Leaching Behavior of Gold and Silver from Concentrated Sulfide Ore Using Ammonium Thiosulfate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Effect of Temperature on Au and Ag Leaching Efficiency
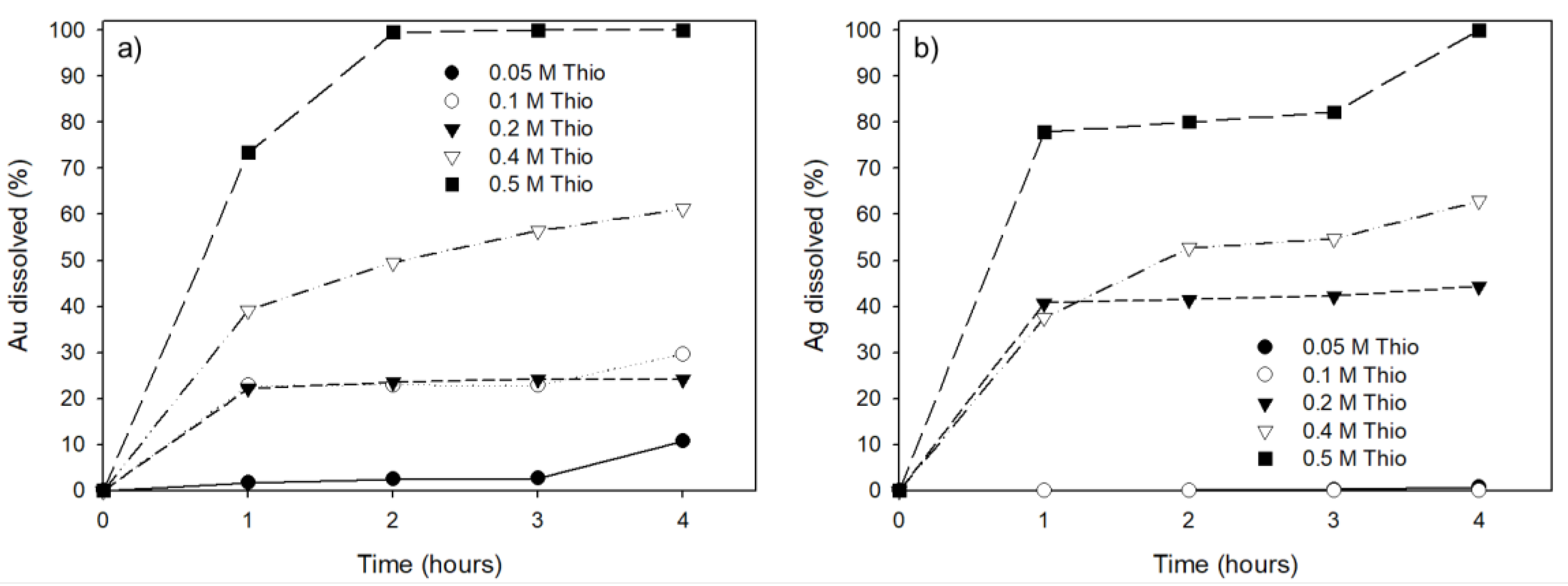
3.2. Effect of Ammonium Thiosulfate Concentration on Au and Ag Leaching Efficiency
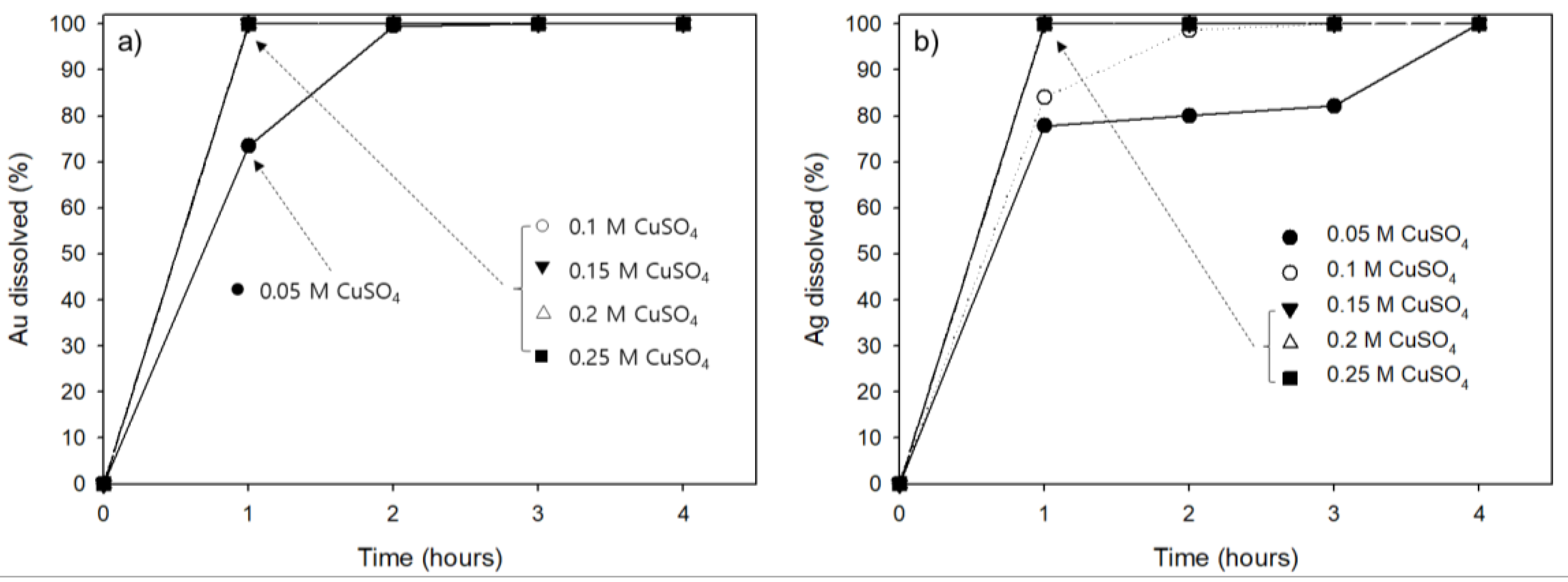
3.3. Effect of Copper Sulfate Concentration on Au and Ag Leaching Efficiency
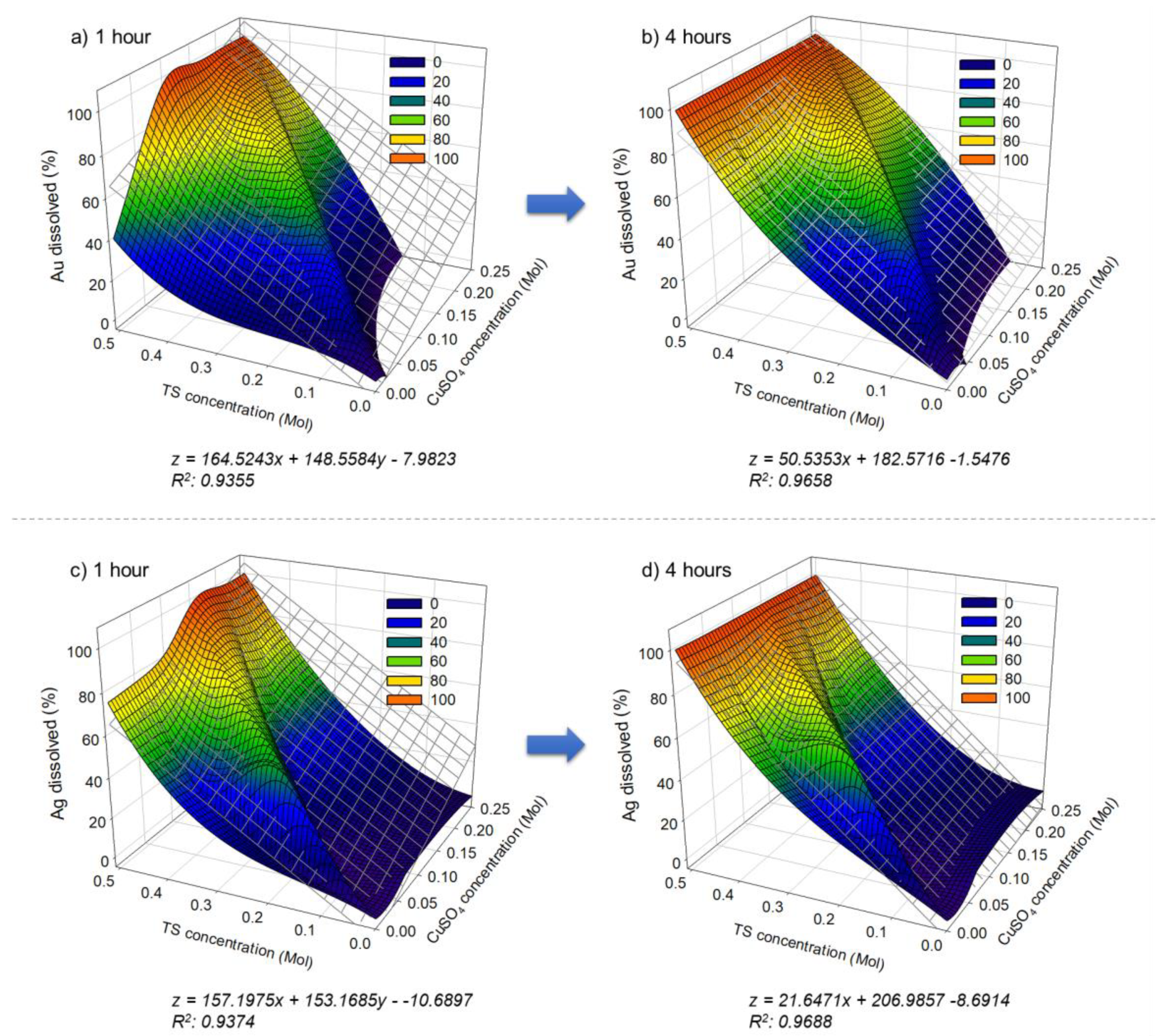
3.4. Correlation between the Concentration of Ammonium Thiosulfate and Copper Sulfate for Au and Ag Leaching
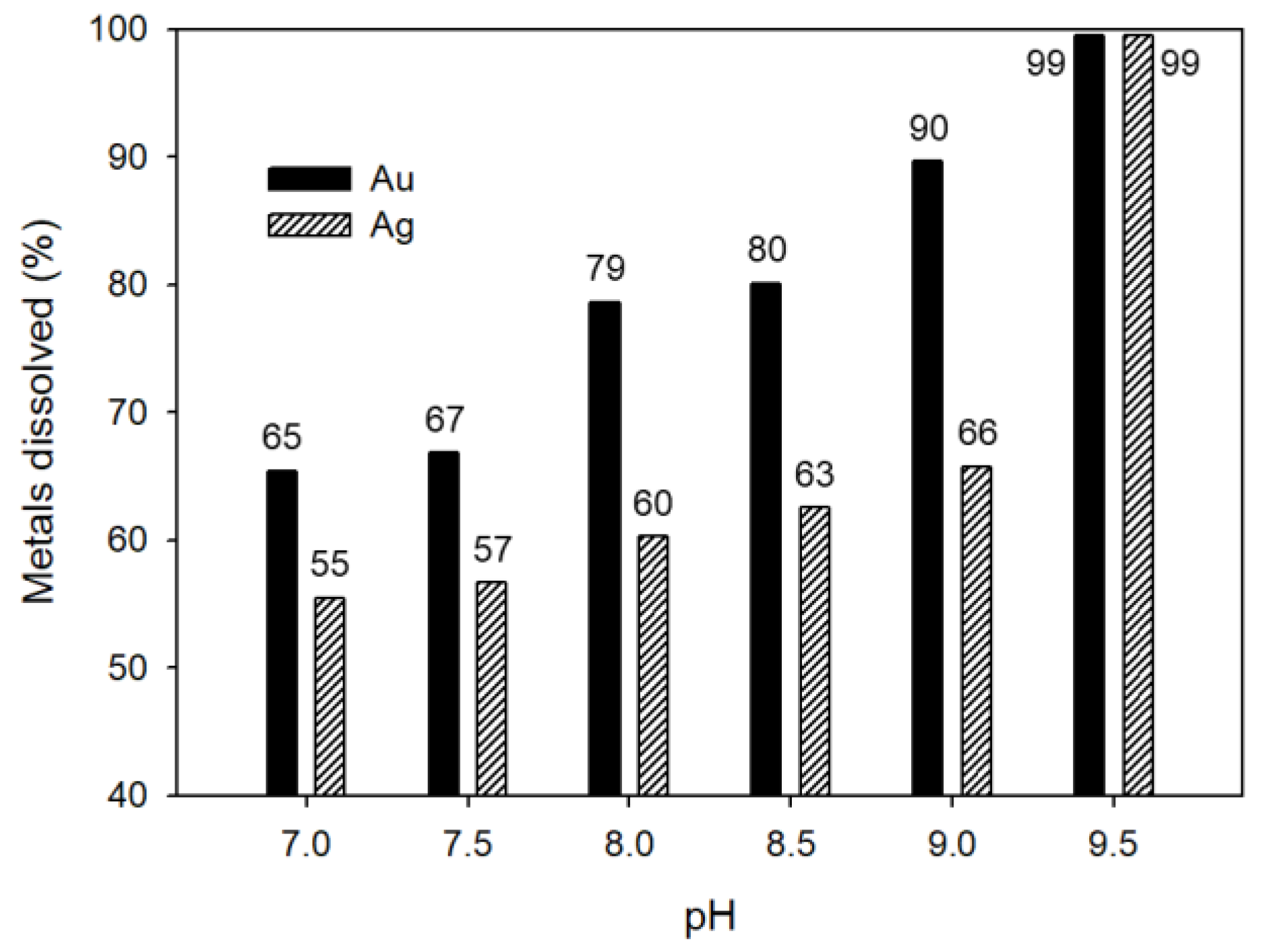
3.5. Effect of pH on Au and Ag Leaching Efficiency
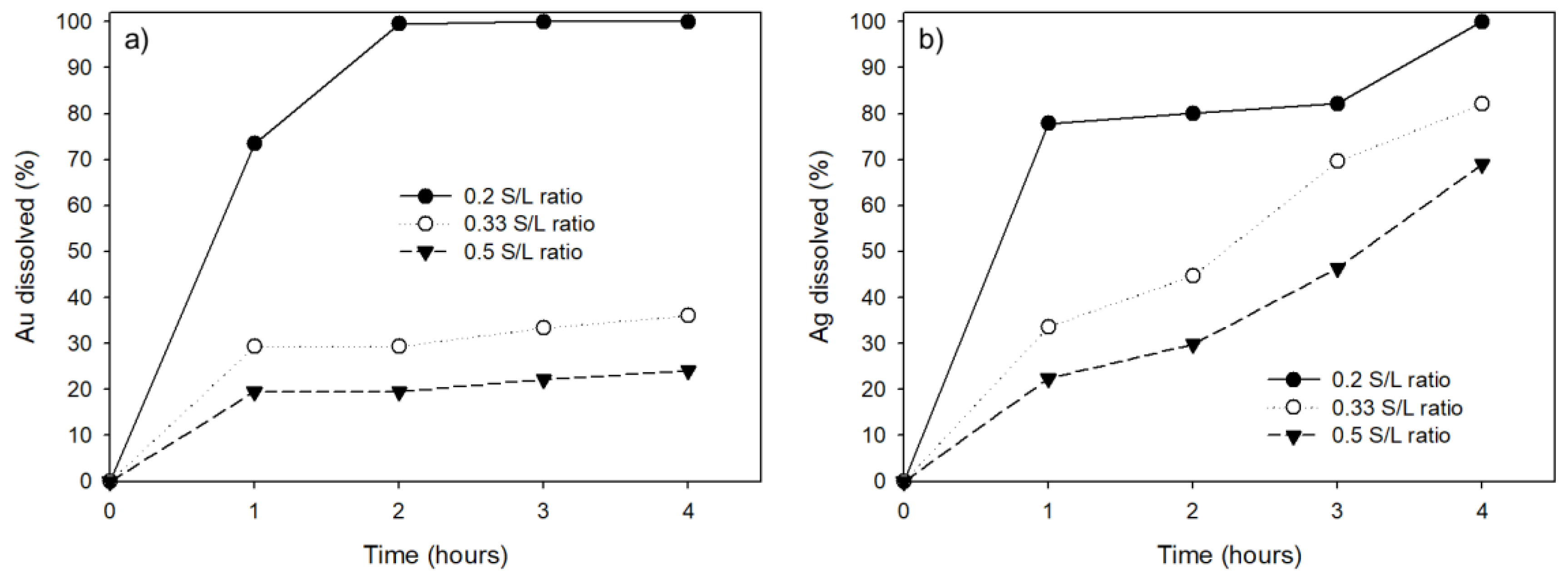
3.6. Effect of Solid/Liquid ratio on Au and Ag Leaching Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Muir, D.M.; Aylmore, M.G. Thiosulphate as an alternative to cyanide for gold processing – issues and impediments. Miner. Process. Extr. Metall. 2004, 113, 2–12. [Google Scholar] [CrossRef]
- Porvali, A.; Rintala, L.; Aromaa, J.; Kaartinen, T.; Forsen, O.; Lundstrom, M. Thiosulfate-copper-ammonia leaching of pure gold and pressure oxidized concentrate. Physicochem. Probl. Miner. Process. 2017. [Google Scholar] [CrossRef]
- Langhans, J.W.; Lei, K.P.V.; Carnahan, T.G. Copper-catalyzed thiosulfate leaching of low-grade gold ores. Hydrometallurgy 1992, 29, 191–203. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Fornari, P.; Massidda, R.; Vegliò, F.; Ubaldini, S. Thiosulphate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Zipperian, D.; Raghavan, S.; Wilson, J.P. Gold and silver extraction by ammoniacal thiosulfate leaching from a rhyolite ore. Hydrometallurgy 1988, 19, 361–375. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by copper(II) in ammoniacal thiosulphate solutions in the presence of additives. Part I: A review of the effect of hard–soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115–116, 1–20. [Google Scholar] [CrossRef]
- Senanayake, G.; Zhang, X.M. Gold leaching by copper(II) in ammoniacal thiosulphate solutions in the presence of additives. Part II: Effect of residual Cu(II), pH and redox potentials on reactivity of colloidal gold. Hydrometallurgy 2012, 115–116, 21–29. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Jiang, T.; Li, Q.; Zhang, X.; Wang, D. Improved thiosulfate leaching of a refractory gold concentrate calcine with additives. Hydrometallurgy 2015, 152, 214–222. [Google Scholar] [CrossRef]
- Mahmoud, M.H.H.; Awad, H.M. Improved recovery of gold and silver from thiosulfate solution on activated carbon in presence of ammonium persulfate. Physicochem. Probl. Miner. Process. 2019. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thermodynamic analysis of gold leaching by ammoniacal thiosulfate using Eh/pH and speciation diagrams. Min. Metall. Explor. 2001, 18, 221–227. [Google Scholar] [CrossRef]
- Senanayake, G. Analysis of reaction kinetics, speciation and mechanism of gold leaching and thiosulfate oxidation by ammoniacal copper(II) solutions. Hydrometallurgy 2004, 75, 55–75. [Google Scholar] [CrossRef]
- Seisko, S.; Lampinen, M.; Aromaa, J.; Laari, A.; Koiranen, T.; Lundström, M. Kinetics and mechanisms of gold dissolution by ferric chloride leaching. Miner. Eng. 2018, 115, 131–141. [Google Scholar] [CrossRef]
- Roldán-Contreras, E.; Salinas-Rodríguez, E.; Hernández-Ávila, J.; Cerecedo-Sáenz, E.; Rodríguez-Lugo, V.; Jeldres, R.I.; Toro, N. Leaching of Silver and Gold Contained in a Sedimentary Ore, Using Sodium Thiosulfate; A Preliminary Kinetic Study. Metals 2020, 10, 159. [Google Scholar] [CrossRef]
- Birich, A.; Stopic, S.; Friedrich, B. Kinetic Investigation and Dissolution Behavior of Cyanide Alternative Gold Leaching Reagents. Sci. Rep. 2019, 9, 7191. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Ficeriová, J.; Baláž, P.; Leon Villachica, C. Thiosulfate leaching of silver, gold and bismuth from a complex sulfide concentrates. Hydrometallurgy 2005, 77, 35–39. [Google Scholar] [CrossRef]
- Alonso-Gómez, A.R.; Lapidus, G.T. Inhibition of lead solubilization during the leaching of gold and silver in ammoniacal thiosulfate solutions (effect of phosphate addition). Hydrometallurgy 2009, 99, 89–96. [Google Scholar] [CrossRef]
- Ayata, S.; Yildiran, H. Optimization of extraction of silver from silver sulphide concentrates by thiosulphate leaching. Miner. Eng. 2005, 18, 898–900. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Leaching behaviour of sulphides in ammoniacal thiosulphate systems. Hydrometallurgy 2002, 63, 189–200. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Li, G. Effect of common associated sulfide minerals on thiosulfate leaching of gold and the role of humic acid additive. Hydrometallurgy 2017, 171, 44–52. [Google Scholar] [CrossRef]
- Molleman, E.; Dreisinger, D. The treatment of copper–gold ores by ammonium thiosulfate leaching. Hydrometallurgy 2002, 66, 1–21. [Google Scholar] [CrossRef]
- Fleming, C.A.; Mezei, A.; Bourricaudy, E.; Canizares, M.; Ashbury, M. Factors influencing the rate of gold cyanide leaching and adsorption on activated carbon, and their impact on the design of CIL and CIP circuits. Miner. Eng. 2011, 24, 484–494. [Google Scholar] [CrossRef]
- Navarro, P.; Vargas, C.; Villarroel, A.; Alguacil, F.J. On the use of ammoniacal/ammonium thiosulphate for gold extraction from a concentrate. Hydrometallurgy 2002, 65, 37–42. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, M.; Sau, D.C.; Agrawal, A.; Chakravarty, S.; Mankhand, T.R. Leaching of Gold from the Waste Mobile Phone Printed Circuit Boards (PCBs) with Ammonium Thiosulphate. IJMEE 2012, 1, 17–21. [Google Scholar] [CrossRef]
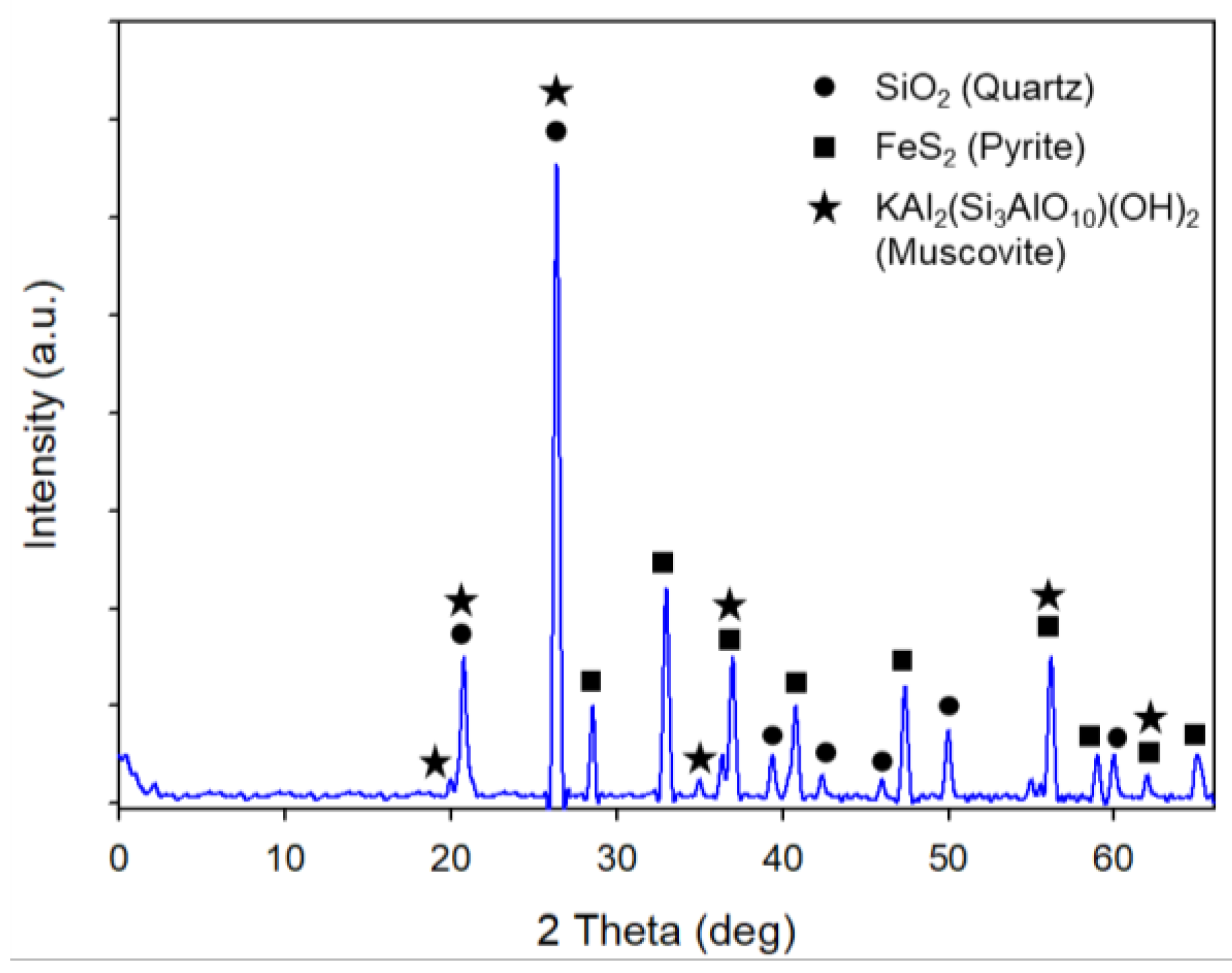


| Element | Au* | Ag* | Al | Fe | Mg | Na | Pb | Cu | Zn | Ti | Si | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents (wt.%) | 84.0 | 852 | 2.42 | 18.9 | 0.0820 | 0.160 | 0.270 | 0.380 | 0.440 | 0.170 | 23.2 | 21.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.; Kim, S.; Sohn, J.; Yang, D.; Lee, H. Leaching Behavior of Gold and Silver from Concentrated Sulfide Ore Using Ammonium Thiosulfate. Metals 2020, 10, 1029. https://doi.org/10.3390/met10081029
Bae M, Kim S, Sohn J, Yang D, Lee H. Leaching Behavior of Gold and Silver from Concentrated Sulfide Ore Using Ammonium Thiosulfate. Metals. 2020; 10(8):1029. https://doi.org/10.3390/met10081029
Chicago/Turabian StyleBae, Mooki, Sookyung Kim, Jeongsoo Sohn, Donghyo Yang, and Hyunju Lee. 2020. "Leaching Behavior of Gold and Silver from Concentrated Sulfide Ore Using Ammonium Thiosulfate" Metals 10, no. 8: 1029. https://doi.org/10.3390/met10081029
APA StyleBae, M., Kim, S., Sohn, J., Yang, D., & Lee, H. (2020). Leaching Behavior of Gold and Silver from Concentrated Sulfide Ore Using Ammonium Thiosulfate. Metals, 10(8), 1029. https://doi.org/10.3390/met10081029





