Abstract
Dry gravity separation using a vibrating zirconia ball bed is proposed in this study to separate aluminum (Al) and stainless steel (STS) scraps obtained from spent hard disk drive recycling. The effects of zirconia ball sizes and vibrating power (vibration amplitude) on the separation efficiency of Al and STS scraps were investigated. The zirconia balls moved down at the center of the vessel and rose with the wall during the vibration test. Although more STS scraps sunk than Al scraps did, the separation efficiency was not maintained because Al scraps also sunk along with balls’ movement. The separation efficiency increased to 86.6% using 1-mm zirconia balls with a 2.5-mm vibration amplitude at 4 min, but it decreased rapidly by ball moving. Therefore, when a ball bed of mixed sizes (2:1 ratio of 1 and 3 mm) was used and arranged, whereby the 3-mm zirconia balls were above the 1-mm ball bed, the separation efficiency increased to 100% for more than 2 min. This dramatic improvement was because the 3-mm ball bed acted as a barrier to prevent sunken STS scraps from rising, and Al scrap cannot sink through the 3-mm ball bed. These results indicate that the separation of Al and STS scraps could be achieved successfully using the dry gravity separation method.
1. Introduction
Hard disk drives (HDDs), the most recognizable electronic storage device [1], have recently been replaced by solid state drives in personal computers [2]. There has been a renewed demand for HDDs in recent years due to their relatively low price that makes them ideal for large cloud data centers. As shown in Table 1, hard disk drives contain NdFeB magnets, aluminum, stainless steel, and printed circuit boards (PCBs) [3], which also contain copper and precious metals, such as gold and silver [4,5,6,7,8,9,10]. The PCB accounts for 82.5% of the economic values of HDD, and rare earth elements (REE) such as Nd have been recognized as a strategic resource [11]. Therefore, recycling has been suggested to recover valuable metals from HDD PCBs and NdFeB magnets.

Table 1.
Average material composition and scrap prices of hard disk drives [3].
A new process for recycling and destroying data in spent HDD was proposed as follows. Firstly, PCB is disassembled from spent HDDs, a relatively straightforward task since it is located outside of these devises. Secondly, HDDs without PCB is scrapped for data security. Finally, scrapped NdFeB magnets are recovered with low-intensity magnetic separator. The aluminum (Al) and stainless steel (STS) scraps generated by the scrapping of HDDs should be separated for recycling because they accounted for about 90% of the weight of HDD scrap, as shown in Table 1 [3]. Gravity separation processes are promising methods to separate Al and STS because the former is lighter than the latter. Generally, gravity separation techniques such as jigging use water as a medium [12], which requires the drying of products after separation. Because this operation consumes huge amounts of energy, dry gravity separation techniques are a more environmentally friendly and sustainable alternative.
The behaviors of particles in vibrating particulate bed have been investigated for predicting size separation of particulates in vibrating vessels [13,14,15,16]. The vibrating particulate bed method was applied recently to recycling processes as follows [17,18,19]. A recycling process using the vibrating ball (1.8–2.6 g·cm–3) with different ratio of soda glass beads (2.44 g·cm–3) and alumina beads (3.57 g·cm–3) was investigated to separate Al and Mg scraps [17,18]. In these tests, the pure Al block (2.69 g·cm−3) sunk, while the pure Mg block (1.72 g·cm−3) floated on the ball bed, resulting in the successful separation of pure Al and Mg blocks [17,18]. Even though the separation efficiency of Al and Mg increased to 100% under certain conditions, however, high efficiency was not maintained during the separation tests.
For spent HDD recycling, the present study is aimed at developing the dry-gravity separation of Al and STS scraps using vibrating ball beds, which has not been studied before. Although the ratio of vessel and ball sizes, the ratio of ball volume and vessel size, the ratio of scrap sample and ball sizes are important factors in separation efficiency [6], in this study, the effects of ball size and vibrating power (i.e., vibration amplitude) on the separation efficiency of Al and STS scraps was investigated using zirconia balls as vibrating media. The behavior of Al and STS scraps was also examined by using a ball bed medium of mixed sizes to maintain high separation efficiency.
2. Materials and Methods
Scraps of spent HDDs were collected from a security company in Korea. As explained above, PCBs and NdFeB magnets were first removed from these devices prior to scrapping, with the remaining consisting mainly of Al and STS. Aluminum and STS scraps were collected from the HDD scraps, and each piece of scrap is about 3 cm long × 1 cm wide. Zirconia balls were used as a ball bed in the separation experiments with sizes of 1, 2 or 3 mm in diameter. The specific gravities of Al scraps, STS scraps and zirconia balls are 2.65, 7.3, and 5.6, respectively, which were determined with a pycnometer before the test. An acryllic vibrating vessel (12 cm height × 14 cm diameter) was set on the vibrator (Haver EML 200 digital plus, Haver & Boecker OHG, Germany) after adding zirconia balls into the vessel with a ball bed height of 5 cm. Each of the four Al and STS scraps were then placed on the ball bed, the vessel was vibrated for 10 min with predetermined vibration amplitudes (i.e., 2, 2.5, and 3 mm). All separation tests were repeated five times. The number of scraps left within the ball bed was counted and the floating ratio was calculated as follows [7,8]:
where F, Nfloat, Nall refer to the floating ratio, the number of scraps remaining on the surface of the ball bed, and the number of scraps initially added into the vessel, respectively. The separation efficiency of the Al scrap was calculated by the following equation [7,8]:
where S, FAl, FSTS represents the separation efficiency, floating ratio of Al scrap, and floating ratio of STS scrap, respectively. The average values of floating ratio are used for floating ratios of Al and STS in Equation (2). When the separation efficiency is close to 1, the Al scrap floated on the surface of the ball bed while the STS scrap sunk.
F = Nfloat/Nall,
S = (FAl × (1 − FSTS))1/2,
3. Results and Discussion
Convection and the ‘Brazil nut effect’ are phenomena observed when vibrating a particle-filled vessel as shown in Figure 1 [13]. Convection represents the circulation of particles within the vessel (Figure 1a), which occurs by frictional interaction of balls and side wall of vessel [13,14,15,16]. The convection could enhance the mixing of balls and size separation [13]. The ‘Brazil nut effect’ is a phenomenon where the largest pieces rise to the top of a particle bed (Figure 1b) [13]. A previous study has also observed that a large particle with higher density tends to sink to the bottom of the particle bed consisting of lower density particles, a phenomenon called the ‘reverse Brazil nut effect’ [20]. Therefore, zirconia balls (5.6 g·cm–3) were used as a medium to separate Al (2.65 g·cm−3) and STS (7.3 g·cm–3) scraps in this study.
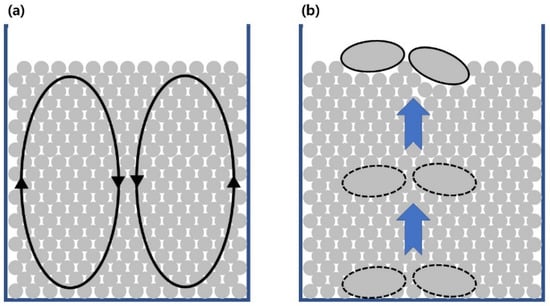
Figure 1.
Schematic diagrams of (a) convection and (b) the ‘Brazil nut effect’ when vibrating a particle-filled vessel.
Figure 2 shows the behaviors of Al and STS scraps during the vibrating test with a 2–3-mm vibration amplitude using 1-mm zirconia balls as the medium. When vibrating the vessel at a vibration amplitude of 2 mm as shown in Figure 2a, the floating ratio of Al scrap was 100% over the entire vibrating test duration while the floating ratio of STS scrap decreased from 100% to 25% (i.e., only one out of four STS scraps floated on the zirconia ball bed). The floating ratio of scrap remains at 25% after 4 min but the floated scrap changed in turn with time by convection during the vibrating test. This result suggests that the heavier scrap (STS) could go downward more easily than the lighter scrap (Al) and indicates that the scraps of STS and Al could be separated by a specific gravity difference. In Figure 2b, both floating ratios fluctuated with time, and it was observed that convection speed increases with increasing vibration amplitude from 2 to 3 mm. Figure 2c shows lower floating ratios of Al and STS scraps with 3 mm than with either 2 or 2.5 mm vibration amplitude. In the test shown in Figure 2c, some scraps appeared on the top of the ball medium but the scraps soon sunk by convection. Figure 3 shows the separation efficiencies of results shown in Figure 2. The separation efficiency with a vibration amplitude of 2 mm increased rapidly to 86.6% after 4 min while the efficiencies with 2.5-mm and 3-mm vibration amplitudes increased and then decreased to 0% and 50%, respectively. This low separation efficiency resulted from the ball convection followed by scraps movements.
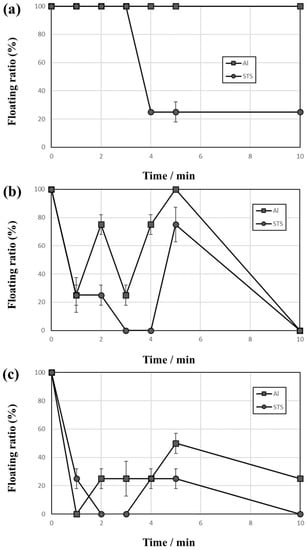
Figure 2.
Floating ratio change with time of Al and stainless steel (STS) scraps during the vibrating test with vibration amplitudes of (a) 2, (b) 2.5, and (c) 3 mm using 1-mm zirconia balls as the medium.
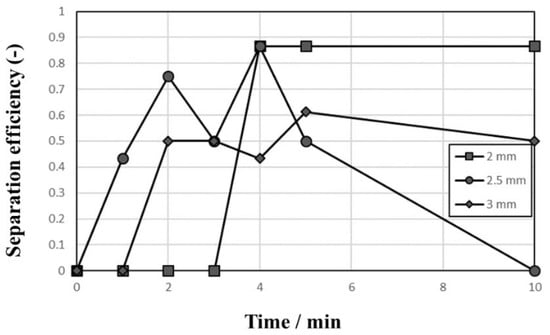
Figure 3.
Separation efficiency of Al and STS scraps using 1-mm zirconia ball medium with vibration amplitudes of 2, 2.5, and 3 mm.
Figure 4 shows the behaviors of Al and STS scraps during the vibrating test with vibration amplitudes of 2.5 mm (Figure 4a) and 3 mm (Figure 4b) using 2-mm zirconia balls as the medium. Conventional studies reported that the ratio of ball diameter to vessel size is one of the key factors of size segregation during the vibrating motion [13]. The 2-mm vibration amplitude was insufficient to vibrate the 2-mm ball bed in this study, and only the balls at the top of the ball bed bounced during the test, so all scraps remained on the top of the ball bed after the test. This kind of phenomena was rarely reported, and the ratio of ball bed volume and vibrating power is also an important factor for the separation. Figure 4a shows the floating ratio of Al and STS scraps with a 2.5-mm vibration amplitude using a 2-mm ball as a medium, and the result indicates that at least one of the scraps remained on the top of the ball bed (no 0% floating ratio). Figure 4b shows the result performed under the condition of a 3-mm vibration amplitude using 2-mm balls as a medium, and the floating ratio fluctuated more than the other tests.
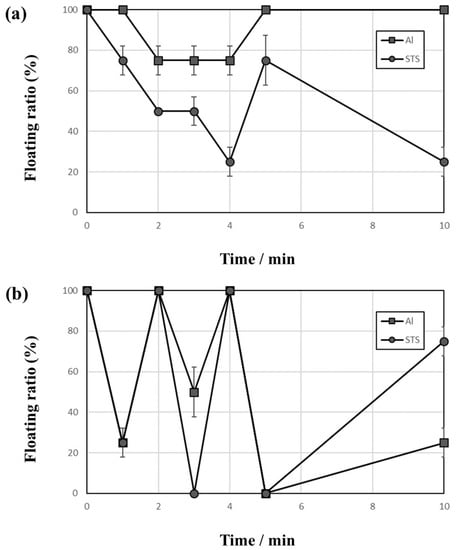
Figure 4.
Floating ratio change with time of Al and STS scraps during vibrating test with vibration amplitudes of (a) 2.5 and (b) 3 mm using 2-mm zirconia balls as the medium.
Different behaviors of convection were observed with ball sizes during the tests, and, as shown in Figure 5, all 1-mm balls moved via convection in the vessel (Figure 5a) but only the upper half of the ball bed moved convectively when 2-mm balls were used as the medium (Figure 5b). Therefore, the scraps move up and down rapidly in the results shown in Figure 4b. When 3-mm balls were used as the medium, convection was not observed and all scraps remained above the ball bed, so the separation of Al and STS scraps was not achieved at all. The ratio of vessel and ball sizes and vibrating power are important factors on the movement of balls [13], so the vessel size and vibrating power would not be enough for 3-mm balls in this study. This fact indicates that the balance of ball bed volume and vibrating power is an important factor to separate the scraps. The separation efficiencies of the tests with a 2-mm ball medium shown in Figure 4 are summarized in Figure 6. As discussed above, the separation efficiency was 0% at the vibration amplitude of 2 mm because the ball bed was not vibrated sufficiently. The separation efficiency increased to 86.6% at 10 min with a 2.5-mm vibration amplitude, and it fluctuated during the test with a vibration amplitude of 3 mm.
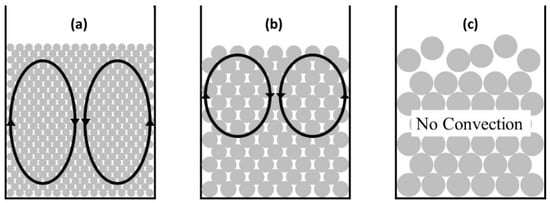
Figure 5.
Convection type as a function of ball size of medium: (a) 1, (b) 2, and (c) 3 mm.
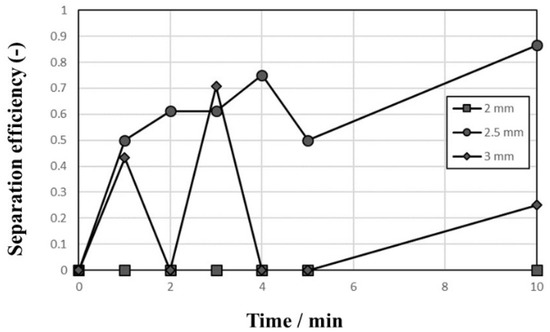
Figure 6.
Separation efficiency of Al and STS scraps using a 2-mm zirconia ball medium with vibration amplitudes of 2, 2.5, and 3 mm.
Although the separation efficiency increased to 86.6% as discussed above, where more Al scraps floated than STS scraps, the efficiency was not maintained due to convection. It is also interesting to note that more power (vibration amplitude) was required to vibrate the larger ball bed. When the ratio of ball diameter to vessel size is larger, no convection was observed but some STS scrap intruded within the 3-mm balls. Therefore, a ball bed with mixed sizes of balls was prepared by mixing 1-mm and 3-mm zirconia balls. The 3-mm balls were placed on top of the 1-mm ball bed by the ‘Brazil nut’ effect. It was expected that this 3-mm ball bed could act as a barrier, preventing the sunken STS scrap from floating again.
Figure 7 shows the behaviors of Al and STS scraps with vibration amplitude of 3 mm using a mixed size ball bed with a 2:1 ratio of 1-mm and 3 mm-balls. All Al scraps remained on top of the mixed-size ball bed during the test because Al scrap cannot sink through the 3-mm ball bed. The floating ratio of STS scrap decreased rapidly to 0%, which remained for 2 min. The STS scraps sank in turn through the 3-mm ball bed, and disappeared before 2 min. However, some of the 1-mm balls began to climb up along the wall after 4 min, so an STS scrap showed up on top of the ball bed at 5 min. As shown in Figure 8, the STS scraps intruded into the 3-mm ball bed and then sank to the bottom of the 1-mm ball bed. Since no convection occurred in the 3-mm ball bed, the STS scraps also remained under the 3-mm ball bed for more than 2 min although the scraps moved up by convection through the 1-mm ball bed. The same behavior was observed five times under the same conditions, so this result indicates that the separation of Al and STS scraps was achieved successfully. Furthermore, 100% of separation efficiency could be maintained longer.
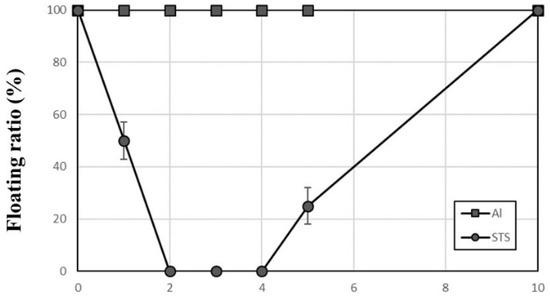
Figure 7.
Floating ratio of Al and STS scraps with time during the vibrating test with a vibration amplitude of 3 mm using a mixed ball size medium consisting of a 2:1 ratio of 1-mm and 3-mm zirconia balls.
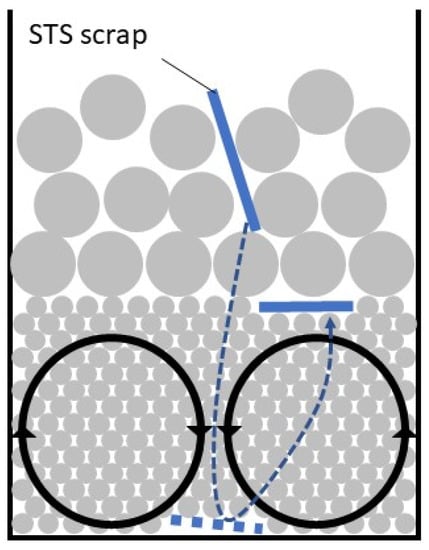
Figure 8.
Schematic diagram of ball vibration in the mixed ball bed.
Figure 9 shows the floating ratios of Al and STS scraps at 4 min by vibrating with amplitudes of 2–3 mm using a ball bed with various ratios of 1-mm and 3-mm balls. When vibrating the vessel with a vibration amplitude of 2 mm (see Figure 9a), most of the scraps remained on top of the ball bed because the vibrating power was insufficient. Figure 9b,c illustrate the results with vibration amplitudes of 2.5 and 3 mm, respectively. With the 2:1 ratio of 1-mm and 3-mm balls, all STS scraps sank but all Al scraps remained on top of the mixed size ball bed, which indicates that Al and STS scraps were separated perfectly. With the 1:1 ratio of 1-mm and 3-mm balls, more 3-mm balls prevented the STS scraps from sinking but the floating ratio of STS scraps decreased from 75 to 50% with increasing vibration amplitude from 2.5 mm to 3 mm. When using the 1:2 ratio of 1-mm and 3-mm balls, the same behavior of STS scraps was not observed in five repeated tests (data not shown) because the intrusion of STS scraps through a 3-mm ball bed occurs randomly while the Al scraps were not sunk during the test. The results shown in Figure 9 are summarized in Figure 10 as separation efficiency. The separation efficiencies at 4 min show 100% with the 2:1 ratio of 1-mm and 3-mm balls using 2.5-mm and 3-mm vibration amplitudes while the efficiency was 0% when the ball bed consisted of only 3-mm balls regardless of the vibration amplitude.
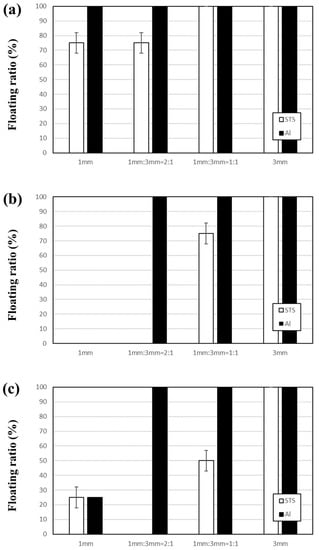
Figure 9.
Floating ratios of Al and STS scraps at 4 min by vibrating with vibration amplitudes of (a) 2, (b) 2.5, and (c) 3 mm using a ball bed with various ratios of 1-mm and 3-mm balls.
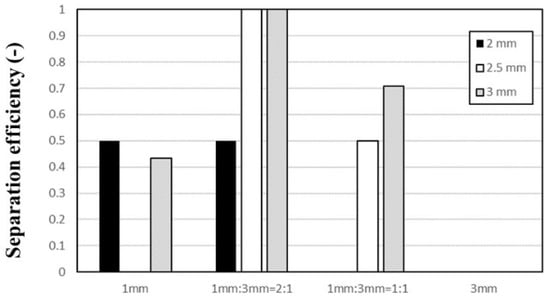
Figure 10.
Separation efficiencies of Al and STS scraps at 4 min by vibrating with vibration amplitudes of 2, 2.5, and 3 mm using a ball bed with various ratio of 1-mm and 3-mm balls.
4. Conclusions
In this study, dry gravity separation was investigated to separate Al and STS scraps using a vibrating zirconia ball bed. The effects of vibration amplitude and zirconia ball size on the separation efficiency was examined, and the results showed separation efficiencies of 86.6% under the following conditions: 1-mm ball size and a vibration amplitude of 2.5 mm. However, the separation efficiency cannot be maintained due to convection. This result is unfavorable for commercialization of the dry gravity separation process because it is difficult to secure process stability. The ratio of medium ball size to vessel is one of the key factors, and, in this study, convection was not observed when the ball bed consisted of only 3-mm balls or when the vibration amplitude was not sufficient to vibrate the vessel. These important observations were not reported in conventional studies. The mixed ball size bed was effective to separate Al and STS scraps and maintain 100% separation efficiency, which could make this dry gravity separation process commercially viable.
Author Contributions
Methodology, H.N. and K.Y.; writing-original draft preparation, C.B.T. and K.Y.; project administration and funding acquisition, K.Y.; data curation, H.N. and K.Y.; writing-review and editing, providing ideas, M.K.J. and K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the R&D Center for Valuable Recycling (Global-Top R&BD Program), Ministry of Environment, Republic of Korea (under the project no. 2019002220001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grochowski, E.; Hoyt, R.F. Future trends in hard disk drives. IEEE Trans. Magn. 1996, 32, 1850–1854. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Fleming, K.; Kim, J. Improving performance and lifetime of solid-state drives using hardware-accelerated compression. IEEE Trans. Consum. Electron. 2011, 57, 1732–1739. [Google Scholar] [CrossRef]
- Manhart, A.; Buchert, M.; Degreif, S.; Mehlhart, G.; Meinel, J. Recycling of Hard Disk Drives–Analysing the Optimal Dismantling Depth for Recyclers in Developing Countries and Emerging Economies. Darmstadt & Accra. Available online: http://www.oeko.de/oekodoc/2447/2015-604-en.pdf/ (accessed on 18 January 2020).
- Calderon, A.R.M.; Alorro, R.D.; Tadesse, B.; Yoo, K.; Tabelin, C.B. Evaluation of maghemite-rich iron oxide composite prepared from magnetite as adsorbent for gold from chloride solution. JOM 2019, 71, 4639–4646. [Google Scholar] [CrossRef]
- Jeon, S.H.; Yoo, K.; Alorro, R.D. Separation of Sn, Bi, Cu from Pb-free solder paste by ammonia leaching followed by hydrochloric acid leaching. Hydrometallurgy 2017, 169, 26–30. [Google Scholar] [CrossRef]
- Jung, M.; Yoo, K.; Alorro, R.D. Dismantling of Electric and Electronic Components from Waste Printed Circuit Boards by Hydrochloric Acid Leaching with Stannic Ions. Mater. Trans. 2017, 58, 1076–1080. [Google Scholar] [CrossRef]
- Jung, M.; Yoo, K.; Alorro, R.D. Regeneration of Sn4+ from Sn2+ solution during electrowinning process using anion exchange membrane. Geosyst. Eng. 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.C.; Yoo, K. Leaching of tin from waste Pb-free solder in hydrochloric acid solution with stannic chloride. Hydrometallurgy 2016, 165, 143–147. [Google Scholar] [CrossRef]
- Lim, Y.; Kwon, O.H.; Lee, J.; Yoo, K. The ammonia leaching of alloy produced from waste printed circuit boards smelting process. Geosyst. Eng. 2013, 16, 216–224. [Google Scholar] [CrossRef]
- Yoo, K.; Park, Y.; Choi, S.; Park, I. Improvement of Copper Metal Leaching in Sulfuric Acid Solution by Simultaneous Use of Oxygen and Cupric Ions. Metals 2020, 10, 721. [Google Scholar] [CrossRef]
- Cho, B.G.; Cho, Y.J.; Lee, J.C.; Yoo, K. Korea’s metal resources recycling research project–valuable recycling. Geosyst. Eng. 2019, 22, 48–58. [Google Scholar] [CrossRef]
- Ito, M.; Takeuchi, M.; Saito, A.; Murase, N.; Phengsaart, T.; Tabelin, C.B.; Hiroyoshi, N.; Tsunekawa, M. Improvement of hybrid jig separation efficiency using wetting agents for the recycling of mixed-plastic wastes. J. Mater. Cycles Waste Manag. 2019, 21, 1376–1383. [Google Scholar] [CrossRef]
- Kudrolli, A. Size separation in vibrated granular matter. Rep. Prog. Phys. 2004, 67, 209–247. [Google Scholar] [CrossRef]
- Gallas, J.A.C.; Herrmann, H.J.; Sokołowski, S. Convection cells in vibrating granular media. Phys. Rev. Lett. 1992, 69, 1371. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Liao, H.H.; Hsiau, S.S. Convection behavior of non-spherical particles in a vibrating bed: Discrete element modeling and experimental validation. Powder Technol. 2013, 237, 53–66. [Google Scholar] [CrossRef]
- Liao, C.C.; Hsiau, S.S.; Huang, T.Y. The effect of vibrating conditions on the electrostatic charge in a vertical vibrating granular bed. Powder Technol. 2011, 208, 1–6. [Google Scholar] [CrossRef]
- Itoh, S.; Tsujita, K.; Morishige, T.; Takenaka, T. The Effect of Vibration Condition for Magnesium Dry Gravity Sorting by Vibrating Particulate Bed. Mater. Sci. Forum. 2018, 941, 1535–1539. [Google Scholar] [CrossRef]
- Morishige, T.; Tsujita, K.; Murasa, S.; Takenaka, T. Magnesium Sorting by Vibrating Particulate Bed from Mixed Metal Scrap. Mater. Trans. 2015, 56, 1756–1758. [Google Scholar] [CrossRef]
- Yoo, J.K.; Lee, M.; Kim, G.H.; Yoo, K. The Gravity Separation of Speiss and Limestone Granules Using Vibrating Zirconia Ball Bed. J. Korean Inst. of Resour. Recycl. 2020, 29, 36–42. [Google Scholar] [CrossRef]
- Hong, D.C.; Quinn, P.V.; Luding, S. Reverse Brazil nut problem: Competition between percolation and condensation. Phys. Rev. Lett. 2001, 86, 3423. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).