Atomistic Simulation of the Strain Driven Phase Transition in Pure Iron Thin Films Containing Twin Boundaries
Abstract
1. Introduction
2. Simulation Method
3. Result
3.1. Film Group 1 with Perpendicular TB-Surface Orientation Relationship
3.1.1. Stress-Strain Curves
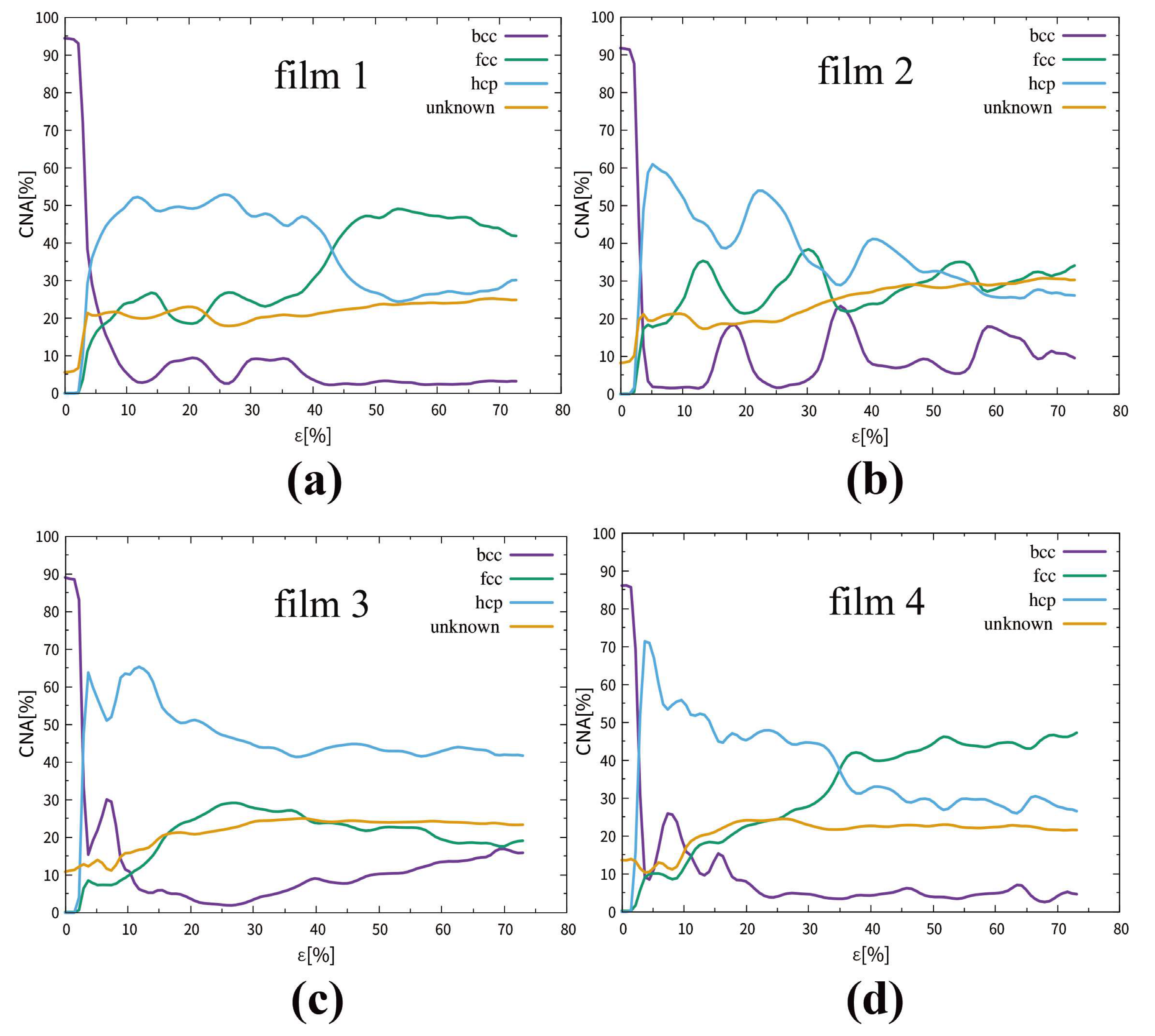
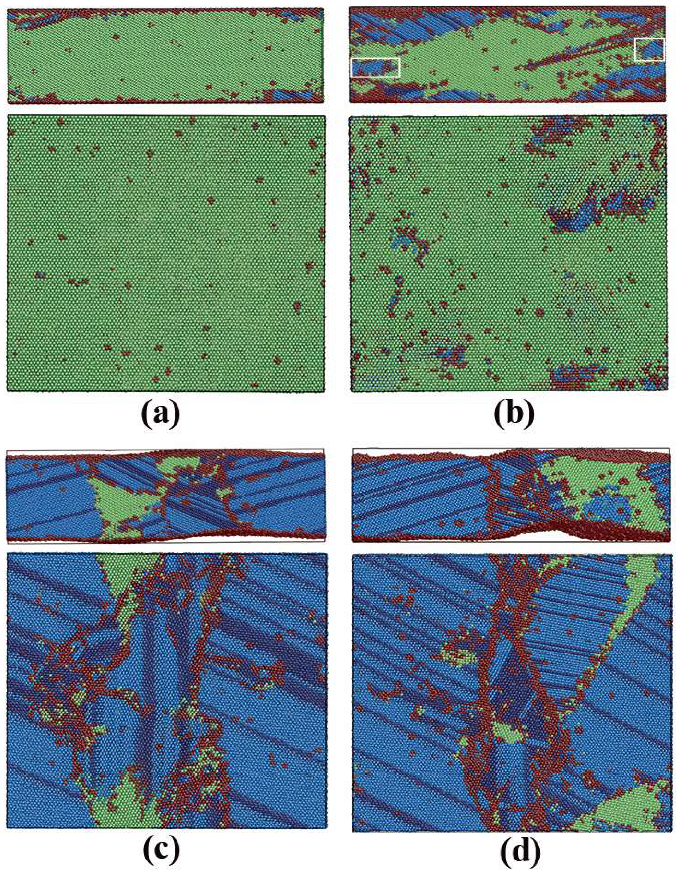
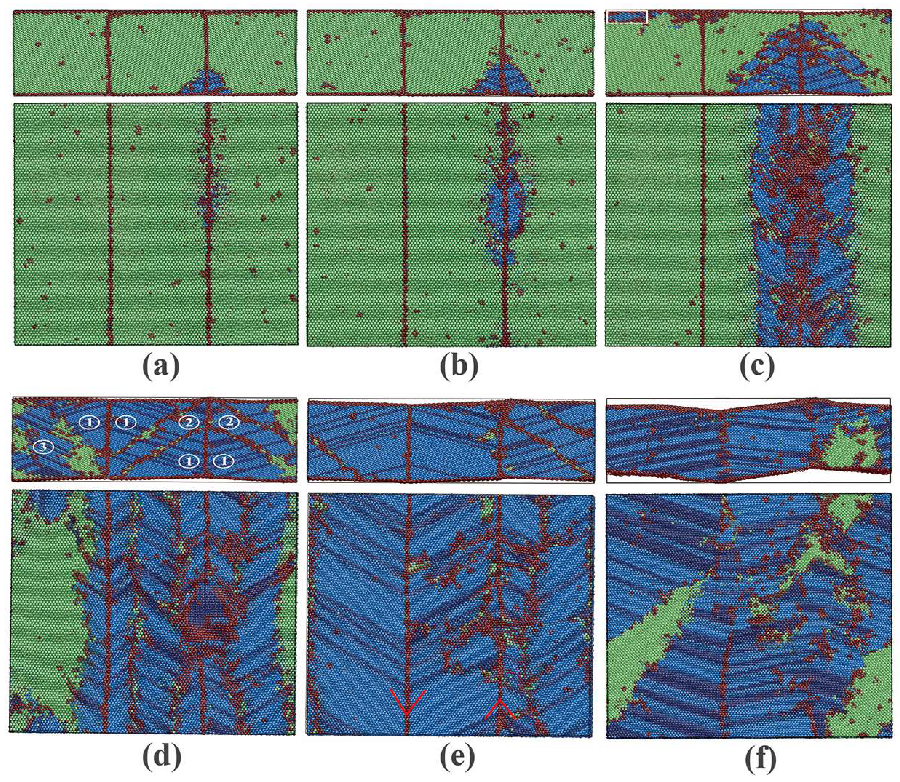
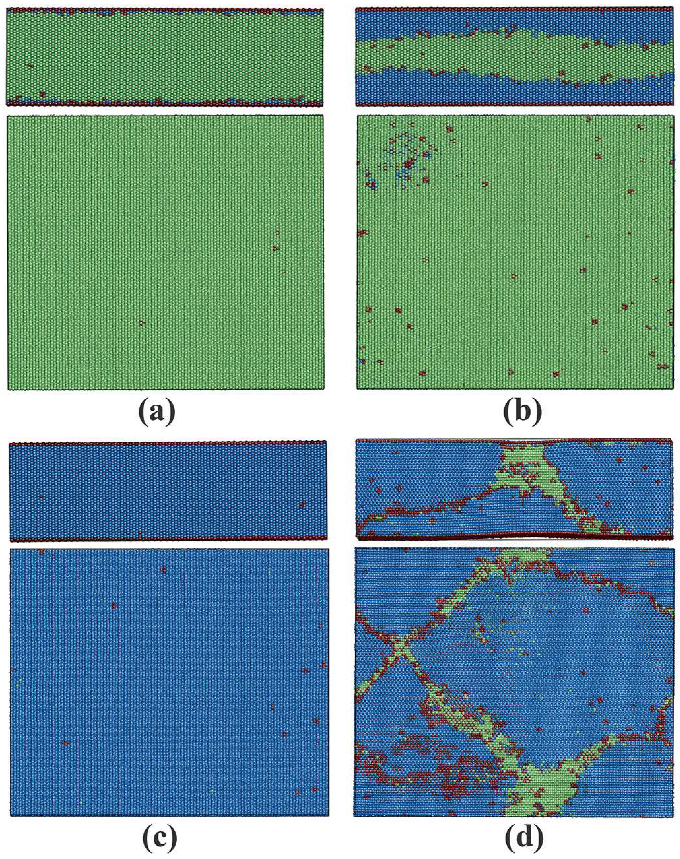
3.1.2. Phase Transitions in Films 1–4
3.2. Film Group 2 with Parallel TB-Surface Orientation Relationship
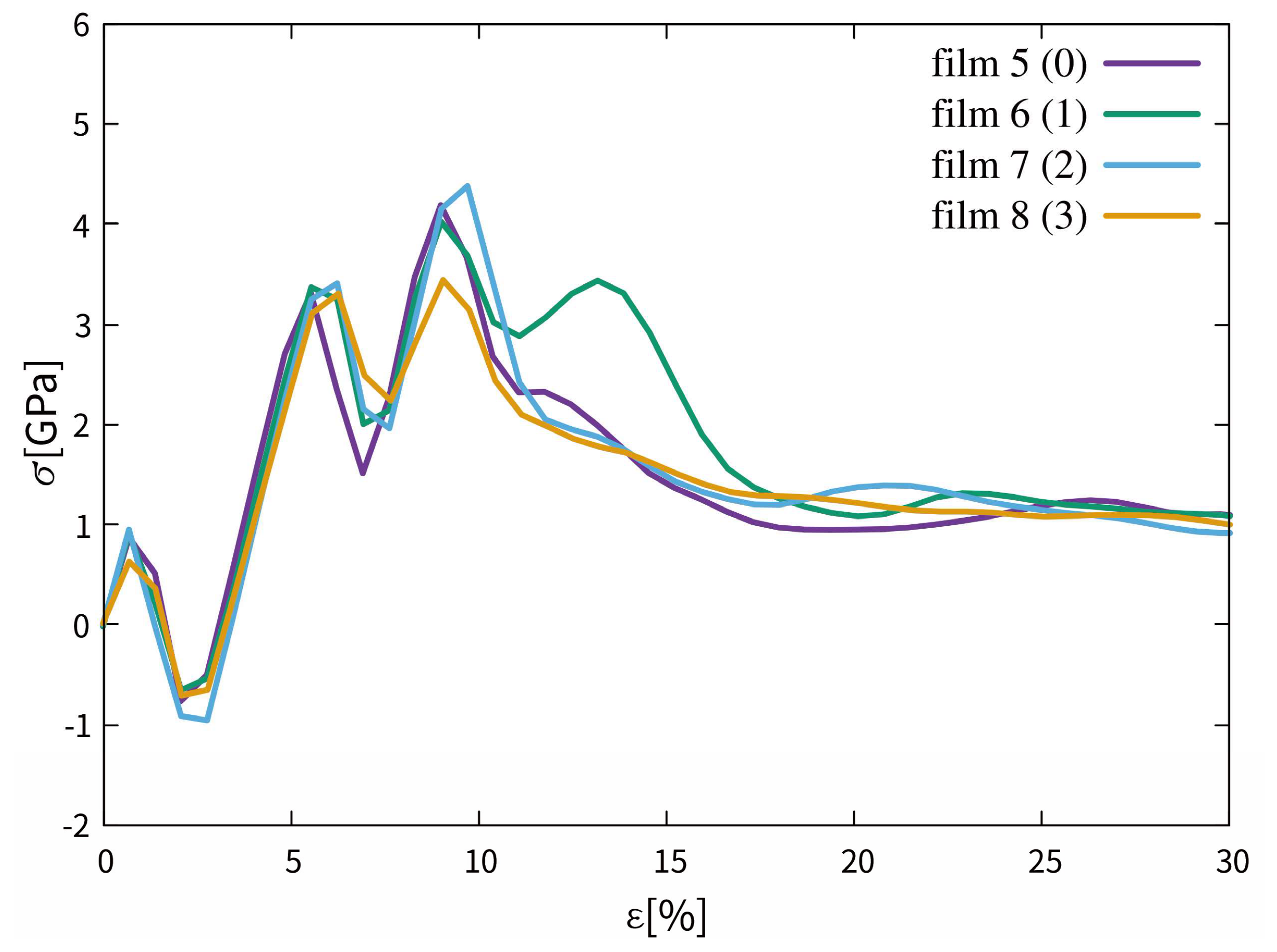
3.2.1. Stress and Strain Curves
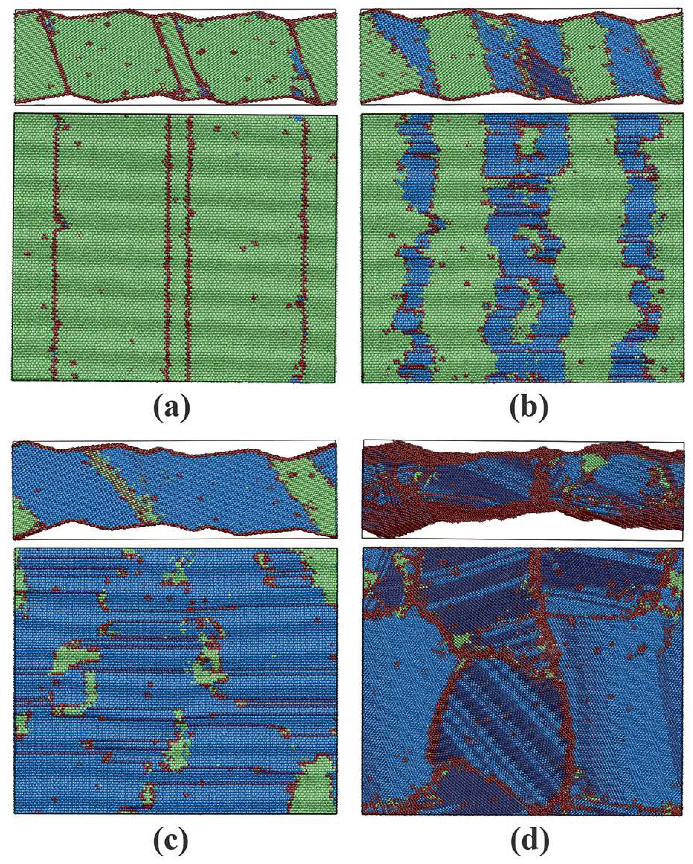
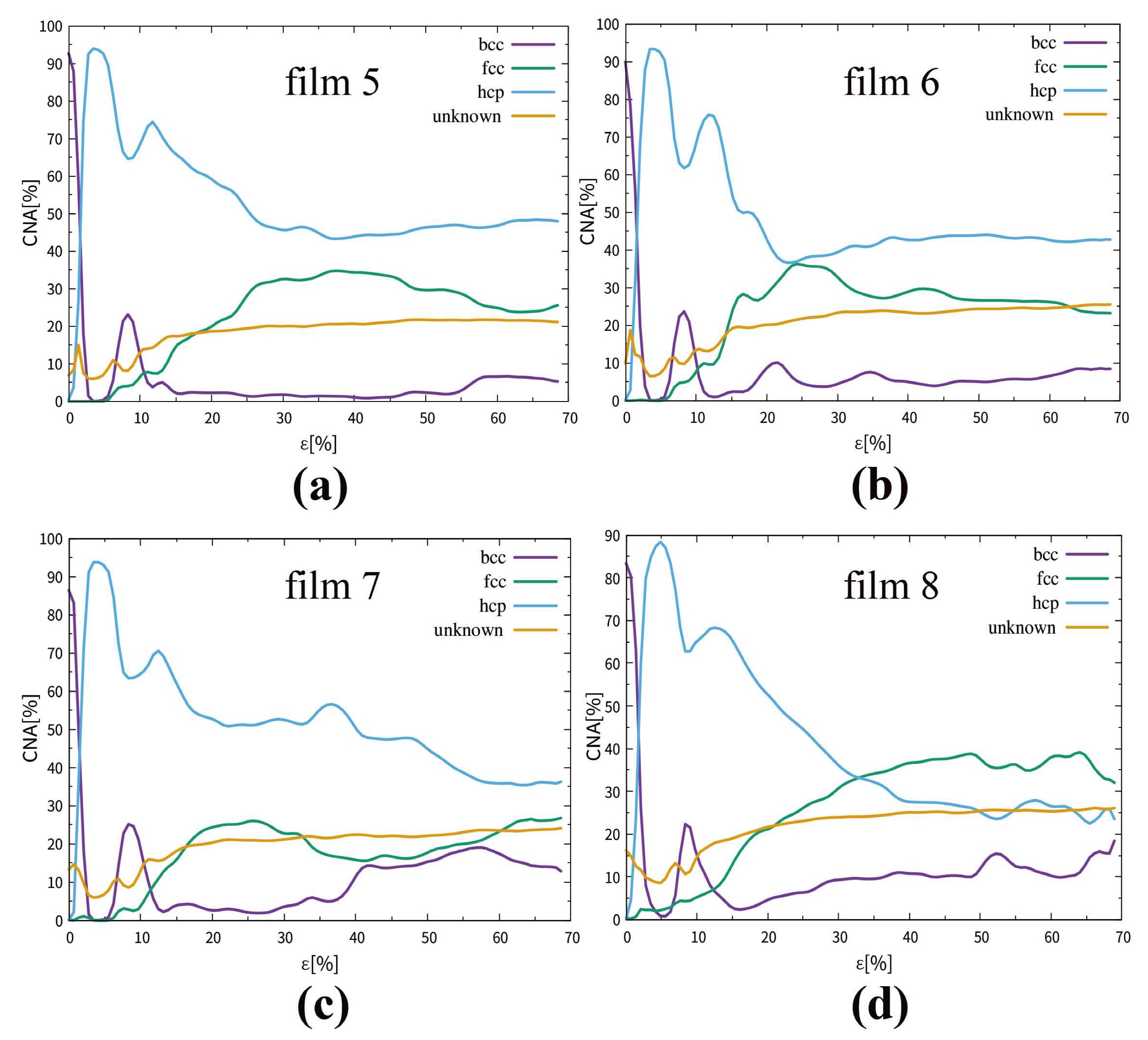
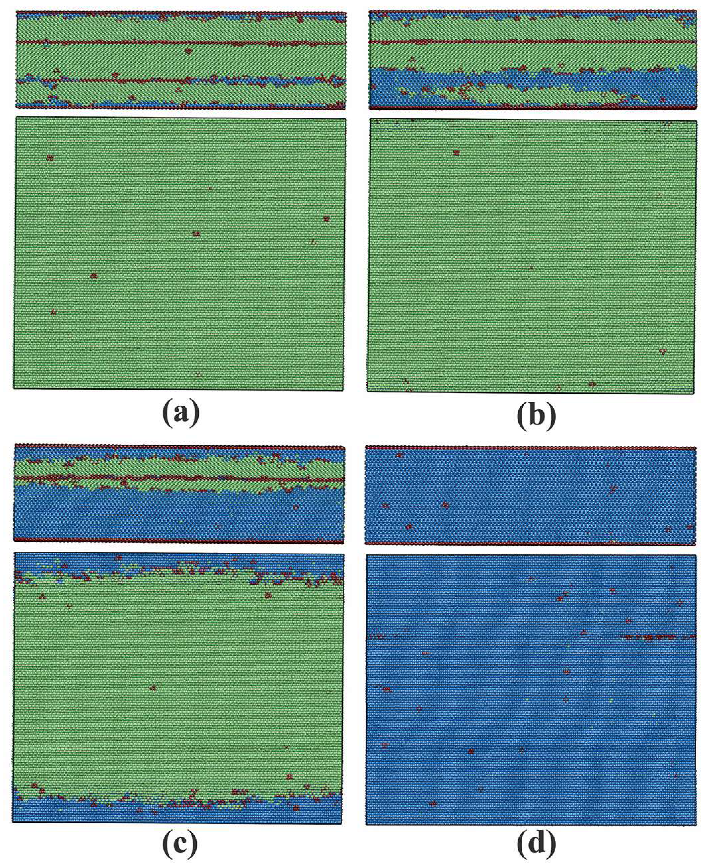
3.2.2. Phase Transitions in Films 5–8
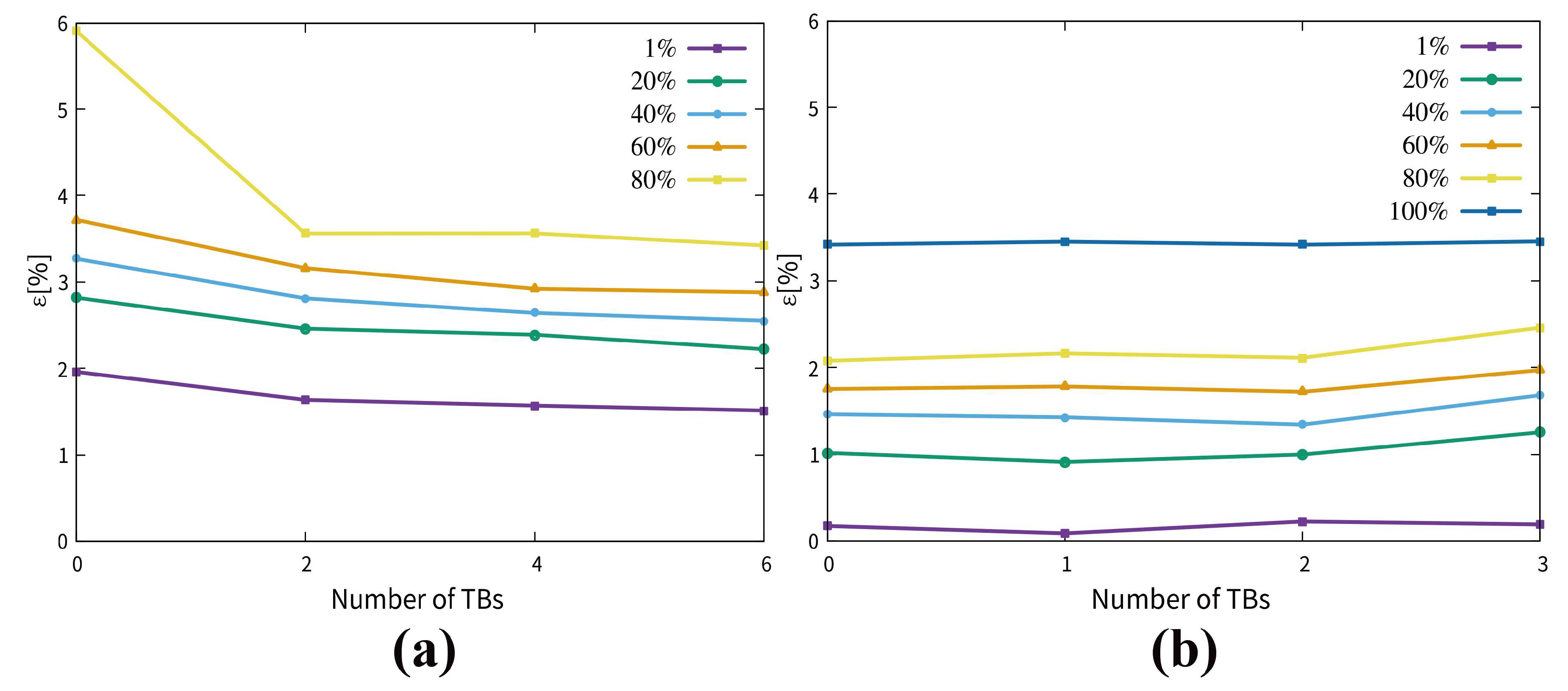
3.3. Dynamics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| x, y, z | Coordinate directions |
| Δx, Δy, Δz | Thicknesses of the films in each direction, Å |
| T | Number of the TBs in the films |
| N | Total number of the atoms |
| σ | Hydrostatic stress, GPa |
| σxx, σyy, σzz | Normal stresses in x, y and z directions, GPa |
| ε | Strain, % |
| CNA (in Figure 4 and Figure 9) | Fractional phase content, analyzed using common neighbor analysis (CNA), % |
References
- Griesser, S.; Bernhard, C.; Dippenaar, R. Effect of nucleation undercooling on the kinetics and mechanism of the peritectic phase transition in steel. Acta Mater. 2014, 81, 111–120. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M. Phase Transformations in Metals and Alloys, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1992; Volume 1, p. 245. [Google Scholar]
- Dupé, B.; Amadon, B.; Pellegrini, Y.P.; Denoual, C. Mechanism for the phase transition in iron. Phys. Rev. B 2013, 87, 024103. [Google Scholar] [CrossRef]
- Pang, W.W.; Zhang, P.; Zhang, G.C.; Xu, A.G.; Zhao, X.G. Morphology and growth speed of hcp domains during shock-induced phase transition in iron. Sci. Rep. 2014, 4, 3628. [Google Scholar] [CrossRef]
- Monza, A.; Meffre, A.; Baudelet, F.; Rueff, J.P.; d’Astuto, M.; Munsch, P.; Huotari, S.; Lachaize, S.; Chaudret, B.; Shukla, A. Iron under pressure: “Kohn Tweezers” and remnant magnetism. Phys. Rev. Lett. 2011, 106, 247201. [Google Scholar] [CrossRef]
- Djohari, H.; Milstein, F.; Maroudas, D. Dynamics of the bcc hcp transition in crystals under uniaxial stress. Phys. Rev. B 2009, 79, 174109. [Google Scholar] [CrossRef]
- Gornostyrev, Y.N.; Katsnel’son, M.I.; Kuznetsov, A.R. Microscopic description of the kinetics of a martensitic transition in real crystals: Bcc-hcp transition in Zr. Jetp Lett. 1999, 70, 380–384. [Google Scholar] [CrossRef]
- Takahashi, T.; Bassett, W.A. High-pressure polymorph of iron. Science 1964, 145, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Clendenen, R.L.; Drickamer, H.G. The effect of pressure on the volume and lattice parameters of ruthenium and iron. J. Phys. Chem. Solids 1964, 25, 865–868. [Google Scholar] [CrossRef]
- Caron, R.N.; Krauss, G. Transformation-induced structures and martensite nucleation. Metall. Trans. 1970, 1, 333–334. [Google Scholar]
- Karewar, S.; Sietsma, J.; Santofimia, M.J. Effect of pre-existing defects in the parent fcc phase on atomistic mechanisms during the martensitic transformation in pure Fe: A molecular dynamics study. Acta Mater. 2018, 142, 71–81. [Google Scholar] [CrossRef]
- Luu, H.T.; Veiga, R.G.A.; Gunkelmann, N. Atomistic study of the role of defects on α → ϵ phase transformations in iron under hydrostatic compression. Metals 2019, 9, 1040. [Google Scholar] [CrossRef]
- Luu, H.T.; Gunkelmann, N. Pressure-induced phase transformations in Fe-C: Molecular dynamics approach. Comput. Mater. Sci. 2019, 162, 295–303. [Google Scholar] [CrossRef]
- Shao, J.L.; Wang, P.; Zhang, F.G.; He, A.M. Hcp/fcc nucleation in bcc iron under different anisotropic compressions at high strain rate: Molecular dynamics study. Sci. Rep. 2018, 8, 7650. [Google Scholar] [CrossRef]
- Wen, Y.H.; Zhu, Z.Z.; Shao, G.F.; Zhu, R.Z. The uniaxial tensile deformation of Ni nanowire: Atomic-scale computer simulations. Physica E 2005, 27, 113–120. [Google Scholar] [CrossRef]
- Ma, F.; Xu, K.W. Size-dependent theoretical tensile strength and other mechanical properties of [001] oriented Au, Ag, and Cu nanowires. J. Mater. Res. 2006, 21, 2810–2816. [Google Scholar] [CrossRef]
- Lu, Y.; Song, J.; Huang, J.Y.; Lou, J. Surface dislocation nucleation mediated deformation and ultrahigh strength in sub-10-nm gold nanowires. Nano Res. 2011, 4, 1261–1267. [Google Scholar] [CrossRef]
- Nizolek, T.; Mara, N.A.; Beyerlein, I.J.; Avallone, J.T.; Pollock, T.M. Enhanced plasticity via kinking in cubic metallic nanolaminates. Adv. Eng. Mater. 2014, 17, 781–785. [Google Scholar] [CrossRef]
- Saha, R.; Nix, W.D. Effects of the substrate on the determination of thin film mechanical properties by nanoindentation. Acta Mater. 2002, 50, 23–38. [Google Scholar] [CrossRef]
- Kontis, P.; Köhler, M.; Evertz, S.; Chen, Y.T.; Schnabel, V.; Soler, R.; Bednarick, J.; Kirchlechner, C.; Dehm, G.; Raabe, D.; et al. Nano-laminated thin film metallic glass design for outstanding mechanical properties. Scr. Mater. 2018, 155, 73–77. [Google Scholar] [CrossRef]
- Hardwick, D.A. The mechanical properties of thin films: A review. Thin Solid Films 1987, 154, 109–124. [Google Scholar] [CrossRef]
- Teodorescu, V.S.; Mihailescu, I.N.; Dinescu, M.; Chitica, N.; Nistor, L.C.; Landuyt, J.V.; Barborica, A. Laser induced phase transition in iron thin films. Le Journal De Physique IV 1994, 04, C4-127-C4-130. [Google Scholar] [CrossRef][Green Version]
- Teodorescu, V.S.; Nistor, L.C.; Landuyt, J.V.; Dinescu, M. TEM study of laser induced phase transition in iron thin films. Mater. Res. Bull. 1994, 29, 63–71. [Google Scholar] [CrossRef]
- Memmel, N.; Detzel, T. Growth, structure and stability of ultrathin iron films on Cu(001). Surf. Sci. 1994, 307–309, 490–495. [Google Scholar] [CrossRef]
- Cuenya, B.R.; Doi, M.; Löbus, S.; Courths, R.; Keune, W. Observation of the fcc-to-bcc Bain transformation in epitaxial Fe ultrathin films on Cu 3Au. Surf. Sci. 2001, 493, 338–360. [Google Scholar] [CrossRef]
- Meiser, J.; Urbassek, H.M. Influence of the crystal surface on the austenitic and martensitic phase transition in pure iron. Crystals 2018, 8, 469. [Google Scholar] [CrossRef]
- Wang, B.; Urbassek, H.M. Computer simulation of strain-induced phase transformations in thin Fe films. Model. Simul. Mater. Sci. Eng. 2013, 21, 085007. [Google Scholar] [CrossRef]
- Sak-Saracino, E.; Urbassek, H.M. Effect of uni- and biaxial strain on phase transformations in Fe thin films. Int. J. Comput. Mater. Sci. Eng. 2016, 5, 1650001.1–1650001.17. [Google Scholar] [CrossRef]
- Meiser, J.; Urbassek, H.M. Dislocations help initiate the α–γ phase transformation in iron-An atomistic study. Metals 2019, 9, 90. [Google Scholar] [CrossRef]
- Finnis, M.W.; Sinclair, J.E. A simple empirical N-body potential for transition metals. Philos. Mag. A 1984, 50, 45–55. [Google Scholar] [CrossRef]
- Mendelev, M.I.; Han, S.; Srolovitz, D.J.; Ackland, G.J.; Sun, D.Y.; Asta, M. Development of new interatomic potentials appropriate for crystalline and liquid iron. Philos. Mag. 2003, 83, 3977–3994. [Google Scholar] [CrossRef]
- Srinivasan, V.S.; Sainath, G.; Choudhary, B.K.; Mathew, M.D.; Jayakumar, T. Effect of temperature and strain amplitude on fatigue behaviour of bcc iron single crystal using molecular dynamics simulation. Procedia Eng. 2013, 55, 742–746. [Google Scholar] [CrossRef][Green Version]
- Mendelev, M.I.; Mishin, Y. Molecular dynamics study of self-diffusion in bcc Fe. Phys. Rev. B 2009, 80, 144111. [Google Scholar]
- Engin, C.; Sandoval, L.; Urbassek, H.M. Characterization of Fe potentials with respect to the stability of the bcc and fcc phase. Model. Simul. Mater. Sci. Eng. 2008, 16, 035005. [Google Scholar] [CrossRef]
- Meyer, R.; Entel, P. Martensite-austenite transition and phonon dispersion curves of Fe_ {1-x} Ni_ {x} studied by molecular-dynamics simulations. Phys. Rev. B 1998, 57, 5140. [Google Scholar] [CrossRef]
- Wang, B.; Urbassek, H.M. Phase transitions in an Fe system containing a bcc/fcc phase boundary: An atomistic study. Phys. Rev. B 2013, 87, 104108. [Google Scholar] [CrossRef]
- Hirel, P. Atomsk: A tool for manipulating and converting atomic data files. Comput. Phys. Commun. 2015, 197, 212–219. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comp. Phys. 1995, 117, 1–19. Available online: https://www.sandia.gov/~sjplimp/papers/jcompphys95.pdf (accessed on 15 July 2020). [CrossRef]
- Stukowski, A.; Albe, K. Extracting dislocations and non-dislocation crystal defects from atomistic simulation data. Model. Simul. Mater. Sci. Eng. 2010, 18, 085001. [Google Scholar] [CrossRef]
- Faken, D.; Jónsson, H. Systematic analysis of local atomic structure combined with 3D computer graphics. Comput. Mater. Sci. 1994, 2, 279–286. [Google Scholar] [CrossRef]
- Li, J. AtomEye: An efficient atomistic configuration viewer. Model. Simul. Mater. Sci. Eng. 2003, 11, 173–177. [Google Scholar] [CrossRef]
- Shibuta, Y.; Takamoto, S.; Suzuki, T. A molecular dynamics study of the energy and structure of the symmetric tilt boundary of iron. ISIJ Int. 2008, 48, 1582–1591. [Google Scholar] [CrossRef]
- Sainath, G.; Choudhary, B.K. Deformation behaviour of body centered cubic iron nanopillars containing coherent twin boundaries. Philos. Mag. 2016, 96, 3502–3523. [Google Scholar] [CrossRef][Green Version]
- Hall, E.O. The deformation and aging of mild steel. Proc. Phys. Soc. Lond. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Ito, Y.; Edalati, K.; Horita, Z. High-pressure torsion of aluminum with ultrahigh purity (99.9999%) and occurrence of inverse Hall-Petch relationship. Mater. Sci. Eng. 2017, 679, 428–434. [Google Scholar] [CrossRef]
- Jeng, Y.R.; Tsai, P.C.; Chiang, S.H. Effects of grain size and orientation on mechanical and tribological characterizations of nanocrystalline nickel films. Wear 2013, 303, 262–268. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Chen, Z.; Long, J.; Yang, T. Phase field crystal simulation of microscopic deformation mechanism of reverse Hall-Petch effect in nanocrystalline materials. Acta Phys. Sin. Chin. Ed. 2013, 62, 118102. [Google Scholar]
- Xu, T.; He, S. Simulation of effect of twin boundary on mechanical property of -Fe. J. Aeronaut. Mater. 2017, 37, 73–79. [Google Scholar]
- Ackland, G.J.; Mendelev, M.I.; Srolovitz, D.J.; Han, S.; Barashev, A.V. Development of an interatomic potential for phosphorus impurities in -iron. J. Phys. Condens. Matter. 2004, 16, S2629–S2642. [Google Scholar] [CrossRef]
- Bain, E.C.; Dunkirk, N.Y. The nature of martensite. Trans. AIME 1924, 70, 25. [Google Scholar]
- Kurdjumov, G.V.; Sachs, G. Über den mechanismus der stahlhärtung. Z. Phys. 1930, 64, 325–343. [Google Scholar] [CrossRef]
- Nishiyama, Z. Mechanism of transformation from face-centred to body-centred cubic lattice. Sci. Rep. Tohoku Imp. Univ. 1934, 23, 637. [Google Scholar]
- Sandoval, L.; Urbassek, H.M.; Entel, P. Solid-solid phase transitions and phonon softening in an embedded-atom method model for iron. Phys. Rev. B 2009, 80, 214108. [Google Scholar] [CrossRef]
- Sandoval, L.; Urbassek, H.M. Finite-size effects in Fe-nanowire solid-solid phase transitions: A molecular dynamics approach. Nano Lett. 2009, 9, 2290–2294. [Google Scholar] [CrossRef]
- Zhang, G.W.; Yang, Z.L.; Luo, G. Investigation of mechanical properties of twin gold crystal nanowires under uniaxial load by molecular dynamics method. Chin. Phys. B 2016, 25, 086203. [Google Scholar] [CrossRef]
- Papon, A.M.; Simon, J.P.; Guyot, P.; Desjonquères, M.C. Calculation of {112 twin and stacking fault energies in b.c.c. transition metals. Philos. Mag. B 1979, 40, 159–172. [Google Scholar] [CrossRef]
- Abe. Introduction to the Structure of Metals and Alloys; Corona Publishing Co. Ltd.: Tokyo, Japan, 1967; p. 64. [Google Scholar]
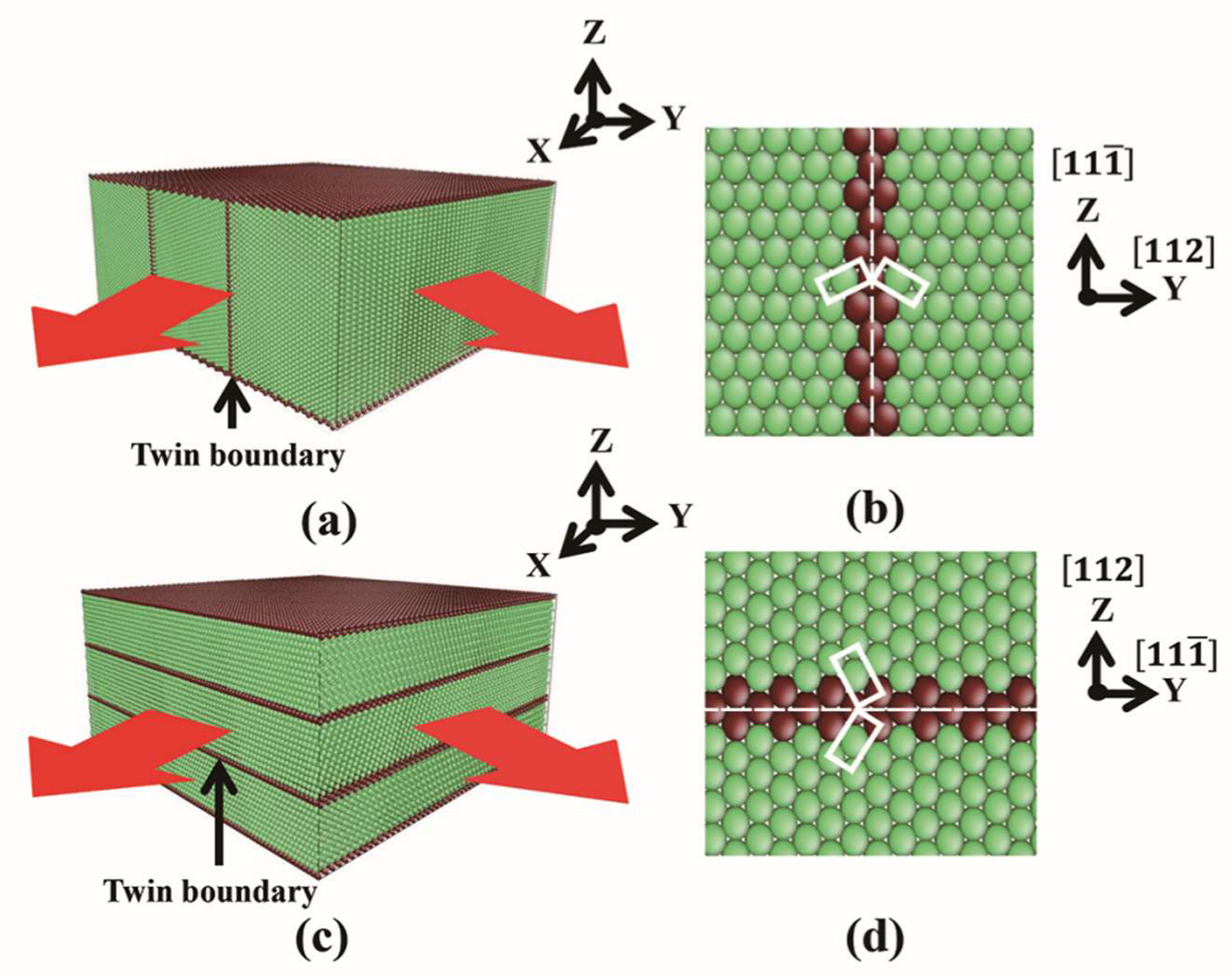
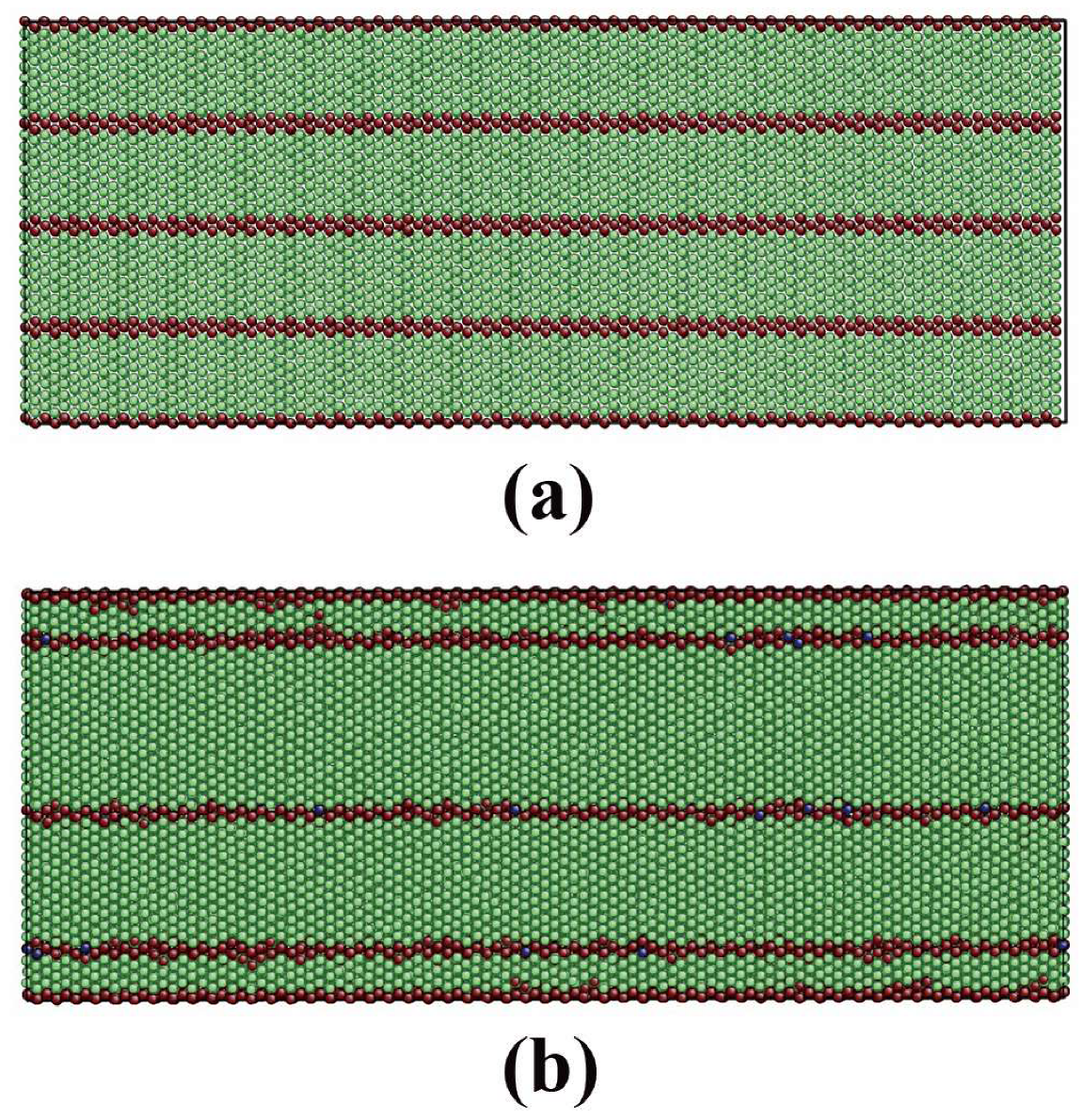

| Film | x | y | z | Δx (Å) | Δy (Å) | Δz (Å) | T | N |
|---|---|---|---|---|---|---|---|---|
| 1 | [0] | [112] | [] | 202.94 | 210.90 | 89.48 | 0 | 324,000 |
| 2 | [0] | [112] | [] | 202.94 | 210.90 | 89.48 | 2 | 324,000 |
| 3 | [0] | [112] | [] | 202.94 | 210.90 | 89.48 | 4 | 324,000 |
| 4 | [0] | [112] | [] | 202.94 | 210.90 | 89.48 | 6 | 324,000 |
| 5 | [0] | [] | [112] | 215.12 | 211.27 | 84.36 | 0 | 324,360 |
| 6 | [0] | [] | [112] | 215.12 | 211.27 | 84.36 | 1 | 324,360 |
| 7 | [0] | [] | [112] | 215.12 | 211.27 | 84.36 | 2 | 324,360 |
| 8 | [0] | [] | [112] | 215.12 | 211.27 | 84.36 | 3 | 324,360 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Wang, B.; Xu, C.; Zhang, J. Atomistic Simulation of the Strain Driven Phase Transition in Pure Iron Thin Films Containing Twin Boundaries. Metals 2020, 10, 953. https://doi.org/10.3390/met10070953
Jiang Y, Wang B, Xu C, Zhang J. Atomistic Simulation of the Strain Driven Phase Transition in Pure Iron Thin Films Containing Twin Boundaries. Metals. 2020; 10(7):953. https://doi.org/10.3390/met10070953
Chicago/Turabian StyleJiang, Yunqiang, Binjun Wang, Chun Xu, and Jianguo Zhang. 2020. "Atomistic Simulation of the Strain Driven Phase Transition in Pure Iron Thin Films Containing Twin Boundaries" Metals 10, no. 7: 953. https://doi.org/10.3390/met10070953
APA StyleJiang, Y., Wang, B., Xu, C., & Zhang, J. (2020). Atomistic Simulation of the Strain Driven Phase Transition in Pure Iron Thin Films Containing Twin Boundaries. Metals, 10(7), 953. https://doi.org/10.3390/met10070953




