Tissue Integration and Biological Cellular Response of SLM-Manufactured Titanium Scaffolds
Abstract
1. Introduction
Background
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection
2.5. Eligible Studies
- (1)
- Inclusion criteria
- (2)
- Exclusion criteria
2.6. Data Extaraction and Synthesis of Results
3. Results
3.1. Study Selection
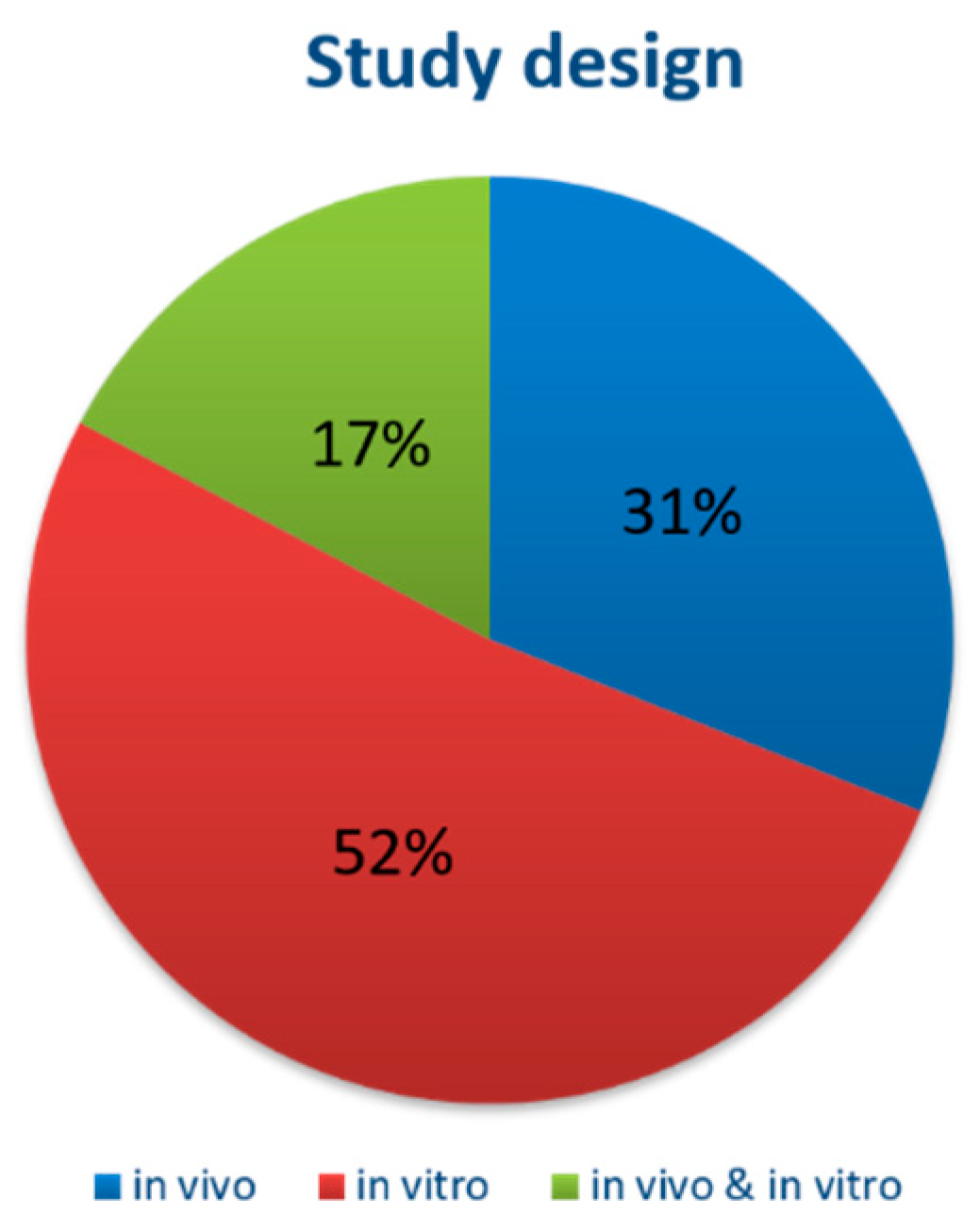
3.2. Study Characteristics
3.3. Overall Characteristics of the Included Studies
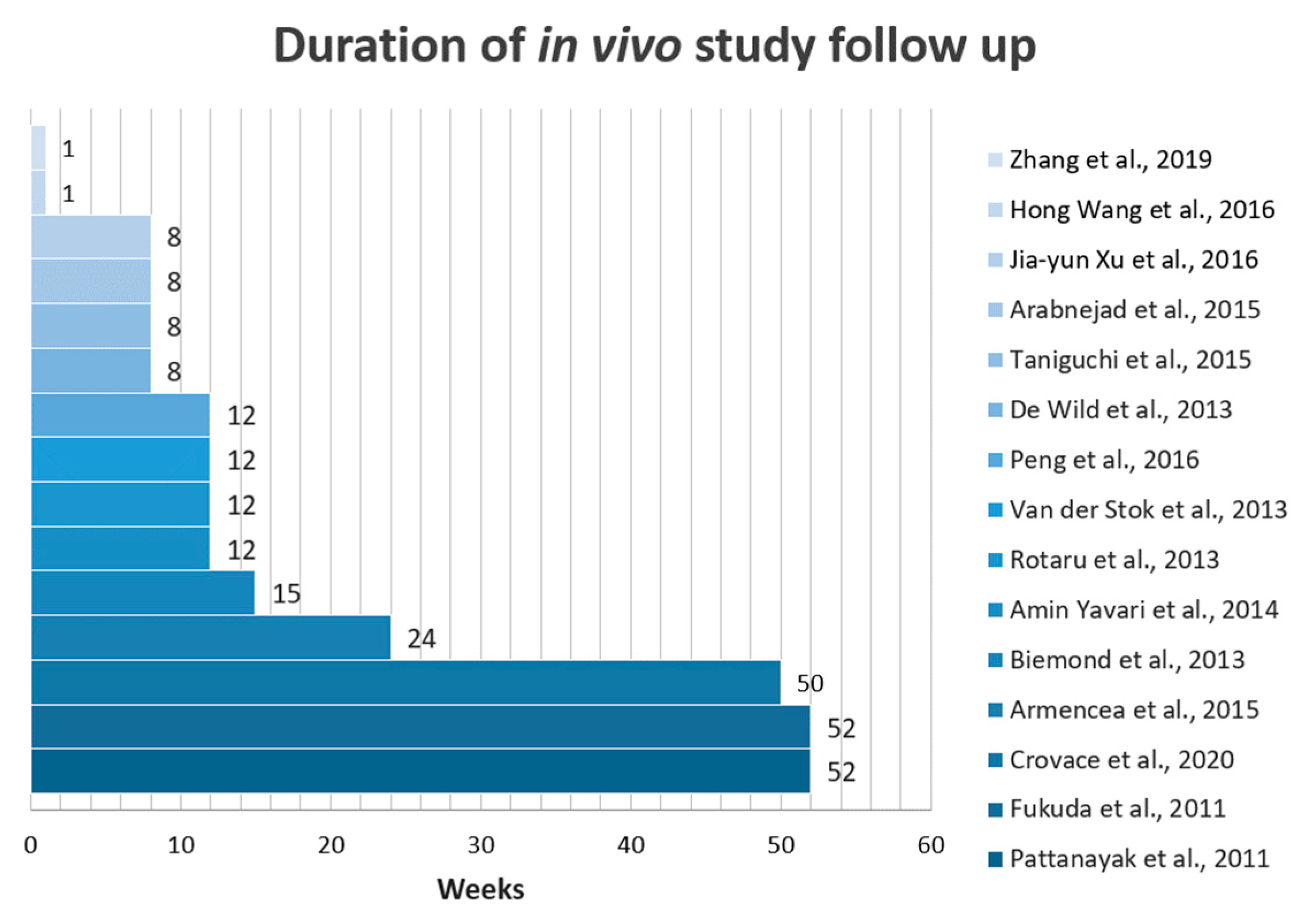
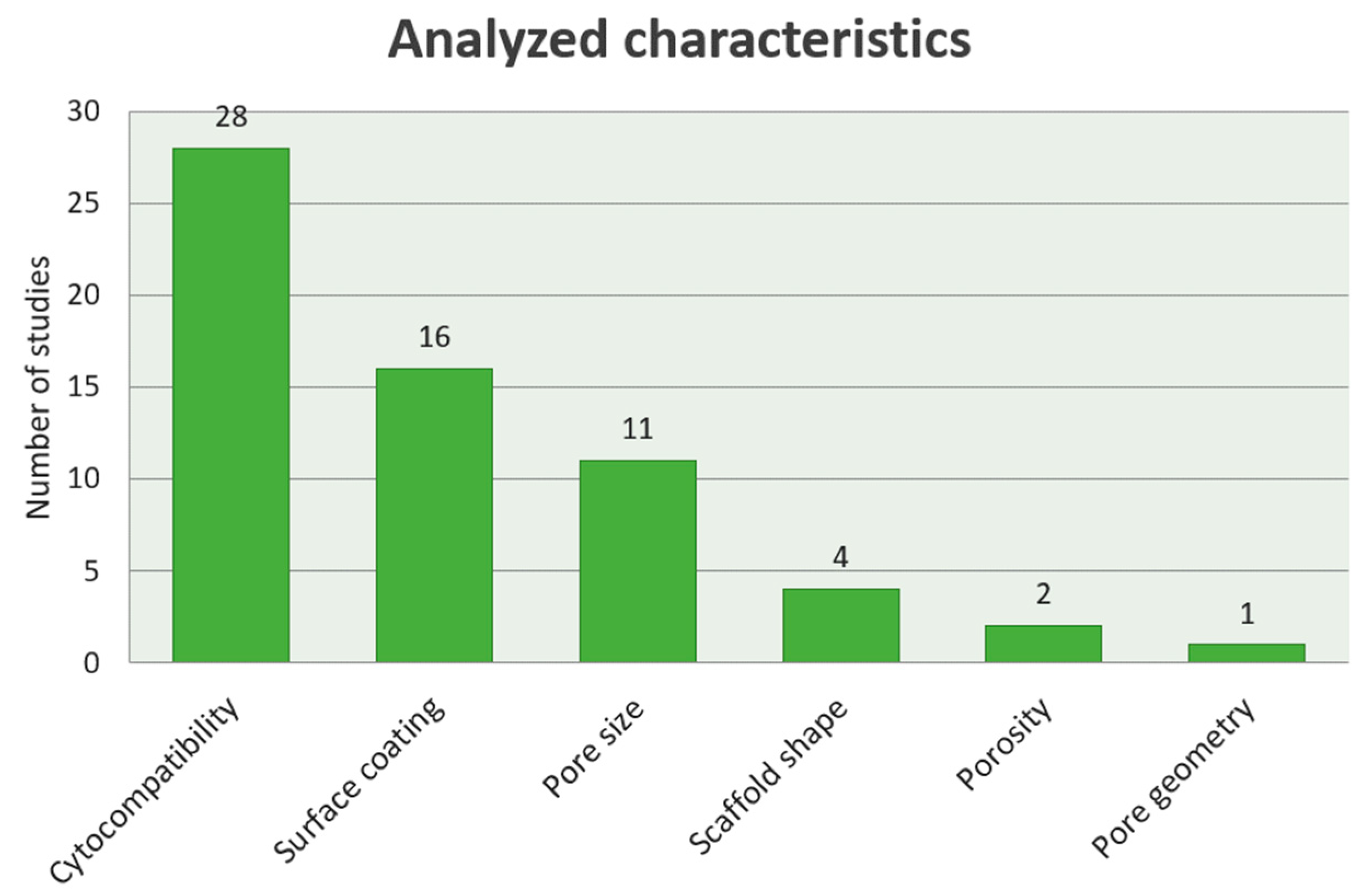
3.4. Specific Characteristics of the Studies
3.4.1. Cytocompatibility of the SLM generated Ti Scaffolds
3.4.2. Scaffold Shape Influence on Bone Ingrowth
3.4.3. Pore Size Influence on the Bone Ingrowth
3.4.4. Influence of the Porosity Degree on the Stability of the Implants
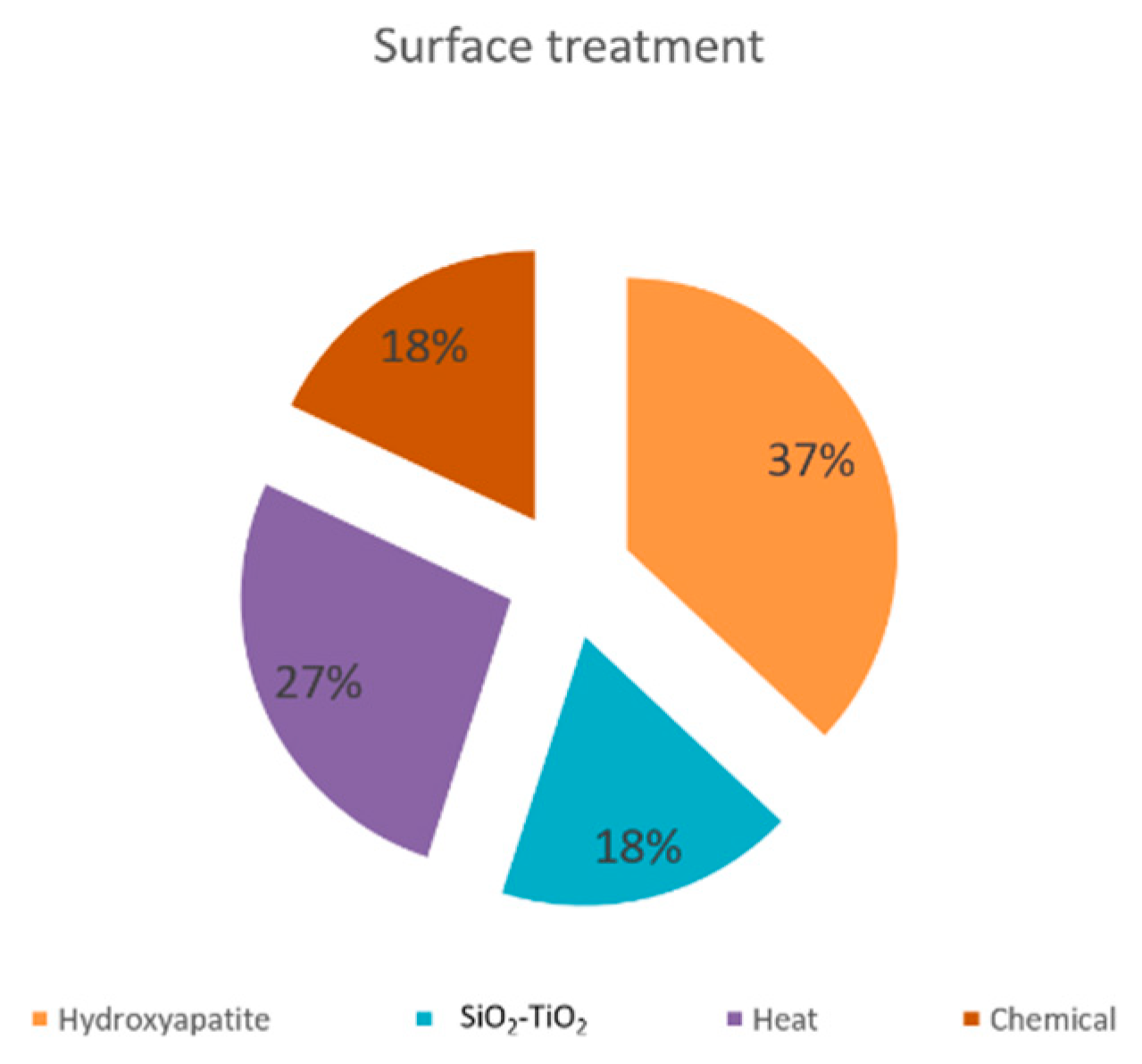
3.4.5. Surface Coating Influence on the Properties of the SLM Implants
3.4.6. Influence of Pore Geometry on Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 13, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, S.H.; Wee, H.; Roush, E.P.; Whitcomb, T.L.; Murter, C.; Kozlansky, G.; Lakhtakia, A.; Kunselman, A.R.; Donahue, H.J.; Armstrong, A.D.; et al. Time course of peri-implant bone regeneration around loaded and unloaded implants in a rat model. J. Orthop. Res. 2017, 35, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Daou, E.E. Biomaterial aspects: A key factor in the longevity of implant overdenture attachment systems. J. Int. Soc. Prev. Community Dent. 2015, 5, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Prado, R.F.D.; Esteves, G.C.; Santos, E.L.D.S.; Bueno, D.A.G.; Cairo, C.A.A.; De Vasconcellos, L.G.O.; Sagnori, R.S.; Tessarin, F.B.P.; Oliveira, F.E.; De Oliveira, L.D.; et al. In vitro and in vivo biological performance of porous Ti alloys prepared by powder metallurgy. PLoS ONE 2018, 13, e0196169. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Cohen, D.J.; Cheng, A.; Sahingur, K.; Clohessy, R.M.; Hopkins, L.B.; Boyan, B.D.; Schwartz, Z. Performance of laser sintered Ti–6Al–4V implants with bone-inspired porosity and micro/nanoscale surface roughness in the rabbit femur. Biomed. Mater. 2017, 12, 025021. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Thijs, L.; Sistiaga, M.L.M.; Wauthle, R.; Xie, Q.-G.; Kruth, J.-P.; Van Humbeeck, J. Strong morphological and crystallographic texture and resulting yield strength anisotropy in selective laser melted tantalum. Acta Mater. 2013, 61, 4657–4668. [Google Scholar] [CrossRef]
- Wysocki, B.; Idaszek, J.; Zdunek, J.; Rożniatowski, K.; Pisarek, M.; Yamamoto, A.; Święszkowski, W. The influence of selective laser melting (SLM) process parameters on in-vitro cell response. Int. J. Mol. Sci. 2018, 19, 1619. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterial 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Bačáková, L.; Filová, E.; Parizek, M.; Ruml, T.; Švorčík, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, Y.; Li, S.; Guo, S.; He, M.; Luo, K.; Lin, J. Copper-modified Ti6Al4V alloy fabricated by selective laser melting with pro-angiogenic and anti-inflammatory properties for potential guided bone regeneration applications. Mater. Sci. Eng. C 2018, 90, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Ganbold, B.; Heo, S.-J.; Koak, J.-Y.; Kim, S.-K.; Cho, J. Human stem cell responses and surface characteristics of 3D printing Co-Cr dental material. Materials 2019, 12, 3419. [Google Scholar] [CrossRef]
- Yuan, W.; He, X.; Zhou, X.; Zhu, Y. Hydroxyapatite nanoparticle-coated 3D-printed porous Ti6Al4V and CoCrMo alloy scaffolds and their biocompatibility to human osteoblasts. J. Nanosci. Nanotechnol. 2018, 18, 4360–4365. [Google Scholar] [CrossRef]
- Costa, M.; Lima, R.; Melo-Fonseca, F.; Bartolomeu, F.; Alves, N.M.; Miranda, A.; Gasik, M.; Silva, F.; Silva, N.A.; Miranda, G. Development of β-TCP-Ti6Al4V structures: Driving cellular response by modulating physical and chemical properties. Mater. Sci. Eng. C 2019, 98, 705–716. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Dourado, N.; Pereira, F.; Alves, N.; Miranda, G.; Silva, F. Additive manufactured porous biomaterials targeting orthopedic implants: A suitable combination of mechanical, physical and topological properties. Mater. Sci. Eng. C 2019, 107, 110342. [Google Scholar] [CrossRef]
- Liu, F.; Ran, Q.; Zhang, T.; Zhang, D.Z.; Su, Z. Additively Manufactured Continuous Cell-Size Gradient Porous Scaffolds: Pore Characteristics, Mechanical Properties and Biological Responses In Vitro. Materials 2020, 13, 2589. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Wally, Z.J.; Haque, A.M.; Feteira, A.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Selective laser melting processed Ti6Al4V lattices with graded porosities for dental applications. J. Mech. Behav. Biomed. Mater. 2018, 90, 20–29. [Google Scholar] [CrossRef]
- Brunette, D.; Tenvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine; Springer: Berlin, Germany, 2001. [Google Scholar]
- Hanawa, T. Titanium-tissue interface reaction and its control with surface treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Smargiassi, A.; Bertacchini, J.; Checchi, M.; Cavani, F.; Ferretti, M.; Palumbo, C. Biocompatibility analyses of Al2O3-treated titanium plates tested with osteocyte and fibroblast cell lines. Biomedicines 2017, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Wu, Z.; Qiu, G.; Wu, G.; Wang, H.; Su, X.; Yin, B.; Ma, S.; Qi, B. Selective laser sintering-produced porous titanium alloy scaffold for bone tissue engineering. Zhonghua Yi Xue Za Zhi 2014, 94, 1499–1502. [Google Scholar]
- Bunney, P.; Zink, A.; Holm, A.; Billington, C.; Kotz, C. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Hirota, M.; Shima, T.; Sato, I.; Ozawa, T.; Iwai, T.; Ametani, A.; Sato, M.; Noishiki, Y.; Ogawa, T.; Hayakawa, T.; et al. Development of a biointegrated mandibular reconstruction device consisting of bone compatible titanium fiber mesh scaffold. Biomaterials 2016, 75, 223–236. [Google Scholar] [CrossRef]
- Ghosh, S.; Abanteriba, S.; Wong, S.; Houshyar, S. Selective laser melted titanium alloys for hip implant applications: Surface modification with new method of polymer grafting. J. Mech. Behav. Biomed. Mater. 2018, 87, 312–324. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Feng, Y.; Yang, X.; Ma, Z.; Shi, L.; Ma, X.; Wang, J.; Ma, T.; Yang, Z.; et al. Evaluation of an artificial vertebral body fabricated by a tantalum-coated porous titanium scaffold for lumbar vertebral defect repair in rabbits. Sci. Rep. 2018, 8, 8927. [Google Scholar] [CrossRef]
- Crovace, A.; Lacitignola, L.; Forleo, D.M.; Staffieri, F.; Francioso, E.; Di Meo, A.; Becerra, J.; Crovace, A.; Santos-Ruiz, L. 3D biomimetic porous titanium (Ti6Al4V ELI) scaffolds for large bone critical defect reconstruction: An experimental study in sheep. Animals 2020, 10, 1389. [Google Scholar] [CrossRef]
- Major, R.; Kowalczyk, P.; Surmiak, M.; Łojszczyk, I.; Podgórski, R.; Trzaskowska, P.; Ciach, T.; Russmueller, G.; Kasperkiewicz, K.; Major, Ł.; et al. Patient specific implants for jawbone reconstruction after tumor resection. Colloids Surf. B Biointerfaces 2020, 193, 111056. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.; Xiao, C.; Zhao, W.; Wu, H.; Tang, J.; Li, Z.; Yu, S.; Li, X.; Min, L.; et al. Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect. Sci. Adv. 2019, 5, eaax6946. [Google Scholar] [CrossRef] [PubMed]
- Maroulakos, M.; Kamperos, G.; Tayebi, L.; Halazonetis, D.; Ren, Y. Applications of 3D printing on craniofacial bone repair: A systematic review. J. Dent. 2019, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, Y.; Liu, X.; Zhang, Q.; Xiong, C. Preparation and properties of a biodegradable poly(lactide-co-glycolide)/poly(trimethylene carbonate) porous composite scaffold for bone tissue engineering. New J Chem 2020, 44, 14632–14641. [Google Scholar] [CrossRef]
- Simon, M.; Lagneau, C.; Moreno, J.; Lissac, M.; Dalard, F.; Grosgogeat, B. Corrosion resistance and biocompatibility of a new porous surface for titanium implants. Eur. J. Oral Sci. 2005, 113, 537–545. [Google Scholar] [CrossRef]
- Fouda, M.F.A.; Nemat, A.; Gawish, A.; Baiuomy, A.R. Does the coating of titanium implants by hydroxyapatite affect the elaboration of free radicals. An experimental study. Aust. J. Basic Appl. Sci. 2009, 3, 1122–1129. [Google Scholar]
- Simmons, C.A.; Valiquette, N.; Pilliar, R.M. Osseointegration of sintered porous-surfaced and plasma spray-coated implants: An animal model study of early postimplantation healing response and mechanical stability. J. Biomed. Mater. Res. 1999, 47, 127–138. [Google Scholar] [CrossRef]
- Cho, S.-B.; Nakanishi, K.; Kokubo, T.; Soga, N.; Ohtsuki, C.; Nakamura, T.; Kitsugi, T.; Yamamuro, T. Dependence of apatite formation on silica gel on its structure: Effect of heat treatment. J. Am. Ceram. Soc. 1995, 78, 1769–1774. [Google Scholar] [CrossRef]
- Xue, W.; Liu, X.; Zheng, X.; Ding, C. In vivo evaluation of plasma-sprayed titanium coating after alkali modification. Biomaterial 2005, 26, 3029–3037. [Google Scholar] [CrossRef]
- Knabe, C.; Klar, F.; Fitzner, R.; Radlanski, R.; Gross, U. In vitro investigation of titanium and hydroxyapatite dental implant surfaces using a rat bone marrow stromal cell culture system. Biomaterial 2002, 23, 3235–3245. [Google Scholar] [CrossRef]
- Guo, L.; Wu, H.; Liu, X.; Zhu, Y.; Gao, J.; Guo, T. Effect of fluoride corrosion on the bonding strenght of Ti-porcelain under statis loads. Mater. Lett. 2009, 63, 2486–2488. [Google Scholar] [CrossRef]
- Katic, V.; Curkovic, L.; Bosnjak, M.U.; Peros, K.; Mandic, D.; Spalj, S. Effect of pH, fluoride and hydrofluoric acid concentration on ion release from NiTi wires with various coatings. Dent. Mater. J. 2017, 36, 149–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Bai, S.; Li, F.; Li, N.; Zhang, J.; Tian, M.; Zhang, Q.; Tong, Y.; Zhang, Z.; Wang, G.; et al. Effect of plasma nitriding and titanium nitride coating on the corrosion resistance of titanium. J. Prosthet. Dent. 2016, 116, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Miyazaki, T.; Suzuki, E.; Miyaji, T. Polishing of titanium prosthetics (Part 6). The chemical polishing baths containing hydrofluoric acid and nitric acid. Shika Zair. Kikai 1989, 8, 103–109. [Google Scholar]
- Wysocki, B.; Idaszek, J.; Buhagiar, J.; Szlązak, K.; Brynk, T.; Kurzydłowski, K.J.; Święszkowski, W. The influence of chemical polishing of titanium scaffolds on their mechanical strength and in-vitro cell response. Mater. Sci. Eng. C Mater. Boil. Appl. 2018, 95, 428–439. [Google Scholar] [CrossRef]
- Ong, J.L.; Chan, D.C.N. Hydroxyapatite and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 2000, 28, 667–707. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Boyan, B.D.; Cheng, A.; Olivares-Navarrete, R.; Schwartz, Z. Implant Surface design regulates mesenchymal stem cell differentiation and maturation. Adv. Dent. Res. 2016, 28, 10–17. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Jones, D.L.; Dudakovic, A.; Thaler, R.; Paradise, C.R.; Kremers, H.M.; Abdel, M.P.; Kakar, S.; Dietz, A.B.; Cohen, R.C.; et al. Osteogenic potential of human adipose-tissue-derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium. Gene 2016, 581, 95–106. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Zhou, Y.; Wang, W.; Cao, X. Study on topology optimization design, manufacturability, and performance evaluation of Ti-6Al-4V porous structures fabricated by selective laser melting (SLM). Materials 2017, 10, 1048. [Google Scholar]
- Attar, H.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Manufacture by selective laser melting and mechanical behavior of commercially pure titanium. Mat. Sci. Eng. 2014, 21, 170–177. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Seifi, M. Metal additive manufacturing: A review of mechanical properties. Annu. Rev. Mater. Res. 2016, 46, 151–186. [Google Scholar] [CrossRef]
- Hrabe, N.W.; Gnäupel-Herold, T.; Quinn, T. Fatigue properties of a titanium alloy (Ti–6Al–4V) fabricated via electron beam melting (EBM): Effects of internal defects and residual stress. Int. J. Fatigue 2017, 94, 202–210. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Kolla, S.; Eckert, J. Additive Manufacturing Processes: Selective Laser Melting, Electron Beam Melting and Binder Jetting—Selection Guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Eckert, J.; Prashanth, K.G. Defining the tensile properties of Al-12Si parts produced by selective laser melting. Acta Mater. 2017, 126, 25–35. [Google Scholar] [CrossRef]
- Trevisan, F.; Calignano, F.; Aversa, A.; Marchese, G.; Lombardi, M.; Biamino, S.; Ugues, D.; Manfredi, D. Additive manufacturing of titanium alloys in the biomedical field: Processes, properties and applications. J. Appl. Biomater. Funct. Mater. 2017, 16, 57–67. [Google Scholar] [CrossRef]
- Bormann, T.; Schumacher, R.; Müller, B.; Mertmann, M.; De Wild, M. Tailoring selective laser melting process parameters for NiTi implants. J. Mater. Eng. Perform. 2012, 21, 2519–2524. [Google Scholar] [CrossRef]
- Šittner, P.; Heller, L.; Pilch, J.; Curfs, C.; Alonso, T.; Favier, D.; Alonso, T. Young’s modulus of austenite and martensite phases in superelastic NiTi wires. J. Mater. Eng. Perform. 2014, 23, 2303–2314. [Google Scholar] [CrossRef]
- Vaithilingam, J.; Kilsby, S.; Goodridge, R.; Christie, S.D.; Edmondson, S.; Hague, R.J. Functionalization of Ti6Al4V components fabricated using selective laser melting with a bioactive compound. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 52–61. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2015, 34, 369–385. [Google Scholar] [CrossRef]
- Ponader, S.; Vairaktaris, E.; Heinl, P.; Wilmowsky, C.V.; Rottmair, A.; Körner, C.; Singer, R.F.; Holst, S.; Schlegel, K.A.; Neukam, F.W.; et al. Effects of topographical surface modifications of electron beam melted Ti-6Al-4V titanium on human fetal osteoblasts. J. Biomed. Mater. Res. Part A 2008, 84, 1111–1119. [Google Scholar] [CrossRef]
- Ou, S.-F.; Peng, B.-Y.; Chen, Y.-C.; Tsai, M.-H. Manufacturing and characterization of NiTi alloy with functional properties by selective laser melting. Metals 2018, 8, 342. [Google Scholar] [CrossRef]
- Yuan, B.; Zhu, M.; Chung, C. Biomedical Porous shape memory alloys for hard-tissue replacement materials. Materials 2018, 11, 1716. [Google Scholar] [CrossRef] [PubMed]
- Bormann, T.; Friess, S.; De Wild, M.; Schumacher, R.; Schulz, G.; Müller, B. Determination of strain fields in porous shape memory alloys using micro-computed tomography. In Developments in X-ray Tomography VII; Stock, S.R., Ed.; SPIE Digital Library: Bellingham, WA, USA, 2010; Volume 7804. [Google Scholar] [CrossRef]
- Sabahi, N.; Chen, W.; Wang, C.-H.; Kruzic, J.J.; Li, X. A Review on additive manufacturing of shape-memory materials for biomedical applications. JOM 2020, 72, 1229–1253. [Google Scholar] [CrossRef]
- Wang, X.; Kustov, S.; Van Humbeeck, J. A Short Review on the microstructure, transformation behavior and functional properties of NiTi shape memory alloys fabricated by selective laser melting. Materials 2018, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Khoo, Z.X.; Liu, Y.; An, J.; Chua, C.K.; Shen, Y.-F.; Kuo, C.-N. A review of selective laser melted NiTi Shape memory alloy. Materials 2018, 11, 519. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Sargeant, T.; Stupp, S.; Dunand, D.C. Porous NiTi for bone implants: A review. Acta Biomater. 2008, 4, 773–782. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhang, X.P. Gradient porosity and large pore size NiTi shape memory alloys. Scr. Mater. 2007, 57, 1020–1023. [Google Scholar] [CrossRef]
- Es-Souni, M.; Es-Souni, M.; Fischer-Brandies, H. Assessing the biocompatibility of NiTi shape memory alloys used for medical applications. Anal. Bioanal. Chem. 2005, 381, 557–567. [Google Scholar] [CrossRef]
- Saedi, S.; Saghaian, S.E.; Jahadakbar, A.; Moghaddam, N.S.; Andani, M.T.; Lu, Y.C.; Elahinia, M.; E Karaca, H.; Saghaian, S.M. Shape memory response of porous NiTi shape memory alloys fabricated by selective laser melting. J. Mater. Sci. Mater. Electron. 2018, 29, 40. [Google Scholar] [CrossRef]
- Bassani, P.; Panseri, S.; Ruffini, A.; Montesi, M.; Ghetti, M.; Zanotti, C.; Tampieri, A.; Tuissi, A. Porous NiTi shape memory alloys produced by SHS: Microstructure and biocompatibility in comparison with Ti2Ni and TiNi3. J. Mater. Sci. Mater. Electron. 2014, 25, 2277–2285. [Google Scholar] [CrossRef]
- Sillberstei, B.; Gyunther, V. Shape-memory implants in spinal surgery: Long-term results. In Shape Memory Implants; Springer: Berlin, Germany, 2000; pp. 147–152. [Google Scholar]
- Shishkovsky, A. Porous biocompatible implants and tissue scaffolds synthesized by selective laser sintering fron Ti and NiTi. J. Mster. Chem. 2008, 18, 1309–1317. [Google Scholar] [CrossRef]
- Xing, H.; Li, R.; Wei, Y.; Ying, B.; Li, D.; Qin, Y. Improved osteogenesis of selective-laser-melted titanium alloy by coating strontium-doped phosphate with high-efficiency air-plasma treatment. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- .Coskun, M.E.; Coskun, K.A.; Tutar, Y. Determination of Optimum Operation Parameters for Low-Intensity Pulsed Ultrasound and Low-Level Laser Based Treatment to Induce Proliferation of Osteoblast and Fibroblast Cells. Photomed Laser Surg 2018, 36, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Alcade, M.P.; Duarte, M.A.H.; Vasconcelos, B.C.; Tanomaru-Filho, M.; Vasconcelos, B.C.; Só, M.V.R.; Vivan, R.R. Torsional fatigue resistance of pathfinding instruments manufactured from several NiTi alloys. Int. Endod. 2018, 51, 697–704. [Google Scholar] [CrossRef]
- Zupanc, J.; Vahdat-Pajouh, N.; Schäfer, E. New thermomechanically treated NiTi alloys—A review. Int. Endod. J. 2018, 51, 1088–1103. [Google Scholar] [CrossRef]
- Pereira, E.S.J.; Peixoto, I.F.C.; Viana, A.C.D.; Oliveira, I.I.; Gonzalez, B.M.; Buono, V.T.L.; Bahia, M.G.D.A. Physical and mechanical properties of a thermomechanically treated NiTi wire used in the manufacture of rotary endodontic instruments. Int. Endod. J. 2011, 45, 469–474. [Google Scholar] [CrossRef]
- Sallica-Leva, E.; Jardini, A.; Fogagnolo, J.B. Microstructure and mechanical behavior of porous Ti–6Al–4V parts obtained by selective laser melting. J. Mech. Behav. Biomed. Mater. 2013, 26, 98–108. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.; Dalgarno, K. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Strauß, S.; Dudziak, S.; Hagemann, R.; Barcikowski, S.; Fliess, M.; Israelowitz, M.; Kracht, D.; Kuhbier, J.W.; Radtke, C.; Reimers, K.; et al. Induction of osteogenic differentiation of adipose derived stem cells by microstructured nitinol actuator-mediated mechanical stress. PLoS ONE 2012, 7, e51264. [Google Scholar] [CrossRef]
- Tsukanaka, M.; Fujibayashi, S.; Takemoto, M.; Matsushita, T.; Kokubo, T.; Nakamura, T.; Sasaki, K.; Matsuda, S. Bioactive treatment promotes osteoblast differentiation on titanium materials fabricated by selective laser melting technology. Dent. Mater. J. 2016, 35, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Yavari, S.A.; Chai, Y.; Böttger, A.J.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Effects of anodizing parameters and heat treatment on nanotopographical features, bioactivity, and cell culture response of additively manufactured porous titanium. Mater. Sci. Eng. C 2015, 51, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Markhoff, J.; Wieding, J.; Weißmann, V.; Pasold, J.; Jonitz, A.; Bader, R. Influence of different three-dimensional open porous titanium scaffold designs on human osteoblasts behavior in static and dynamic cell investigations. Materials 2015, 8, 5490–5507. [Google Scholar] [CrossRef] [PubMed]
- Nover, A.B.; Lee, S.L.; Georgescu, M.S.; Howard, D.R.; Saunders, R.A.; Yu, W.T.; Klein, R.W.; Napolitano, A.P.; Ateshian, G.A.; Hung, C.T. Porous titanium bases for osteochondral tissue engineering. Acta Biomater. 2015, 27, 286–293. [Google Scholar] [CrossRef]
- Wysocki, B.; Idaszek, J.; Szlązak, K.; Strzelczyk, K.; Brynk, T.; Kurzydłowski, K.J.; Święszkowski, W. Post processing and biological evaluation of the titanium scaffolds for bone tissue engineering. Materials 2016, 9, 197. [Google Scholar] [CrossRef]
- Fousova, M.; Vojtěch, D.; KUBÁSEK, J.; Jablonska, E.; Fojt, J. Promising characteristics of gradient porosity Ti-6Al-4V alloy prepared by SLM process. J. Mech. Behav. Biomed. Mater. 2017, 69, 368–376. [Google Scholar] [CrossRef]
- Warnke, P.H.; Douglas, T.E.L.; Wollny, P.; Sherry, E.; Steiner, M.; Galonska, S.; Becker, S.T.; Springer, I.N.; Wiltfang, J.; Sivananthan, S. Rapid prototyping: Porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng. Part C Methods 2009, 15, 115–124. [Google Scholar] [CrossRef]
- Vaithilingam, J.; Prina, E.; Goodridge, R.; Hague, R.J.; Edmondson, S.; Rose, F.R.A.J.; Christie, S.D.R. Surface chemistry of Ti6Al4V components fabricated using selective laser melting for biomedical applications. Mater. Sci. Eng. C 2016, 67, 294–303. [Google Scholar] [CrossRef]
- Matena, J.; Petersen, S.; Gieseke, M.; Kampmann, A.; Teske, M.; Beyerbach, M.; Escobar, H.M.; Haferkamp, H.; Gellrich, N.-C.; Nolte, I. SLM produced porous titanium implant improvements for enhanced vascularization and osteoblast seeding. Int. J. Mol. Sci. 2015, 16, 7478–7492. [Google Scholar] [CrossRef]
- Van Bael, S.; Chai, Y.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Biemond, J.E.; Hannink, G.; Verdonschot, N.; Buma, P. Bone ingrowth potential of electron beam and selective laser melting produced trabecular-like implant surfaces with and without a biomimetic coating. J. Mater. Sci. Mater. Electron. 2012, 24, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, D.K.; Fukuda, A.; Matsushita, T.; Takemoto, M.; Fujibayashi, S.; Sasaki, K.; Nishida, N.; Nakamura, T.; Kokubo, T. Bioactive Ti metal analogous to human cancellous bone: Fabrication by selective laser melting and chemical treatments. Acta Biomater. 2011, 7, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- De Wild, M.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.S.; Bredell, M.; Gujer, A.K.; Grätz, K.W.; Weber, F.E. Bone regeneration by the osteoconductivity of porous titanium implants manufactured by selective laser melting: A histological and micro computed tomography study in the rabbit. Tissue Eng. Part A 2013, 19, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Arabnejad, S.; Johnston, R.B.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef]
- Rotaru, H.; Armencea, G.; Spîrchez, D.; Berce, C.; Marcu, T.; Leordean, D.; Kim, S.G.; Dinu, C.; Băciuț, G.; Băciuț, M. In vivo behavior of surface modified Ti6Al7Nb alloys used in selective laser melting for custom-made implants. A preliminary study. Rom. J. Morphol. Embryol. 2013, 54, 791–796. [Google Scholar]
- Van Der Stok, J.; Van Der Jagt, O.P.; Yavari, S.A.; De Haas, M.F.P.; Waarsing, J.H.; Jahr, H.; Van Lieshout, E.M.; Patka, P.; Verhaar, J.A.N.; Zadpoor, A.A.; et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J. Orthop. Res. 2012, 31, 792–799. [Google Scholar] [CrossRef]
- Armencea, G.; Berce, C.; Rotaru, H.; Bran, S.; Leordean, D.; Coada, C.; Todea, M.; Jula, C.A.; Gheban, D.; Băciuţ, G.; et al. Micro-CT and histological analysis of Ti6Al7Nb custom made implants with hydroxyapatite and SiO2-TiO2 coatings in a rabbit model. Clujul Med. 2015, 88, 408–414. [Google Scholar] [CrossRef]
- Peng, W.; Xu, L.-W.; You, J.; Fang, L.; Zhang, Q. Selective laser melting of titanium alloy enables osseointegration of porous multi-rooted implants in a rabbit model. Biomed. Eng. Online 2016, 15, 85. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Liu, C.; Wang, C.; Tan, X.; Hu, M. A Comparison of Biocompatibility of a Titanium Alloy Fabricated by Electron Beam Melting and Selective Laser Melting. PLoS ONE 2016, 11, e0158513. [Google Scholar] [CrossRef]
- Yavari, S.A.; Van Der Stok, J.; Chai, Y.C.; Wauthle, R.; Birgani, Z.T.; Habibović, P.; Mulier, M.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Bone regeneration performance of surface-treated porous titanium. Biomaterial 2014, 35, 6172–6181. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Chen, X.-S.; Zhang, C.-Y.; Liu, Y.; Wang, J.; Deng, F.-L. Improved bioactivity of selective laser melting titanium: Surface modification with micro-/nano-textured hierarchical topography and bone regeneration performance evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Ilea, A.; Timuş, D.; Petrescu, N.B.; SoriŢău, O.; Boşca, B.A.; Mager, V.; Barbu-Tudoran, L.; Băbţan, A.M.; Câmpian, R.S.; Barabás, R. An in vitro Study on the biocompatibility of titanium implants made by selective laser melting. Biotechnol. Bioprocess Eng. 2019, 24, 782–792. [Google Scholar] [CrossRef]
- Ilea, A.; Vrabie, O.-G.; Băbțan, A.-M.; Miclăuş, V.; Ruxanda, F.; Sárközi, M.; Barbu-Tudoran, L.; Mager, V.; Berce, C.; Boșca, A.B.; et al. Osseointegration of titanium scaffolds manufactured by selective laser melting in rabbit femur defect model. J. Mater. Sci. Mater. Med. 2019, 30, 26. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Hagihara, K.; Hisamoto, K.; Sun, S.-H.; Nakano, T. Crystallographic texture control of beta-type Ti–15Mo–5Zr–3Al alloy by selective laser melting for the development of novel implants with a biocompatible low Young’s modulus. Scr. Mater. 2017, 132, 34–38. [Google Scholar] [CrossRef]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Hori, T.; Nakano, T. Microstructure of equiatomic and non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. J. Alloys Compd. 2018, 753, 412–421. [Google Scholar] [CrossRef]
- Rondelli, G.; Vicentini, B. Evaluation by electrochemical tests of the passive film stability of equiatomic Ni-Ti alloy also in presence of stress-induced martensite. J. Biomed. Mater. Res. 2000, 51, 47–54. [Google Scholar] [CrossRef]
- Nagase, T.; Hori, T.; Todai, M.; Sun, S.-H.; Nakano, T. Additive manufacturing of dense components in beta-titanium alloys with crystallographic texture from a mixture of pure metallic element powders. Mater. Des. 2019, 173, 107771. [Google Scholar] [CrossRef]
- Toffoli, A.; Parisi, L.; Bianchi, M.G.; Lumetti, S.; Bussolati, O.; Macaluso, G.M. Thermal treatment to increase titanium wettability induces selective proteins adsorption from blood serum thus affecting osteoblasts adhesion. Mater. Sci. Eng. C 2019, 107, 110250. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today BIO 2019, 3, 100024. [Google Scholar] [CrossRef] [PubMed]



| Author, Year, Reference | Study Design | Scaffold Size, Dimension | Pore Size, Architecture | Porosity | Material | Surface Treatment | Number of Probes | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Bartolomeu et al., 2020 [17] | in vitro | Ø 4.3 mm × height 4.8 mm × 5.6 | 500, 600 µm | 64–93% | Ti6Al4V | - | L929 murine fibroblast cell line | 1, 7, 14, 21, 28 days |
| Bartolomeu et al., 2020 [17] | in vitro | Ø 4.3 mm × height 4.8 mm × 5.6 | 500, 600 µm | 64–93% | Ti6Al4V | - | L929 murine fibroblast cell line | 1, 7, 14, 21, 28 days |
| Liu et al., 2020 [18] | in vitro | Ø 10 mm height × 20 mm, Ø 5 mm height × 15 mm | Gyroid, diamond | - | Ti6Al4V | - | Primary osteoblasts | 7 days |
| Hanawa et al., 2020 [22] | in vitro | Ø 6.76 height × 4.33 mm | 400–650 µm | - | Ti6Al4V | - | Primary osteoblasts | 1, 7, 14, 21, 28 days |
| Crovace et al., 2020 [30] | in vivo | Ø 40 cm × 12 mm diameter | 1.5–2.4 mm, Gyroid | - | Ti6Al4V | - | 12 sheep | 1 year |
| Zhang et al., 2019 [32] | in vitro in vivo | - | 504 µm | 65% | Ti6Al4V | - | VX2 tumor cells, L929 murine cells, Rabbits | 1, 3, 5 days, 5 weeks |
| Strauss et al., 2012 [83] | in vitro | Ø 2.5 mm × height 10 mm | - | - | TiNi (Nitinol) | - | 105 human ASCs from fat tissue/well | 24 h, 48 h, 6 weeks |
| Tsukanaka et al., 2016 [84] | in vitro | Ø 1 mm × height 14 mm × thickness 14 mm | 20–180 nm, Plate | - | CpTi | diluted HCl, alkali and heat | 105 osteoblast from neonatal mouse calvaria/12 scaffolds | 2, 7, 14 days |
| Amin Yavari et al., 2015 [85] | in vitro | disk: Ø 8 mm × height 3 mm | 500 μm, Disk | - | Ti6Al4V | 500 °C heat | 50,000 hPDC/scaffold | 21 days |
| Markhoff et al., 2015 [86] | in vitro | - | C: 700 μm, P: 400–620 μm, D: 400–1000 μm, cubic, pyramidal, diagonal | C: 51%, P: 76%, D: 75% | Ti-6Al-4V | - | 4 × 105 human, osteoblasts/scaffold | 1, 4, 8 days |
| Nover et al., 2015 [87] | in vitro | Ø 4 and 10 mm × height 7 mm | 600, 900, and 1200 μm, Cylindrical | - | commercially pure Ti | - | 30 × 106 articular cartilage harvested from adult canine knees cells/mL | 14, 28, 35 days |
| Wysocki et al., 2016 [88] | in vitro | Ø 6 mm × height 4 mm | 200, 500 and 200 + 500 μm, Cylindrical | 70% | commercially pure Ti | HF and HF-HNO3 acid solutions | 2.5 × 105 hMSCs/scaffold | 7 days |
| Fousová et al., 2017 [89] | in vitro | 2 mm | 1 and 2 mm, Rhombic dodecahedron | - | Ti-6Al-4V | - | 24,000 Human Bone Osteosarcoma Epithelial Cells/scaffold | 5 days |
| Warnke et al., 2009 [90] | in vitro | side length 50 mm | 0.45–1.2 mm, Cubic | - | Ti6Al4V | - | 105 human osteoblasts/scaffold | 6 weeks |
| Vaithilingam et al., 2016 [91] | in vitro | Ø 3 mm × height 10 mm × thickness 10 mm | Cuboidal | - | Ti6Al4V | - | indirect study: 800 μL;direct study: 80,000 immortalized NIH 3T3 mouse embryonic fibroblast cells/mL | indirect study: 72 h; direct study: 7 days |
| Matena et al., 2015 [92] | in vitro | width 3.5 mm × depth 3.5 mm × height 1.25 mm | 250 μm | - | TiAl6V4 | Polycaprolactone (PCL) | 2.5 × 104 Green Fluorescent protein (GFP)–osteoblasts/scaffold (9 scaffolds) | 7 days |
| Van Bael et al., 2012 [93] | in vitro | Ø 6 mm × height 6 mm | 500 and 1000 μm, Triangular, hexagonal, rectangular: | - | Ti6Al4V | - | 200,000 hPDC/scaffold | 14 days |
| Biemond et al., 2013 [94] | in vivo | Ø 4 mm × height 10 mm | 250–800 μm, Cylindrical | 63% | Ti6Al4V | calcium phosphate | 14 goats | 4, 15 weeks |
| Pattanayak et al., 2011 [95] | in vivo | Ø 6 mm × height 15 mm | 400–800 μm, Cubic | 55–75% | Ti6Al4V | NaOH, HCl and heat | 25 rabbits | 3, 6, 12, 26, 52 weeks |
| De Wild et al., 2013 [96] | in vivo | Ø 6 and 7.5 mm × height 3.8 mm | 700 μm, Cylindrical | 83.5% | Ti-powder | sandblasted, sandblasted and acid-etched | 5 rabbits | 8 weeks |
| Taniguchi et al., 2015 [97] | in vivo | - | 300, 600, and 900 μm diamond crystal lattice | 65% | pure Titanium powder | - | 36 rabbits | 2, 4, 8 weeks |
| Arabnejad et al., 2015 [98] | in vivo | - | 500 μm and 770 μm, tetrahedron and octet | 55.51% and 69.88% | Ti6Al4V | - | 2 dogs | 4,8 weeks |
| Rotaru et al., 2013 [99] | in vivo | Ø 5 mm × thickness 1 mm | Disc | 24–25% | Ti6Al7Nb | hydroxyapatite and SiO2–TiO2 solution | 36 rats | 1, 2, 3 months |
| Van der Stok et al., 2013 [100] | in vivo | 6 mm | 490 μm, Femur-shapoed | 88% and 68% | Ti6Al4V | chemical and heat | 27 rats | 4, 8, 12 weeks |
| Armencea et al., 2015 [101] | in vivo | Ø 3.3 mm × length 10 mm | Cylindrical screw-type | 24–25% | Ti6Al7Nb | hydroxyapatite, SiO2-TiO2 | 18 rabbits | 1, 3, 6 months |
| Peng et al., 2016 [102] | in vivo | Ø 4 mm × height 10 mm | ~290 + ~390 µm Multi-rooted implant | - | Ti6Al4V | - | 33 rabbits | 4, 8, 12 weeks |
| Hong Wang et al., 2016 [103] | in vivo + in vitro | Ø 10 mm × thickness 2 mm | Quadrate | - | Ti6Al4V | - | 4 dogs, 3 rabbits, 30 guinea pigs | 1, 3, 7 days |
| Amin Yavari et al.,2014 [104] | in vitro + in vivo | Ø 8 mm × height 3 mm | 500 μm, Disc | 88% | Ti6Al4V | acidealkali, alkalieacide heat and anodizing-heat | in vitro: 50,000 human periosteum-derived cells (hPDC)/scaffold; in vivo: 30 rats | in vitro: 1, 7, 21 days; in vivo: 12 weeks |
| Jia-yun Xu et al., 2016 [105] | in vitro + in vivo | disc:Ø 1 mm × height 10 mm × thickness 10 mm | 600 μm | - | pure Ti powder | sandblasting, anodization and alkali-heat | in vitro: 2 × 104 osteoblast from neonatal mouse calvaria; in vivo: 12 rabbits | in vitro: 1, 3, 5, 7 days; in vivo: 4, 8 weeks |
| Fukuda et al., 2011 [106] | In vitro+in vivo | Ø 3.3 mm × length 15 mm | 500, 600, 900, and 1200 μm, Cylindrical | - | Ti powder | Chemical and heat | in vitro: 10 samples of simulated body fluid; in vivo: 8 dogs | in vitro: 3–7 days; in vivo: 16, 26, or 52 weeks |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Băbțan, A.-M.; Timuș, D.; Sorițău, O.; Boșca, B.A.; Barabas, R.; Ionel, A.; Petrescu, N.B.; Feurdean, C.N.; Bordea, I.R.; Saraci, G.; et al. Tissue Integration and Biological Cellular Response of SLM-Manufactured Titanium Scaffolds. Metals 2020, 10, 1192. https://doi.org/10.3390/met10091192
Băbțan A-M, Timuș D, Sorițău O, Boșca BA, Barabas R, Ionel A, Petrescu NB, Feurdean CN, Bordea IR, Saraci G, et al. Tissue Integration and Biological Cellular Response of SLM-Manufactured Titanium Scaffolds. Metals. 2020; 10(9):1192. https://doi.org/10.3390/met10091192
Chicago/Turabian StyleBăbțan, Anida-Maria, Daniela Timuș, Olga Sorițău, Bianca Adina Boșca, Reka Barabas, Anca Ionel, Nausica Bianca Petrescu, Claudia Nicoleta Feurdean, Ioana Roxana Bordea, George Saraci, and et al. 2020. "Tissue Integration and Biological Cellular Response of SLM-Manufactured Titanium Scaffolds" Metals 10, no. 9: 1192. https://doi.org/10.3390/met10091192
APA StyleBăbțan, A.-M., Timuș, D., Sorițău, O., Boșca, B. A., Barabas, R., Ionel, A., Petrescu, N. B., Feurdean, C. N., Bordea, I. R., Saraci, G., Vesa, Ş. C., & Ilea, A. (2020). Tissue Integration and Biological Cellular Response of SLM-Manufactured Titanium Scaffolds. Metals, 10(9), 1192. https://doi.org/10.3390/met10091192











