The Effect of 45S5 Bioglass and Ag, Cu, or Zn Addition on the Crystal Structure, Properties, and Antibacterial Effect of Bulk Ti23Zr25Nb Biocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Materials Characterization
- -
- Ti (α) (ref. code 01-071-4632) hexagonal P63/mmc
- -
- Ti (β) (ref. code 01-074-7075) cubic Im-3m
- -
- Ti2ZrO (ref. code 01-072-1881) hexagonal P6/mmm
- -
- Nb5Si3P (ref. code 00-051-0788) hexagonal P63/mcm
2.3. Assessment of Biofilm Formation Inhibition
3. Results
4. Discussion
5. Conclusions
- –
- the content of Ti (β) increases with milling time and 45S5 Bioglass decreases it,
- –
- Ti23Zr25Nb-3BG and Ti23Zr25Nb-6BG need longer milling time (16 h) than Ti23Zr25Nb (10 h) to produce fully single-phase β-type powders,
- –
- 45S5 Bioglass limits the content of Ti (β) as a beta non-stabilizer in the bulk samples,
- –
- Cu and Zn decrease the content of Ti (β) despite their β–stabilizing properties in the bulk samples,
- –
- the corrosion, mechanical properties, and wettability of the produced materials are beneficial in contrast to the Ti23Zr25Nb alloy,
- –
- Ti23Zr25Nb-9BG-Ag, Ti23Zr25Nb-9BG-Cu, Ti23Zr25Nb-9BG-Zn are proved to have high antibacterial activity against S. mutans being present in the human oral cavity,
- –
- produced materials based on the performed experiments are highly recommended for biomedical use (especially as dental implants) because of their high corrosion resistance, low Young modulus, and good antibacterial activity.
Author Contributions
Funding
Conflicts of Interest
References
- Marczewski, M.; Miklaszewski, A.; Jurczyk, M. Structure evolution analysis in ultrafine-grained Zr and Nb-based beta titanium alloys. J. Alloy. Compd. 2018, 765, 459–469. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. Development of β-type Ti-x at. % Mo alloys by mechanical alloying and powder metallurgy: Phase evolution and mechanical properties (10 ≤ x ≤ 35). J. Alloy. Compd. 2019, 776, 370–378. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Kowalski, K.; Jurczyk, M. Influence of the processing method on the properties of Ti-23 at.% mo alloy. Metals 2019, 9, 931. [Google Scholar] [CrossRef]
- Jurczyk, K.; Kubicka, M.M.; Ratajczak, M.; Jurczyk, M.U.; Niespodziana, K.; Nowak, D.M.; Gajecka, M.; Jurczyk, M. Antibacterial activity of nanostructured Ti-45S5 bioglass-Ag composite against Streptococcus mutans and Staphylococcus aureus. Trans. Nonferrous Met. Soc. 2016, 26, 118–125. [Google Scholar] [CrossRef]
- Jurczyk, K.; Niespodziana, K.; Jurczyk, M.U.; Jurczyk, M. Synthesis and characterization of titanium-45S5 Bioglass nanocomposites. Mater. Des. 2011, 32, 2554–2560. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Seuss, S.; Boccaccini, A.R. Microstructure and properties of composite polyetheretherketone/ Bioglass® coatings deposited on Ti-6Al-7Nb alloy for medical applications. Appl. Surf. Sci. 2013, 273, 62–67. [Google Scholar] [CrossRef]
- Ananth, K.P.; Suganya, S.; Mangalaraj, D.; Ferreira, J.M.F.; Balamurugan, A. Electrophoretic bilayer deposition of zirconia and reinforced bioglass system on Ti6Al4V for implant applications: An in vitro investigation. Mater. Sci. Eng. C 2013, 33, 4160–4166. [Google Scholar] [CrossRef]
- López, M.M.M.; Fauré, J.; Cabrera, M.I.E.; García, M.E.C. Structural characterization and electrochemical behavior of 45S5 bioglass coating on Ti6Al4V alloy for dental applications. Mater. Sci. Eng. B 2016, 206, 30–38. [Google Scholar] [CrossRef]
- Xue, B.; Guo, L.; Chen, X.; Fan, Y.; Ren, X.; Li, B.; Ling, Y.; Qiang, Y. Electrophoretic deposition and laser cladding of bioglass coating on Ti. J. Alloy. Compd. 2017, 710, 663–669. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Peters, C.; Roether, J.A.; Eifler, D.; Misra, S.K.; Minay, E.J. Electrophoretic deposition of polyetheretherketone (PEEK) and PEEK/Bioglass® coatings on NiTi shape memory alloy wires. J. Mater. Sci. 2006, 41, 8152–8159. [Google Scholar] [CrossRef]
- Estrada-Cabrera, E.; Torres-Ferrer, L.R.; Aztatzi-Aguilar, O.G.; De Vizcaya-Ruiz, A.; Meraz-Rios, M.A.; Zarate-Triviño, D.G.; Arizmendi-Morquecho, A.; Bugallo, A.D.L.; Prokhorov, E.; Luna-Barcenas, G. Chitosan-bioglass coatings on partially nanostructured anodized Ti-6Al-4V alloy for biomedical applications. Surf. Coatings Technol. 2019, 375, 468–476. [Google Scholar] [CrossRef]
- Jugowiec, D.; Łukaszczyk, A.; Cieniek, Ł.; Kot, M.; Reczyńska, K.; Cholewa-Kowalska, K.; Pamuła, E.; Moskalewicz, T. Electrophoretic deposition and characterization of composite chitosan-based coatings incorporating bioglass and sol-gel glass particles on the Ti-13Nb-13Zr alloy. Surf. Coatings Technol. 2017, 319, 33–46. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Kot, M.; Seuss, S.; Kędzierska, A.; Czyrska-Filemonowicz, A.; Boccaccini, A.R. Electrophoretic deposition and characterization of HA/chitosan nanocomposite coatings on Ti6Al7Nb alloy. Met. Mater. Int. 2015, 21, 96–103. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Enculescu, M.; Tanase, C.; Tulyaganov, D.U.; Ferreira, J.M.F. Superior biofunctionality of dental implant fixtures uniformly coated with durable bioglass films by magnetron sputtering. J. Mech. Behav. Biomed. Mater. 2015, 51, 313–327. [Google Scholar] [CrossRef]
- Stan, G.E.; Popescu, A.C.; Mihailescu, I.N.; Marcov, D.A.; Mustata, R.C.; Sima, L.E.; Petrescu, S.M.; Ianculescu, A.; Trusca, R.; Morosanu, C.O. On the bioactivity of adherent bioglass thin films synthesized by magnetron sputtering techniques. Thin Solid Films 2010, 518, 5955–5964. [Google Scholar] [CrossRef]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed Electron Deposition of nanostructured bioactive glass coatings for biomedical applications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- Goller, G. The effect of bond coat on mechanical properties of plasma sprayed bioglass-titanium coatings. Ceram. Int. 2004, 30, 351–355. [Google Scholar] [CrossRef]
- Chalisgaonkar, V.; Das, M.; Balla, V.K. Laser processing of Ti composite coatings reinforced with hydroxyapatite and bioglass. Addit. Manuf. 2018, 20, 134–143. [Google Scholar] [CrossRef]
- Szesz, E.M.; Lepienski, C.M. Anodic bonding of titanium alloy with bioactive glass. J. Non. Cryst. Solids 2017, 471, 19–27. [Google Scholar] [CrossRef]
- Krause, D.; Thomas, B.; Leinenbach, C.; Eifler, D.; Minay, E.J.; Boccaccini, A.R. The electrophoretic deposition of Bioglass® particles on stainless steel and Nitinol substrates. Surf. Coatings Technol. 2006, 200, 4835–4845. [Google Scholar] [CrossRef]
- Durgalakshmi, D.; Balakumar, S.; Raja, C.A.; George, R.P.; Mudali, U.K. Structural, morphological and antibacterial investigation of Ag-impregnated sol-gel-derived 45S5 nanoBioglass systems. J. Nanosci. Nanotechnol. 2015, 15, 4285–4295. [Google Scholar] [CrossRef] [PubMed]
- Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S. Stacked Bioglass/TiO2 nanocoatings on titanium substrate for enhanced osseointegration and its electrochemical corrosion studies. Appl. Surf. Sci. 2015, 349, 561–569. [Google Scholar] [CrossRef]
- Raja, C.A.; Balakumar, S.; Bargavi, P.; Rajashree, P.; Anandkumar, B.; George, R.P. Decoration of 1-D nano bioactive glass on reduced graphene oxide sheets: Strategies and in vitro bioactivity studies. Mater. Sci. Eng. C 2018, 90, 85–94. [Google Scholar]
- Raja, C.A.; Balakumar, S.; Durgalakshmi, D.; George, R.P.; Anandkumar, B.; Kamachi Mudali, U. Reduced graphene oxide/nano-Bioglass composites: Processing and super-anion oxide evaluation. RSC Adv. 2016, 6, 19657–19661. [Google Scholar] [CrossRef]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surfaces B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Candidato, R.T.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coatings Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Li, G.; Zhang, S.; Zhao, R.; Dong, A.; Zhang, R. Preparation and in vitro antibacterial properties of anodic coatings co-doped with Cu, Zn, and P on a Ti–6Al–4V alloy. Mater. Chem. Phys. 2020, 241, 122360. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Mushtovatova, L.S.; Karpova, M.R.; Sheikin, V.V.; Litvinova, L.S.; Khlusov, I.A. Zn-, Cu- or Ag-incorporated micro-arc coatings on titanium alloys: Properties and behavior in synthetic biological media. Surf. Coatings Technol. 2019, 369, 52–68. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, X.; Chen, M.; Hou, B. Effect of the existing form of Cu element on the mechanical properties, bio-corrosion and antibacterial properties of Ti-Cu alloys for biomedical application. Mater. Sci. Eng. C 2016, 69, 1210–1221. [Google Scholar] [CrossRef]
- Ma, Z.; Li, M.; Liu, R.; Ren, L.; Zhang, Y.; Pan, H.; Zhao, Y.; Yang, K. In vitro study on an antibacterial Ti-5Cu alloy for medical application. J. Mater. Sci. Mater. Med. 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, H.; Liu, J.; Qin, G.; Chen, D.; Zhang, E. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater. Sci. Eng. C 2019, 100, 38–47. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liu, C.; Wang, H.; Ren, B.; Yang, K.; Zhang, E. Effect of Cu content on the antibacterial activity of titanium-copper sintered alloys. Mater. Sci. Eng. C 2014, 35, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Ren, J.; Li, S.; Yang, L.; Qin, G. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of as-cast Ti-Cu alloys. Biomed. Mater. 2016, 11, 065001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium-copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, G.; Li, H.; Yang, L.; Wang, X.; Qin, G.; Zhang, E. Anti-bacterium influenced corrosion effect of antibacterial Ti-3Cu alloy in Staphylococcus aureus suspension for biomedical application. Mater. Sci. Eng. C 2019, 94, 376–384. [Google Scholar] [CrossRef]
- Liu, R.; Tang, Y.; Zeng, L.; Zhao, Y.; Ma, Z.; Sun, Z.; Xiang, L.; Ren, L.; Yang, K. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent. Mater. 2018, 34, 1112–1126. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, E. Biocorrosion properties of antibacterial Ti-10Cu sintered alloy in several simulated biological solutions. J. Mater. Sci. Mater. Med. 2015, 26, 142. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef]
- Zhang, E.L.; Fu, S.; Wang, R.X.; Li, H.X.; Liu, Y.; Ma, Z.Q.; Liu, G.K.; Zhu, C.S.; Qin, G.W.; Chen, D.F. Role of Cu element in biomedical metal alloy design. Rare Met. 2019, 38, 476–494. [Google Scholar] [CrossRef]
- Chen, M.; Yang, L.; Zhang, L.; Han, Y.; Lu, Z.; Qin, G.; Zhang, E. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater. Sci. Eng. C 2017, 75, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhang, H.; Zhang, E.; You, J.; Ma, X.; Bai, X. Antibacterial activities and biocompatibilities of Ti-Ag alloys prepared by spark plasma sintering and acid etching. Mater. Sci. Eng. C 2018, 92, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.C.; Pereira, P.R.; Barbosa, A.M.; Torrado, E.; Alves, N.M. Optimization of silver-containing bioglass nanoparticles envisaging biomedical applications. Mater. Sci. Eng. C 2019, 94, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sotoudehbagha, P.; Sheibani, S.; Khakbiz, M.; Ebrahimi-Barough, S.; Hermawan, H. Novel antibacterial biodegradable Fe-Mn-Ag alloys produced by mechanical alloying. Mater. Sci. Eng. C 2018, 88, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Marczewski, M.; Miklaszewski, A.; Maeder, X.; Jurczyk, M. Crystal Structure Evolution, Microstructure Formation, and Properties of Mechanically Alloyed Ultrafine-Grained Ti-Zr-Nb Alloys at 36 ≤ Ti ≤ 70 (at. %). Materials 2020, 13, 587. [Google Scholar] [CrossRef]
- Kowalski, K.; Jurczyk, M.U.; Wirstlein, P.K.; Jakubowicz, J.; Jurczyk, M. Influence of 45S5 Bioglass addition on microstructure and properties of ultrafine grained (Mg-4Y-5.5Dy-0.5Zr) alloy. Mater. Sci. Eng. B 2017, 219, 28–36. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M.; Kaczmarek, M.; Paszel-Jaworska, A.; Romaniuk, A.; Lipińska, N.; Żurawski, J.; Urbaniak, P.; Jurczyk, M. Nanoscale size effect in in situ titanium based composites with cell viability and cytocompatibility studies. Mater. Sci. Eng. C 2017, 73, 525–536. [Google Scholar] [CrossRef]
- Boyan, B.D.; Cohen, D.J.; Schwartz, Z. Bone Tissue Grafting and Tissue Engineering Concepts. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 7, pp. 298–313. ISBN 9780128035818. [Google Scholar]
- Van Vugt, T.A.; Geurts, J.A.P.; Arts, J.J.; Lindfors, N.C. Biomaterials in treatment of orthopedic infections. In Management of Periprosthetic Joint Infections (PJIs); Woodhead Publishing: Sawston, UK, 2017; pp. 41–68. ISBN 9780081002421. [Google Scholar]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Adamek, G.; Jakubowicz, J. Microstructure of the mechanically alloyed and electrochemically etched Ti-6Al-4V and Ti-15Zr-4Nb nanocrystalline alloys. Mater. Chem. Phys. 2010, 124, 1198–1204. [Google Scholar] [CrossRef]
- Jurczyk, K.; Miklaszewski, A.; Jurczyk, M.U.; Jurczyk, M. Development of β type Ti23Mo-45S5 bioglass nanocomposites for dental applications. Materials 2015, 8, 8032–8046. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.L. The Cu-Ti (Copper-Titanium) system. Bull. Alloy Phase Diagrams 1983, 4, 81–95. [Google Scholar] [CrossRef]
- Okamoto, H. Ti-Zn (Titanium-Zinc). J. Phase Equilibria Diffus. 2008, 29, 211–212. [Google Scholar] [CrossRef]
- Murray, J.L. The Nb-Ti (Niobium-Titanium) System. Bull. Alloy Phase Diagrams 1981, 2, 55–61. [Google Scholar] [CrossRef]
- Lin, K.L.; Lin, C.C. Ti2ZrO phases formed in the titanium and zirconia interface after reaction at 1550 °C. J. Am. Ceram. Soc. 2005, 88, 1268–1272. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Z.; Shao, H.; Liu, X.; Ji, Y. Mechanical properties of porous Ti-Mo and Ti-Nb alloys for biomedical application by gelcasting. Procedia Eng. 2012, 36, 160–167. [Google Scholar] [CrossRef]
- Cullinane, D.M.; Einhorn, T.A. Chapter 2—Biomechanics of Bone. In Principles of Bone Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 17–32. ISBN 978-0-12-098652-1. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Rodriguez-Florez, N.; Oyen, M.L.; Shefelbine, S.J. Insight into differences in nanoindentation properties of bone. J. Mech. Behav. Biomed. Mater. 2013, 18, 90–99. [Google Scholar] [CrossRef]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty: A review. J. Int. Med. Res. 2018, 46, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Thevenot, P.; Hu, W. Surface Chemistry Influences Implant Biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.G.; Leeflang, M.A.; Zhou, J.; Duszczyk, J. ZK30-bioactive glass composites for orthopedic applications: A comparative study on fabrication method and characteristics. Mater. Sci. Eng. B 2011, 176, 1644–1652. [Google Scholar] [CrossRef]
- Zaludin, M.A.F.; Jamaludin, S.B.; Idris, M.S.; Llah, N.A. Effect of 45S5 Bio-Glass Particles on Physical Properties and Corrosion Resistance of the Mg-5Zn Matrix Composite. Open J. Met. 2014, 04, 1–8. [Google Scholar] [CrossRef][Green Version]
- Tomashov, N.D.; Altovsky, R.M.; Chernova, G.P. Passivity and Corrosion Resistance of Titanium and Its Alloys. J. Electrochem. Soc. 1961, 108, 113. [Google Scholar] [CrossRef]
- Gu, J.L.; Shao, Y.; Zhao, S.F.; Lu, S.Y.; Yang, G.N.; Chen, S.Q.; Yao, K.F. Effects of Cu addition on the glass forming ability and corrosion resistance of Ti-Zr-Be-Ni alloys. J. Alloys Compd. 2017, 725, 573–579. [Google Scholar] [CrossRef]
- Clarke, J.K. On the Bacterial Factor in the Aetiology of Dental Caries. Br. J. Exp. Pathol. 1924, 5, 141. [Google Scholar]
- Kumar, R.; Münstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Wong, M.S.; Chu, W.C.; Sun, D.S.; Huang, H.S.; Chen, J.H.; Tsai, P.J.; Lin, N.T.; Yu, M.S.; Hsu, S.F.; Wang, S.L.; et al. Visible-light-induced bactericidal activity of a nitrogen-doped titanium photocatalyst against human pathogens. Appl. Environ. Microbiol. 2006, 72, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Zain, N.M. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J. 2018, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Cossellu, G.; Motta, V.; Dioni, L.; Angelici, L.; Vigna, L.; Farronato, G.; Pesatori, A.C.; Bollati, V. Titanium and Zirconium levels are associated with changes in MicroRNAs expression: Results from a human cross-sectional study on obese population. PLoS ONE 2016, 11, e0161916. [Google Scholar] [CrossRef]





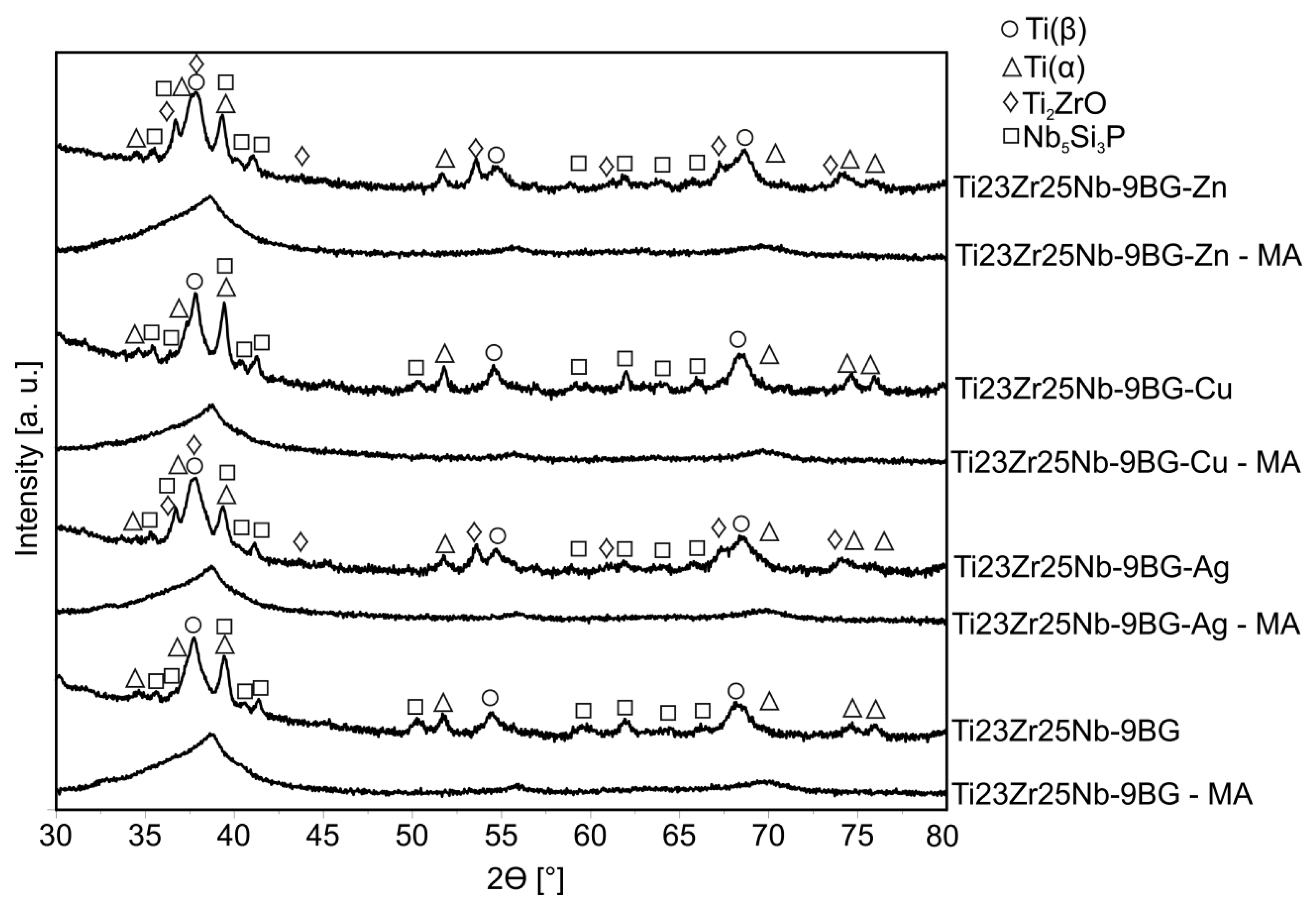







| Material | BG/TNZ [%] | AB/TNZ-BG [%] | T [h] | CP [MPa] | S [°C] |
|---|---|---|---|---|---|
| Ti23Zr25Nb-3BG | 3 | - | 16 | 600 | 800 |
| Ti23Zr25Nb-6BG | 6 | - | |||
| Ti23Zr25Nb-9BG | 9 | - | |||
| T23Zr25Nb-9B-Ag | 9 | 1 | |||
| T23Zr25Nb-9B-Cu | 9 | 1 | |||
| T23Zr25Nb-9B-Zn | 9 | 1 |
| Sample | Tiα | Tiβ | Ti2ZrO | Nb5Si3P | Rwp [%] | Rexp [%] | S | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a [Å] | c [Å] | V [Å3] | PA [%] | a [Å] | V [Å3] | PA [%] | a [Å] | c [Å] | V [Å3] | PA [%] | a [Å] | c [Å] | V [Å3] | PA [%] | ||||
| Ti23Zr25Nb | - | - | - | - | 3.3524 (2) | 37.68 (1) | 100 | - | - | - | - | - | - | - | - | 6.28 | 3.65 | 1.72 |
| Ti23Zr25Nb 3BG | 2.9987 (35) | 4.8192 (136) | 37.53 (20) | 2.08 | 3.3602 (2) | 37.94 (1) | 96.38 | - | - | - | - | 7.7633 (145) | 5.3173 (135) | 277.53 (1.74) | 1.54 | 4.65 | 3.4 | 1.37 |
| Ti23Zr25Nb 6BG | 2.9967 (9) | 4.7992 (35) | 37.32 (5) | 9.83 | 3.3577 (2) | 37.86 (1) | 75.63 | 4.7683 (11) | 3.0281 (7) | 59.62 (4) | 10.47 | 7.7296 (34) | 5.3123 (27) | 274.87 (38) | 4.07 | 3.83 | 3.22 | 1.19 |
| Ti23Zr25Nb 9BG | 3.0003 (6) | 4.8085 (21) | 37.49 (3) | 28.13 | 3.3720 (4) | 38.34 (2) | 65.02 | - | - | - | - | 7.7132 (25) | 5.2967 (20) | 272.9 (28) | 6.86 | 4.52 | 3.44 | 1.31 |
| Ti23Zr25Nb 9BG-Ag | 3.0011 (8) | 4.7970 (32) | 37.42 (5) | 15.3 | 3.3579 (4) | 37.86 (2) | 65.98 | 4.7858 (10) | 3.0329 (8) | 60.16 (4) | 13.73 | 7.7575 (29) | 5.3213 (22) | 277.33 (32) | 4.99 | 3.97 | 3.1 | 1.28 |
| Ti23Zr25Nb 9BG-Cu | 2.9946 (4) | 4.8229 (12) | 37.46 (2) | 31.89 | 3.3629 (3) | 38.03 (1) | 59.1 | - | - | - | - | 7.7398 (27) | 5.3117 (21) | 275.57 (30) | 9.00 | 5.02 | 3.38 | 1.49 |
| Ti23Zr25Nb 9BG-Zn | 3.0033 (5) | 4.8188 (21) | 37.64 (3) | 17.13 | 3.3557 (3) | 37.79 (1) | 54.68 | 4.7897 (7) | 3.0301 (6) | 60.20 (3) | 19.47 | 7.7671 (24) | 5.3331 (18) | 278.63 (26) | 8.73 | 3.64 | 2.96 | 1.23 |
| Material | Porosity [%] |
|---|---|
| Ti23Zr25Nb-3BG | 15.4 ± 3.6 |
| Ti23Zr25Nb-6BG | 22.1 ± 8.9 |
| Ti23Zr25Nb-9BG | 22.8 ± 6.5 |
| Ti23Zr25Nb-9BG-Ag | 14.0 ± 4.1 |
| Ti23Zr25Nb-9BG-Cu | 18.3 ± 7.8 |
| Ti23Zr25Nb-9BG-Zn | 13.7 ± 4.6 |
| Sample | HV0.3 | CA [M] Diiodomethane [°] | CA [M] Glycerol [°] | SFE [mN/m] | Disperse [mN/m] | Polar [mN/m] |
|---|---|---|---|---|---|---|
| Ti23Zr25Nb | 375 ± 33 | 62.2 ± 9.0 | 64.6 ± 4.9 | 35.1 ± 10.0 | 27.4 ± 5.6 | 7.7 ± 4.4 |
| Ti23Zr25Nb-3BG | 315 ± 36 | 50.8 ± 7.4 | 70.2 ± 4.5 | 36.1 ± 6.5 | 33.8 ± 4.4 | 2.3 ± 2.1 |
| Ti23Zr25Nb-6BG | 321 ± 22 | 56.0 ± 6.2 | 68.1 ± 7.4 | 34.6 ± 5.7 | 30.9 ± 3.7 | 3.7 ± 2.0 |
| Ti23Zr25Nb-9BG | 218 ± 23 | 57.7 ± 4.4 | 61.5 ± 4.2 | 36.9 ± 3.3 | 29.9 ± 2.5 | 7.0 ± 0.8 |
| Ti23Zr25Nb-9BG-Ag | 322 ± 24 | 44.9 ± 3.8 | 57.5 ± 2.4 | 42.3 ± 4.2 | 37.0 ± 2.2 | 5.3 ± 2.0 |
| Ti23Zr25Nb-9BG-Cu | 329 ± 49 | 49.3 ± 2.4 | 66.7 ± 4.7 | 37.5 ± 2.5 | 34.7 ± 1.3 | 2.8 ± 1.2 |
| Ti23Zr25Nb-9BG-Zn | 387 ± 49 | 50.5 ± 6.6 | 77.8 ± 3.1 | 34.6 ± 4.4 | 33.9 ± 3.9 | 0.7 ± 0.5 |
| Sample | CFU/mL After 4 h of Incubation | CFU/mL After 20 h of Incubation | RF % |
|---|---|---|---|
| Ti23Zr25Nb | <1.0 × 103 | 3.2 × 104 | 78.67 |
| Ti23Zr25Nb-9BG | <1.0 × 103 | 2.4 × 104 | 84 |
| Ti23Zr25Nb-9BG-Ag | <1.0 × 103 | 3.1 × 103 | 97.93 |
| Ti23Zr25Nb-9BG-Cu | <1.0 × 103 | 8.5 × 103 | 94.33 |
| Ti23Zr25Nb-9BG-Zn | <1.0 × 103 | 3.5 × 103 | 97.67 |
| microcrystalline Ti (control) | <1.0 × 103 | 2.0 × 105 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marczewski, M.; Jurczyk, M.; Pecyna, P.; Ratajczak, M.; Gajecka, M.; Jurczyk, M.U. The Effect of 45S5 Bioglass and Ag, Cu, or Zn Addition on the Crystal Structure, Properties, and Antibacterial Effect of Bulk Ti23Zr25Nb Biocomposites. Metals 2020, 10, 1115. https://doi.org/10.3390/met10091115
Marczewski M, Jurczyk M, Pecyna P, Ratajczak M, Gajecka M, Jurczyk MU. The Effect of 45S5 Bioglass and Ag, Cu, or Zn Addition on the Crystal Structure, Properties, and Antibacterial Effect of Bulk Ti23Zr25Nb Biocomposites. Metals. 2020; 10(9):1115. https://doi.org/10.3390/met10091115
Chicago/Turabian StyleMarczewski, M., M. Jurczyk, P. Pecyna, M. Ratajczak, M. Gajecka, and M. U. Jurczyk. 2020. "The Effect of 45S5 Bioglass and Ag, Cu, or Zn Addition on the Crystal Structure, Properties, and Antibacterial Effect of Bulk Ti23Zr25Nb Biocomposites" Metals 10, no. 9: 1115. https://doi.org/10.3390/met10091115
APA StyleMarczewski, M., Jurczyk, M., Pecyna, P., Ratajczak, M., Gajecka, M., & Jurczyk, M. U. (2020). The Effect of 45S5 Bioglass and Ag, Cu, or Zn Addition on the Crystal Structure, Properties, and Antibacterial Effect of Bulk Ti23Zr25Nb Biocomposites. Metals, 10(9), 1115. https://doi.org/10.3390/met10091115







