Microstructural Investigations of Novel High Temperature Alloys Based on NiAl-(Cr,Mo)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
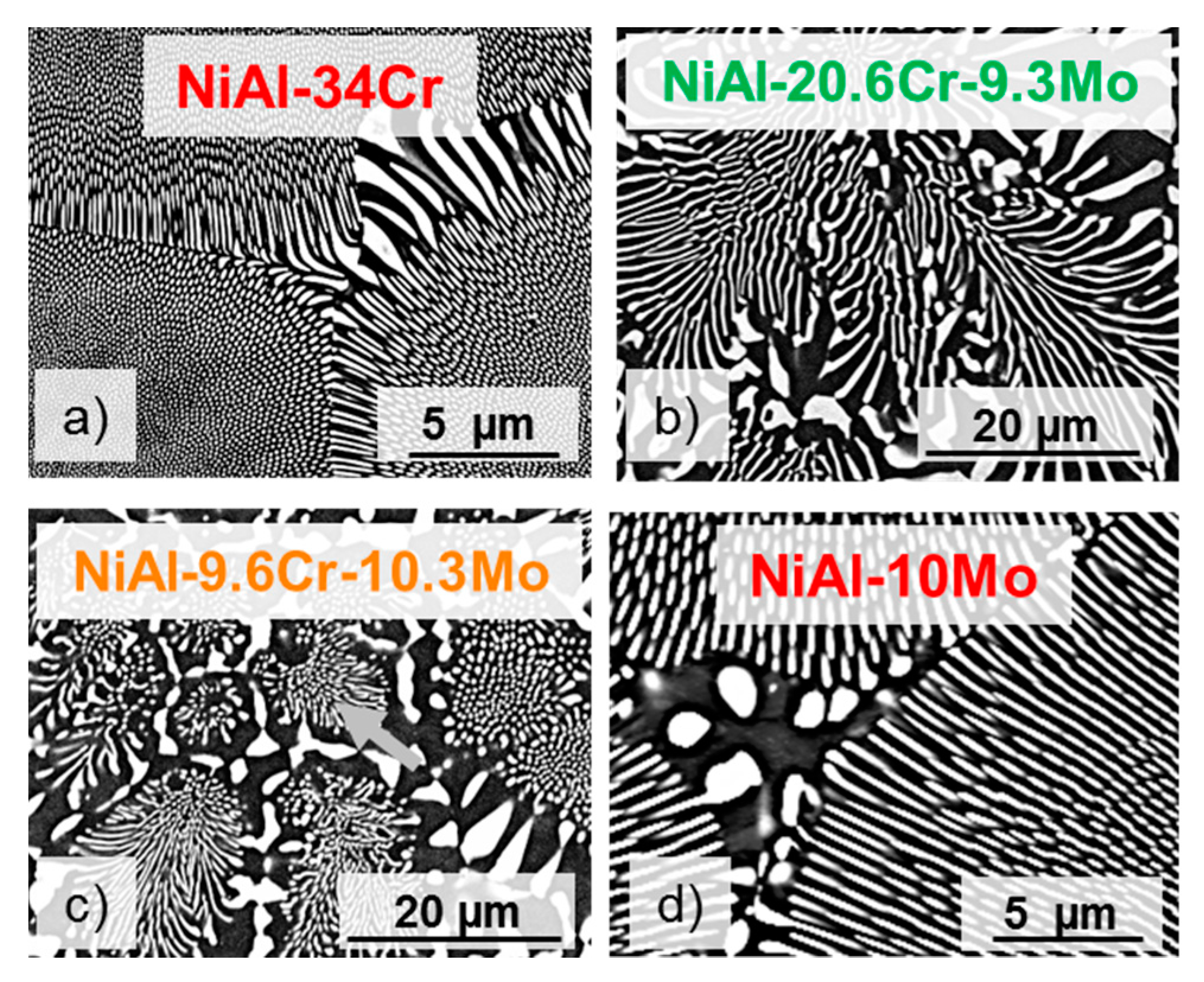
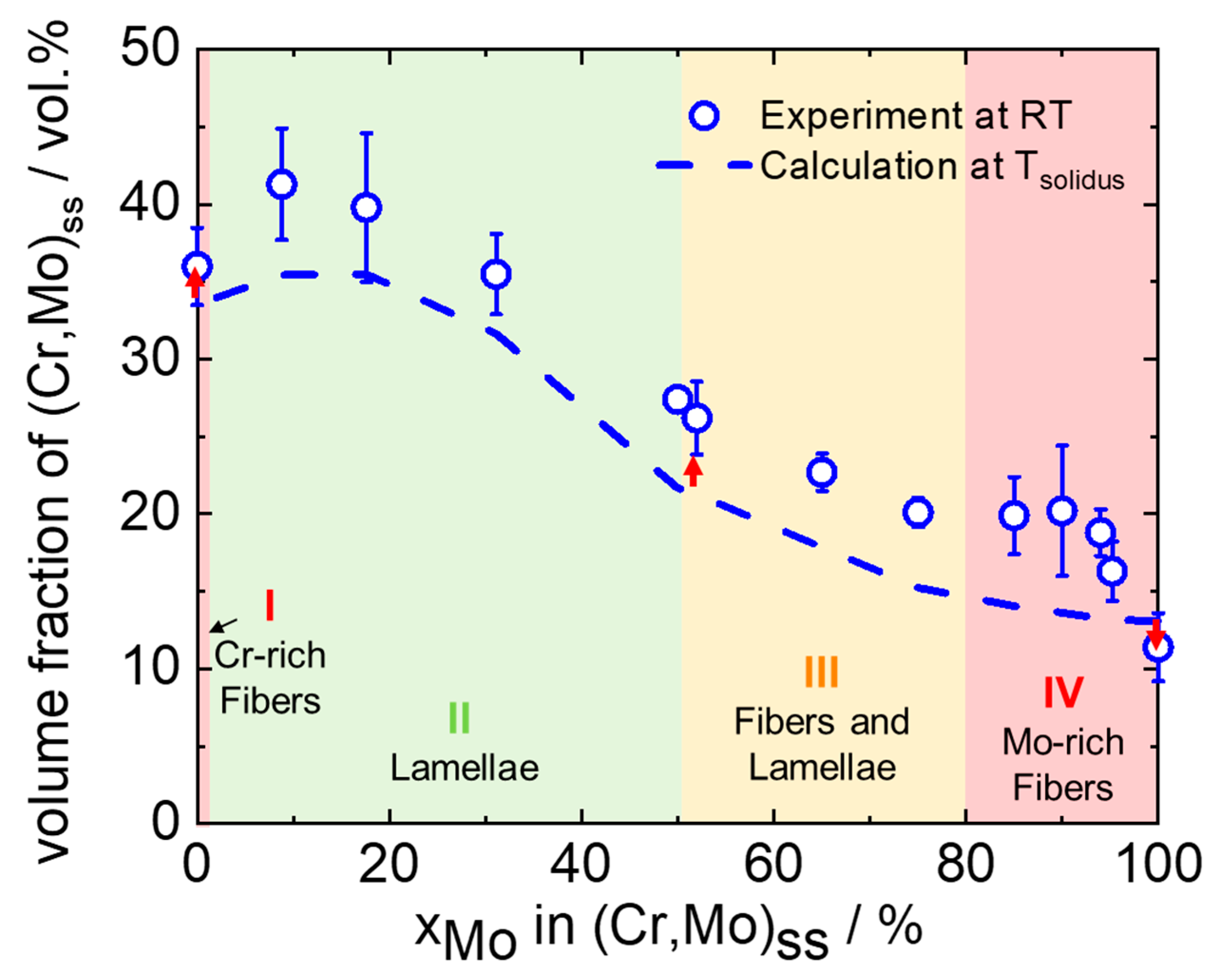
3.1. Morphology and Volume Fraction of the Solid Solution
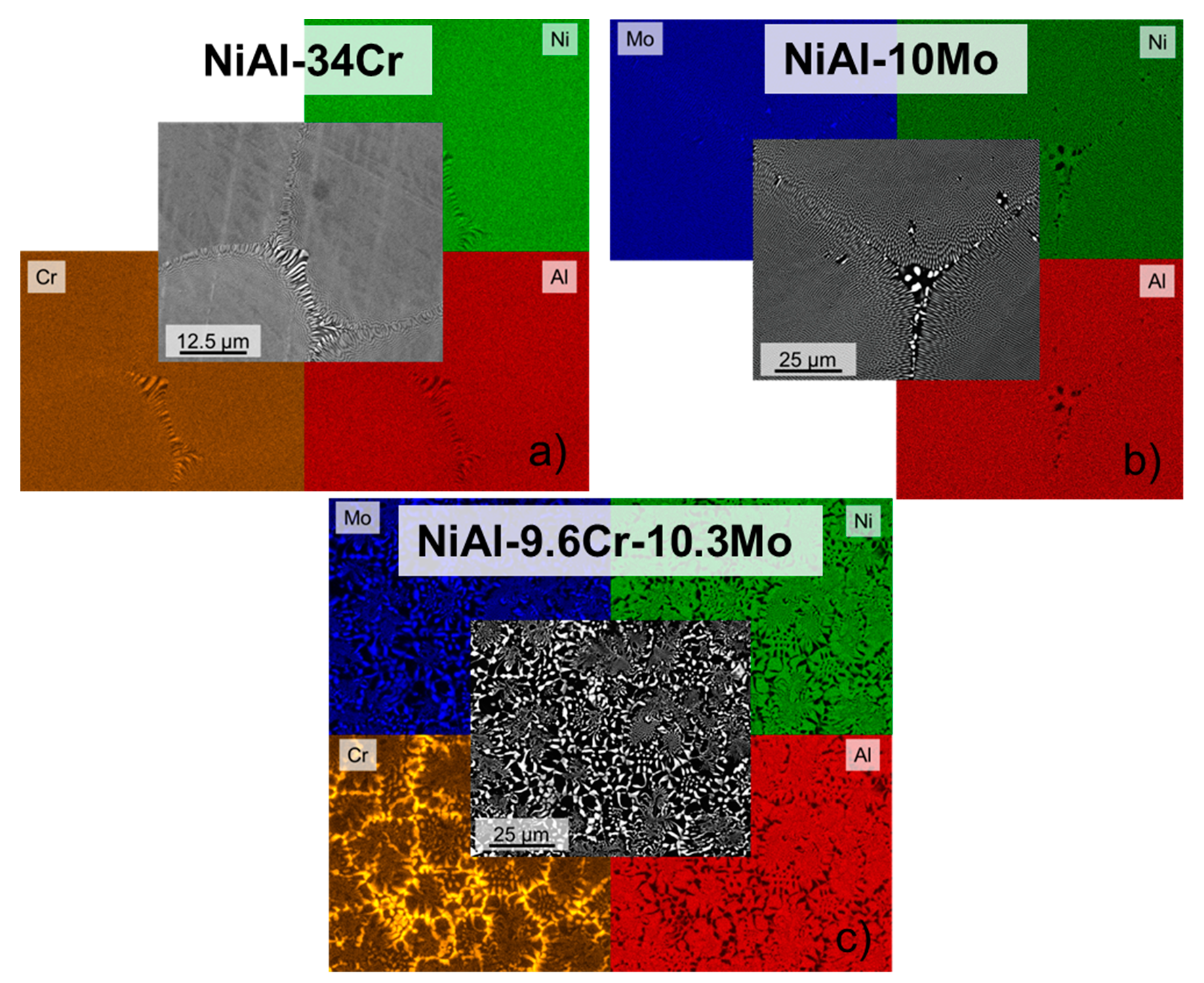
3.2. Element Distribution within the Microstructure
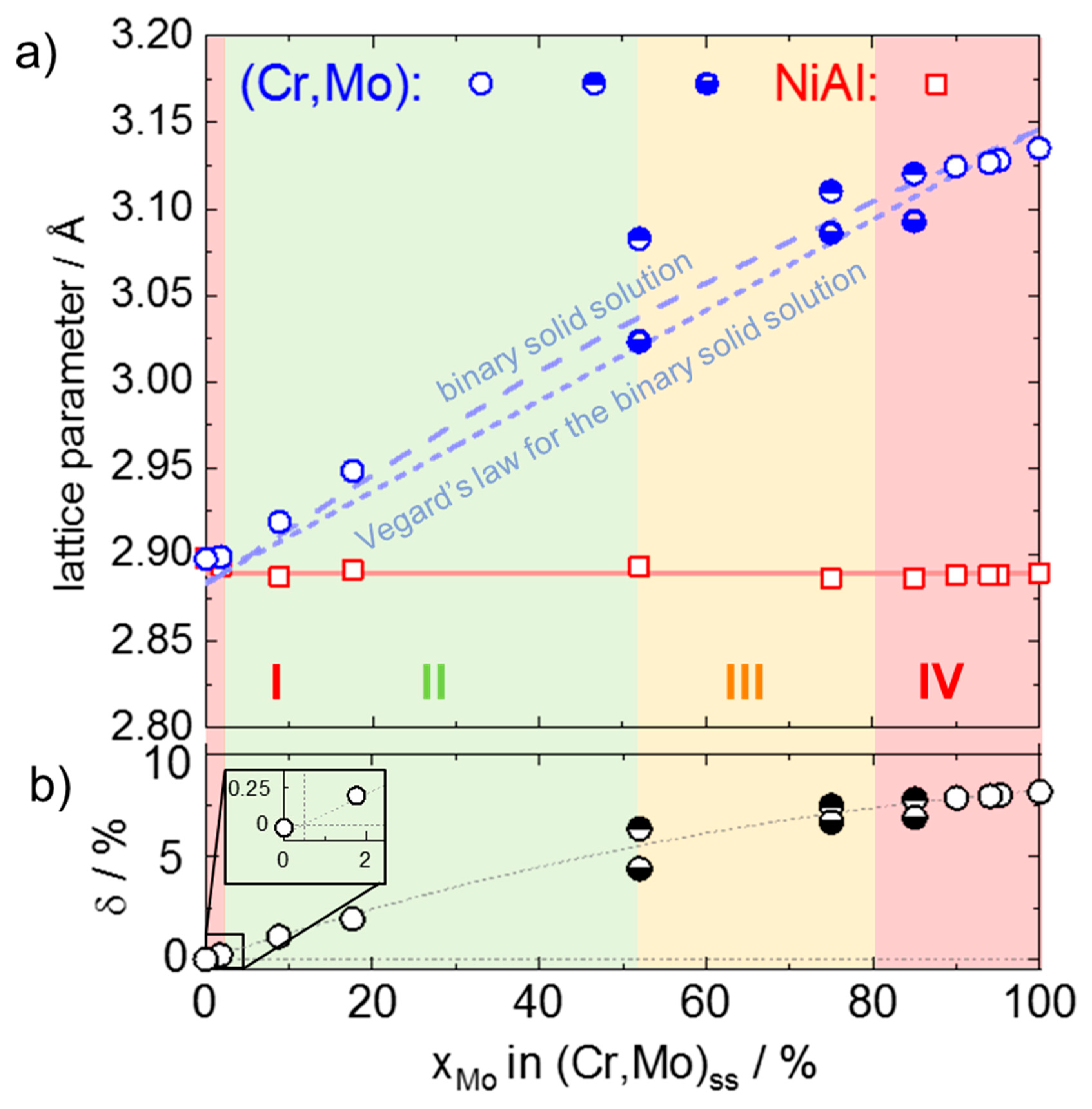
3.3. Room Temperature Lattice Parameter of NiAl and (Cr,Mo)ss
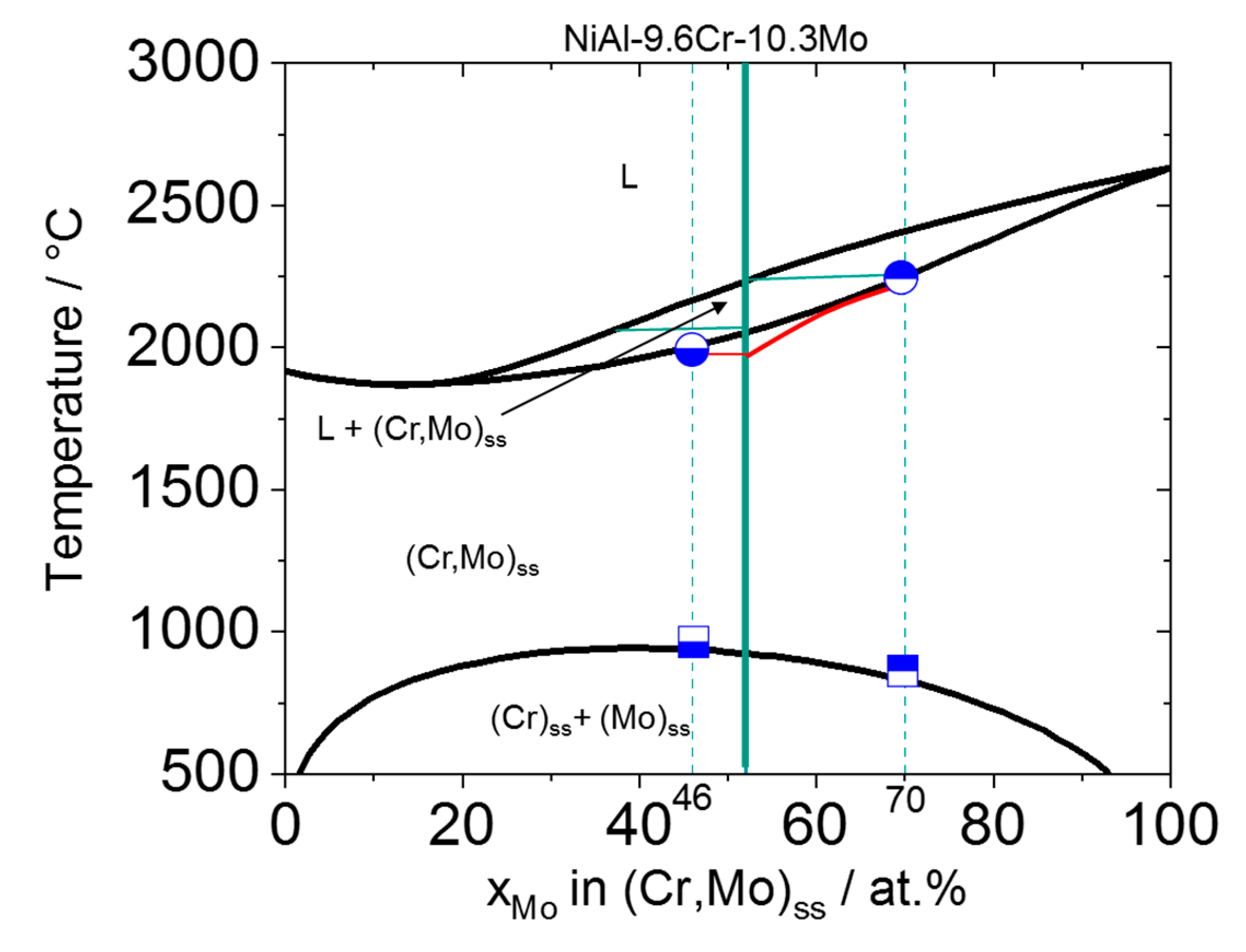
3.4. Evolution of Mo-Rich and Cr-Rich (Cr,Mo)ss during the Solidification Sequence
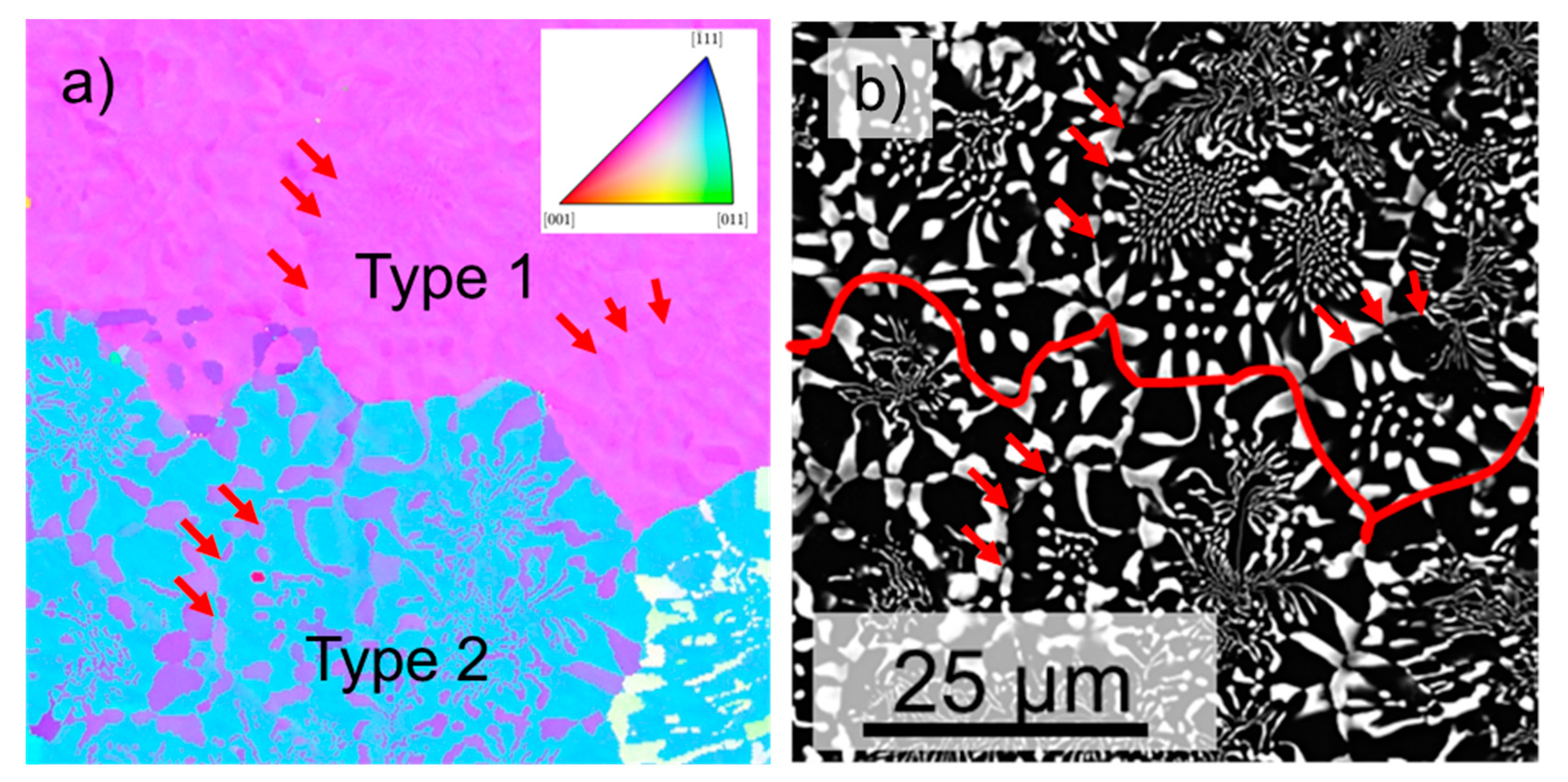
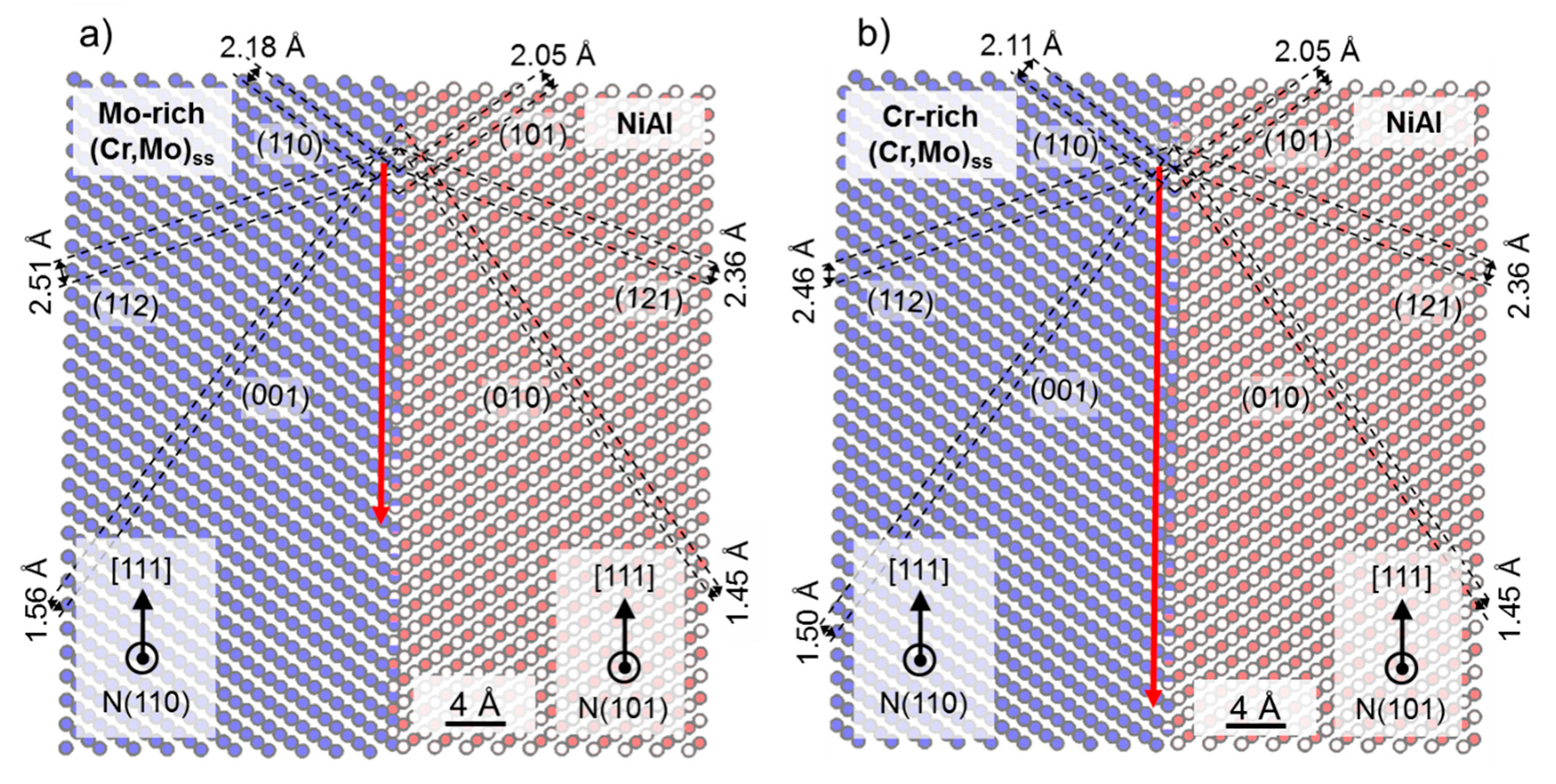
3.5. Crystallographic Relationship in As-Cast NiAl-9.6Cr-10.3Mo Compared to As-Cast NiAl-34Cr and NiAl-10Mo
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frommeyer, G.; Rablbauer, R.; Schaefer, H.J. Elastic properties of B2-ordered NiAl and NiAl-X (Cr, Mo, W) alloys. Intermetallics 2010, 18, 299–305. [Google Scholar] [CrossRef]
- Johnson, D.R.; Chen, X.F.; Oliver, B.F.; Noebe, R.D.; Whittenberger, J.D. Processing and mechanical properties of in-situ composites from the NiAI-Cr and the NiAI-(Cr,Mo) eutectic systems. Intermetallics 1995, 3, 99–113. [Google Scholar] [CrossRef]
- Bei, H.; George, E.P. Microstructures and mechanical properties of a directionally solidified NiAl–Mo eutectic alloy. Acta Mater. 2005, 53, 69–77. [Google Scholar] [CrossRef]
- Cline, H.E.; Walter, J.L.; Lifshin, E.; Russell, R.R. Structures, faults, and the rod-plate transition in eutectics. Metall. Trans. 1971, 2, 189–194. [Google Scholar] [CrossRef]
- Miracle, D.B. The physical and mechanical properties of NiAl. Acta Metall. Mater. 1993, 41, 649–684. [Google Scholar] [CrossRef]
- Noebe, R.D.; Bowman, R.R.; Nathal, M.V. Review of the physical and mechanical properties of the B2 compound NiAl. Int. Mater. Rev. 1993, 38, 193–232. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Shrivastava, S. Brittle-to-ductile transaction in NiAl single crystal. Acta Mater. 1998, 46, 1493–1502. [Google Scholar] [CrossRef]
- Zhang, J.F.; Shen, J.; Shang, Z.; Feng, Z.R.; Wang, L.S.; Fu, H.Z. Microstructure and room temperature fracture toughness of directionally solidified NiAl-Mo eutectic in situ composites. Intermetallics 2012, 21, 18–25. [Google Scholar] [CrossRef]
- Misra, A.; Gibala, R. Plasticity in multiphase intermetallics. Intermetallics 2000, 8, 1025–1034. [Google Scholar] [CrossRef]
- Dudová, M.; Kuchařová, K.; Barták, T.; Bei, H.; George, E.P.; Somsen, C.; Dlouhý, A. Creep in directionally solidified NiAl–Mo eutectics. Scr. Mater. 2011, 65, 699–702. [Google Scholar] [CrossRef]
- Seemüller, C.; Heilmaier, M.; Haenschke, T.; Bei, H.; Dlouhy, A.; George, E.P. Influence of fiber alignment on creep in directionally solidified NiAl–10Mo in-situ composites. Intermetallics 2010, 35, 110–115. [Google Scholar] [CrossRef]
- Haenschke, T.; Gali, A.; Heilmaier, M.; Krüger, M.; Bei, H.; George, E.P. Synthesis and characterization of lamellar and fibre-reinforced NiAl-Mo and NiAl-Cr. J. Phys. Conf. Ser. 2010, 240. [Google Scholar] [CrossRef]
- Albiez, J.; Sprenger, I.; Seemüller, C.; Weygand, D.; Heilmaier, M.; Böhlke, T. Physically motivated model for creep of directionally solidified eutectics evaluated for the intermetallic NiAl–9Mo. Acta Mater. 2016, 110, 377–385. [Google Scholar] [CrossRef]
- Cline, H.E.; Walter, J.L. The effect of alloy additions on the rod-plate transition in the eutectic NiAl-Cr. Metall. Trans. 1971, 1, 2907–2917. [Google Scholar] [CrossRef]
- Kellner, M.; Sprenger, I.; Steinmetz, P.; Hötzer, J.; Nestler, B.; Heilmaier, M. Phase-field simulation of the microstructure evolution in the eutectic NiAl-34Cr system. Comput. Mater. Sci. 2017, 128, 379–387. [Google Scholar] [CrossRef]
- Kumar, A.; Ensslen, C.; Krüger, A.; Klimenkov, M.; Kraft, O.; Schwaiger, R. Micromechanical study on the deformation behavior of directionally solidified NiAl–Cr eutectic composites. J. Mater. Res. 2017, 32, 2127–2134. [Google Scholar] [CrossRef]
- Whittenberger, J.D.; Raj, S.V.; Locci, I.E.; Salem, J.A. Effect of growth rate on elevated temperature plastic flow and room temperature fracture toughness of directionally solidified NiAl-31Cr-3Mo. Intermetallics 1999, 7, 1159–1168. [Google Scholar] [CrossRef]
- Whittenberger, J.D.; Raj, S.V.; Locci, I.E.; Salem, J.A. Elevated temperature strength and room-temperature toughness of directionally solidified Ni-33Al-33Cr-1Mo. Metall. Mater. Trans. A 2002, 33, 1385–1397. [Google Scholar] [CrossRef]
- Chen, X.F.; Johnson, D.R.; Noebe, R.D.; Oliver, B.F. Deformation and fracture of a directionally solidified NiAl-28Cr-6Mo eutectic alloy. J. Mater. Res. 1995, 10, 1159–1170. [Google Scholar] [CrossRef]
- Peng, J.; Franke, P.; Seifert, H.J. Experimental Investigation and CALPHAD Assessment of the Eutectic Trough in the System NiAl-Cr-Mo. J. Phase Equilibria Diffus. 2016, 37, 592–600. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Rashkova, B.; Clemens, H.; Stark, A.; Mayer, S. Effect of carbon addition on solidification behavior, phase evolution and creep properties of an intermetallic β-stabilized γ-TiAl based alloy. Intermetallics 2014, 46, 173–194. [Google Scholar] [CrossRef]
- Hammersley, A.P. ESRF Internal Report, ESRF97HA02T, FIT2D: An Introduction and Overview; ESRF: Grenoble, France, 1997. [Google Scholar]
- Hammersley, A.P. ESRF Internal Report, ESRF98HA01T, FIT2D V9.129 Reference Manual V3.1998, 1; ESRF: Grenoble, France, 1998. [Google Scholar]
- Lutterotti, L.; Bortolotti, M.; Ischia, G.; Lonardelli, I.; Wenk, H.-R. Rietveld texture analysis from diffraction images. Z. Krist. Suppl. 2007, 26, 125–130. [Google Scholar] [CrossRef]
- Nelson, J.B.; Riley, D.P. An experimental investigation of extrapolation methods in the derivation of accurate unit-cell dimensions of crystals. Proc. Phys. Soc. 1945, 57, 160–177. [Google Scholar] [CrossRef]
- Peng, J.; Franke, P.; Manara, D.; Watkins, T.; Konings, R.; Seifert, H.J. Experimental investigation and thermodynamic re-assessment of the Al–Mo–Ni system. J. Alloys Compd. 2016, 674, 305–314. [Google Scholar] [CrossRef]
- Peng, J. Experimental Investigation and Thermodynamic modeling of the Al-Cr-Mo-Ni System and Its Sub-Systems; Dissertation, Karlsruhe Institute of Technology: Karlsruhe, Germany, 2016. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Sutliff, J.A.; Jackson, M.R.; Chang, K.M. Processing, Microstructures, and Properties of Cr-Cr3Si, Nb-Nb3Si, and V-V3Si Eutectics. Mater. Manuf. Process. 1994, 9, 89–109. [Google Scholar] [CrossRef]
- Misra, A.; Gibala, R.; Noebe, R.D. Optimization of toughness and strength in multiphase intermetallics. Intermetallics 2001, 9, 971–978. [Google Scholar] [CrossRef]
- Hughes, T.; Lautenschlager, E.P.; Cohen, J.B.; Brittain, J.O. X-Ray Diffraction Investigation of β′-NiAl Alloys. J. Appl. Phys. 1971, 42, 3705–3716. [Google Scholar] [CrossRef]
- Taylor, A.; Doyle, N.J. Further studies on the Nickel-Aluminum system. I. The β-NiAI and δ-Ni2AI3 phase fields. J. Appl. Cryst. 1972, 5, 201–209. [Google Scholar] [CrossRef]
- Noebe, R.D.; Bowman, R.R.; Nathal, M.V. NASA Technical Memorandum 105598; NASA: Washington, DC, USA, 1992. [Google Scholar]
- Rablbauer, R.; Fischer, R.; Frommeyer, G. Mechanical properties of NiAl–Cr alloys in relation to microstructure and atomic defects. Z. Met. 2004, 95, 525–535. [Google Scholar] [CrossRef]
- Baen, S.R.; Duwez, P. Constitution of Iron-Chromium-Molydenum alloys. J. Met. 1951, 331–335. [Google Scholar] [CrossRef]
- Trzebiatowski, W.; Ploszek, H.; Lobzowski, J. X-Ray analysis of Chromium-Molybdenum and Chromium-Tungsten alloys. Anal. Chemustry 1947, 19, 93–95. [Google Scholar] [CrossRef]
- Venkatraman, M.; Neumann, J.P. The Cr-Mo (Chromium-Molybdenum) system. Bull. Alloy Phase Diagr. 1987, 8, 216–220. [Google Scholar] [CrossRef]
- Hahn, J.D.; Wu, F.; Bellon, P. Cr-Mo solid solutions forced by high-energy ball milling. Metall. Mater. Trans. A 2004, 35, 1105–1111. [Google Scholar] [CrossRef]
- Naidu, S.V.N.; Sriramamurthy, A.M.; Rao, P.R. The Cr-W (Chromium-Tungsten) system. Bull. Alloy Phase Diagr. 1984, 5, 289–292. [Google Scholar] [CrossRef]
- Laffitte, M.; Kubaschewski, O. Activities of Chromium in Chromium-Molybdenum solid solutions. Trans. Faraday Soc. 1960, 57, 932–934. [Google Scholar] [CrossRef]
- Milenkovic, S.; Caram, R. Growth morphology of the NiAl-V in situ composites. J. Mater. Process. Technol. 2003, 144, 629–635. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Edge-to-edge matching model for predicting orientation and habit planes- the improvements. Scr. Mater. 2005, 52, 963–968. [Google Scholar] [CrossRef]


| Alloy | Element | Overall Composition * | NiAl Matrix | (Cr,Mo)ss | ||
|---|---|---|---|---|---|---|
| Calc. | Exp. ** | Calc. | Exp. ** | |||
| 90% Mo in (Cr,Mo)ss | Al/at.% | 44.31 | 49.51 | 48.7 | 4.18 | 10.4 |
| Cr/at.% | 1.06 | 0.46 | 2.4 | 6.24 | 1.4 | |
| Ni/at.% | 44.86 | 50.03 | 46.1 | 0.02 | 4.4 | |
| Mo/at.% | 9.74 | 0 | 2.8 | 89.56 | 83.8 | |
| 95% Mo in (Cr,Mo)ss | Al/at.% | 44.6 | 49.64 | 50.9 | 4.04 | 10.0 |
| Cr/at.% | 0.5 | 0.23 | 0.1 | 2.77 | 0.7 | |
| Ni/at.% | 45.1 | 50.13 | 48.6 | 0.01 | 4.5 | |
| Mo/at.% | 9.8 | 0 | 0.4 | 93.17 | 84.8 | |
| Alloy | Colony Type 1 | Colony Type 2 |
|---|---|---|
| NiAl-34Cr | (1.9 ± 0.8)° about arb. axis | - |
| NiAl-9.6Cr-10.3Mo | (0.95 ± 0.5)° about arb. axis | (0.95 ± 0.5)° about |
| NiAl-10Mo | (0.9 ± 0.6)° about arb. axis | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gombola, C.; Kauffmann, A.; Geramifard, G.; Blankenburg, M.; Heilmaier, M. Microstructural Investigations of Novel High Temperature Alloys Based on NiAl-(Cr,Mo). Metals 2020, 10, 961. https://doi.org/10.3390/met10070961
Gombola C, Kauffmann A, Geramifard G, Blankenburg M, Heilmaier M. Microstructural Investigations of Novel High Temperature Alloys Based on NiAl-(Cr,Mo). Metals. 2020; 10(7):961. https://doi.org/10.3390/met10070961
Chicago/Turabian StyleGombola, Camelia, Alexander Kauffmann, Golnar Geramifard, Malte Blankenburg, and Martin Heilmaier. 2020. "Microstructural Investigations of Novel High Temperature Alloys Based on NiAl-(Cr,Mo)" Metals 10, no. 7: 961. https://doi.org/10.3390/met10070961
APA StyleGombola, C., Kauffmann, A., Geramifard, G., Blankenburg, M., & Heilmaier, M. (2020). Microstructural Investigations of Novel High Temperature Alloys Based on NiAl-(Cr,Mo). Metals, 10(7), 961. https://doi.org/10.3390/met10070961







