2. Materials and Methods
Equiatomic, high-entropy alloy powders with chemical composition of CoCrFeNi (ratio of 1:1:1:1) were prepared by mechanical alloying (MA) followed by spark plasma sintering (SPS).
The CoCrFeNi alloy powders were prepared from pure elemental powders, whereas the information on the powders’ characteristics are given in
Table 1 and
Table 2. For reference, the 316 L alloy atomized powder was also milled with the same parameters. The powders were milled using a 10:1 ball-to-powder weight ratio, with 10 mm diameter balls. Each starting powder mixture weighed 30 g. Milling balls and powder mixtures were introduced into a high-carbon steel hardened steel milling container. The milling container was then sealed and flushed with Ar or N
2 gas (purity 99.998%) to limit powder oxidation during the milling process. Dry milling was performed in Pulverisette 6 ball mill (Fritsch GmbH, Germany) with a milling rate (ratational) of 300 rounds per minute. The total milling time was selected from 5–50 h for different millings. In the end of the dry-milling process, the free powders were removed from the milling bowl and stored under protective atmosphere. The scheme of the parameters pertaining to powder material preparation is presented in
Figure 1.
Additionally, empty bowls still containing part of the powders in the form of an adhesive layer stuck to its walls (
Figure 2) were filled with 200 mL of ethanol and wet-milled for 30 min. This was done to fully remove the powders stuck to the milling equipment. The extracted powders were then filtered and dried. The wet-milled powders were measured separately only to evaluate the concentration of C, N, and O. This measurement was performed to evaluate the level of contamination caused by wet milling, compared to dry-milled powders.
The “0 h of milling” condition represents the unmilled alloys, i.e., the ones that simply were not subjected to the MA process. Instead, the input powders Co, Cr, Fe, and Ni were manually mixed in an equiatomic ratio, before being inserted into the milling bowl.
MA powders were subsequently sintered by SPS dr. Sinter SPS625 in a 12-mm-diameter graphite die using a graphite foil interlayer between the powder and die to ensure proper electrical contact. The amount of used powders was calculated to obtain 5-mm-thick cylindrical samples. The sintering was performed from room temperature with a heating rate of 100 °C/min till final temperature of 900 °C with a dwell of 10 min and uniaxial pressure of 50 MPa in vacuum atmosphere. At the end of sintering, the setup was left to naturally cool down, until it was opened at ~200 °C. Sintered samples were then cut and prepared by standard metallographic procedures. The porosity, as well as particle size and area, were measured using ImageJ software on micrographs taken by scanning electron microscope (SEM). ULTRA PLUS (Carl Zeiss GmbH, Germany) with the Energy-dispersive X-ray spectroscopy (EDS) detector were utilized for analysis of powder and bulk microstructures and chemical composition determination. The exact concentrations of C element were measured in the milled powders and bulk materials using combustion analyzers G4 ICARUS (Bruker, Billerica, Massachusetts, MA, USA), whereas O and N were measured using an TC-336 analyzer (LECO Corporation, St. Joseph, Michigan, MI, USA).
4. Discussion
To summarize the effects of milling and SPS densification on carbon, oxygen, and nitrogen contamination of the materials, the results for the CoCrFeNi HEA sample milled for 30 h in N
2 atmosphere are shown in
Figure 9.
Regarding the role of the PCA during processing, the results showed that even a short wet milling continuing for only 30 min in ethanol (C
2H
5OH) contributes to significant contamination of powders by oxygen and, to a larger extent, by carbon. We may speculate that the use of oxygen-free PCA, such as toluene, could decrease the oxygen contamination, but it would still lead to carbon contamination [
23]. Therefore, it is advisable to completely avoid using PCA during milling.
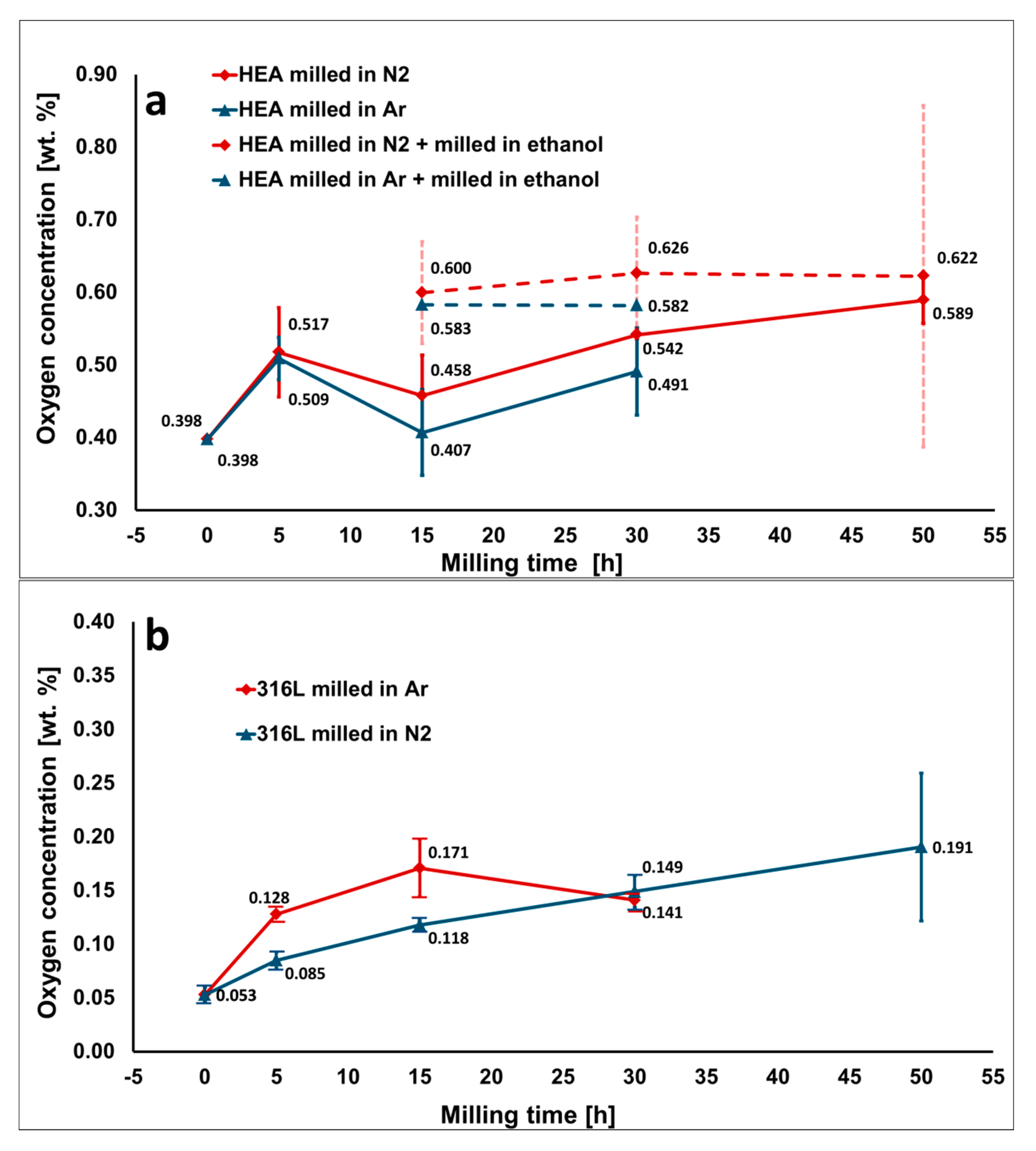
Oxygen causes the most significant contamination in the unmilled powders. At the respective concentrations measured inside the produced HEA materials (up to ~0.5 wt.%), oxygen contamination can induce significant changes of properties and microstructures. Oxygen is already present on the surfaces of raw powders in the form of various oxides. The oxides form due to reaction of metallic surfaces with ambient air. The layer of oxides is formed on the surfaces of metals already during powder manufacturing and can grow during the storage [
24]. Elements with relatively high affinity for oxygen, such as chromium and iron in our case, are very prone to oxidation [
25]. This pre-processing oxidation of the raw elemental powders is one of the most significant contributions to the contamination of the final bulks. It should be noted that suppression of contamination during elemental powder preparation is extremely difficult. In some cases, it can be induced by selection of specialized methods, such as vacuum atomization and powder handling under protective atmospheres [
26]. The oxygen contamination slightly increases also after milling for 30 h, even despite the use of protective N
2 atmosphere (
Section 3.3). The contamination can be explained by the leak of air, due to their not being a 100% air tighten milling bowl. However, a more probable explanation is the impurities contained in the N
2 gas, where some of the remaining oxygen is always presented. The new surfaces created by milling are then immediately oxidized by oxygen impurities inside the bowl, as well as during opening of the milling bowl at the end of milling and feeding the powder to the SPS die. The densification inside SPS either retains the oxygen concentration of the milled powders, or it can slightly decrease it. The slight oxygen decrease observed in some cases can be rationalized on the basis of the nature of sintering environment (slight reducing atmosphere of vacuum and C-rich gasses), combined with relatively high processing temperature. The latter setup could result in a tendency to degas the powders, comparable to degassing by use of vacuum during melting (VIM and VAM process). The use of high-vacuum during the SPS process could also decrease the extent of oxygen contamination in the materials with low affinity to it.
Carbon contamination is even more problematic than oxygen. The initially mixed powders contain a relatively low concentration of carbon. However, even such a low concentration of carbon can cause issues for materials prone to sensitization [
27]. The carbon contamination is considerably increased by the milling process, positively increasing with prolonging milling time. Such carbon concentration is tenths of wt.% and would significantly alter the properties of FCC HEAs [
22], by formation of carbides and interactions inside a matrix solid solution. Carbon contamination during milling is caused by the abrasive wear of the milling bowl and balls, made from hardened high-carbon steel. This type of contamination will be observed in various types of milling equipment, because hardened steel (bearing steel, tool steel, etc.) is the most common material used for the milling bowl [
7,
28]. The use of steel equipment also results in contamination by iron [
29]. On the other hand, iron contamination levels during milling are not significant in cases of iron-containing materials, such as the CoCrNiFe HEA. The contamination from the milling equipment has been observed in all types of materials prepared with milling equipment made from metals and ceramics such as, for instance, WC [
23], SiC [
30], and stainless steel [
31]. The general rule to eliminate said contamination induced by equipment abrasion is to reduce the milling time to the largest possible extent. The best way to completely eliminate the contamination of the powders from the milling equipment would be to use the equipment (milling balls and bowl) made from the material identical to that which is being milled. However, this option will be possible only in very limited number of cases due to the sheer variety of milled materials and, on the other hand, limited amount of materials used for the milling equipment. Finally, the largest contamination by carbon results from graphite die-processed powder interaction in SPS. Such contamination has been documented before [
32]. However, it is not clear if the contamination is caused by the reaction of the powders in the direct vicinity of graphite mold, or if it is carried by a carbon-containing gas emanating during SPS. The carbon contamination from the graphite die can be reduced by application of protective coating on the die walls in the form of spray (BN powder) or use of different die materials (tungsten). Additionally, the layer of protective material can be added in some cases in the form of thin sheet (steel, Nb), preventing the direct contact of the powder with the graphite die.
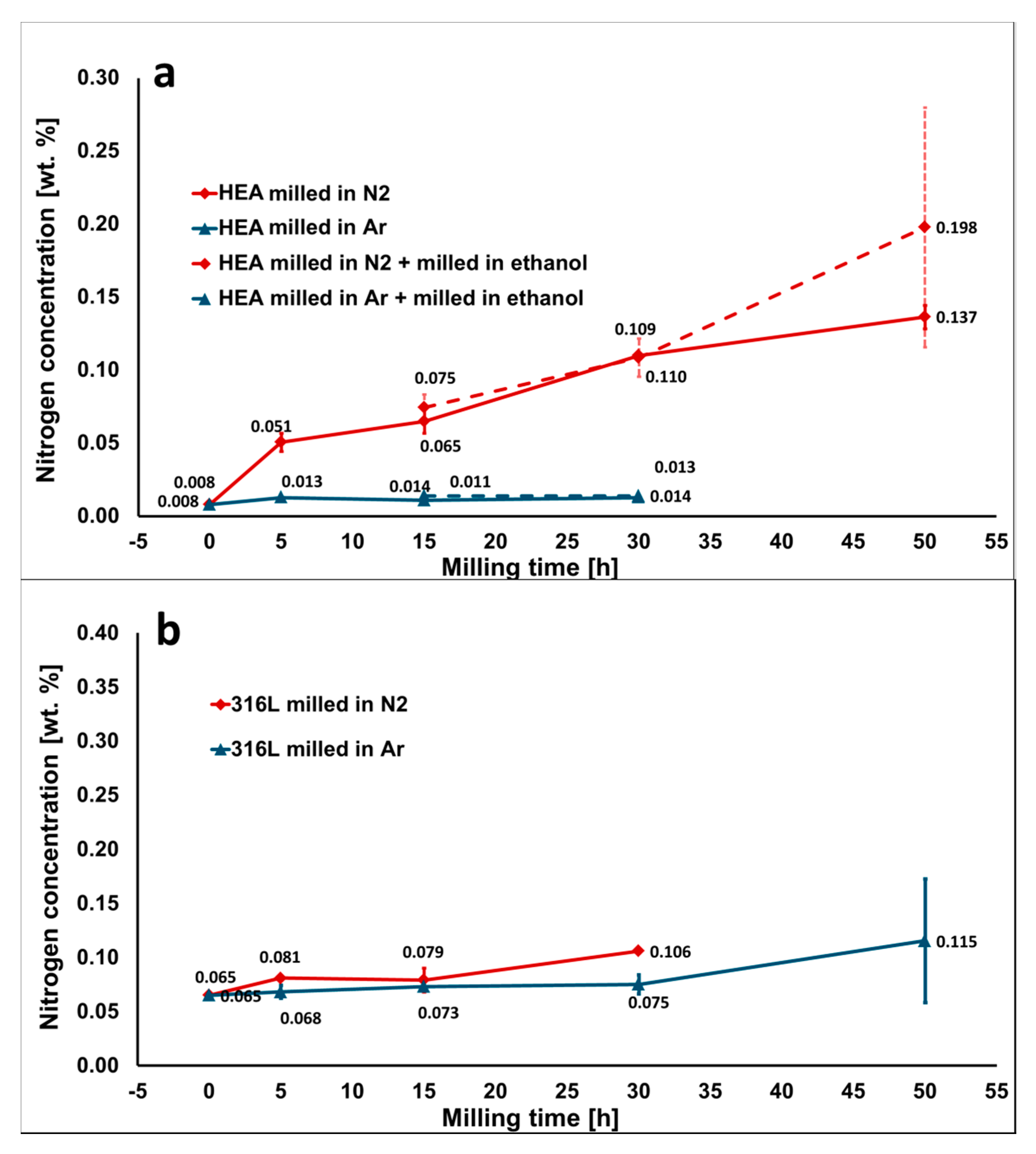
The least significant contribution to overall contamination is caused by nitrogen. The nitrogen contamination of the initial powders is very low. Additionally, the utilization of Ar inert gas during milling secures stable values of nitrogen concentration throughout the whole processing. In case the nitrogen level has to be decreased further, the use of high-vacuum during the SPS process could decrease nitrogen contamination even further.
One of the most significant observations of the study comes from the comparison between preparation by milling from elemental powders (CoCrFeNi HEA) and milling of pre-alloyed powder (316L steel). It is evident that the use of pre-alloyed gas atomized powder results in significantly purer materials, when compared to preparation from mixed elemental powders with desired composition. This is caused by lower initial contamination of pre-alloyed powder (especially by oxygen), resulting in consistently less contaminated materials prepared from pre-alloyed powders.
There are slight differences between the extent of contamination of the 316 L steel and the HEA caused during milling, presumably caused by the differences in the chemical composition. The largest difference is visible in more significant increase of carbon concentration of 316 L steel during milling and subsequent sintering. It can be explained by the chemical composition of the 316 L steel, containing larger concentration of Fe. The HEA shows lower affinity towards carbon, due to lower content of Fe and higher content of Ni and Co, i.e., elements with lower affinity for carbon (less negative enthalpy of mixing of Ni and Co compared to Fe [
33]). As mentioned previously, the differences in the total concentrations of oxygen and nitrogen in the 316 L steel and HEA are mostly caused by the differences in their initial content in used raw materials. However, the extent of contamination caused during preparation is comparable in both materials.
In conclusion, the use of inert-gas atomized, pre-alloyed powder handled in protective atmosphere, avoidance of PCA, and minimization of mechanical milling time results in minimal contamination of the final bulks. In special cases, the use of an SPS die treated by protective layers (BN) and utilization of high-vacuum during sintering could be also beneficial. The preparation of alloy powder by mechanical milling of elemental powders produces more contaminated bulks, compared to preparation from atomized powders. On the other hand, the use of elemental powders offers a better versatility and timeliness for preparation of various arrays of materials, compared to atomization. For the atomization, the desired material has to be prepared in the form of ingot prior to atomization, complicating the processing. Therefore, preparation of powders by milling (alloying) of elemental powders is the process of choice for instances when shorter time for preparation of final bulk outweighs the issues of contamination, such as initial alloy screening.
5. Conclusions
In this study, the CoCrFeNi high-entropy alloy (HEA) powder and bulk forms were subject to a systematic chemistry analysis in order to evaluate the extent of contamination at every single stage of the powder metallurgy production process. For comparison purposes, the same procedure was performed in a 316 L stainless steel material. The powders were mechanically alloyed and subsequently spark plasma sintered (SPS). The influence of milling times, selected protective atmosphere during milling (Ar or N2), use of PCA, and sintering process were discussed in terms of oxygen, nitrogen, and carbon contents. The main outcomes of this investigation can be summarized as follows.
Abrasion of the high-carbon steel milling bowl and balls has been identified as a significant source of contamination of powder materials in the mechanical alloying (MA) process. Due to the increased carbon contents, a considerable amount of carbides was observed in the structures of both HEA and steel samples. These inclusions can significantly affect the mechanical, corrosion and other properties of the final products.
The most significant contamination by oxygen is induced by the initial oxidation of raw powder surfaces. Oxygen contamination also slightly increases with milling time.
In the case of the HEA prepared from elemental powder, the oxygen contamination caused by oxidation of raw powder before milling induces formation of significant volume of oxides in the sintered bulks.
The bulk of Cr-containing alloy (HEA) prepared using pure elemental powders shows significantly higher oxygen contamination than bulks prepared from pre-alloyed powder (316 L steel).
The protective atmosphere does not seem to play an important role in the amount of oxygen and carbon contents during milling, even though the use of Ar resulted in slightly lower oxygen contamination.
The nitrogen contamination is significantly higher when using N2 atmosphere, compared to use of Ar.
Wet milling in 200 mL of ethanol led to an increase in carbon and oxygen contents of all samples as compared to dry milling.
The significant contribution to the overall carbon contamination of the bulk materials is also caused by the SPS process, as the powders possibly react with the carbon-rich environment inside the graphite die, substantially increasing the carbon levels of the sintered bulk products.
Author Contributions
Conceptualization: I.M., A.K., and I.D.; methodology: I.M., A.K., and V.K.; validation: I.M., A.K., and V.K.; formal analysis: I.M., A.K., L.M.-G., and O.A.; investigation: I.M. and A.K.; resources: V.P., A.Z., and I.D.; data curation: I.M., A.K., and V.K.; writing—original draft preparation: I.M., A.K., L.M.-G., and O.A.; writing—review and editing: I.M., L.M.-G., and O.A.; visualization: I.M., A.K., L.M.-G., and O.A.; supervision: I.M. and I.D.; project administration: I.M. and I.D.; funding acquisition: I.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Czech Science Foundation Project No. 19–22016S and the project ArMADit no. CZ.02.1.01/0.0/0.0/16_025/0007304.
Acknowledgments
This work was financially supported by Czech Science Foundation Project No. 19–22016S and the project ArMADit no. CZ.02.1.01/0.0/0.0/16_025/0007304.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moravcikova-Gouvea, L.; Moravcik, I.; Omasta, M.; Veselý, J.; Cizek, J.; Minárik, P.; Cupera, J.; Záděra, A.; Jan, V.; Dlouhy, I. High-strength Al0.2Co1.5CrFeNi1.5Ti high-entropy alloy produced by powder metallurgy and casting: A comparison of microstructures, mechanical and tribological properties. Mater. Charact. 2020, 159, 110046. [Google Scholar] [CrossRef]
- Moravcik, I.; Gamanov, S.; Moravcikova-Gouvea, L.; Kovacova, Z.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Influence of Ti on the Tensile Properties of the High-Strength Powder Metallurgy High Entropy Alloys. Materials 2020, 13, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Guo, Y.; Wang, H.; Shan, J.; Chang, Y. Microstructures and mechanical properties of oxide dispersion strengthened CoCrFeNi high-entropy alloy produced by mechanical alloying and spark plasma sintering. Intermetallics 2020, 123, 106819. [Google Scholar] [CrossRef]
- Moravcik, I.; Gouvea, L.; Hornik, V.; Kovacova, Z.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Synergic strengthening by oxide and coherent precipitate dispersions in high-entropy alloy prepared by powder metallurgy. Scr. Mater. 2018, 157, 24–29. [Google Scholar] [CrossRef]
- Guo, L.; Ou, X.; Ni, S.; Liu, Y.; Song, M. Effects of carbon on the microstructures and mechanical properties of FeCoCrNiMn high entropy alloys. Mater. Sci. Eng. A 2019, 746, 356–362. [Google Scholar] [CrossRef]
- Abbaschian, R.; Abbaschian, L.; Reed-Hill, R.E. Physical Metallurgy Principles, 4th ed.; Cengage Learning: Stamford, CT, USA, 2008; ISBN1 10: 0495082546. ISBN2 13: 9780495082545. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Moravcik, I.; Cizek, J.; Zapletal, J.; Kovacova, Z.; Vesely, J.; Minarik, P.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Microstructure and mechanical properties of Ni1,5Co1,5CrFeTi0,5 high entropy alloy fabricated by mechanical alloying and spark plasma sintering. Mater. Des. 2017, 119, 141–150. [Google Scholar] [CrossRef]
- Moravcik, I.; Gouvea, L.; Cupera, J.; Dlouhy, I. Preparation and properties of medium entropy CoCrNi/boride metal matrix composite. J. Alloys Compd. 2018, 748, 979–988. [Google Scholar] [CrossRef]
- Gouvea, L.; Moravcik, I.; Cizek, J.; Krajnakova, P.; Jan, V.; Dlouhy, I. Characterization of powder metallurgy high-entropy alloys prepared by spark plasma sintering. In Materials Science Forum; Trans Tech Publication Ltd.: Cham, Switzerland, 2018; Volume 941, pp. 1053–1058. ISBN 9783035712087. [Google Scholar]
- Waseem, O.A.; Ryu, H.J. Powder Metallurgy Processing of a W(x)TaTiVCr High-Entropy Alloy and Its Derivative Alloys for Fusion Material Applications. Sci. Rep. 2017, 7, 1926. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. Enhanced Wear Behaviour of Spark Plasma Sintered AlCoCrFeNiTi High-Entropy Alloy Composites. Materials 2018, 11, 2225. [Google Scholar] [CrossRef] [Green Version]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Moravcik, I.; Hadraba, H.; Li, L.; Dlouhy, I.; Raabe, D.; Li, Z. Yield strength increase of a CoCrNi medium entropy alloy by interstitial nitrogen doping at maintained ductility. Scr. Mater. 2020, 178, 391–397. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- Su, J.; Raabe, D.; Li, Z. Hierarchical microstructure design to tune the mechanical behavior of an interstitial TRIP-TWIP high-entropy alloy. Acta Mater. 2019, 163, 40–54. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Thurston, K.V.S.; Bei, H.; Wu, Z.; George, E.P.; Ritchie, R.O. Exceptional damage-tolerance of a medium-entropy alloy CrCoNi at cryogenic temperatures. Nat. Commun. 2016, 7, 10602. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Moravcik, I.; Hornik, V.; Minárik, P.; Li, L.; Dlouhy, I.; Janovska, M.; Raabe, D.; Li, Z. Interstitial doping enhances the strength-ductility synergy in a CoCrNi medium entropy alloy. Mater. Sci. Eng. A 2020, 781, 139242. [Google Scholar] [CrossRef]
- Vaidya, M.; Karati, A.; Marshal, A.; Pradeep, K.G.; Murty, B.S. Phase evolution and stability of nanocrystalline CoCrFeNi and CoCrFeMnNi high entropy alloys. J. Alloys Compd. 2019, 770, 1004–1015. [Google Scholar] [CrossRef]
- Hryha, E.; Shvab, R.; Bram, M.; Bitzer, M.; Nyborg, L. Surface chemical state of Ti powders and its alloys: Effect of storage conditions and alloy composition. Appl. Surf. Sci. 2016, 388, 294–303. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Li, W.; Che, S.; Zheng, J.; Qiao, L.; Ying, Y. The preparation and magnetic performance of the iron-based soft magnetic composites with the Fe@Fe3O4 powder of in situ surface oxidation. J. Magn. Magn. Mater. 2018, 454, 103–109. [Google Scholar] [CrossRef]
- Deffley, R. Vacuum vs. Non-Vacuum Melted Gas Atomized Powders; Carpenter technology: Philadelphia, PA, USA, 2017. [Google Scholar]
- Mújica Roncery, L.; Weber, S.; Theisen, W. Nucleation and precipitation kinetics of M23C6 and M2N in an Fe–Mn–Cr–C–N austenitic matrix and their relationship with the sensitization phenomenon. Acta Mater. 2011, 59, 6275–6286. [Google Scholar] [CrossRef]
- Luo, X.-T.; Li, C.-J.; Yang, G.-J. Correlations between milling conditions and iron contamination, microstructure and hardness of mechanically alloyed cubic BN particle reinforced NiCrAl matrix composite powders. J. Alloys Compd. 2013, 548, 180–187. [Google Scholar] [CrossRef]
- Fernandes, B.B.; Rodrigues, G.; Coelho, G.C.; Ramos, A.S. On iron contamination in mechanically alloyed Cr–Si powders. Mater. Sci. Eng. A 2005, 405, 135–139. [Google Scholar] [CrossRef]
- Castle, E.; Csanádi, T.; Grasso, S.; Dusza, J.; Reece, M. Processing and Properties of High-Entropy Ultra-High Temperature Carbides. Sci. Rep. 2018, 8, 8609. [Google Scholar] [CrossRef] [Green Version]
- Le Claire, J.J.; Laitila, E.A.; Mikkola, D.E. Forming nanostructured cubic trialuminide/carbide composites by mechanical milling followed by thermal processing. Scr. Mater. 2004, 50, 95–98. [Google Scholar] [CrossRef]
- Mackie, A.J.; Hatton, G.D.; Hamilton, H.G.C.; Dean, J.S.; Goodall, R. Carbon uptake and distribution in Spark Plasma Sintering (SPS) processed Sm(Co, Fe, Cu, Zr)z. Mater. Lett. 2016, 171, 14–17. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
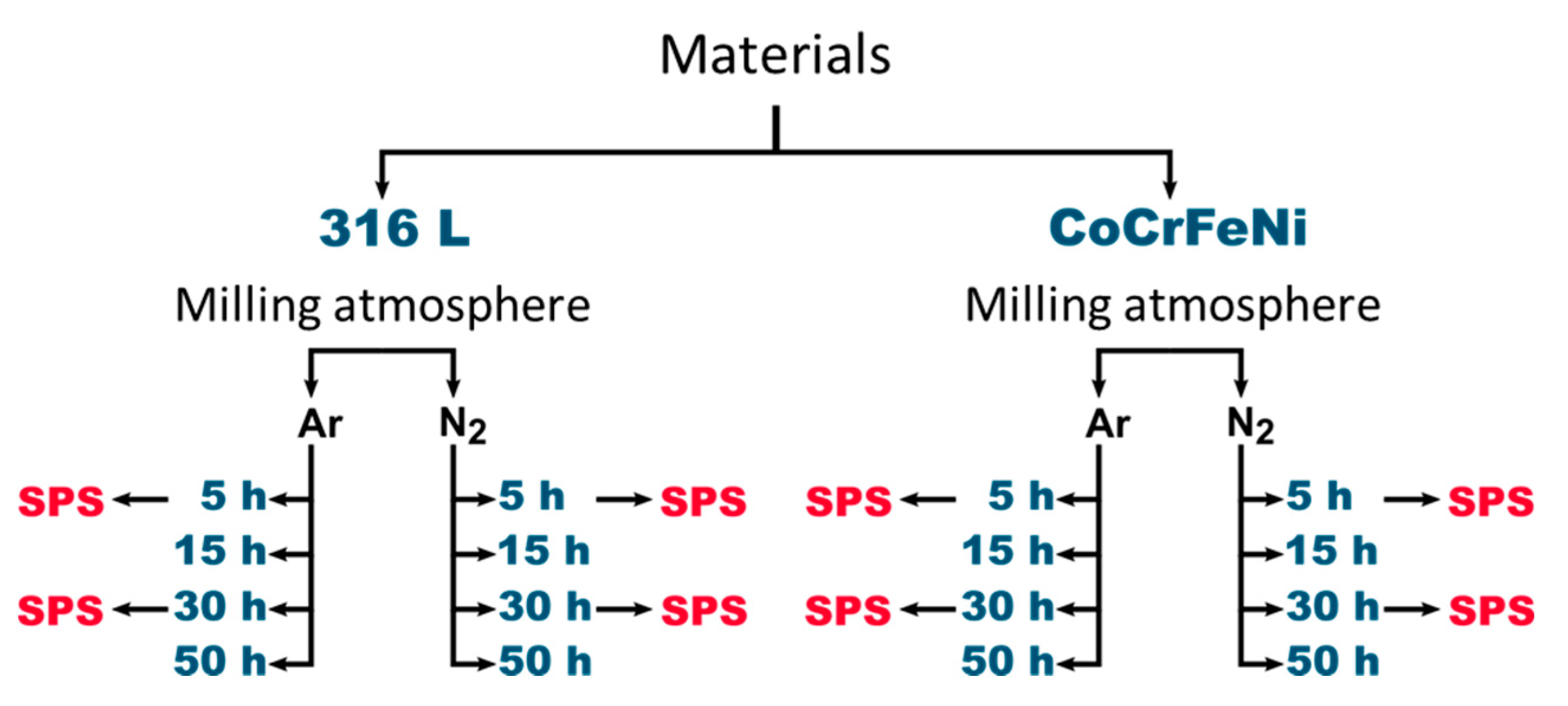
Figure 1.
Schematic representation of various powder materials prepared using a set of variable milling parameters (time, atmosphere). The 316 L alloy atomized powder and CoCrFeNi powder prepared from pure elements was used for milling experiments. Powder samples designated by the spark plasma sintering (SPS) arrow were subsequently densified.
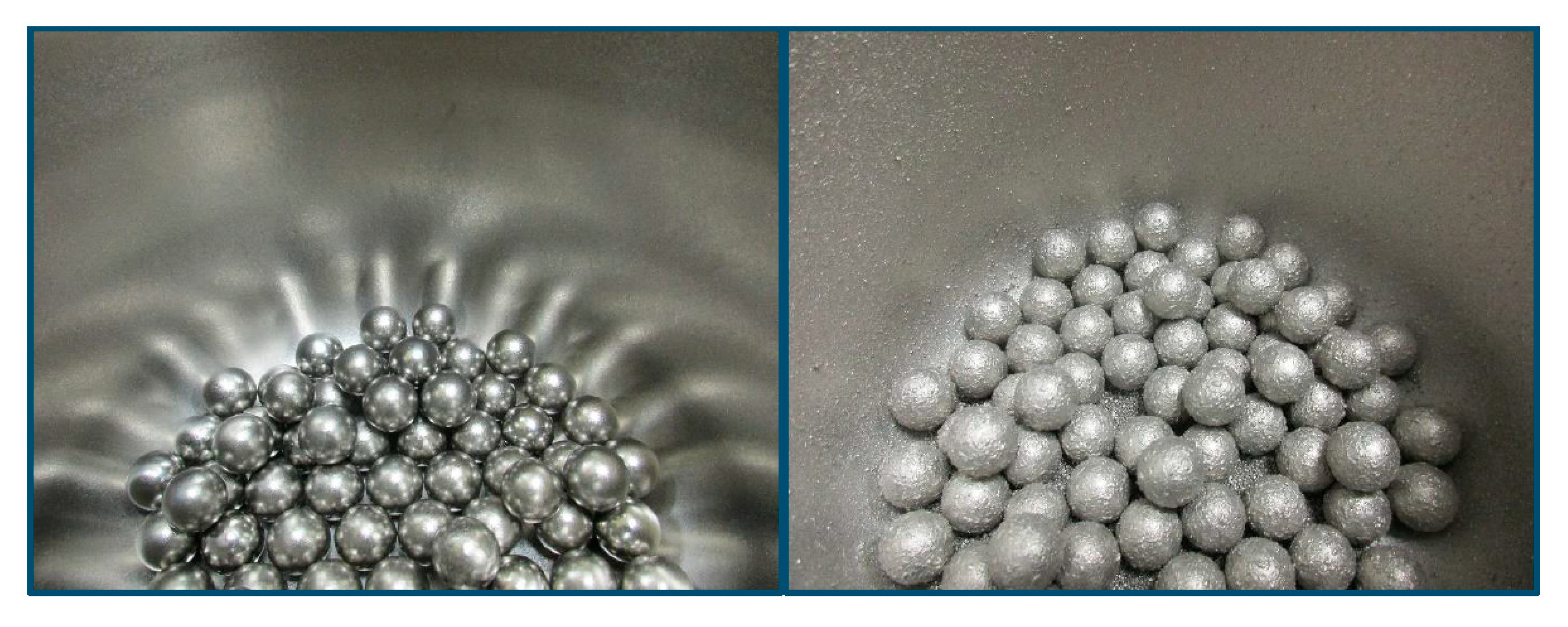
Figure 2.
Inside of the milling bowl before (left) and after (right) the dry milling process of the CoCrFeNi powders milled for 15 h. Apart from the free powder at the bottom, the inside of the bowl still contains significant amount of powder stuck to its walls and to the milling balls, which were removed by 30 min of wet milling.
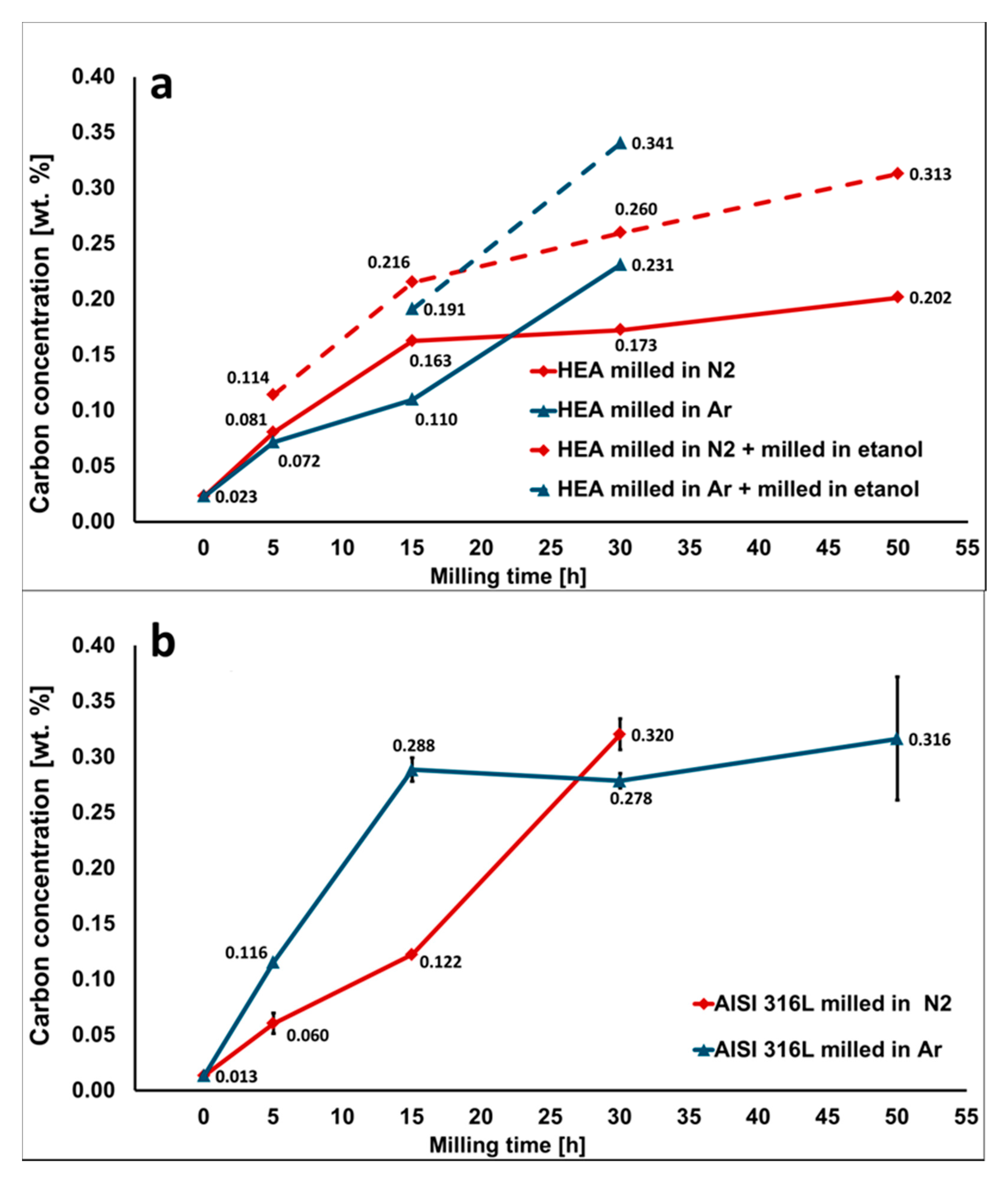
Figure 3.
Carbon concentration of powders milled in Ar and N2 atmosphere as a function of the milling time: (a) high-entropy alloy (HEA) powder, the dashed line represents powders additionally milled in process control agents (PCA) (ethanol) for 30 min; and (b) 316 L steel powder. The carbon contamination caused by the wear of high-carbon steel milling equipment is increasing with milling time.
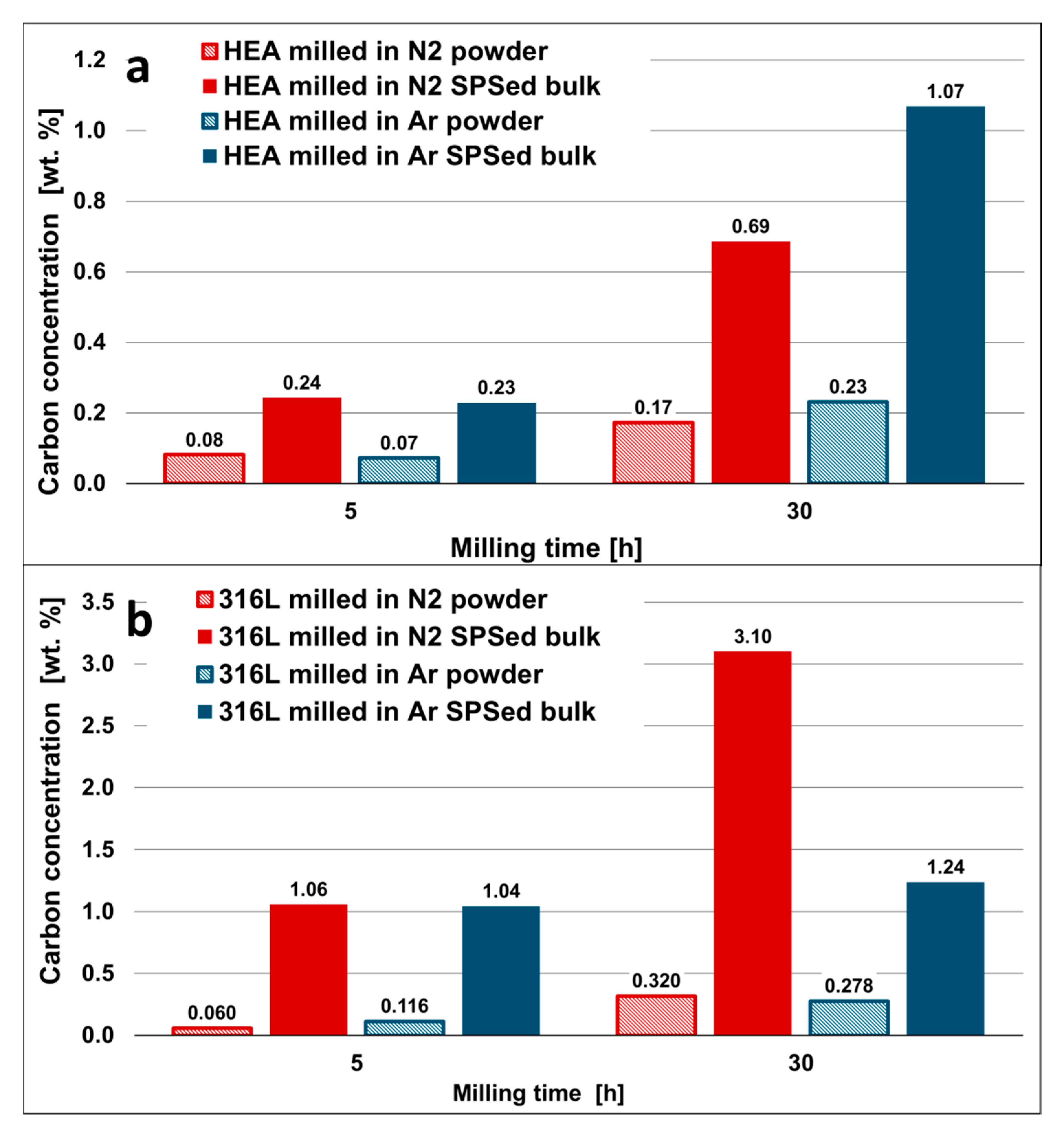
Figure 4.
Carbon concentration of selected powders milled in Ar and N2 atmosphere as compared to concentration of the same materials after the SPS process (SPSed bulks); (a) HEA and (b) 316 steel. The SPS densification in graphite die significantly increases carbon contamination.
Figure 5.
Oxygen concentration of the powders milled in Ar and N2 atmosphere as a function of the milling time: (a) HEA powder, the dashed line represents powders additionally milled in PCA (ethanol) for 30 min; and (b) 316 L steel powder. The oxygen contamination gradually increases with milling time. Relatively high-oxygen concentration in unmilled HEA powder (0 h) is caused by initial oxidation of surfaces of elemental pure powders prior to milling.
Figure 6.
Nitrogen concentration of powders milled in Ar and N2 atmosphere as a function of the milling time: (a) HEA powder, the dashed line represents powders additionally milled in PCA (ethanol) for 30 min; and (b) 316 L steel powder. The milling in Ar atmosphere prevents nitrogen contamination, while milling of the HEA powders in N2 atmosphere causes significant nitrogen pick-up.
Figure 7.
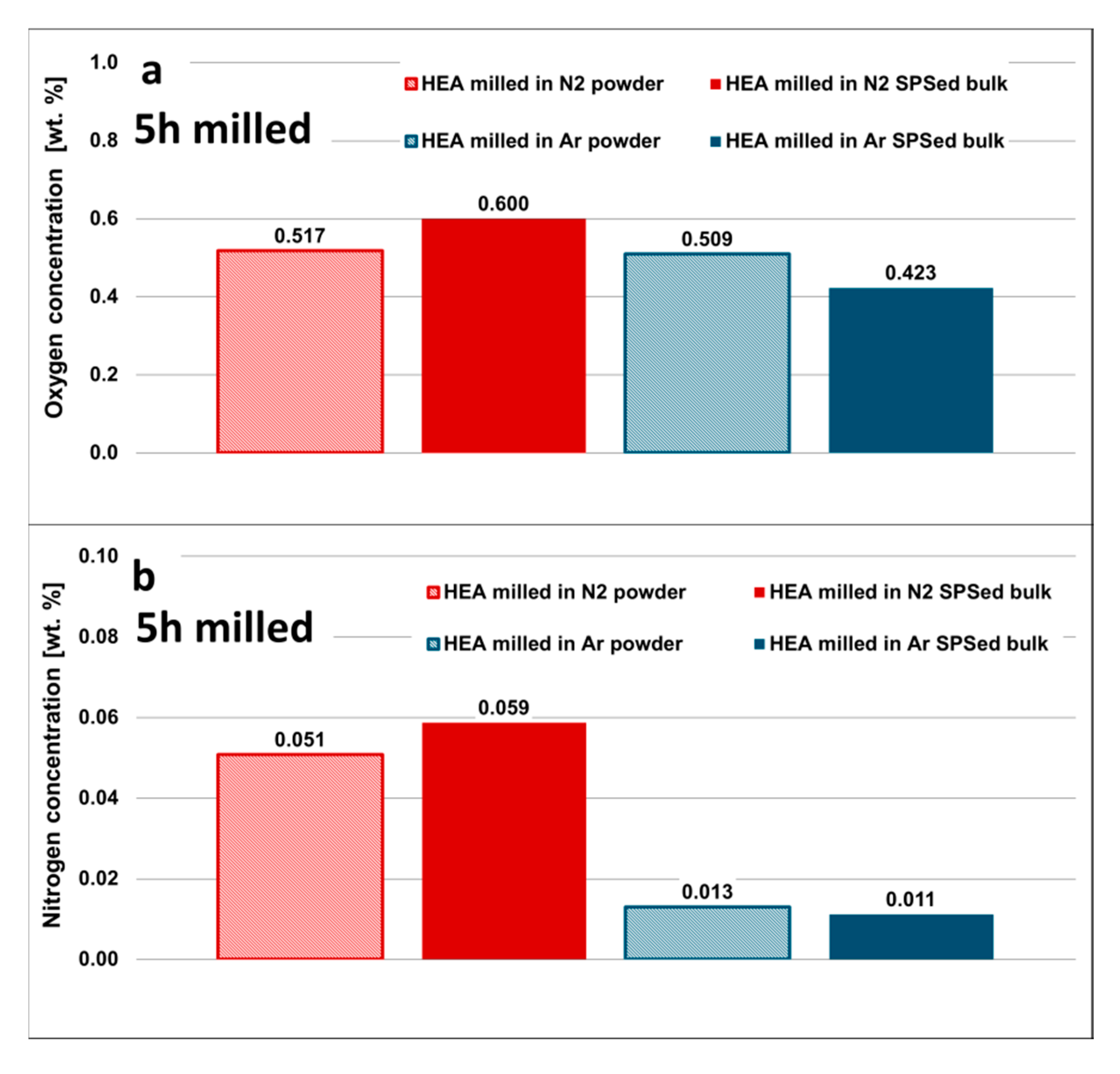
Comparison between the powder and bulk materials of HEA milled for 5 h in Ar and N2 atmosphere; (a) oxygen concentration and (b) nitrogen concentration. The nitrogen and oxygen concentrations are retained after the SPS, within the scatter of the measurements and their accuracy.
Figure 8.
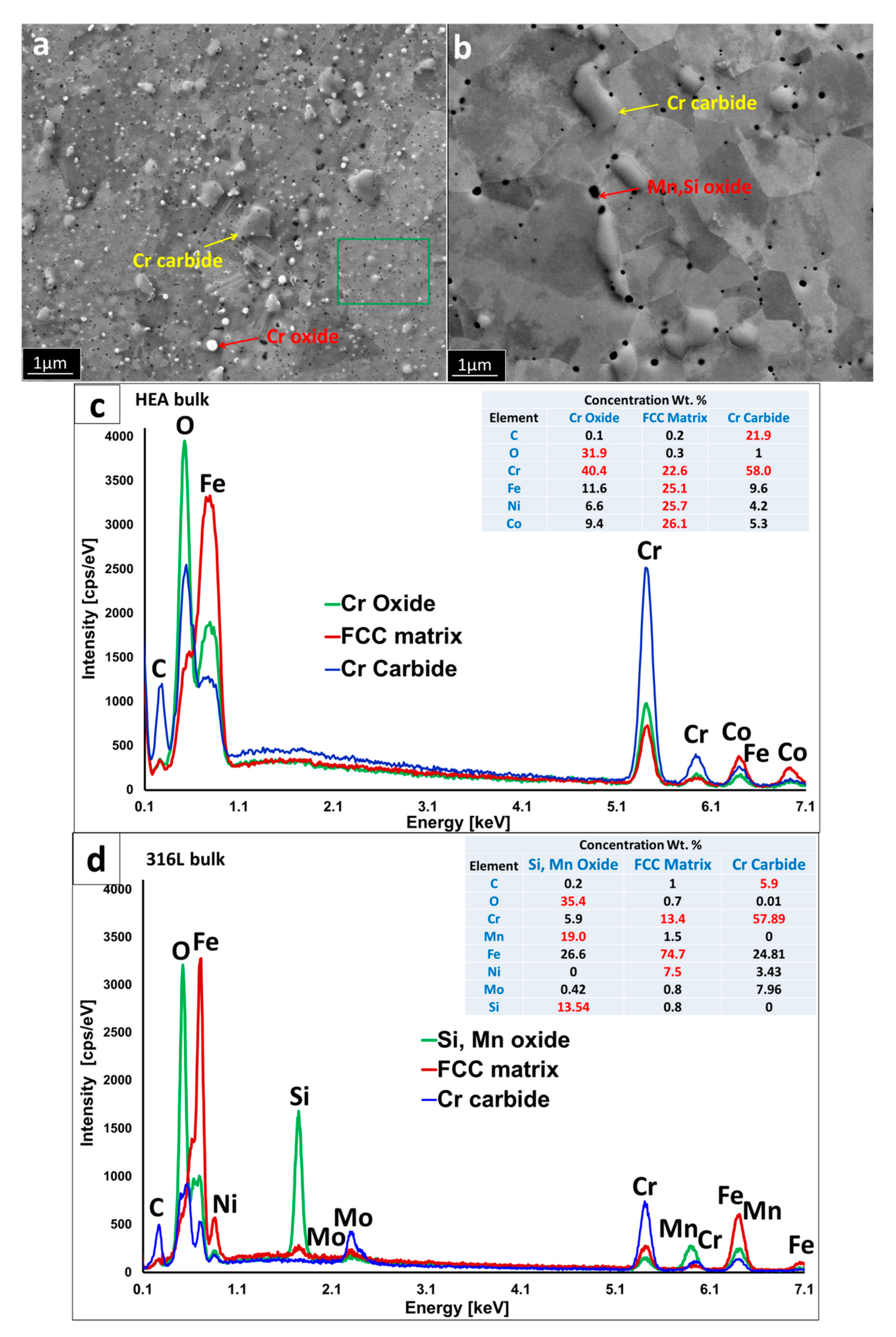
Representative microstructures of the bulk materials prepared from powders milled in Ar atmosphere for 30 h, taken with SEM in secondary electrons mode; (a) HEA; (b) 316L steel; (c) EDS analysis of representative particles in HEA; and (d) EDS analysis of representative particles in 316 L steel. The microstructures are composed from FCC matrix grains, oxide particles with varying chemistry and size, and larger chromium carbide particles. It should be noted that due to the insensitivity of EDS to light elements, the exact quantities of C and O elements presented in tables are only for informative purposes.
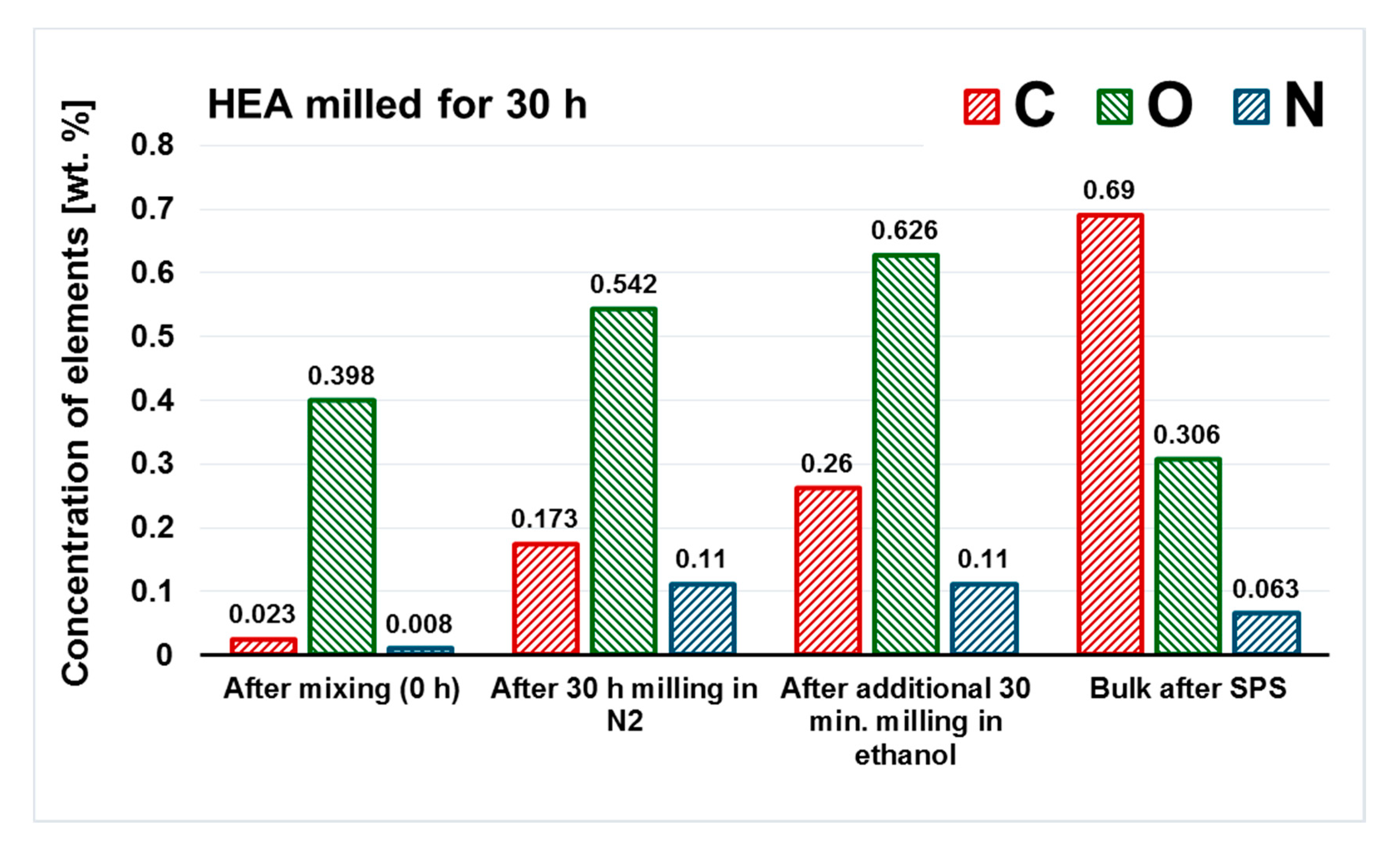
Figure 9.
The evolution of contamination in the representative case of HEA powder milled for 30 h and additional 30 min in ethanol. The SPSed bulk was prepared from the powder after 30 h of milling.
Table 1.
Manufacturers and product parameters of powders used for preparation of mechanically alloyed CoCrFeNi alloy.
| Element | Manufacturer | Product Identification |
|---|
| Co | Alfa Aesar | Particle size < 44 µm, purity 99.5% |
| Cr | Alfa Aesar | Particle size < 44 µm, purity 99% |
| Fe | Alfa Aesar | Particle size < 10 µm, purity ≥ 99.5% |
| Ni | GTV | Particle size 15–53 µm, purity 99% |
| 316L | Höganäs AB | 53–150 µm, purity 99.5% |
Table 2.
Chemical composition and of 316L alloy powder from
Table 1.
| Element | Fe | C | Mo | Ni | Mn | Cr | Si | O |
|---|
| wt.% | 65.83 | 0.014 | 2.4 | 12.3 | 1.6 | 17.0 | 0.8 | 0.059 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).