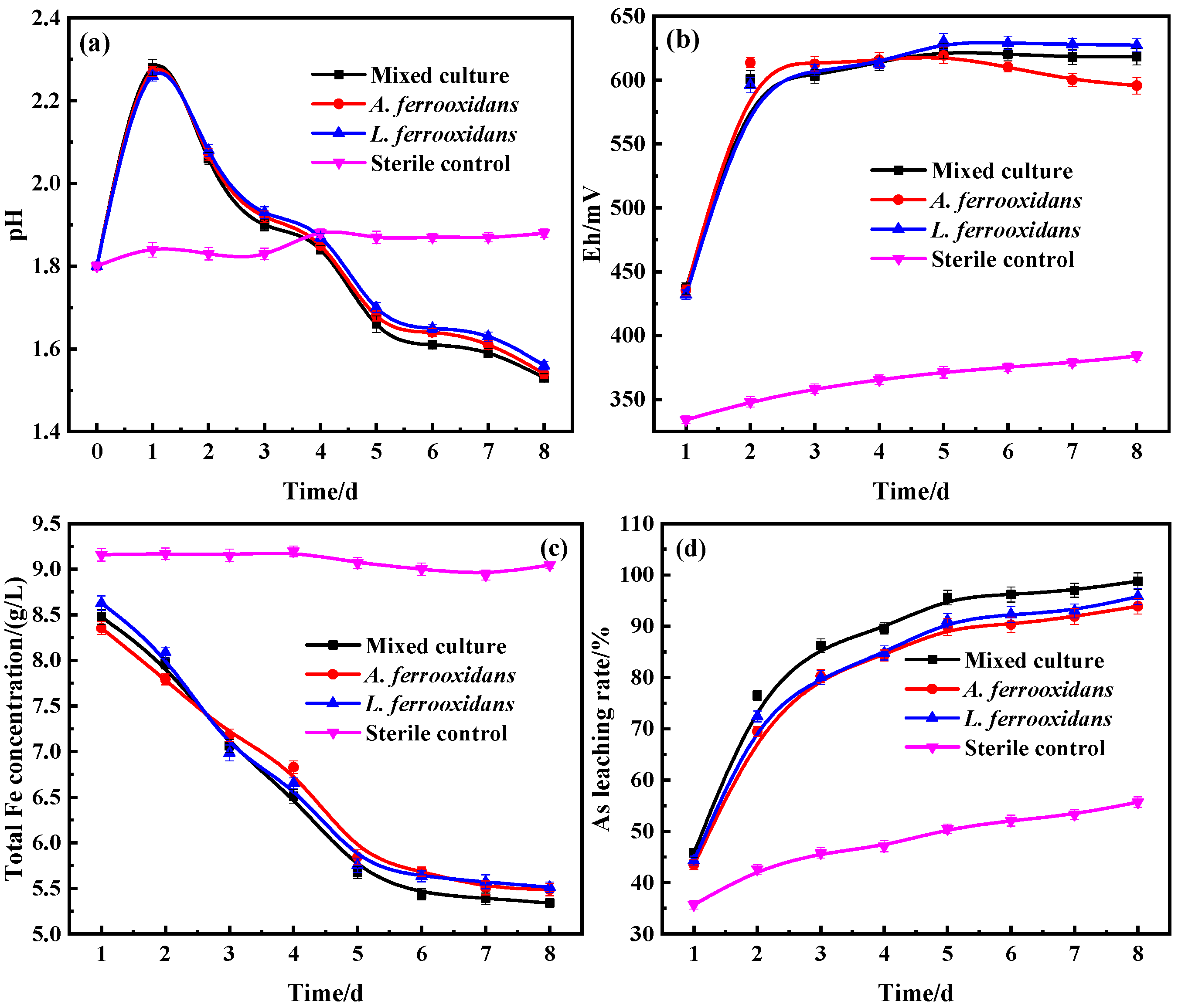
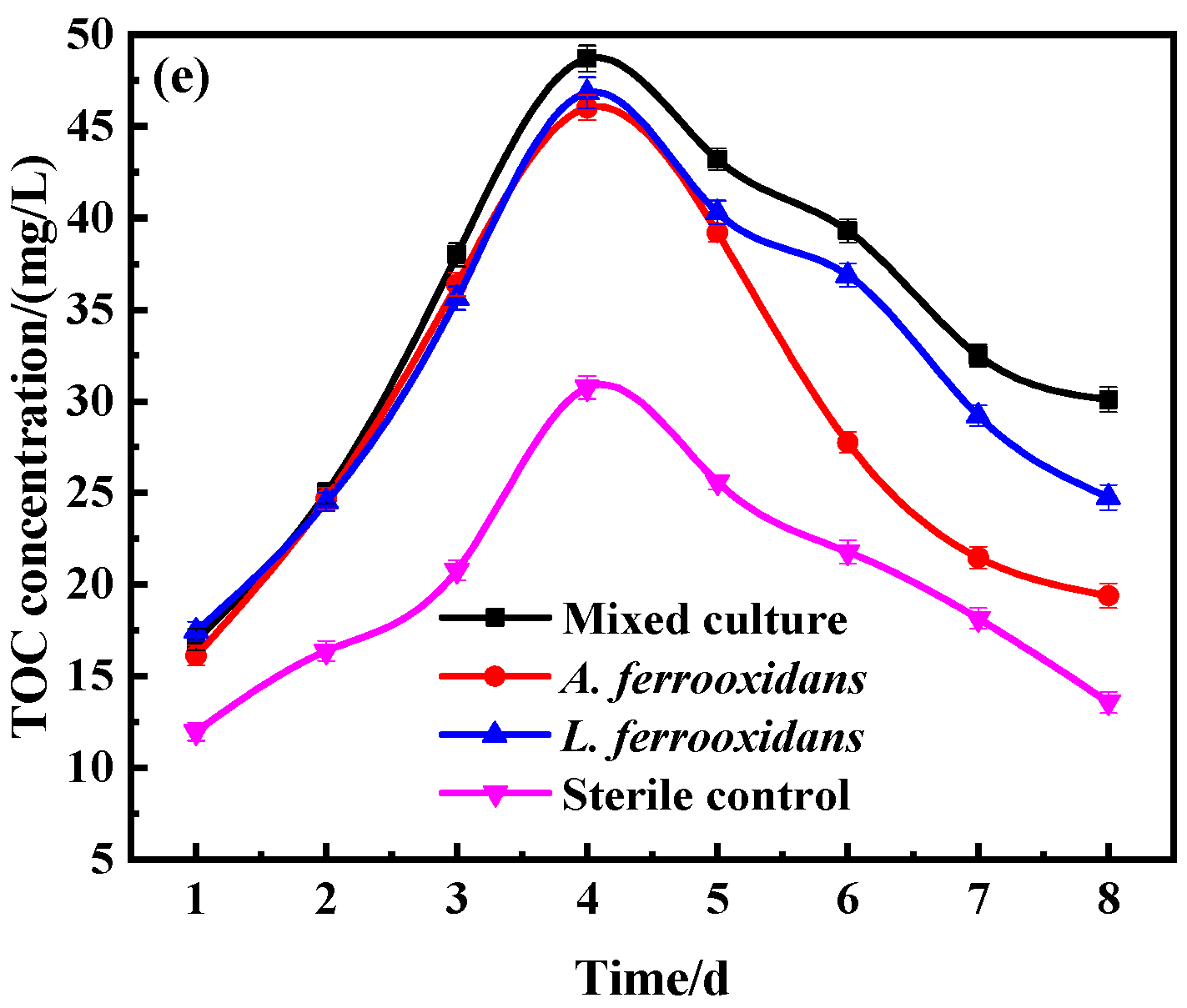
3.1. Bio-Oxidation Behavior of DRGO
3.2. Characterization of DRGO
3.2.1. Mineralogical Composition Analysis by XRD
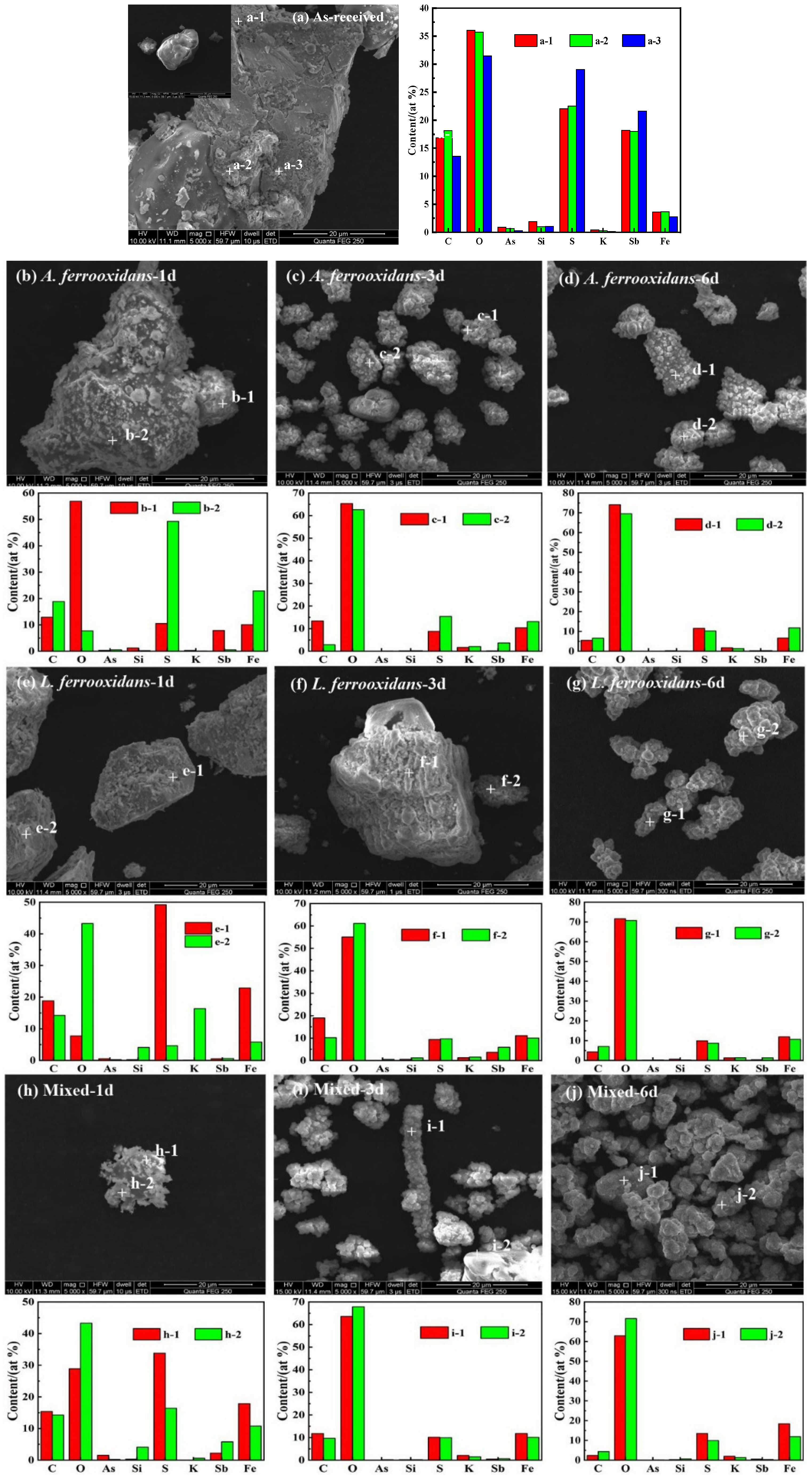
3.2.2. Morphology Analysis by SEM-EDS
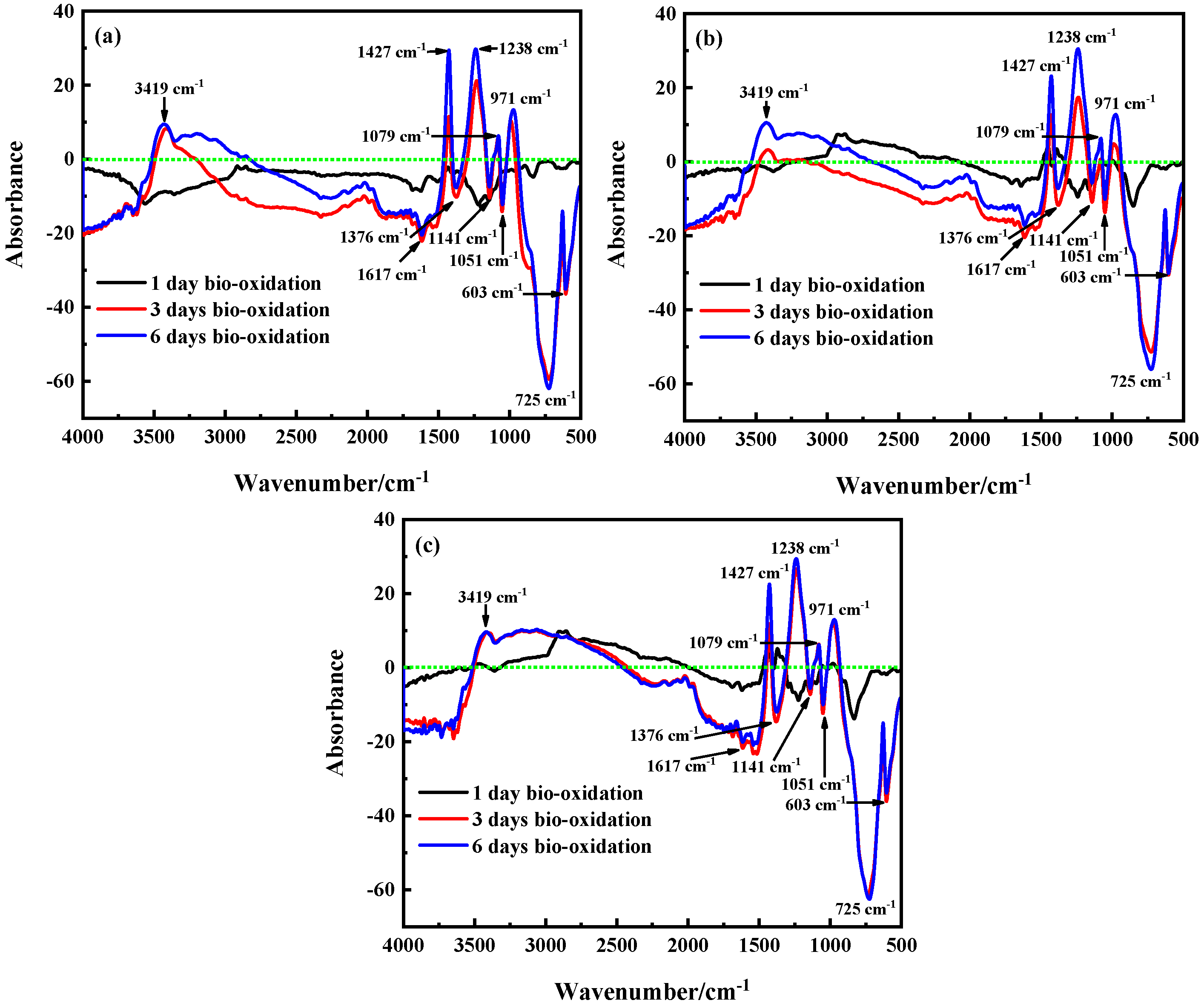
3.2.3. Chemical Bonds and Functional Groups Analysis by FTIR
3.3. The Process of Sulfides Bio-Oxidation
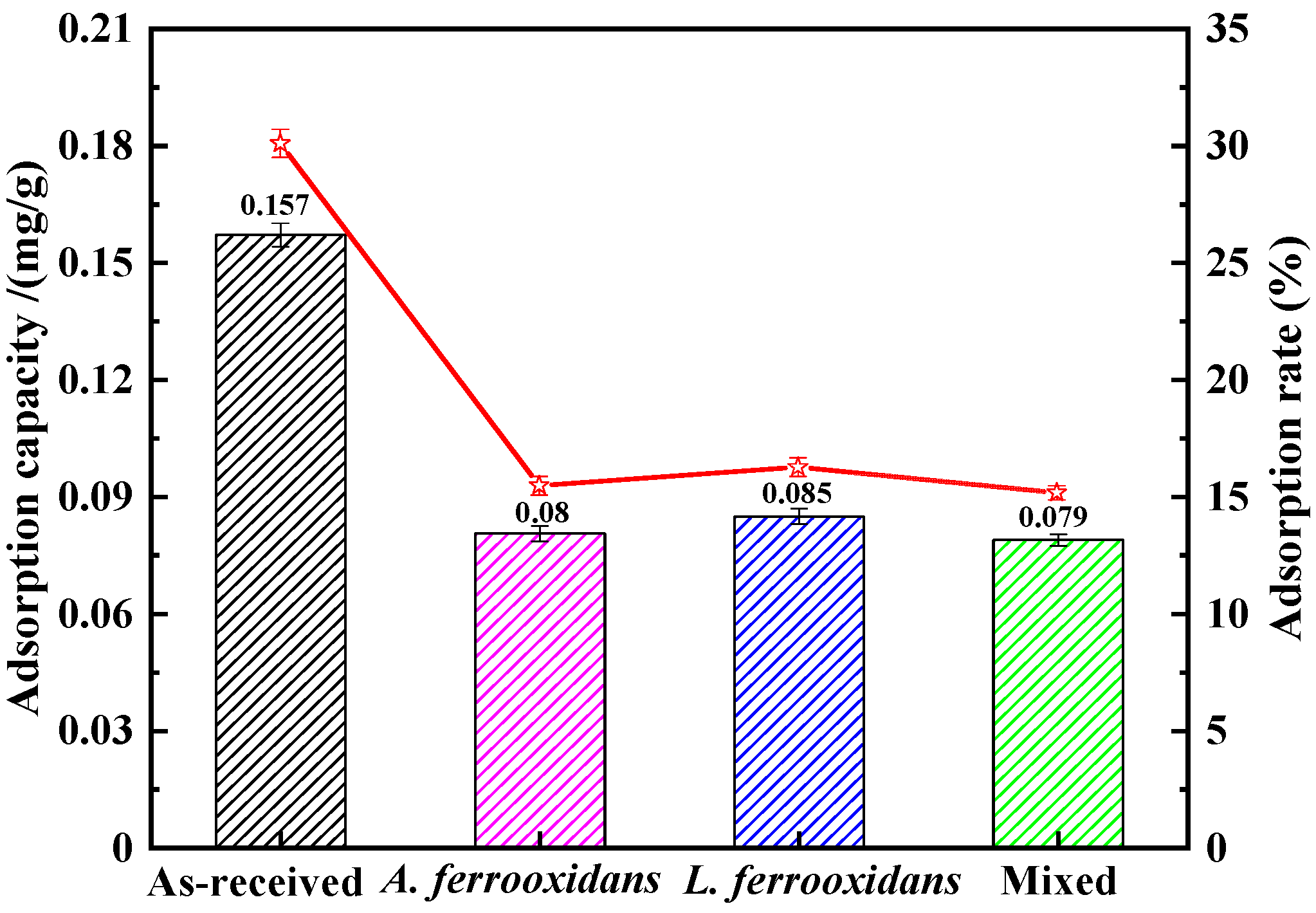
3.4. Preg-Robbing Studies of DRGO in Thiourea System
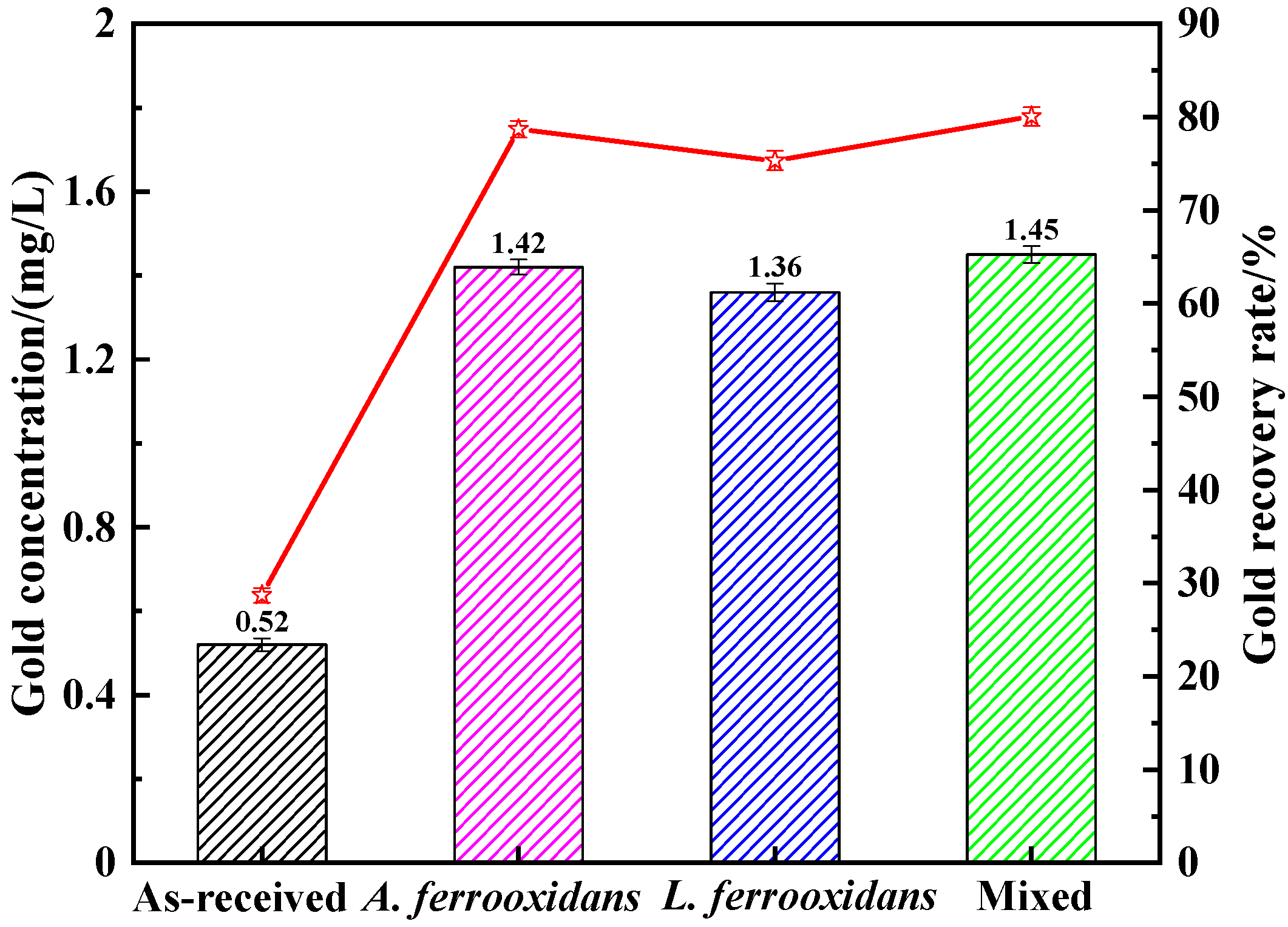
3.5. Thiourea Leaching
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Bio-Oxidation of Arsenopyrite and Pyrite | ∆G0298/(kcal/mol) | No. |
|---|---|---|
| FeAsS + 5Fe3+ + 3H2O = 6Fe2+ + H3AsO3 + S0 + 3H+ | −81.66 | 1 |
| 2FeAsS + 16Fe3+ + 9H2O = 18Fe2+ + 2HAsO3− + HS2O3− + 15H+ | −211.199 | 2 |
| 6FeAsS + 12Fe3+ = 3As2S2 + 18Fe2+ | −322.269 | 3 |
| FeAsS + 3H2O + 6Fe3+ = 7Fe2+ + S0 + HAsO3− + 5H+ | −91.489 | 4 |
| 4FeAsS + 4H2O + 12Fe3+ = As2S3 + S2− + 16Fe2+ + 2AsO2− + 8H+ | −194.989 | 5 |
| 2FeAsS + 6H2O + 10Fe3+ = S0 + 2HAsO3− + S2− + 12Fe2+ + 10H+ | −126.894 | 6 |
| 2As2S2 + 15H2O + 16Fe3+ = 4HAsO3− + 2S2− + HS2O3− + 16Fe2+ + 25H+ | −67.163 | 7 |
| As2S3 + 9H2O + 10Fe3+ = 2HAsO3− + S2− + HS2O3− + 10Fe2+ + 15H+ | −42.212 | 8 |
| FeS2 + 14Fe3+ + 8H2O = 15Fe2+ + 16H+ + 2SO42− | −134.77 | 9 |
| FeS2 + 2Fe3+ = 3Fe2+ + 2S0 | −19.164 | 10 |
| FeS2 + 3H2O + 6Fe3+ = 7Fe2+ + HS2O3− + 5H+ | −47.385 | 11 |
| S0 + 4H2O + 6Fe3+ = SO42− + 8H+ + 6Fe2+ | −57.803 | 12 |
| Fe3+ + 3H2O = Fe(OH)3 + 3H+ | 5.503 | 13 |
| 2Fe3+ + 4H2O = 2FeO·OH + 6H+ | 1.07 | 14 |
References
- Agorhom, E.A.; Skinner, W.; Zanin, M. Upgrading of low-grade gold ore samples for improved particle characterisation using Micro-CT and SEM/EDX. Adv. Powder Technol. 2012, 23, 498–508. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Perrot, F.; Morris, C.; Rea, S.; Benvie, B.; Austin, P.; Hackl, R. Evaluation of submerged bio-oxidation concept for refractory gold ores. Hydrometallurgy 2013, 141, 117–125. [Google Scholar] [CrossRef]
- Wang, G.H.; Xie, S.B.; Liu, X.X.; Wu, Y.H.; Liu, Y.J.; Zeng, T.T. Bio-oxidation of a high-sulfur and high-arsenic refractory gold concentrate using a two-stage process. Miner. Eng. 2018, 120, 94–101. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, Q.; Song, X.L.; Dong, J.K. Research status of carbonaceous matter in carbonaceous gold ores and bio-oxidation pretreatment. Trans. Nonferrous Met. Soc. China 2013, 23, 3405–3411. [Google Scholar] [CrossRef]
- Nanthakumar, B.; Pickles, C.A.; Kelebek, S. Microwave pretreatment of a double refractory gold ore. Miner. Eng. 2007, 20, 1109–1119. [Google Scholar] [CrossRef]
- Luo, W.J.; Yang, H.Y.; Jin, Z.A. Study on the Gold Recovery of Double Refractory Gold Ore Concentrate by Biological Oxidation Pretreatment. Adv. Mat. Res. 2015, 1130, 379–382. [Google Scholar] [CrossRef]
- Pyke, B.L.; Johnston, R.F.; Brooks, P. Characterisation and behaviour of carbonaceous material in a refractory gold bearing ore. Miner. Eng. 1999, 12, 851–862. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.B.; Li, Q.; Jiang, T.; Liu, S.Q.; Li, G.H. The development of an environmentally friendly leaching process of a high C, As and Sb bearing sulfide gold concentrate. Miner. Eng. 2016, 89, 138–147. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Dew, D.; du Plessis, C. Biomineralization of metal-containing ores and concentrates. Trends Biotechnol. 2003, 21, 38–44. [Google Scholar] [CrossRef]
- Brierley, C.L. Biohydrometallurgical prospects. Hydrometallurgy 2010, 104, 324–328. [Google Scholar] [CrossRef]
- Ma, S.J.; Luo, W.J.; Mo, W.; Su, X.J.; Liu, P.; Yang, J.L. Removal of arsenic and sulfur from a refractory gold concentrate by microwave heating. Miner. Eng. 2010, 23, 61–63. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Gentina, J.C.; Acevedo, F. Biooxidation of a gold concentrate in a continuous stirred tank reactor: Mathematical model and optimal configuration. Biochem. Eng. J. 2004, 19, 33–42. [Google Scholar] [CrossRef]
- Sand, W.; Gerke, T.; Hallmann, R.; Schippers, A. Sulfur chemistry, biofilm, and the (in)direct attack mechanism ? a critical evaluation of bacterial leaching. Appl. Microbiol. Biotechnol. 1995, 43, 961–966. [Google Scholar] [CrossRef]
- Glenn, J.K.; Morgan, M.A.; Mayfield, M.B.; Kuwahara, M.; Gold, M.H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem. Biophys. Res. Commun. 1983, 114, 1077–1083. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science 1983, 221, 661–663. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin Peroxidase of Phanerochaete Chrysosporium. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1988; Volume 161, pp. 238–249. [Google Scholar]
- Ofori-Sarpong, G.; Tien, M.; Osseo-Asare, K. Myco-hydrometallurgy: Coal model for potential reduction of preg-robbing capacity of carbonaceous gold ores using the fungus, Phanerochaete chrysosporium. Hydrometallurgy 2010, 102, 66–72. [Google Scholar] [CrossRef]
- Ofori-Sarpong, G.; Osseo-Asare, K. Preg-robbing of gold from cyanide and non-cyanide complexes: Effect of fungi pretreatment of carbonaceous matter. Int. J. Miner. Process. 2013, 119, 27–33. [Google Scholar] [CrossRef]
- Amankwah, R.K.; Yen, W.T.; Ramsay, J.A. A two-stage bacterial pretreatment process for double refractory gold ores. Miner. Eng. 2005, 18, 103–108. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Ni, Y.; Yang, X.; Li, H. Bioleaching of arsenic from medicinal realgar by pure and mixed cultures. Process Biochem. 2007, 42, 1265–1271. [Google Scholar] [CrossRef]
- Akcil, A.; Ciftci, H.; Deveci, H. Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate. Miner. Eng. 2007, 20, 310–318. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, M.H.; Park, H.J.; Lee, J.U. Bioleaching of arsenic and heavy metals from mine tailings by pure and mixed cultures of Acidithiobacillus spp. J. Ind. Eng. Chem. 2015, 21, 451–458. [Google Scholar] [CrossRef]
- Anderson, C.G. Alkaline sulfide gold leaching kinetics. Miner. Eng. 2016, 92, 248–256. [Google Scholar] [CrossRef]
- Sun, C.B.; Zhang, X.L.; Kou, J.; Xing, Y. A review of gold extraction using noncyanide lixiviants: Fundamentals, advancements, and challenges toward alkaline sulfur-containing leaching agents. Int. J. Miner. Metall. Mater. 2020, 27, 417–431. [Google Scholar] [CrossRef]
- Zhou, H.; Song, Y.S.; Li, W.J.; Song, K. Electrochemical behavior of gold and its associated minerals in alkaline thiourea solutions. Int. J. Miner. Metall. Mater. 2018, 25, 737–743. [Google Scholar] [CrossRef]
- Syna, N.; Valix, M. Assessing the potential of activated bagasse as gold adsorbent for gold–thiourea. Miner. Eng. 2003, 16, 511–518. [Google Scholar] [CrossRef]
- Chen, C.K.; Lung, T.N.; Wan, C.C. A study of the leaching of gold and silver by acidothioureation. Hydrometallurgy 1980, 5, 207–212. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Yannopoulos, J.C. The Extractive Metallurgy of Gold; Springer: New York, NY, USA, 1991. [Google Scholar]
- Li, J.S.; Miller, J.D. Reaction kinetics of gold dissolution in acid thiourea solution using ferric sulfate as oxidant. Hydrometallurgy 2007, 89, 279–288. [Google Scholar] [CrossRef]
- Gallagher, N.P.; Hendrix, J.L.; Milosavljevic, E.B.; Nelson, J.H.; Solujic, L. Affinity of activated carbon towards some gold(I) complexes. Hydrometallurgy 1990, 25, 305–316. [Google Scholar] [CrossRef]
- Nakbanpote, W.; Thiravetyan, P.; Kalambaheti, C. Preconcentration of gold by rice husk ash. Miner. Eng. 2000, 13, 391–400. [Google Scholar] [CrossRef]
- Konadu, K.T.; Huddy, R.J.; Harrison, S.T.L.; Osseo-Asare, K.; Sasaki, K. Sequential pretreatment of double refractory gold ore (DRGO) with a thermophilic iron oxidizing archeaon and fungal crude enzymes. Miner. Eng. 2019, 138, 86–94. [Google Scholar] [CrossRef]
- Guo, Y.J.; Guo, X.; Wu, H.Y.; Li, S.P.; Wang, G.H.; Liu, X.X.; Qiu, G.Z.; Wang, D.Z. A novel bio-oxidation and two-step thiourea leaching method applied to a refractory gold concentrate. Hydrometallurgy 2017, 171, 213–221. [Google Scholar] [CrossRef]
- Kirby, C.S.; Thomas, H.M.; Southam, G.; Donald, R. Relative contributions of abiotic and biological factors in Fe(II) oxidation in mine drainage. Appl. Geochem. 1999, 14, 511–530. [Google Scholar] [CrossRef]
- Schippers, A.; Sand, W. Bacterial Leaching of Metal Sulfides Proceeds by Two Indirect Mechanisms via Thiosulfate or via Polysulfides and Sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Nazari, B.; Jorjani, E.; Hani, H.; Manafi, Z.; Riahi, A. Formation of jarosite and its effect on important ions for Acidithiobacillus ferrooxidans bacteria. Trans. Nonferrous Met. Soc. China 2014, 24, 1152–1160. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Licheri, C.; Atzei, D.; Loi, G.; Elsener, B.; Rossi, G.; Rossi, A. Arsenopyrite and pyrite bioleaching: Evidence from XPS, XRD and ICP techniques. Anal. Bioanal. Chem. 2011, 401, 2237–2248. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Wincott, P.L.; Lloyd, J.R.; Vaughan, D.J. The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans. Geochim. Cosmochim. Acta 2008, 72, 5616–5633. [Google Scholar] [CrossRef]
- Wang, H.M.; Bigham, J.M.; Jones, F.S.; Tuovinen, O.H. Synthesis and properties of ammoniojarosites prepared with iron-oxidizing acidophilic microorganisms at 22–65 degrees C. Geochim. Cosmochim. Acta 2007, 71, 155–164. [Google Scholar] [CrossRef]
- Marchevsky, N.; Barroso Quiroga, M.M.; Giaveno, A.; Donati, E. Microbial oxidation of refractory gold sulfide concentrate by a native consortium. Trans. Nonferrous Met. Soc. China 2017, 27, 1143–1149. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, X.D.; Chen, A.J.; Liu, H.W.; Yin, H.Q.; Qiu, G.Z.; Hao, X.D.; Liang, Y.L. Comparative study on chalcopyrite bioleaching with assistance of different carbon materials by mixed moderate thermophiles. Trans. Nonferrous Met. Soc. China 2019, 29, 1294–1303. [Google Scholar] [CrossRef]
- Nguyen, T.; Martin, J.; Byrd, E.; Embree, N. Relating laboratory and outdoor exposure of coatings: II. J. Coat. Technol. 2002, 74, 65–80. [Google Scholar] [CrossRef]
- Martin, J.W.; Nguyen, T.; Byrd, E.; Dickens, B.; Embree, N. Relating laboratory and outdoor exposures of acrylic melamine coatings: I. Cumulative damage model and laboratory exposure apparatus. Polym. Degrad. Stab. 2002, 75, 193–210. [Google Scholar] [CrossRef]
- Mazzetti, L.; Thistlethwaite, P.J. Raman spectra and thermal transformations of ferrihydrite and schwertmannite. J. Raman Spectrosc. 2002, 33, 104–111. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and Infrared Spectra of Carbonates of Calcite Structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Lazaroff, N.; Melanson, L.; Lewis, E.; NicholasSantoro; CurtPueschel. Scanning electron microscopy and infrared spectroscopy of iron sediments formed by Thiobacillus ferrooxidans. Geomicrobiol. J. 1985, 4, 231–268. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. The Kinetics of Calcite Dissolution in Acetic Acid Solutions. Chem. Eng. Sci. 1998, 53, 3863–3874. [Google Scholar] [CrossRef]
- Painter, P.C.; Starsinic, M.; Squires, E.; Davis, A.A. Concerning the 1600 cm−1 region in the i.r. spectrum of coal. Fuel 1983, 62, 742–744. [Google Scholar] [CrossRef]
- Sakthivel, R.; Das, B.; Satpati, B.; Mishra, B.K. Gold supported iron oxide-hydroxide derived from iron ore tailings for CO oxidation. Appl. Surf. Sci. 2009, 255, 6577–6581. [Google Scholar] [CrossRef]
- Ofori-Sarpong, G.; Amankwah, R.K.; Osseo-Asare, K. Reduction of preg-robbing by biomodified carbonaceous matter—A proposed mechanism. Miner. Eng. 2013, 42, 29–35. [Google Scholar] [CrossRef]
- Liu, J.Y.; Tao, X.X.; Cai, P. Study of Formation of Jarosite Mediated by Thiobacillus Ferrooxidans in 9K Medium. In Proceedings of the International Conference on Mining Science & Technology, Amsterdam, The Netherlands, 12 October 2009; Ge, S., Liu, J., Guo, C., Eds.; Elsevier Science Bv: Amsterdam, The Netherlands, 2009; Volume 1, pp. 706–712. [Google Scholar]
- Koslides, T. Pressure oxidation of arsenopyrite and pyrite in alkaline solutions. Hydrometallurgy 1992, 30, 87–106. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. The effect of sulphur species on thiosulphate leaching of gold. Miner. Eng. 2007, 20, 273–281. [Google Scholar] [CrossRef]
- Twum Konadu, K.; Sasaki, K.; Ofori-Sarpong, G.; Osseo-Asare, K.; Kaneta, T. Bio-Modification of Carbonaceous Matters in Gold Ore: Model Experiments Using Powdered Activated Charcoal and Cell-Free Extracts of Phanerochaete chrysosporium. Hydrometallurgy 2017, 168, 76–83. [Google Scholar] [CrossRef]
- Moreira, M.T.; Feijoo, G.; Lema, J.M. Fungal Bioreactors: Applications to White-Rot Fungi. Rev. Environ. Sci. Biotechnol. 2003, 2, 247–259. [Google Scholar] [CrossRef]
- Li, Q.; Shen, H.; Xu, R.; Zhang, Y.; Yang, Y.B.; Xu, B.; Jiang, T.; Yin, H.Q. Effect of Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans on preg-robbing of gold by graphite from thiourea leaching solution. J. Clean. Prod. 2020, 261, 9. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Tributsch, H.; Hansford, G.S. Reasons why ‘Leptospirillum’-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for the biooxidation of pyrite and related ores. Microbiol. Read. 1999, 145, 5–13. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron X-ray photoelectron spectroscopic study of the chalcopyrite leached by moderate thermophiles and mesophiles. Miner. Eng. 2014, 69, 185–195. [Google Scholar] [CrossRef]
- Falco, L.; Pogliani, C.; Curutchet, G.; Donati, E. A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 2003, 71, 31–36. [Google Scholar] [CrossRef]

| Constituent | Au * | C | As | Fe | SiO2 | CaO | MgO | S | ||||||||||
| Content/wt% | 18.05 | 6.95 | 1.62 | 19.6 | 27.68 | 4.26 | 2.00 | 20.76 | ||||||||||
| Phase of C | Elemental carbon | Organic carbon | Carbonate | Total | ||||||||||||||
| Content/wt% | 4.74 | 0.50 | 1.71 | 6.95 | ||||||||||||||
| Distribution/% | 68.20 | 7.20 | 24.60 | 100.00 | ||||||||||||||
| Phase of Au | Exposed gold | Encapsulated in arsenopyrite | Encapsulated in pyrite | Encapsulated in oxides | Encapsulated in silicates | Total | ||||||||||||
| Content * | 0.31 | 12.07 | 3.69 | 1.87 | 0.11 | 18.05 | ||||||||||||
| Distribution/% | 1.72 | 66.87 | 20.44 | 10.36 | 0.61 | 100.00 | ||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Li, Q.; Meng, F.; Yang, Y.; Xu, B.; Yin, H.; Jiang, T. Bio-Oxidation of a Double Refractory Gold Ore and Investigation of Preg-Robbing of Gold from Thiourea Solution. Metals 2020, 10, 1216. https://doi.org/10.3390/met10091216
Xu R, Li Q, Meng F, Yang Y, Xu B, Yin H, Jiang T. Bio-Oxidation of a Double Refractory Gold Ore and Investigation of Preg-Robbing of Gold from Thiourea Solution. Metals. 2020; 10(9):1216. https://doi.org/10.3390/met10091216
Chicago/Turabian StyleXu, Rui, Qian Li, Feiyu Meng, Yongbin Yang, Bin Xu, Huaqun Yin, and Tao Jiang. 2020. "Bio-Oxidation of a Double Refractory Gold Ore and Investigation of Preg-Robbing of Gold from Thiourea Solution" Metals 10, no. 9: 1216. https://doi.org/10.3390/met10091216
APA StyleXu, R., Li, Q., Meng, F., Yang, Y., Xu, B., Yin, H., & Jiang, T. (2020). Bio-Oxidation of a Double Refractory Gold Ore and Investigation of Preg-Robbing of Gold from Thiourea Solution. Metals, 10(9), 1216. https://doi.org/10.3390/met10091216




