Effect of Aging Treatment on Microstructure and Properties of the Fe55(CoCrNi)10(MoV)5C5 Medium-Entropy Alloy
Abstract
1. Introduction
2. Experimental Procedures
3. Results and Discussion
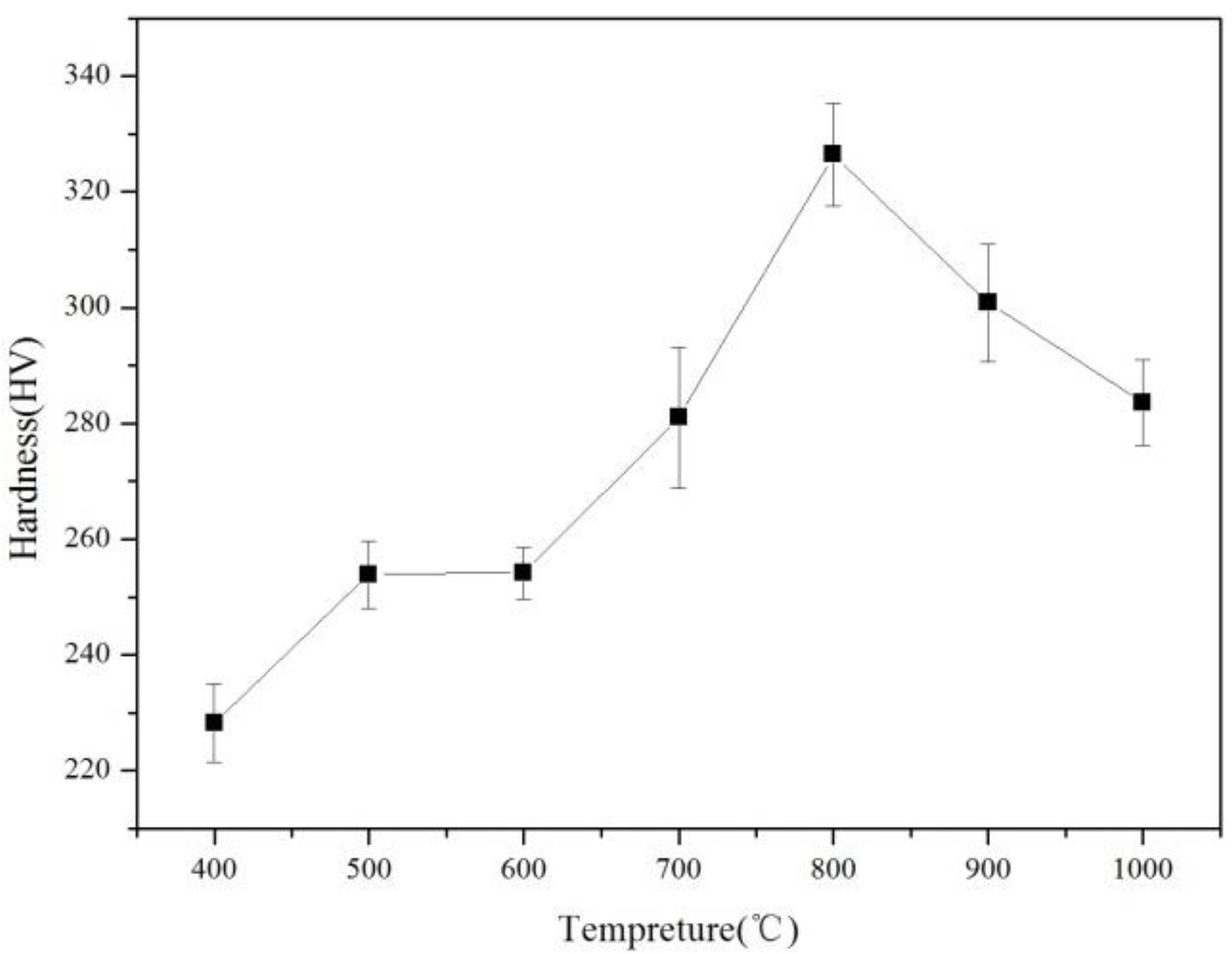
3.1. Age-Hardening Curve
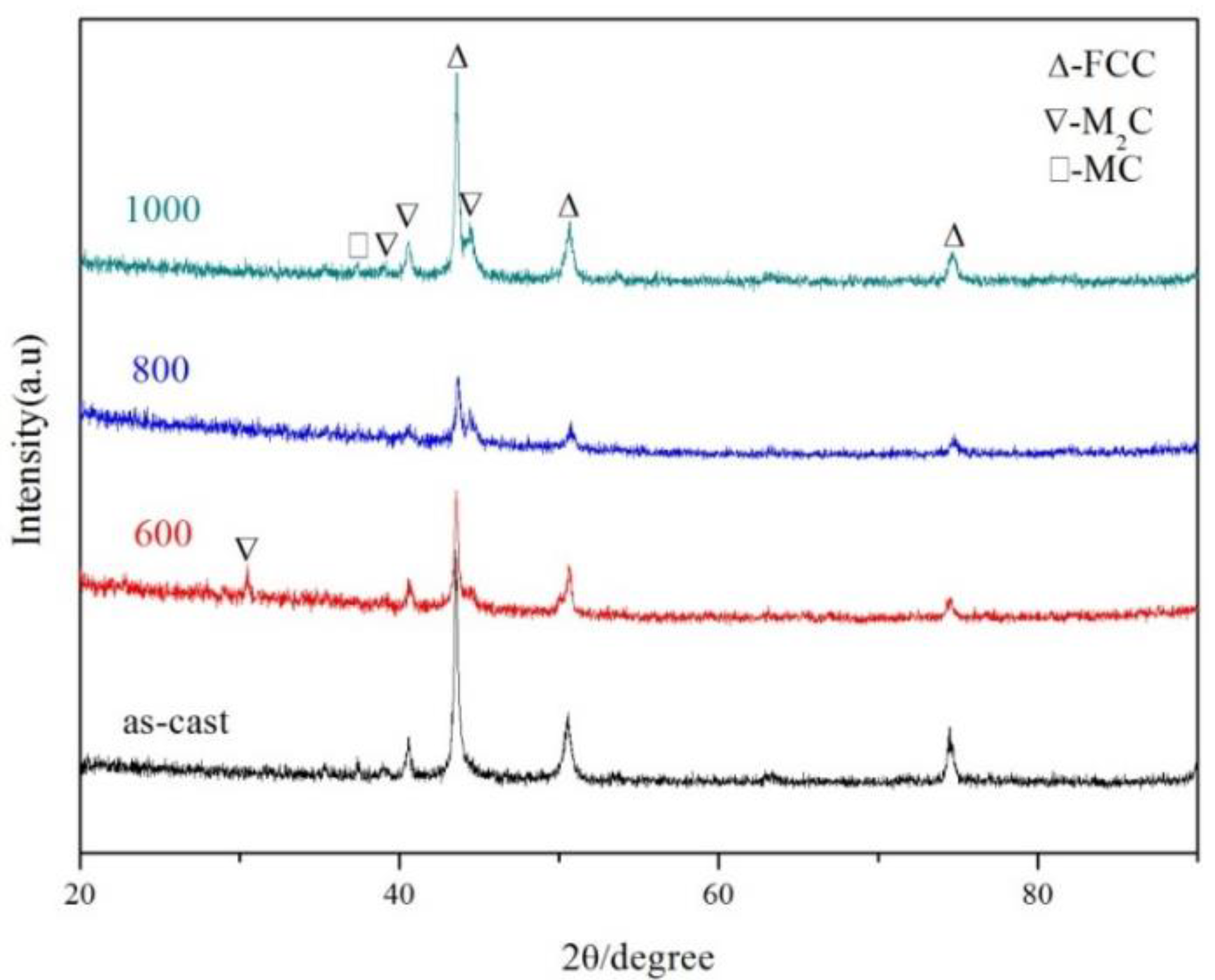
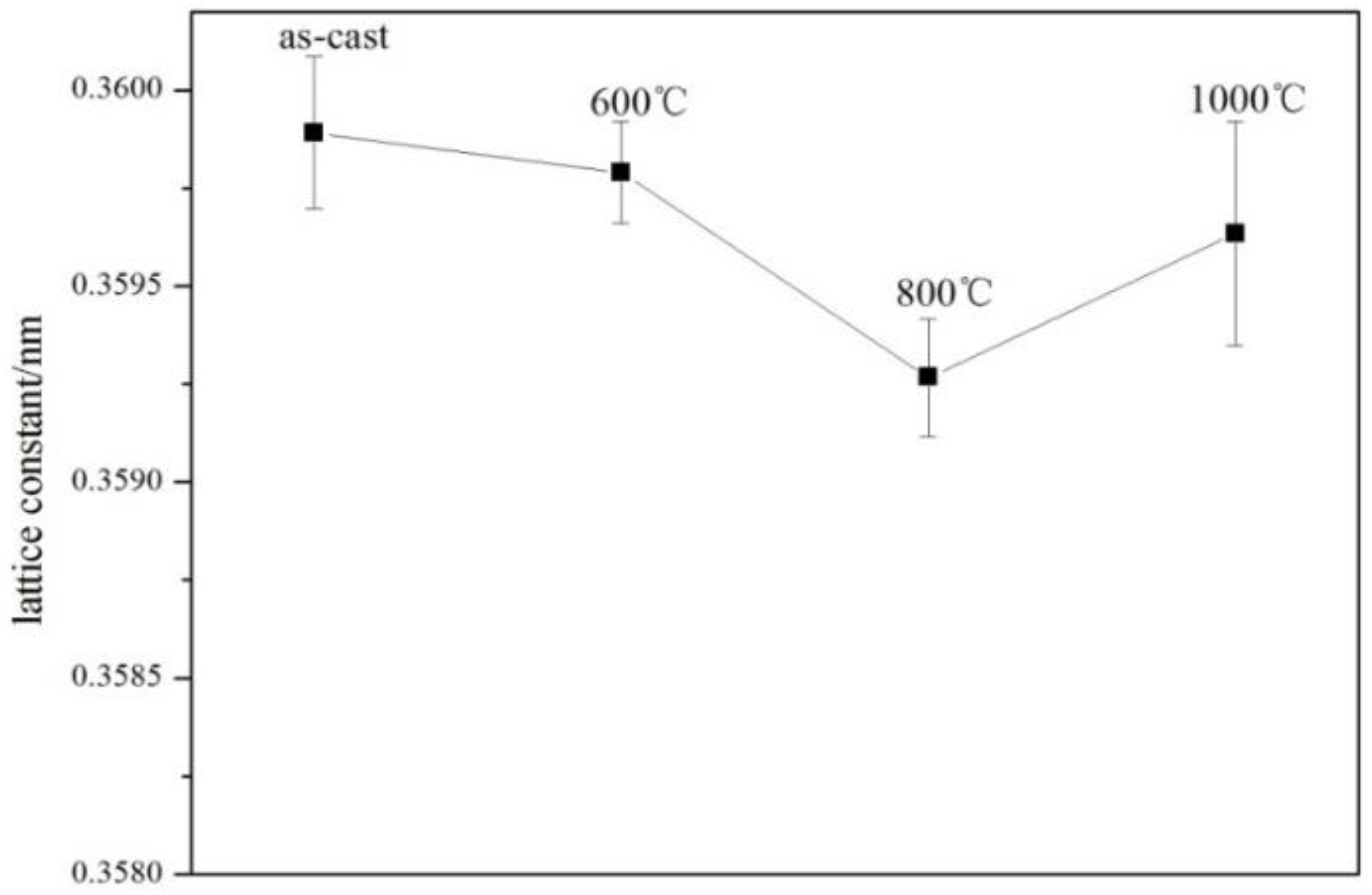
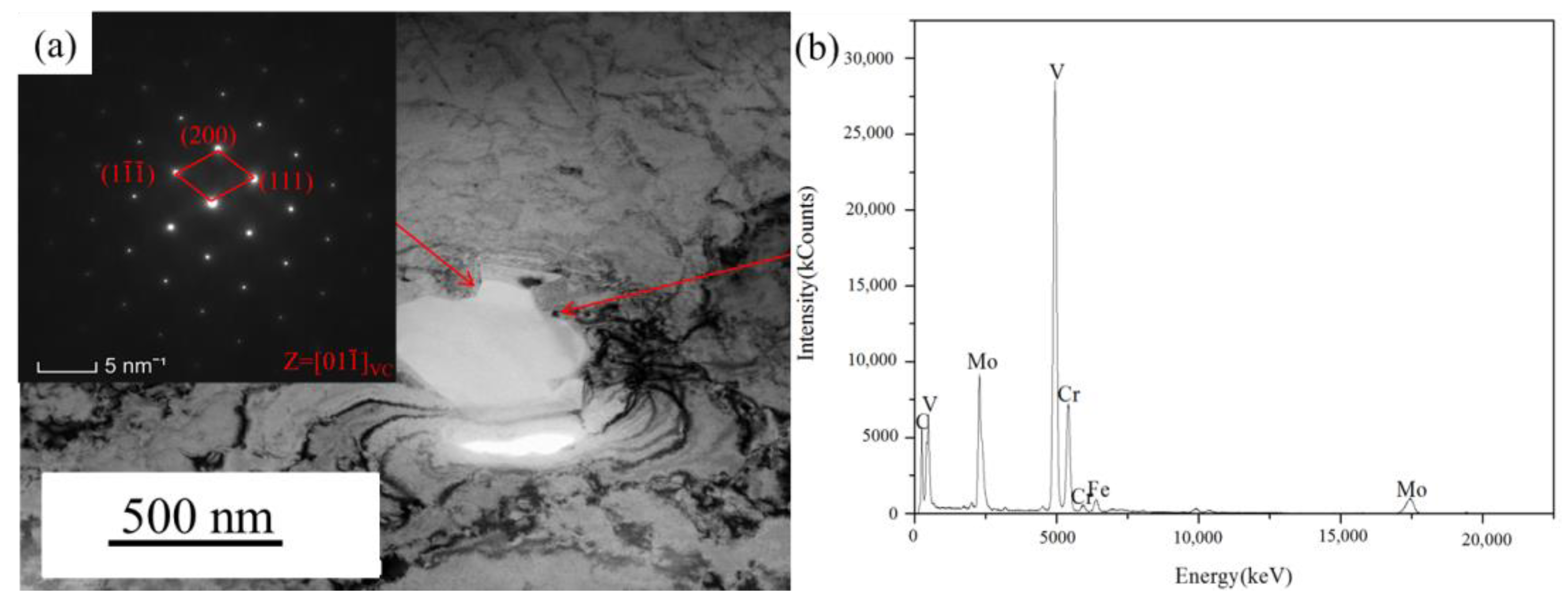
3.2. Phase Analysis
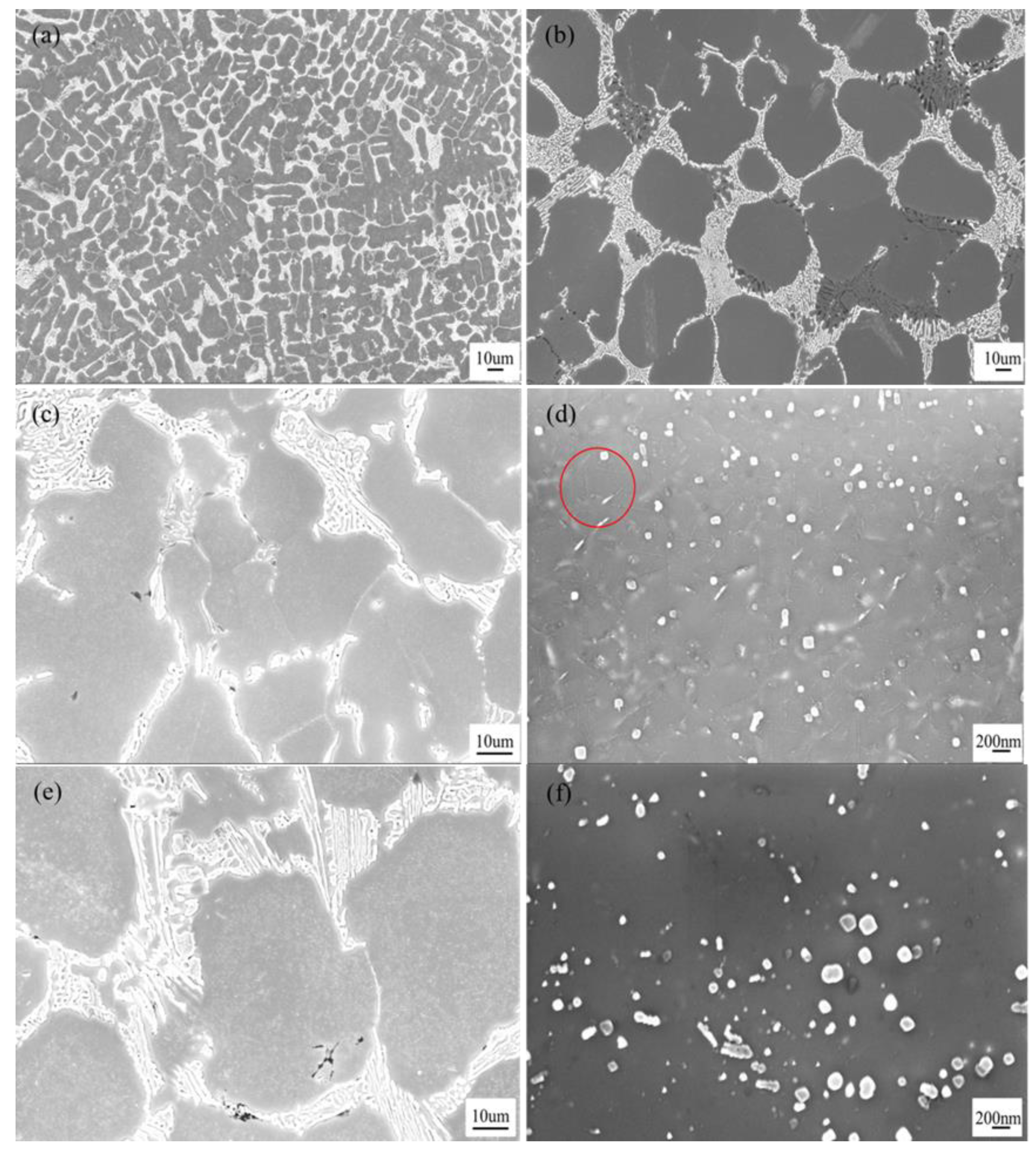
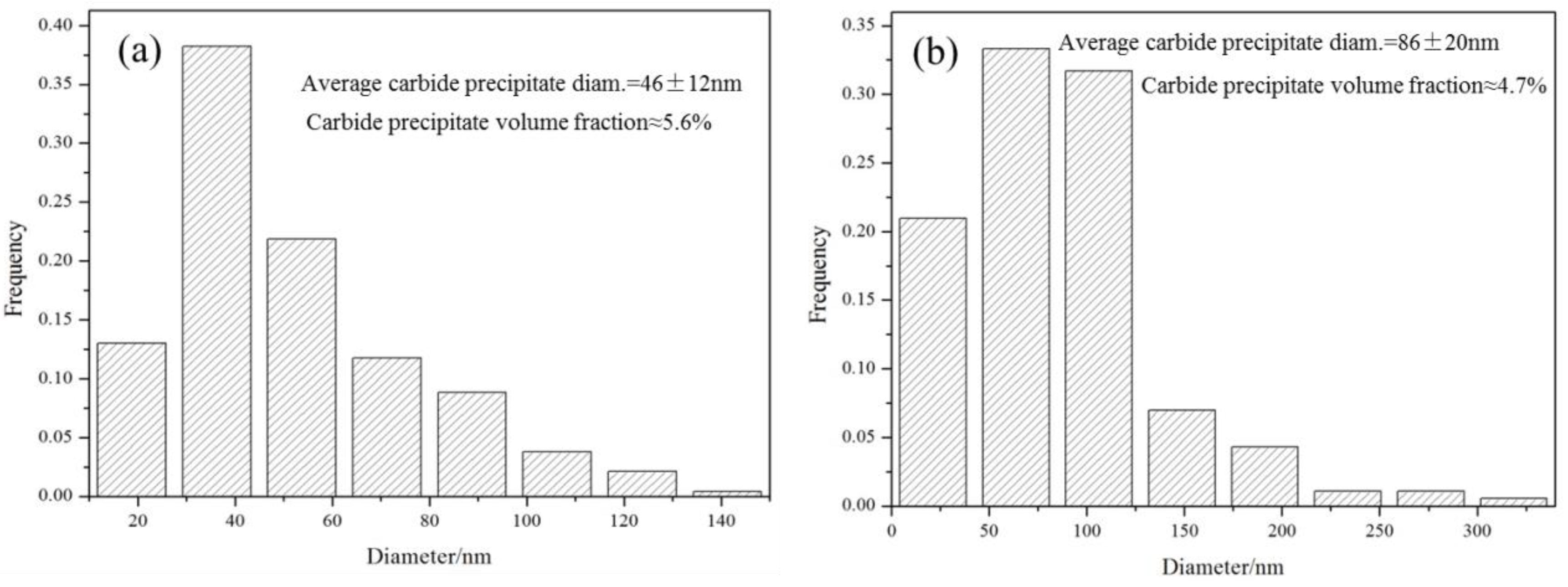
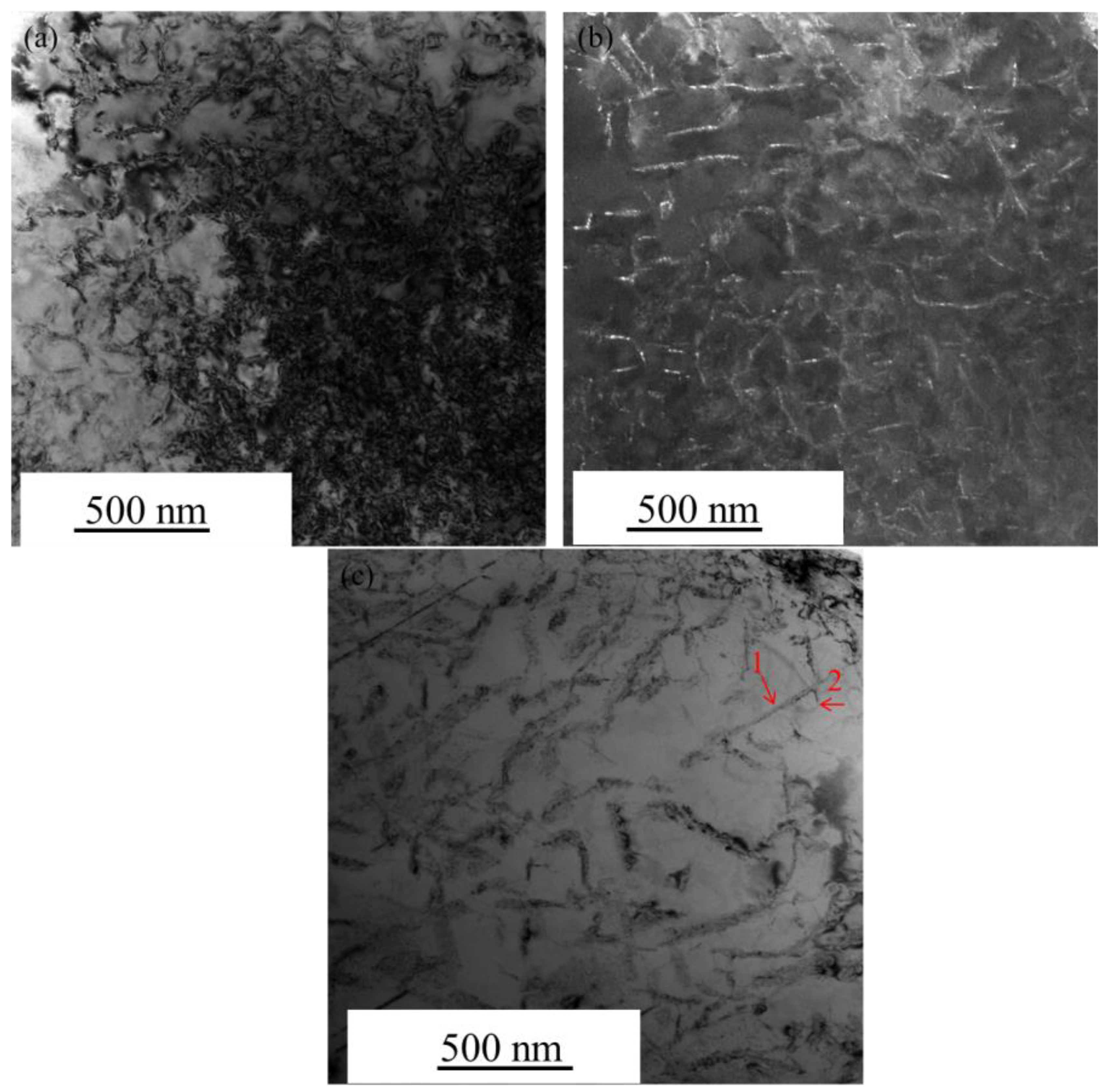
3.3. Microstructures
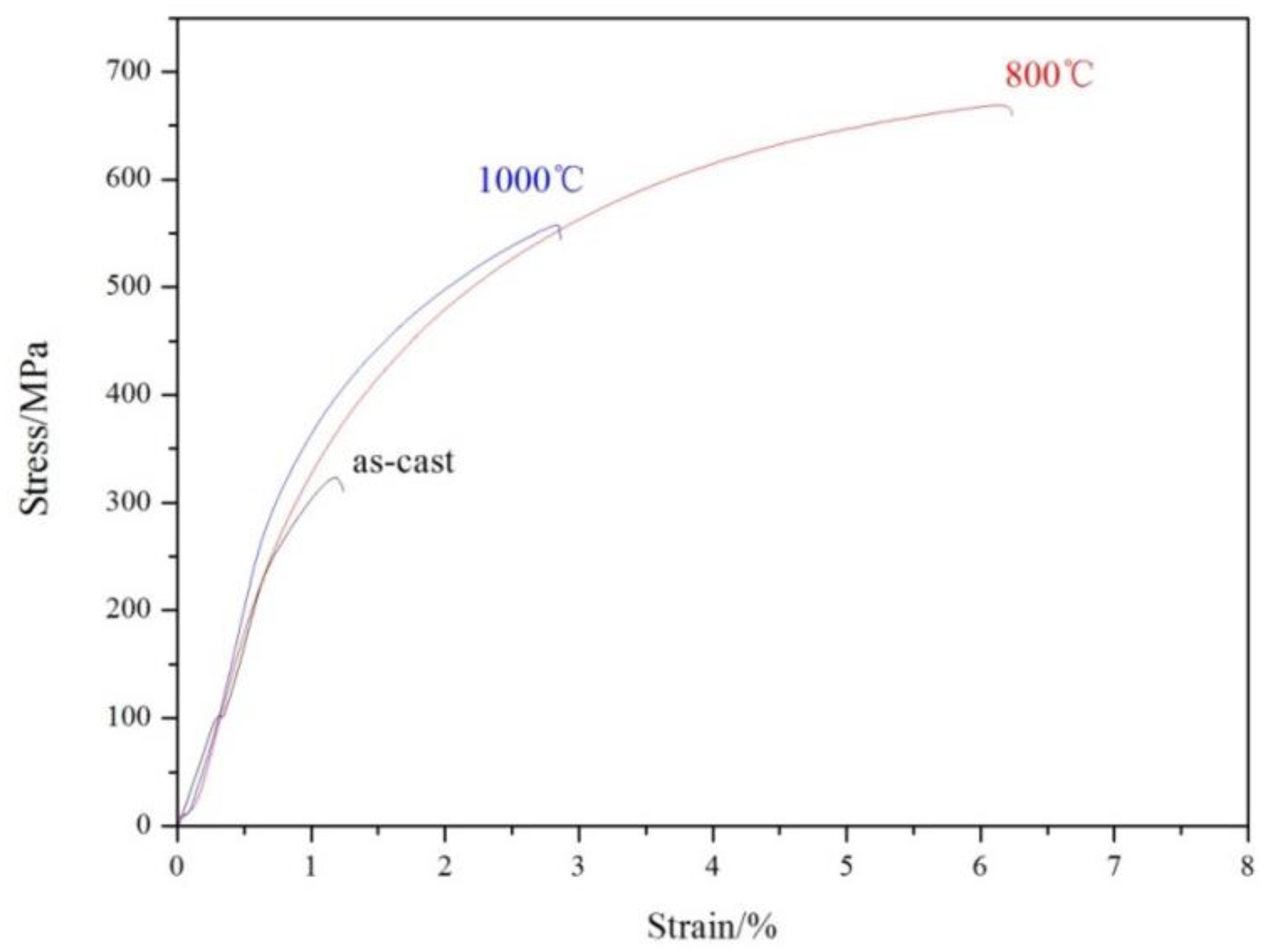
3.4. Tensile Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Wu, Q.; Niu, S.; Li, J.; Wang, J.; Liu, C.T. Solid solution is land of the Co-Cr-Fe-Ni high entropy alloy system. Scr. Mater. 2017, 131, 42–46. [Google Scholar] [CrossRef]
- Zhang, W.R.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Chernichenko, R.S.; Yurchenko, N.Y.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Effect of thermomechanical processing on microstructure and mechanical properties of the carbon-containing CoCrFeNiMn high entropy alloy. J. Alloys Compd. 2017, 693, 394–405. [Google Scholar] [CrossRef]
- Qiu, X.W.; Wu, M.J.; Liu, C.G.; Zhang, Y.P.; Huang, C.X. Corrosion performance of Al2CrFeCoxCuNiTi high-entropy alloy coatings in acid liquids. J. Alloys Compd. 2017, 708, 353–357. [Google Scholar] [CrossRef]
- Seol, J.B.; Bae, J.W.; Li, Z.; Han, J.C.; Kim, J.G.; Raabe, D.; Kim, H.S. Boron doped ultrastrong and ductile high-entropy alloys. Acta Mater. 2018, 151, 366–376. [Google Scholar] [CrossRef]
- Riva, S.; Tudball, A.; Mehraban, S.; Lavery, N.P.; Brown, S.G.R.; Yusenko, K.V. A novel High-Entropy Alloy-based composite material. J. Alloys Compd. 2018, 730, 544–551. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L.; Bor, H.Y. Effect of aging treatment on microstructure and properties of high-entropy Cu0.5CoCrFeNi alloy. Intermetallics 2010, 18, 1244–1250. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.X.; Li, D.M.; Shi, L.; Cai, B.; Wang, M.X. Corrigendum to “Effect of elemental interaction on microstructure of CuCrFeNiMn high-entropy alloy system”. J. Alloys Compd. 2010, 503, 538. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Li, X.F.; Feng, Y.H.; Liu, B.; Yi, D.H.; Yang, X.H.; Zhang, W.D.; Chen, G.; Liu, Y.; Bai, P.K. Influence of NbC particles on microstructure and mechanical properties of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. J. Alloys Compd. 2019, 788, 485–494. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.M.; Qiao, D.X.; Lu, Y.P.; Chao, Z.Q.; Li, T.J. Effects of Ta Addition on the Microstructures and Mechanical Properties of CoCrFeNi High Entropy Alloy. Mater. Chem. Phys. 2018, 210, 43–48. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Age-hardening of the CoCrFeNiMo0.85 high-entropy alloy. Mater. Charact. 2013, 81, 92–96. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.J.; Liu, S.Z.; Wu, Q.F.; Li, J.J.; Wang, J.C.; Liu, C.T.; Dang, Y.Y. Strengthening the CoCrFeNiNb0.25 high entropy alloy by FCC precipitate. J. Alloys Compd. 2016, 667, 53–57. [Google Scholar] [CrossRef]
- Saenarjhan, N.; Kang, J.H.; Kim, S.J. Effects of carbon and nitrogen on austenite stability and tensile deformation behavior of 15Cr-15Mn-4Ni based austenitic stainless steels. Mater. Sci. Eng. A 2019, 742, 608–616. [Google Scholar] [CrossRef]
- Conrad, H. Effect of interstitial solutes on the strength and ductility of titanium. Prog. Mater. Sci. 1981, 26, 123–403. [Google Scholar] [CrossRef]
- Urrutia, I.G.; Raabe, D. Multistage strain hardening through dislocation substructure and twinning in a high strength and ductile weight-reduced Fe-Mn-Al-C steel. Acta Mater. 2012, 60, 5791–5802. [Google Scholar] [CrossRef]
- Gutierrez, I.; Raabe, D. Microbanding mechanism in a Fe-Mn-C high-Mn twinning-induced plasticity steel. Scr. Mater. 2013, 69, 53–56. [Google Scholar] [CrossRef]
- Ou, P.; Xing, H.; Sun, J. Precipitation of nanosized MX at coherent Cu-rich phases in Super304H austenitic steel. Metall. Mater. Trans. A 2015, 46, 1–5. [Google Scholar] [CrossRef]
- Ou, P.; Li, L.; Xie, X.F.; Sun, J. Steady-state creep behavior of Super304H austenitic steel at elevated temperatures. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 1336–1343. [Google Scholar] [CrossRef]
- Anburaj, J.; Nazirudeen, S.S.M.; Narayanan, R.; Anandavel, B.; Chandrasekar, A. Ageing of forged superaustenitic stainless steel: Precipitate phases and mechanical properties. Mater. Sci. Eng. A 2012, 535, 99–107. [Google Scholar] [CrossRef]
- Wu, Z.; Parish, C.M.; Bei, H. Nano-twin mediated plasticity in carbon-containing FeNiCoCrMn high entropy alloys. J. Alloys Compd. 2015, 647, 815–822. [Google Scholar] [CrossRef]
- Gao, N.; Lu, D.H.; Zhao, Y.Y.; Liu, X.W.; Liu, G.H.; Wu, Y.; Liu, G.; Fan, Z.T.; Lu, Z.P.; George, E.P. Strengthening of a CrMnFeCoNi high-entropy alloy by carbide precipitation. J. Alloys Compd. 2019, 792, 1028–1035. [Google Scholar] [CrossRef]
- Tasan, C.C.; Deng, Y.; Pradeep, K.G.; Yao, M.J.; Springer, H.; Raabe, D. Composition Dependence of Phase Stability, Deformation Mechanisms, and Mechanical Properties of the CoCrFeMnNi High-Entropy Alloy System. JOM 2014, 66, 1993–2001. [Google Scholar] [CrossRef]
- Ma, D.C.; Yao, M.J.; Pradeep, K.G.; Tasan, C.C.; Springer, H.; Raabe, D. Phase stability of non-equiatomic CoCrFeMnNi high entropy alloys. Acta Mater. 2015, 98, 288–296. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Tikhonovsky, M.A.; Salishchev, G.A. Tensile properties of the Cr-Fe-Ni-Mn non-equiatomic multicomponent alloys with different Cr contents. Mater. Des. 2015, 87, 60–65. [Google Scholar] [CrossRef]
- Scott, C.; Remy, B.; Collet, J.L.; Cael, A.; Bao, C.; Danoix, F.; Malard, B.; Curfs, C. Precipitation strengthening in high manganese austenitic TWIP steels. Int. J. Mater. Res. 2011, 102, 538–549. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.Y.; Han, B.; Han, T.; Cheng, Y.Y. Influence of secondary carbides precipitation and transformation on the secondary hardening of laser melted high chromium steel. J. Mater. Sci. 2010, 45, 3442–3447. [Google Scholar] [CrossRef]
- Xu, W.; Rivera-Diaz-del-Castillo, P.E.J.; Yan, W.; Yang, K.; Martin, D.S.; Kestens, L.A.I.; van der Zwaag, S. A new ultrahigh-strength stainless steel strengthened by various coexisting nanoprecipates. Acta Mater. 2010, 58, 4067–4075. [Google Scholar] [CrossRef]
- Wang, H.B.; Hou, L.G.; Li, Y.B.; Ou, P.; Shen, L.; Wen, X.E.; Cui, H.; Zhang, J.S. Effect of niobium on the secondary precipitates and tempering resistance of spray-formed M3:2 high-speed steel. J. Mater. Eng. Perform. 2019, 28, 926–937. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, T.; Li, L.; Zhao, Y.H.; Huang, J.F.; Liaw, P.K.; Zhang, Y. A low-cost lightweight entropic alloy with high strength. J. Mater. Eng. Perform. 2018, 27, 6648–6656. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Transm. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Zhong, Q.P.; Zhao, Z.H. Fractography; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Kamikawa, N.; Abe, Y.; Miyamoto, G.; Funakawa, Y.; Furuhara, T. Erratum to “Tensile Behavior of Ti, Mo-added Low Carbon Steels with Interphase Precipitation”. ISIJ Int. 2014, 54, 212–221. [Google Scholar] [CrossRef]
- Hao, L.H.; Ji, X.; Zhang, G.Q.; Zhao, W.; Sun, M.Y.; Peng, Y. Carbide precipitation behavior and Mechanical properties of micro-alloyed medium Mn steel. J. Mater. Sci. Technol. 2020, 47, 122–130. [Google Scholar] [CrossRef]


| Elements (at %) | Fe | Co | Cr | Ni | Mo | V | C |
|---|---|---|---|---|---|---|---|
| Nominal composition | 55 | 10 | 10 | 10 | 5 | 5 | 5 |
| Real composition | 49.56 | 12.05 | 11.02 | 11.56 | 5.35 | 5.69 | 5.1 |
| Alloy | δ/% | VEC | Ω | |||
|---|---|---|---|---|---|---|
| Fe55(CoCrNi)10(MoV)5C5 | 10.967 | −3.23 | 0.14 | 5.9 | 7.45 | 6.15 |
| Element | C | V | Cr | Fe | Co | Ni | Mo |
|---|---|---|---|---|---|---|---|
| C | - | −82 | −61 | −50 | −42 | −39 | −67 |
| V | - | −2 | −7 | −14 | −18 | 0 | |
| Cr | - | −1 | −4 | −7 | 0 | ||
| Fe | - | −1 | −2 | −2 | |||
| Co | - | 0 | −5 | ||||
| Ni | - | −7 | |||||
| Mo | - |
| States | Yield Strength /MPa | Ultimate Tensile Strength /MPa | Elongation ε/% |
|---|---|---|---|
| As-cast | 313 | 322 | 1.2 |
| 800 °C | 349 | 626 | 6.2 |
| 1000 °C | 374 | 548 | 2.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, D.; Wang, H.; Hou, L.; Ou, P.; Wang, Y.; Zhao, H. Effect of Aging Treatment on Microstructure and Properties of the Fe55(CoCrNi)10(MoV)5C5 Medium-Entropy Alloy. Metals 2020, 10, 1093. https://doi.org/10.3390/met10081093
Hong D, Wang H, Hou L, Ou P, Wang Y, Zhao H. Effect of Aging Treatment on Microstructure and Properties of the Fe55(CoCrNi)10(MoV)5C5 Medium-Entropy Alloy. Metals. 2020; 10(8):1093. https://doi.org/10.3390/met10081093
Chicago/Turabian StyleHong, Da, Hebin Wang, Longgang Hou, Ping Ou, Yiqi Wang, and Hongjin Zhao. 2020. "Effect of Aging Treatment on Microstructure and Properties of the Fe55(CoCrNi)10(MoV)5C5 Medium-Entropy Alloy" Metals 10, no. 8: 1093. https://doi.org/10.3390/met10081093
APA StyleHong, D., Wang, H., Hou, L., Ou, P., Wang, Y., & Zhao, H. (2020). Effect of Aging Treatment on Microstructure and Properties of the Fe55(CoCrNi)10(MoV)5C5 Medium-Entropy Alloy. Metals, 10(8), 1093. https://doi.org/10.3390/met10081093




