The Effect of the Processing Parameters on the Properties of the Liquid Phase Spark Plasma Sintered 80Fe-20(Al + MWCNT) Magnetic Metal Matrix Nanocomposites
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Sample Preparations
2.2. Characterization
3. Results and Discussion
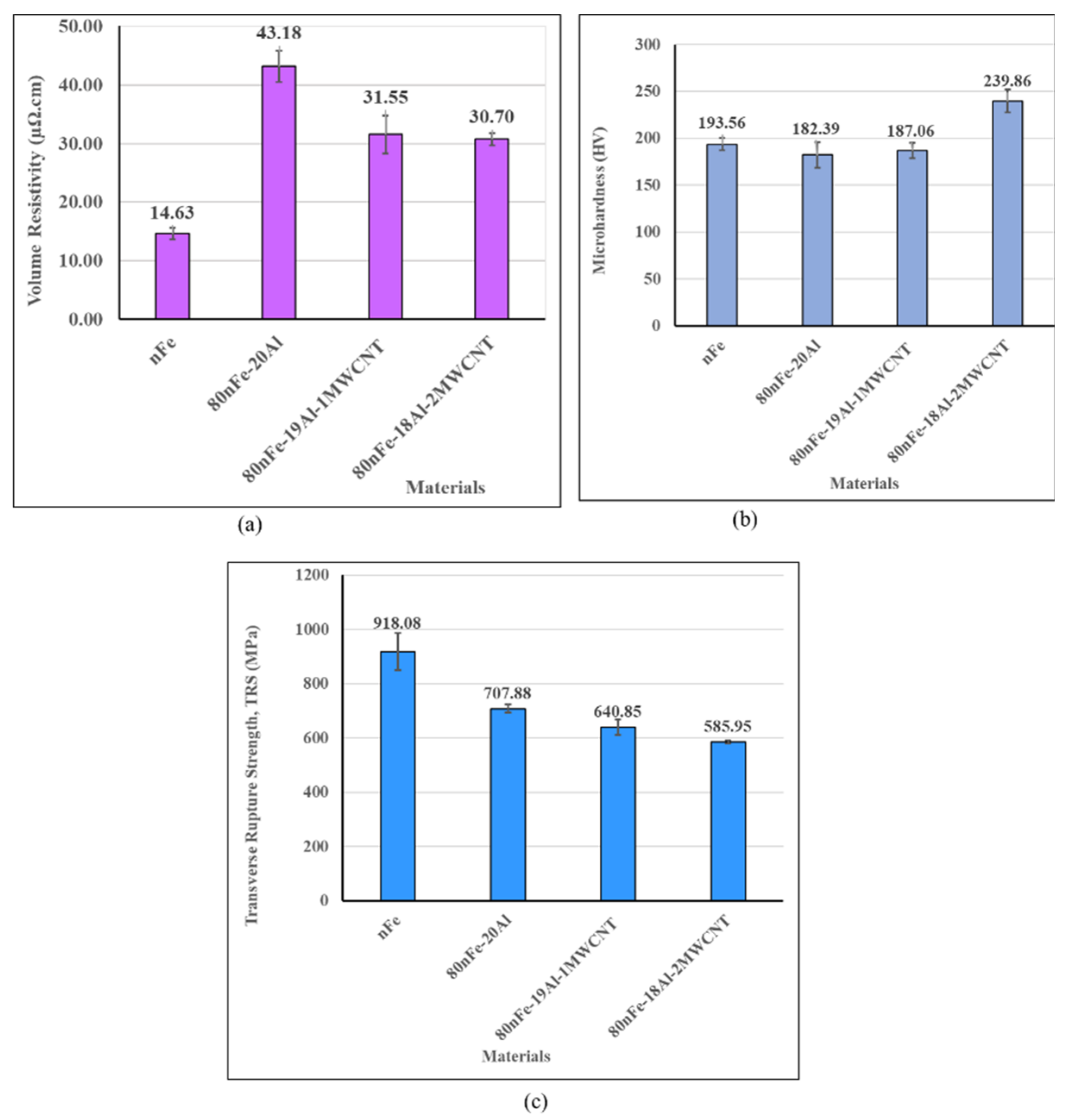
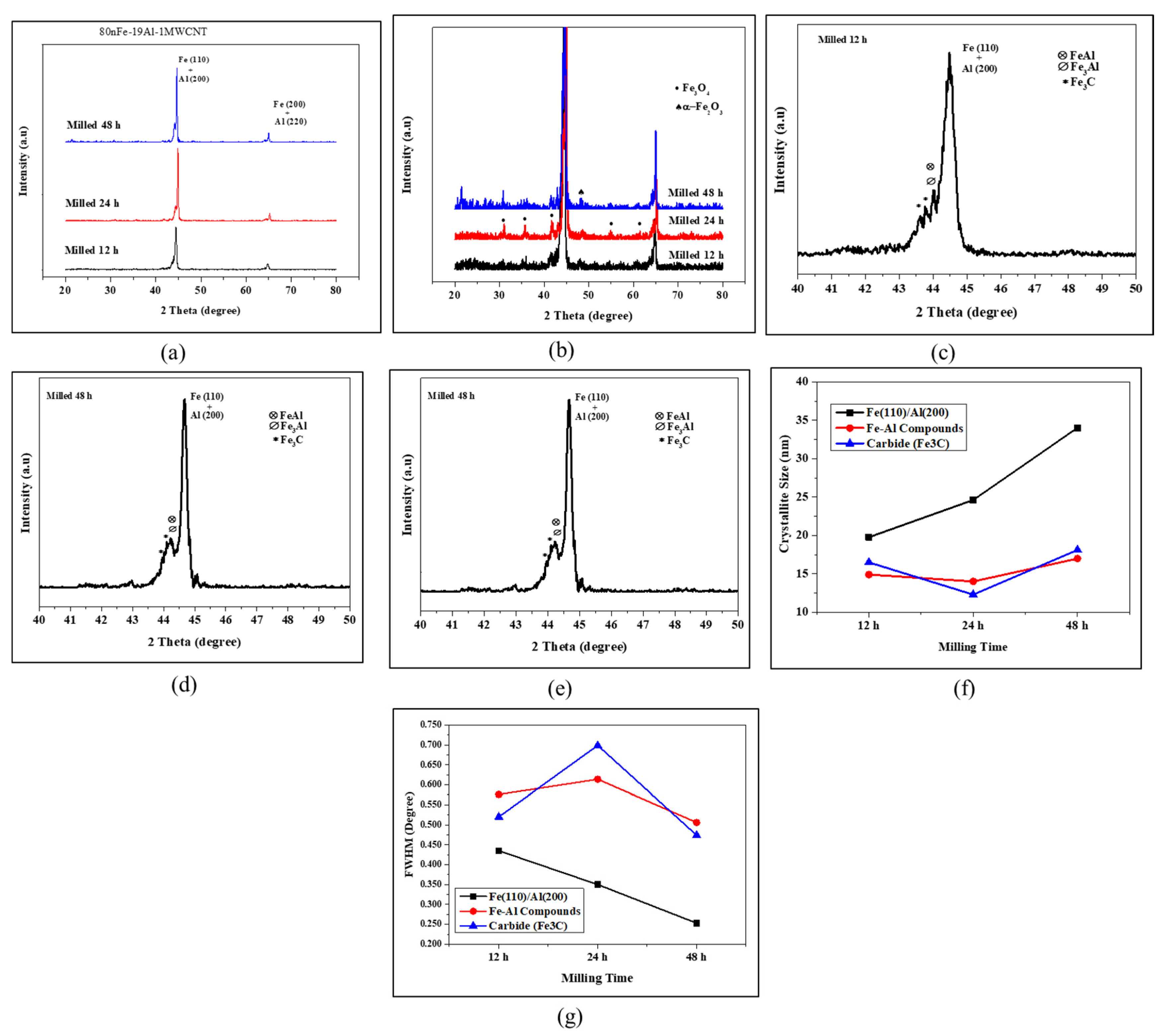
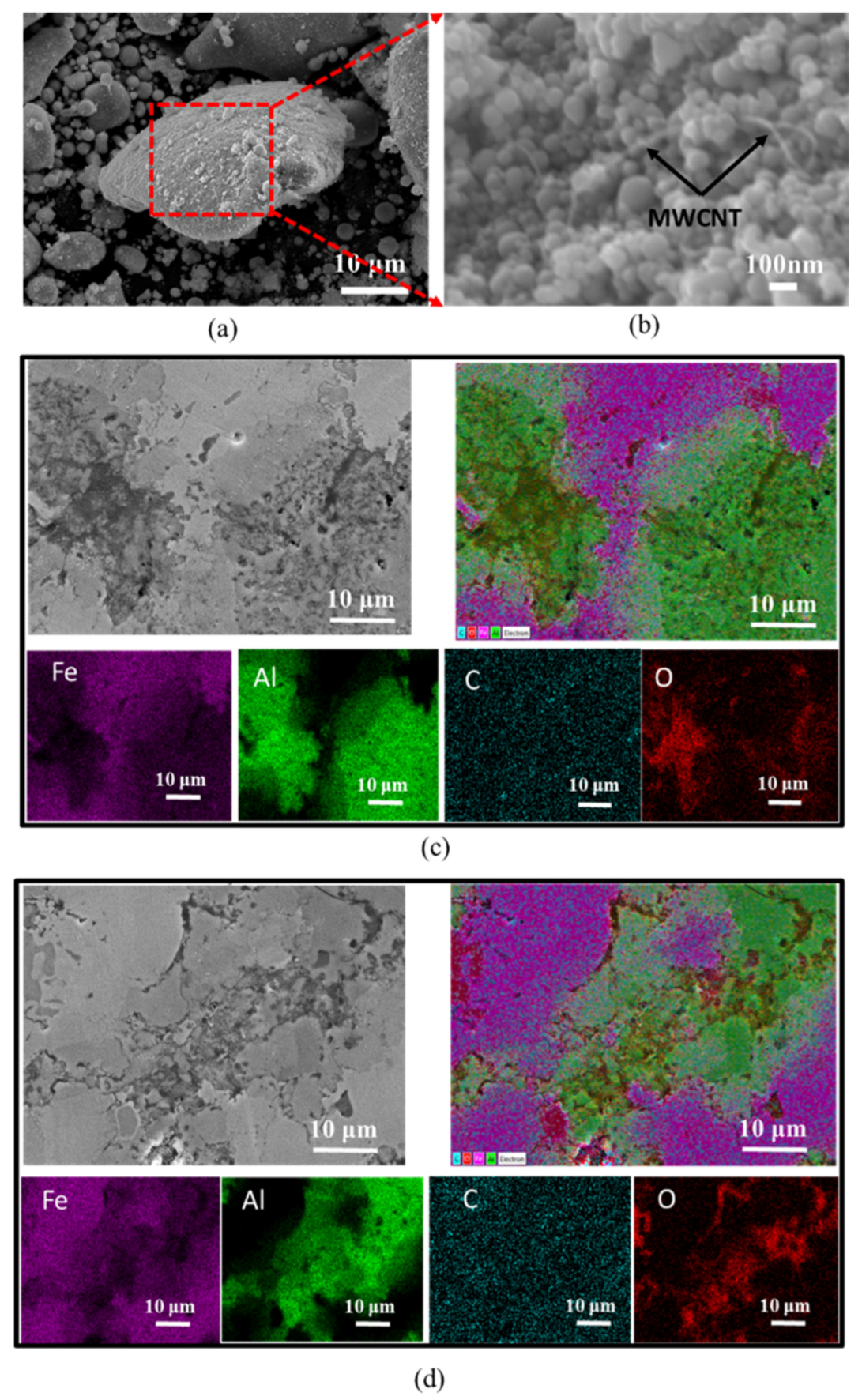
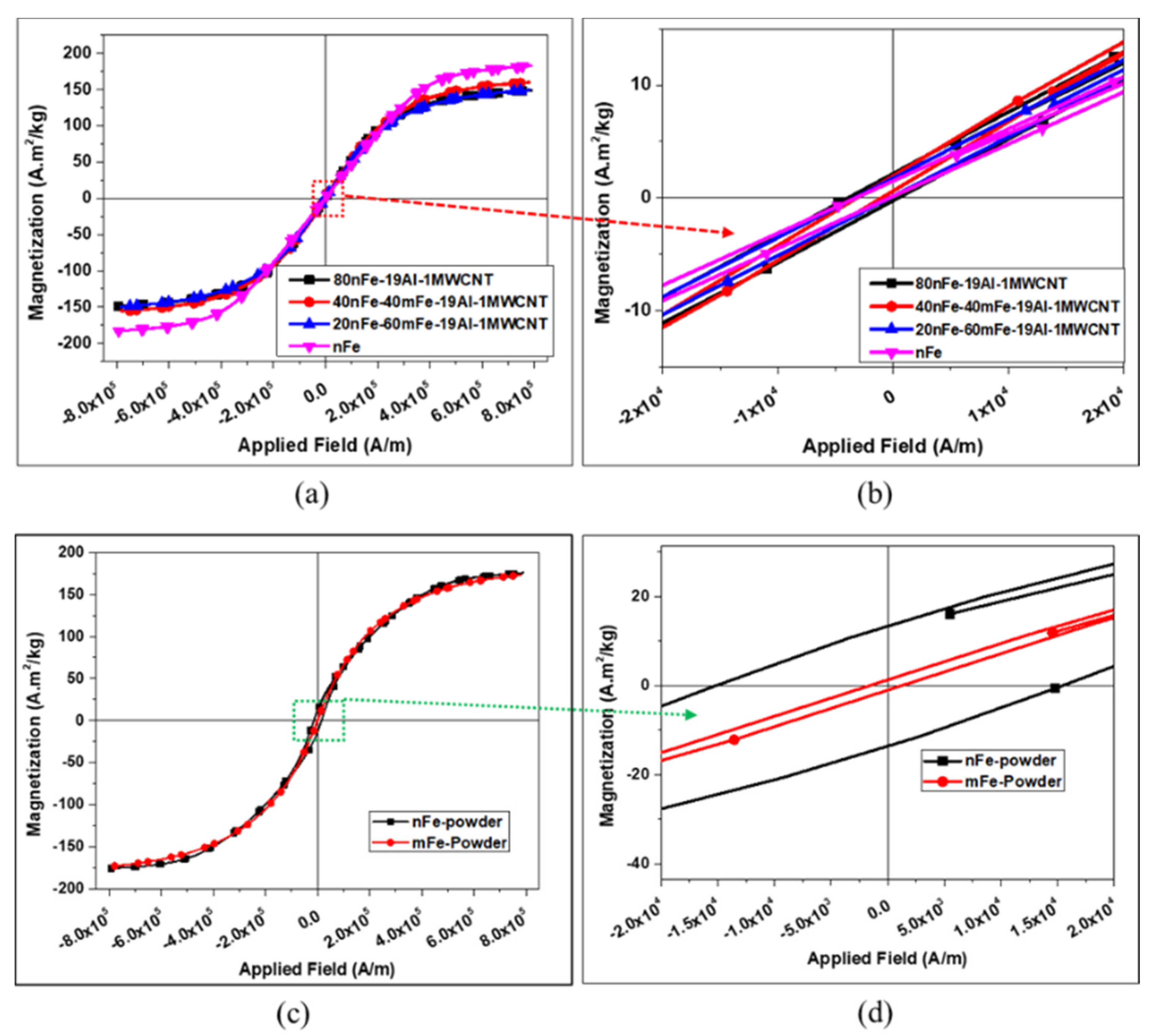
3.1. Effect of MWCNT Content on the Properties of the Nanocomposites
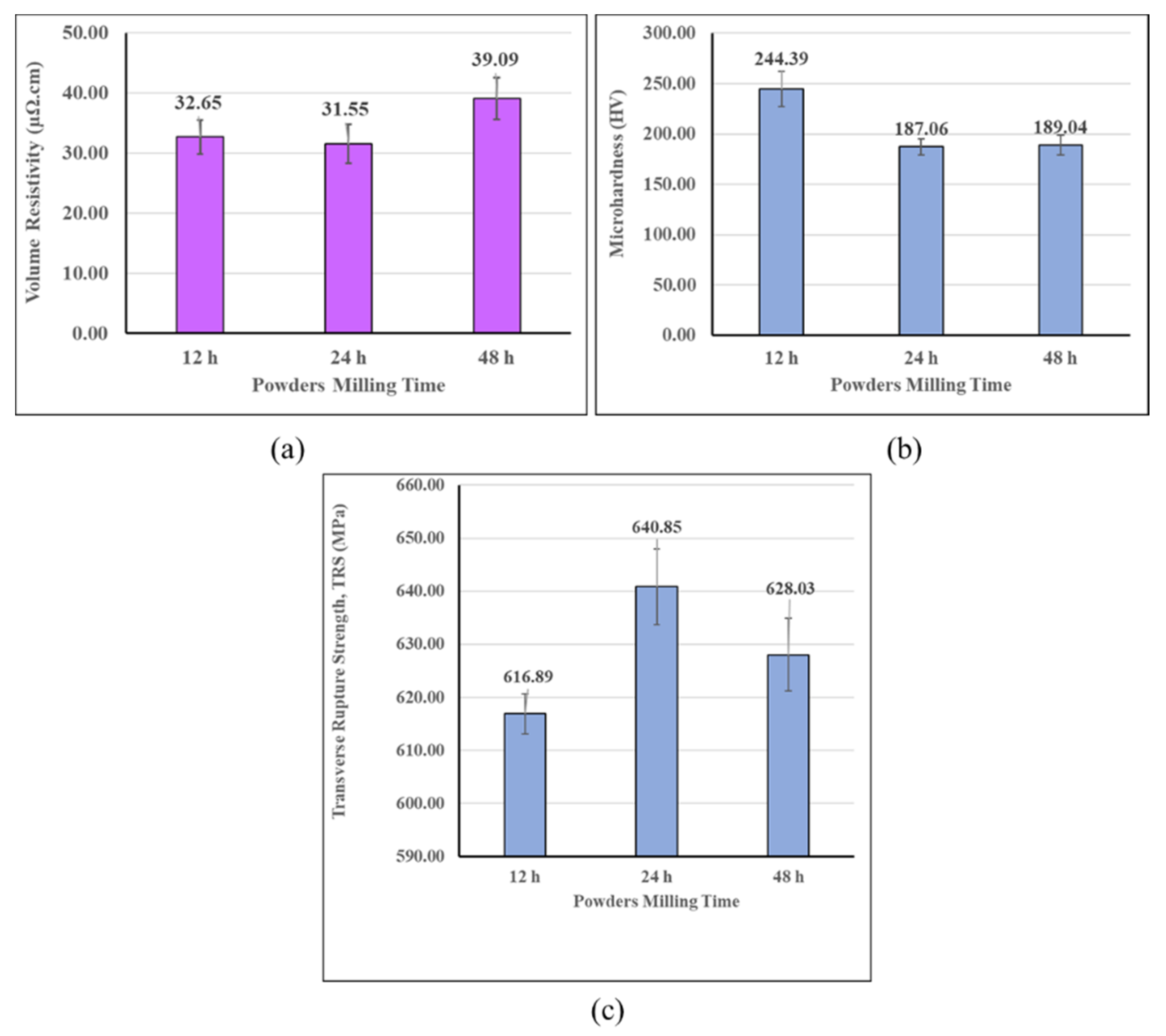
3.2. Effect of Powder Milling Time
3.3. Effect of Combining Fe Nanoparticles and Fe Microparticles
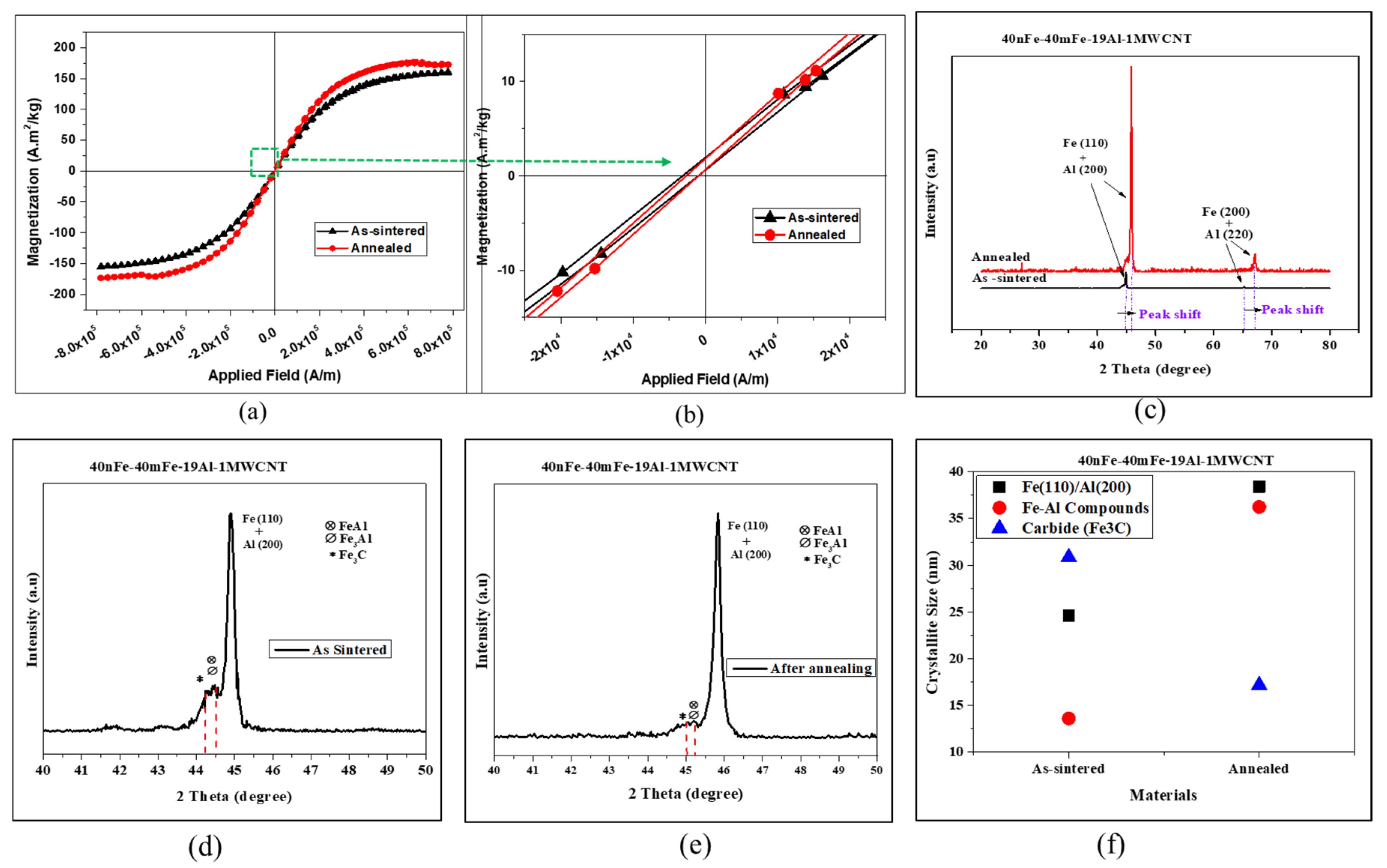
3.4. Effect of Post-Sintering Annealing Heat Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malaki, M.; Xu, W.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced metal matrix nanocomposites. Metals 2019, 9, 330. [Google Scholar] [CrossRef]
- Yu, W.H.; Sing, S.L.; Chua, C.K.; Kuo, C.N.; Tian, X.L. Particle-reinforced metal matrix nanocomposites fabricated by selective laser melting: A state of the art review. Prog. Mater. Sci. 2019, 104, 330–379. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Hamim, S.U.; Karbalaei Akbari, M.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Munir, K.S.; Jia, S.; et al. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Compos. Part A Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Kumar, N.; Chittappa, H.C.; Ezhil Vannan, S. Development of Aluminium-Nickel Coated Short Carbon Fiber Metal Matrix Composites. Mater. Today Proc. 2018, 5, 11336–11345. [Google Scholar] [CrossRef]
- Ma, P.; Jia, Y.; Gokuldoss, P.K.; Yu, Z.; Yang, S.; Zhao, J.; Li, C. Effect of Al2O3 nanoparticles as reinforcement on the tensile behavior of Al-12Si composites. Metals 2017, 7, 359. [Google Scholar] [CrossRef]
- Esawi, A.M.K.; Morsi, K.; Sayed, A.; Gawad, A.A.; Borah, P. Fabrication and properties of dispersed carbon nanotube-aluminum composites. Mater. Sci. Eng. A 2009, 508, 167–173. [Google Scholar] [CrossRef]
- Kalra, C.; Tiwari, S.; Sapra, A.; Mahajan, S.; Gupta, P. Processing and Characterization of Hybrid Metal Matrix Composites. J. Mater. Environ. Sci. Environ. Sci. 2018, 9, 1979–1986. [Google Scholar]
- Ceschini, L.; Dahle, A.; Gupta, M.; Jarfors, A.E.W.; Jayalakshmi, S.; Morri, A.; Rotundo, F.; Toschi, S.; Singh, R.A. Aluminum and Magnesium Metal Matrix Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-981-10-2680-5. [Google Scholar]
- Gupta, P.; Kumar, D.; Jha, A.K.; Parkash, O. Effect of height to diameter (h/d) ratio on the deformation behaviour of Fe-Al2O3 metal matrix nanocomposites. Bull. Mater. Sci. 2016, 39, 1245–1258. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, D.; Quraishi, M.A.; Parkash, O. Effect of sintering parameters on the corrosion characteristics of Iron-Alumina metal matrix nanocomposites. J. Mater. Environ. Sci. 2015, 6, 155–167. [Google Scholar]
- Gupta, P.; Kumar, D.; Quraishi, M.A.; Parkash, O. Influence of processing parameters on corrosion behavior of metal matrix nanocomposites. J. Mater. Environ. Sci. 2016, 7, 3930–3937. [Google Scholar]
- Khosla, P.; Singh, H.K.; Katoch, V.; Dubey, A.; Singh, N.; Kumar, D.; Gupta, P. Synthesis, mechanical and corrosion behaviour of iron–silicon carbide metal matrix nanocomposites. J. Compos. Mater. 2018, 52, 91–107. [Google Scholar] [CrossRef]
- Krings, A.; Boglietti, A.; Cavagnino, A.; Sprague, S. Soft Magnetic Material Status and Trends in Electric Machines. IEEE Trans. Ind. Electron. 2017, 64, 2405–2414. [Google Scholar] [CrossRef]
- Wu, C.; Huang, M.; Luo, D.; Jiang, Y.; Yan, M. SiO2 nanoparticles enhanced silicone resin as the matrix for Fe soft magnetic composites with improved magnetic, mechanical and thermal properties. J. Alloys Compd. 2018, 741, 35–43. [Google Scholar] [CrossRef]
- Difference Between Alloy and Composite. Available online: http://www.differencebetween.net/science/difference-between-alloy-and-composite/ (accessed on 12 March 2020).
- Tugirumubano, A.; Go, S.H.; Shin, H.J.; Kwac, L.K.; Kim, H.G. Magnetic, Electrical, and Mechanical Behavior of Fe-Al-MWCNT and Fe-Co-Al-MWCNT Magnetic Hybrid Nanocomposites Fabricated by Spark Plasma Sintering. Nanomaterials 2020, 10, 436. [Google Scholar] [CrossRef]
- Holladay, J.W. Review of Developments in Iron-Aluminum-Base Alloys; Battelle Memorial Institute, Defense Metals Information Center: Colombus, OH, USA, 1961. [Google Scholar]
- Tugirumubano, A. Development and Characterization of Magnetic Particulate Composites for Magnetic Core Applications. Ph.D. Thesis, Jeonju University, Jeonju, Korea, 2020. [Google Scholar]
- Albaaji, A.J.; Castle, E.G.; Reece, M.J.; Hall, J.P.; Evans, S.L. Mechanical and magnetic properties of spark plasma sintered soft magnetic FeCo alloy reinforced by carbon nanotubes. J. Mater. Res. 2016, 31, 3448–3458. [Google Scholar] [CrossRef]
- Pang, L.X.; Xing, D.J.; Zhang, A.Q.; Xu, J.; Sun, S.S. Magnetic Properties of MWNTs-Fe3Al Composite. Adv. Mater. Res. 2009, 79–82, 1351–1354. [Google Scholar] [CrossRef]
- Phuong, D.D.; Van Trinh, P.; Van An, N.; Van Luan, N.; Minh, P.N.; Khisamov, R.K.; Nazarov, K.S.; Zubairov, L.R.; Mulyukov, R.R.; Nazarov, A.A. Effects of carbon nanotube content and annealing temperature on the hardness of CNT reinforced aluminum nanocomposites processed by the high pressure torsion technique. J. Alloys Compd. 2014, 613, 68–73. [Google Scholar] [CrossRef]
- Kurita, H.; Kwon, H.; Estili, M.; Kawasaki, A. Multi-Walled Carbon Nanotube-Aluminum Matrix Composites Prepared by Combination of Hetero-Agglomeration Method, Spark Plasma Sintering and Hot Extrusion. Mater. Trans. 2011, 52, 1960–1965. [Google Scholar] [CrossRef]
- Singla, D.; Amulya, K.; Murtaza, Q. CNT Reinforced Aluminium Matrix Composite-A Review. Mater. Today Proc. 2015, 2, 2886–2895. [Google Scholar] [CrossRef]
- Shadakshari, R.; Mahesha, K.; Niranjan, H.B. Carbon Nanotube Reinforced Aluminium Matrix Composites – A Review. Int. J. Innov. Res. Sci. Eng. Technol. 2012, 1, 206–213. [Google Scholar]
- Kumar, A.; Banerjee, M.K. Production of Iron—Multiwall Carbon Nanotubes (CNT) Composite by Mechanical Alloying. Int. J. Eng. Technol. Sci. Res. 2017, 4, 469–479. [Google Scholar]
- Everett, R.K.; Arsenault, R.J. (Eds.) Metal Matrix Composites: Processing and Interfaces; Academic Press: Cambridge, MA, USA, 1991; ISBN 0123418321. [Google Scholar]
- Vani, V.V.; Chak, S.K. The effect of process parameters in Aluminum Metal Matrix Composites with Powder Metallurgy. Manuf. Rev. 2018, 5, 7. [Google Scholar] [CrossRef]
- Chang, I.; Yuyuan, Z. (Eds.) Advances in Powder Metallurgy: Properties, Processing and Applications; Woodhead Publishing: Amsterdam, The Netherlands, 2013; ISBN 9781845695316. [Google Scholar]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Al Aqeeli, N.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark plasma sintering of metals and metal matrix nanocomposites: A review. J. Nanomater. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Amin, K. Carbon Nanotubes Reinforced Aluminum Matrix Composites—A Review of Processing Techniques. Pertanika J. Sch. Res. Rev. 2017, 3, 70–92. [Google Scholar]
- Franceschin, G.; Flores-Martínez, N.; Victorio, G.V.; Ammar, S.; Valenzuela, R. Sintering and Reactive Sintering by Spark Plasma Sintering (SPS). In Sintering of Functional Materials; InTech: Burlington, MA, USA, 2018. [Google Scholar]
- Chen, B.; Shen, J.; Ye, X.; Imai, H.; Umeda, J.; Takahashi, M.; Kondoh, K. Solid-state interfacial reaction and load transfer efficiency in carbon nanotubes (CNTs)-reinforced aluminum matrix composites. Carbon N. Y. 2017, 114, 198–208. [Google Scholar] [CrossRef]
- Aliyu, I.; Saheb, N.; Hassan, S.; Al-Aqeeli, N. Microstructure and Properties of Spark Plasma Sintered Aluminum Containing 1 wt.% SiC Nanoparticles. Metals 2015, 5, 70–83. [Google Scholar] [CrossRef]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- He, K.; Chen, N.; Wang, C.; Wei, L.; Chen, J. Method for Determining Crystal Grain Size by X-ray Diffraction. Cryst. Res. Technol. 2018, 53, 1–6. [Google Scholar] [CrossRef]
- ASTM, A. B528-99: Standard Test Methods for Transverse Rupture Strength of Metal Powder Specimens. In ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Neamţu, B.V.; Chicinaş, I.; Isnard, O.; Ciascai, I.; Chiriac, H.; Lostun, M. Magnetic properties of nanocrystalline Ni3Fe compacts prepared by spark plasma sintering. Intermetallics 2013, 35, 98–103. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Lahiri, D.; Agarwal, A. Carbon nanotube reinforced metal matrix composites—A review. Int. Mater. Rev. 2010, 55, 41–64. [Google Scholar] [CrossRef]
- Shishkovsky, I.; Missemer, F.; Kakovkina, N.; Smurov, I. Intermetallics synthesis in the Fe-Al system via layer by layer 3D laser cladding. Crystals 2013, 3, 517–529. [Google Scholar] [CrossRef]
- Paris, S.; Gaffet, E.; Bernard, F. Control of FeAl Composition Produced by SPS Reactive Sintering from Mechanically Activated Powder Mixture. J. Nanomater. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Sunday, K.J. Development of Ferrite-coated Soft Magnetic Composites: Correlation of Microstructure to Magnetic Properties. Ph.D. Thesis, Drexel University, Drexel, PA, USA, 2017. [Google Scholar]
- Ryl, J.; Wysocka, J.; Cieslik, M.; Gerengi, H.; Ossowski, T.; Krakowiak, S.; Niedzialkowski, P. Understanding the origin of high corrosion inhibition efficiency of bee products towards aluminium alloys in alkaline environments. Electrochim. Acta 2019, 304, 263–274. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Luo, F.; Fan, X.; Luo, Z.; Hu, W.; Li, G.; Li, Y.; Liu, X.; Wang, J. Ultra-low inter-particle eddy current loss of Fe3Si/Al2O3 soft magnetic composites evolved from FeSiAl/Fe3O4 core-shell particles. J. Magn. Magn. Mater. 2019, 484, 218–224. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, X.; Hu, W.; Luo, F.; Wang, J.; Wu, Z.; Liu, X.; Li, G.; Li, Y. Properties of Fe2SiO4/SiO2 coated Fe-Si soft magnetic composites prepared by sintering Fe-6.5wt%Si/Fe3O4 composite particles. J. Magn. Magn. Mater. 2020, 499. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Yan, M.; Wang, J. Correlating the microstructure, growth mechanism and magnetic properties of FeSiAl soft magnetic composites fabricated via HNO3 oxidation. Acta Mater. 2018, 146, 294–303. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Davydov, V.; Rakhmanina, A.; Kireev, I.; Alieva, I.; Zhironkina, O.; Strelkova, O.; Dianova, V.; Samani, T.D.; Mireles, K.; Yahia, L.H.; et al. Solid state synthesis of carbon-encapsulated iron carbide nanoparticles and their interaction with living cells. J. Mater. Chem. B 2014, 2, 4250–4261. [Google Scholar] [CrossRef]
- Fähler, S.; Schultz, L. Magnetic Films: Hard. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 4767–4771. [Google Scholar]
- Yu, R.H.; Basu, S.; Zhang, Y.; Parvizi-Majidi, A.; Xiao, J.Q. Pinning effect of the grain boundaries on magnetic domain wall in FeCo-based magnetic alloys. J. Appl. Phys. 1999, 85, 6655–6659. [Google Scholar] [CrossRef]
- Chapman, J.S. Electric Machinery Fundamentals, 5th ed.; McGraw-Hill: New York, NY, USA, 2012; ISBN 9780073529547. [Google Scholar]
- Goldman, A. Magnetic Components for Power Electronics, 1st ed.; Springer: New York, NY, USA, 2002; ISBN 9781461352808. [Google Scholar]
- Taghvaei, A.H.; Shokrollahi, H.; Janghorban, K.; Abiri, H. Eddy current and total power loss separation in the iron-phosphate-polyepoxy soft magnetic composites. Mater. Des. 2009, 30, 3989–3995. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskshi, S.R.; Lahiri, D. Carbon Nanotubes: Reinforced Metal Matrix Composites, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781138113732. [Google Scholar]
- Li, Y.; Kröger, M. A theoretical evaluation of the effects of carbon nanotube entanglement and bundling on the structural and mechanical properties of buckypaper. Carbon N. Y. 2012, 50, 1793–1806. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling; CRC Press: New York, NY, USA, 2004; ISBN 0203020642, 9780203020647. [Google Scholar]
- Hanejko, F.G.; Ellis, G.W.; Hale, T.J. Application of High Performance Material Processing—Electormagnetic Products. In Proceedings of the International Conference on Powder Metallurgy & Particulate Materials, Las Vegas, NV, USA, 31 May–4 June 1998; pp. 1–16. [Google Scholar]
- Prasanna, A.A. Effect of crystallite size on Vickers microhardness in nanostructured Heusler Ni39+xMn50Sn11−x (x ≤ 2)alloys. In Proceedings of the IEEE International Conference on Nanoscience, Engineering and Technology (ICONSET 2011), Chennai, India, 28–30 November 2011; pp. 424–428. [Google Scholar]
- Lei, J.; Zheng, J.; Zheng, H.; Qiao, L.; Ying, Y.; Cai, W.; Li, W.; Yu, J.; Lin, M.; Che, S. Effects of heat treatment and lubricant on magnetic properties of iron-based soft magnetic composites with Al2O3 insulating layer by one-pot synthesis method. J. Magn. Magn. Mater. 2019, 472, 7–13. [Google Scholar] [CrossRef]
- Yi, Y.; Peng, Y.; Xia, C.; Wu, L.; Ke, X.; Nie, J. Influence of heat treatment on microstructures and magnetic properties of Fe-based soft magnetic composites prepared by co-precipitation method. J. Magn. Magn. Mater. 2019, 476, 100–105. [Google Scholar] [CrossRef]
- Muhammed Shafi, P.; Chandra Bose, A. Impact of crystalline defects and size on X-ray line broadening: A phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 2015, 5, 057137. [Google Scholar] [CrossRef]
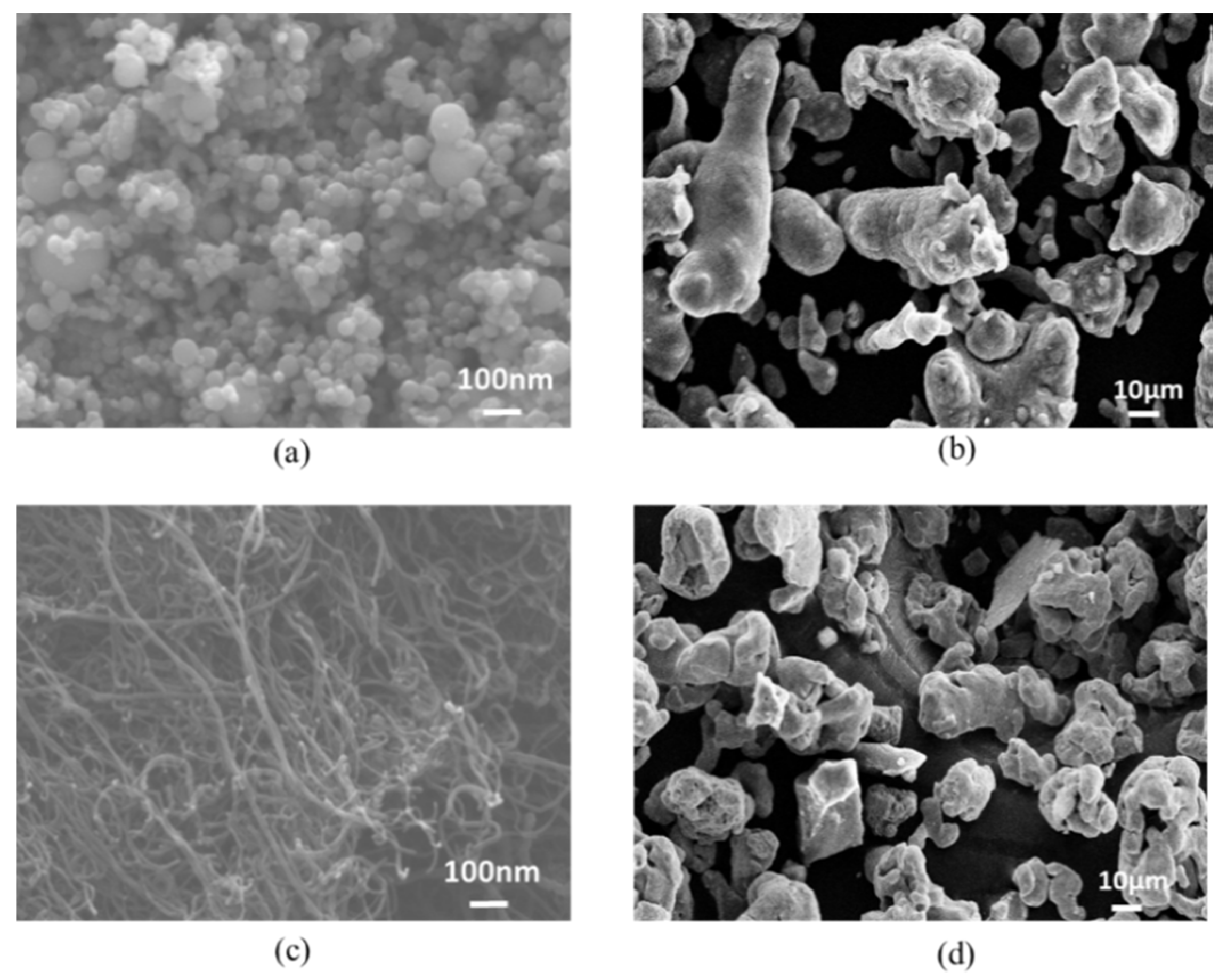
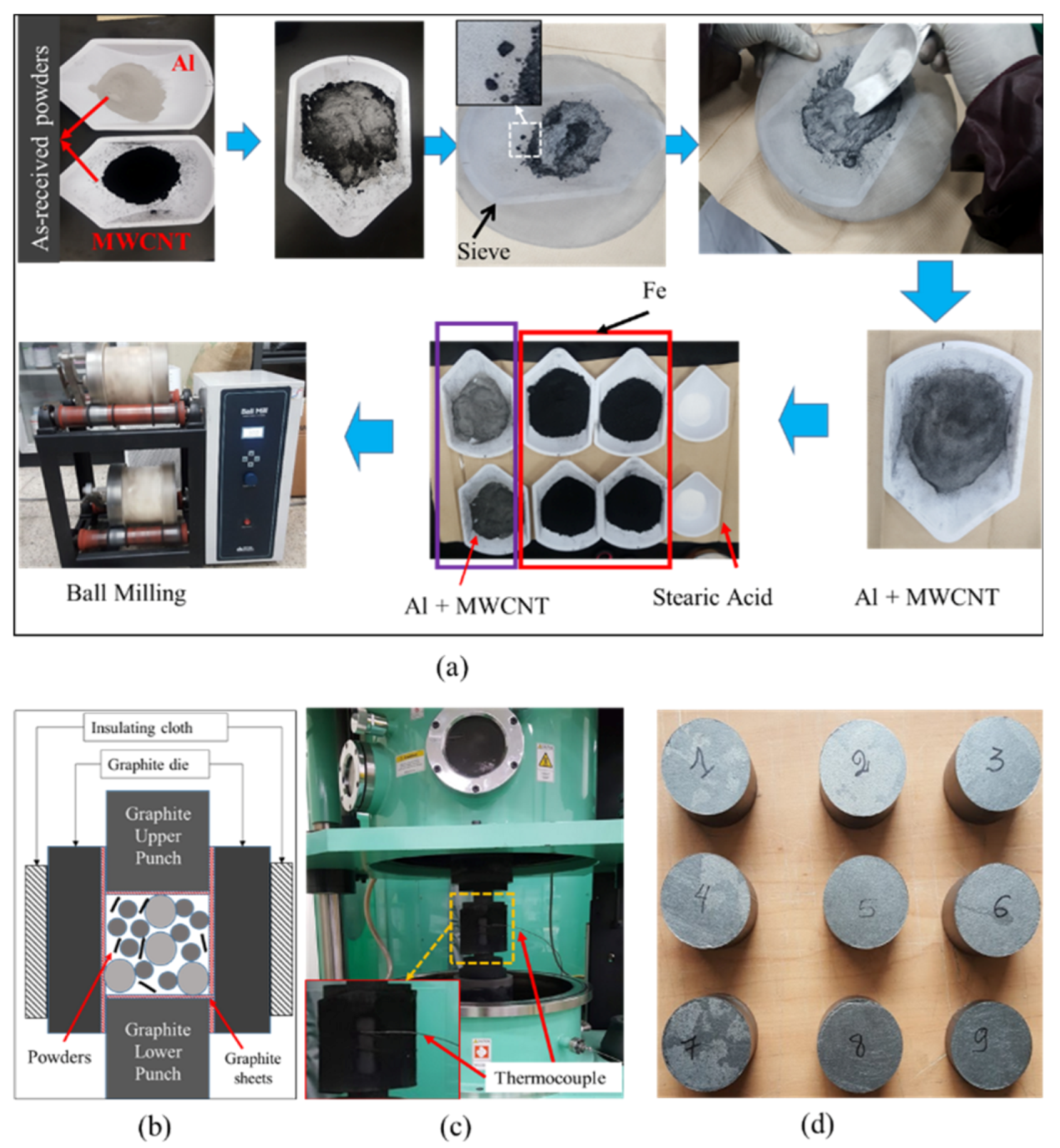
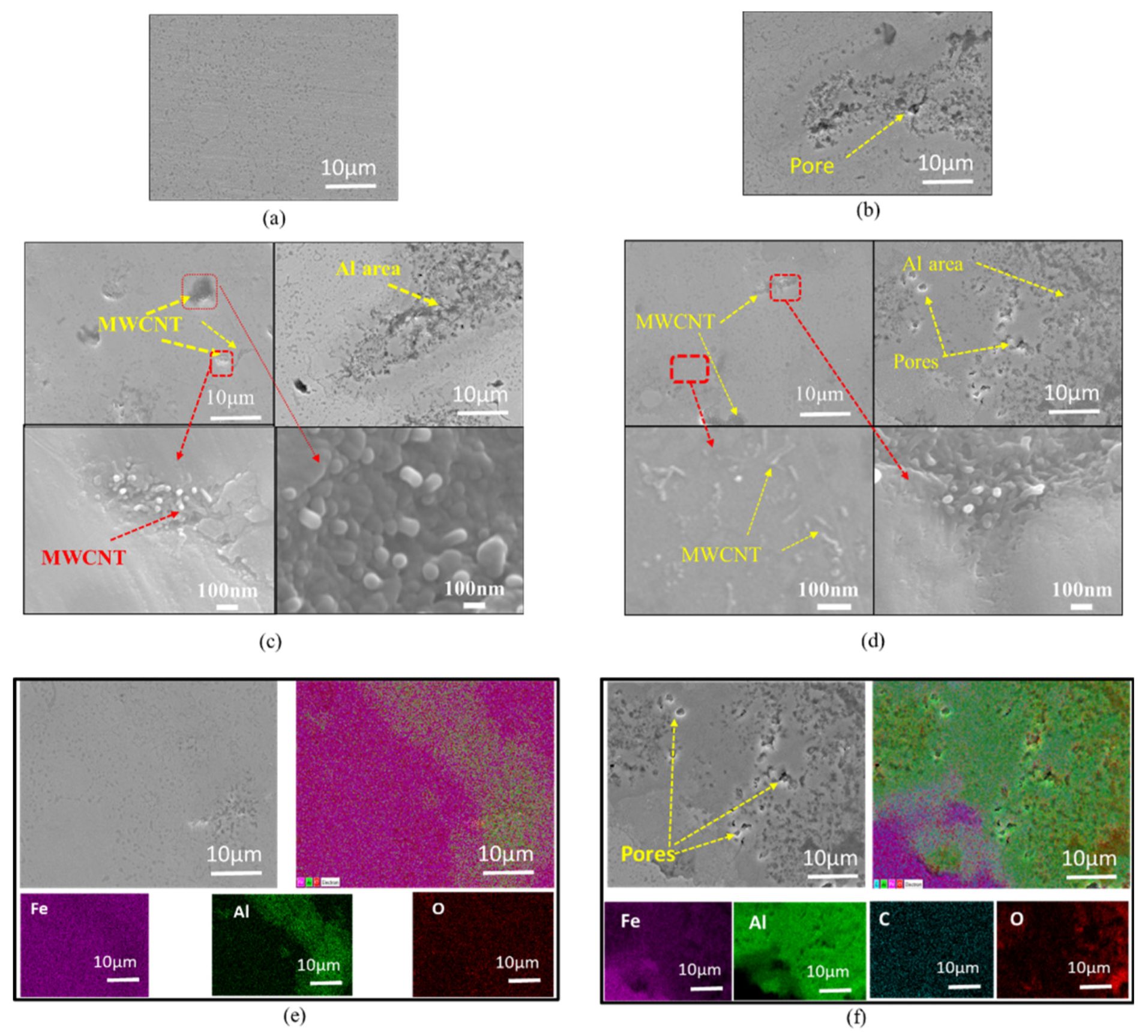


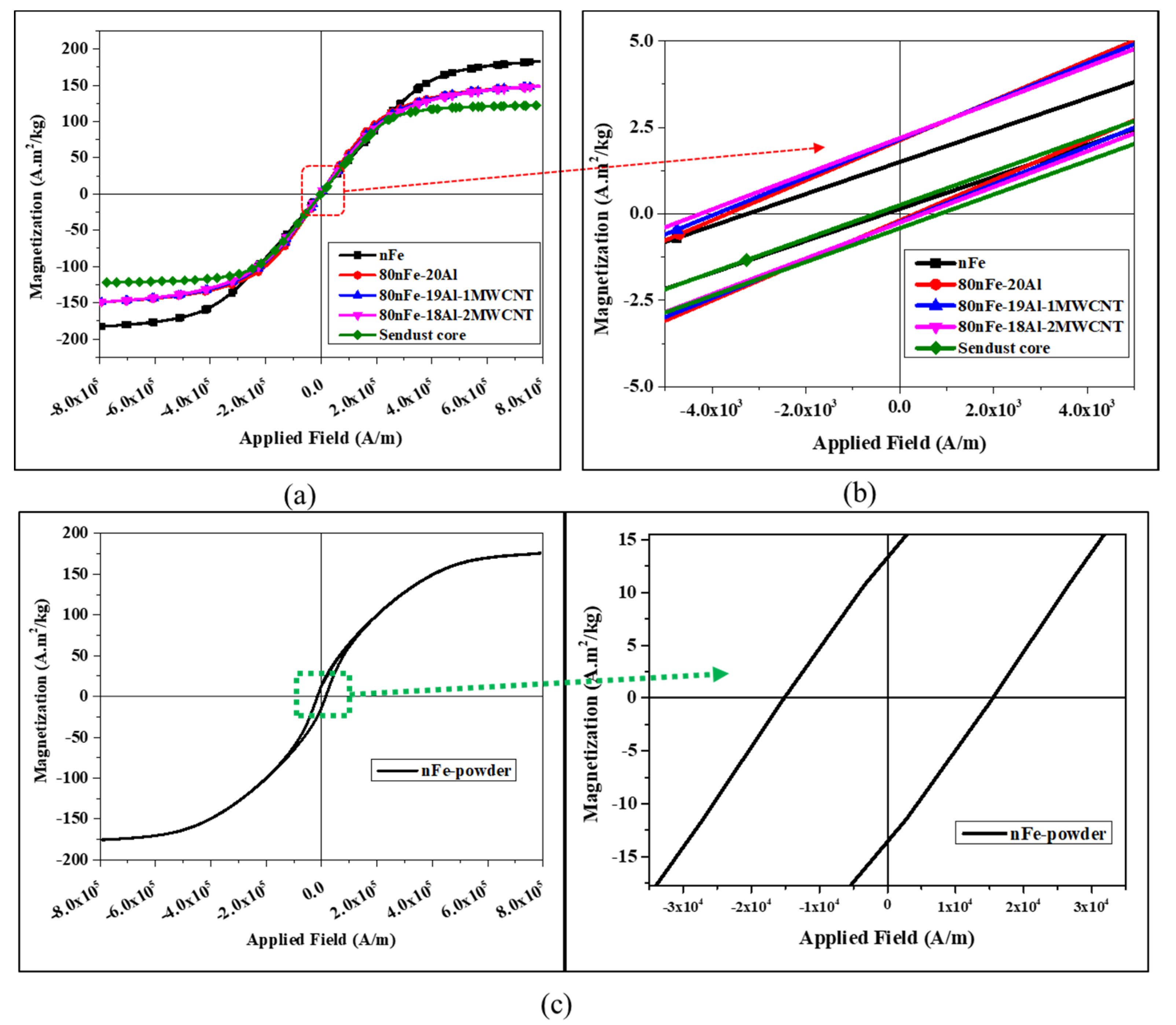




| Composites | Volume Fraction (vol%) | |||
|---|---|---|---|---|
| Fe | Al | MWCNT | ||
| nFe | mFe | |||
| 80nFe-20Al | 80 | 20 | ||
| 80nFe-19Al-1MWCNT | 80 | 19 | 1 | |
| 80nFe-18Al-2MWCNT | 80 | 18 | 2 | |
| 40nFe-40mFe-19Al-1MWCNT | 40 | 40 | 19 | 1 |
| 20nFe-60mFe-19Al-1MWCNT | 20 | 60 | 19 | 1 |
| nFe | 100 | |||
| Composites | Density (g/cm3) | Relative Density (%) | Coercivity, Hc (A/m) | Magnetization, Ms (A·m2/kg) | Remanence, Mr (A·m2/kg) |
|---|---|---|---|---|---|
| nFe | 7.47 | 94.93 | 1466.24 | 182.76 | 0.68 |
| 80nFe-20Al | 6.30 | 92.16 | 1995.70 | 148.83 | 1.16 |
| 80nFe-19Al-1MWCNT | 6.28 | 91.98 | 2175.64 | 148.82 | 1.19 |
| 80nFe-18Al-2MWCNT | 6.21 | 91.09 | 2368.63 | 148.83 | 1.22 |
| Sendust Core | 6.03 | - | 685.10 | 122.20 | 0.33 |
| nFe (as-received powders) | 15,309.71 | 175.97 | 13.44 |
| Milling Time | Density (g/cm3) | Relative Density (%) | Coercivity, Hc (A/m) | Magnetization, Ms (A·m2/kg) | Remanence, Mr (A·m2/kg) |
|---|---|---|---|---|---|
| 12 h | 6.22 | 91.08 | 2125.64 | 143.04 | 1.12 |
| 24 h | 6.28 | 91.98 | 2175.64 | 148.82 | 1.19 |
| 48 h | 6.35 | 93.03 | 2094.19 | 143.86 | 1.05 |
| Composites | Density (g/cm3) | Relative Density (%) | Coercivity, Hc (A/m) | Magnetization, Ms (A·m2/kg) | Remanence, Mr (A·m2/kg) |
|---|---|---|---|---|---|
| 80nFe-19Al-1MWCNT | 6.28 | 91.98 | 2175.64 | 148.82 | 1.19 |
| 40nFe-40mFe-19Al-1MWCNT | 6.24 | 91.36 | 1083.36 | 157.82 | 0.68 |
| 20nFe-60mFe-19Al-1MWCNT | 6.08 | 89.08 | 1514.41 | 149.61 | 0.79 |
| nFe Compact | 7.47 | 94.93 | 1466.24 | 182.76 | 0.68 |
| nFe (as-received powders) | 15309.71 | 175.97 | 13.44 | ||
| mFe (as-received powders) | 1409.24 | 173.12 | 1.15 |
| 40nFe-40mFe-19Al-1MWCNT | Density (g/cm3) | Coercivity, Hc (A/m) | Magnetization, Ms (A·m2/kg) | Remanence, Mr (A·m2/kg) |
|---|---|---|---|---|
| As sintered | 6.24 | 1083.36 | 157.82 | 0.68 |
| Annealed | 6.27 | 854.29 | 175.11 | 0.58 |
| 40nFe-40mFe-19Al-1MWCNT | TRS (MPa) | Electrical Resistivity (μΩ·cm) |
|---|---|---|
| As sintered | 590.39 | 34.99 |
| Annealed | 460.43 | 30.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tugirumubano, A.; Go, S.H.; Kwac, L.K.; Shin, H.J.; Kim, H.G. The Effect of the Processing Parameters on the Properties of the Liquid Phase Spark Plasma Sintered 80Fe-20(Al + MWCNT) Magnetic Metal Matrix Nanocomposites. Metals 2020, 10, 962. https://doi.org/10.3390/met10070962
Tugirumubano A, Go SH, Kwac LK, Shin HJ, Kim HG. The Effect of the Processing Parameters on the Properties of the Liquid Phase Spark Plasma Sintered 80Fe-20(Al + MWCNT) Magnetic Metal Matrix Nanocomposites. Metals. 2020; 10(7):962. https://doi.org/10.3390/met10070962
Chicago/Turabian StyleTugirumubano, Alexandre, Sun Ho Go, Lee Ku Kwac, Hee Jae Shin, and Hong Gun Kim. 2020. "The Effect of the Processing Parameters on the Properties of the Liquid Phase Spark Plasma Sintered 80Fe-20(Al + MWCNT) Magnetic Metal Matrix Nanocomposites" Metals 10, no. 7: 962. https://doi.org/10.3390/met10070962
APA StyleTugirumubano, A., Go, S. H., Kwac, L. K., Shin, H. J., & Kim, H. G. (2020). The Effect of the Processing Parameters on the Properties of the Liquid Phase Spark Plasma Sintered 80Fe-20(Al + MWCNT) Magnetic Metal Matrix Nanocomposites. Metals, 10(7), 962. https://doi.org/10.3390/met10070962





