Recent Progress in Transient Liquid Phase and Wire Bonding Technologies for Power Electronics
Abstract
1. Introduction
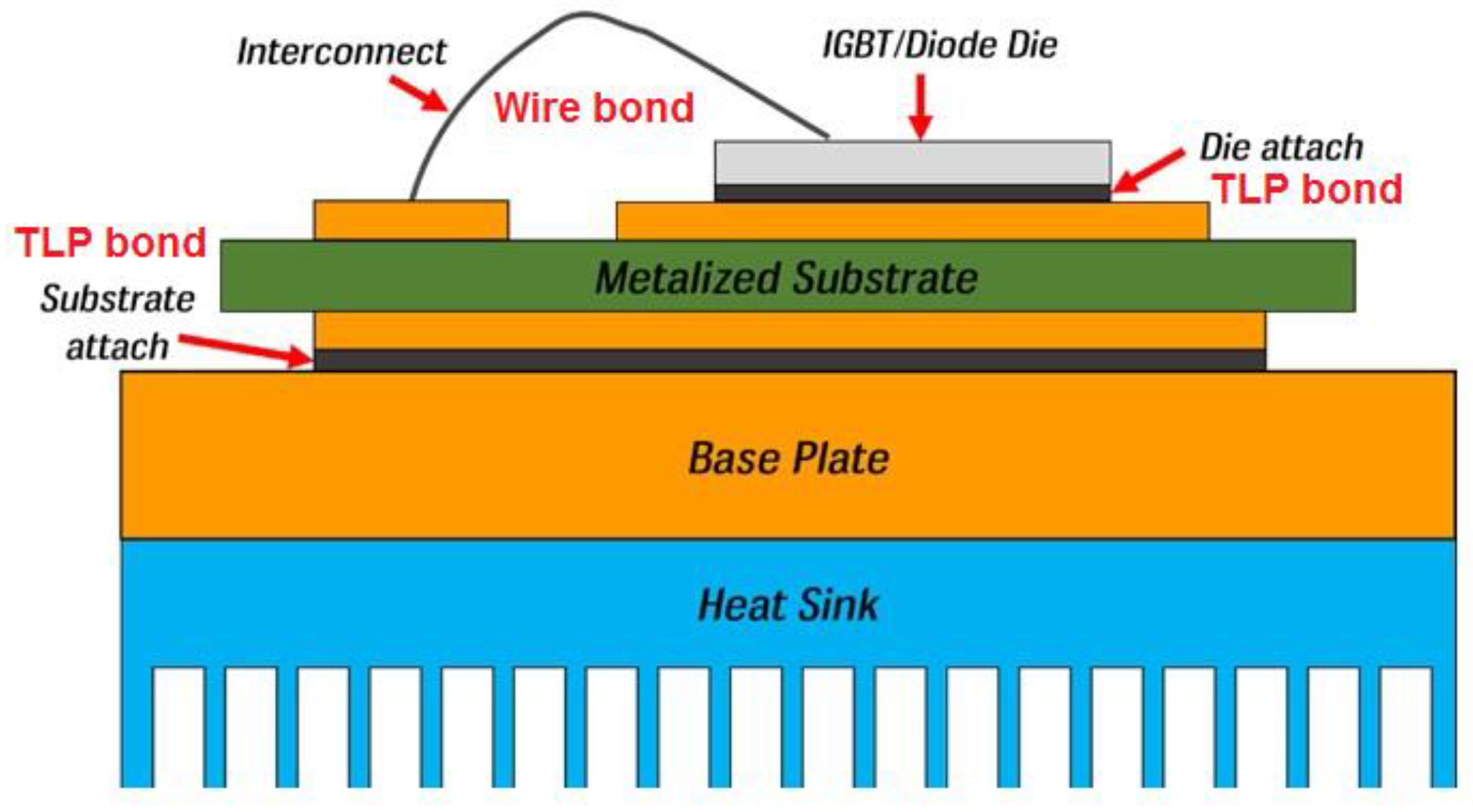
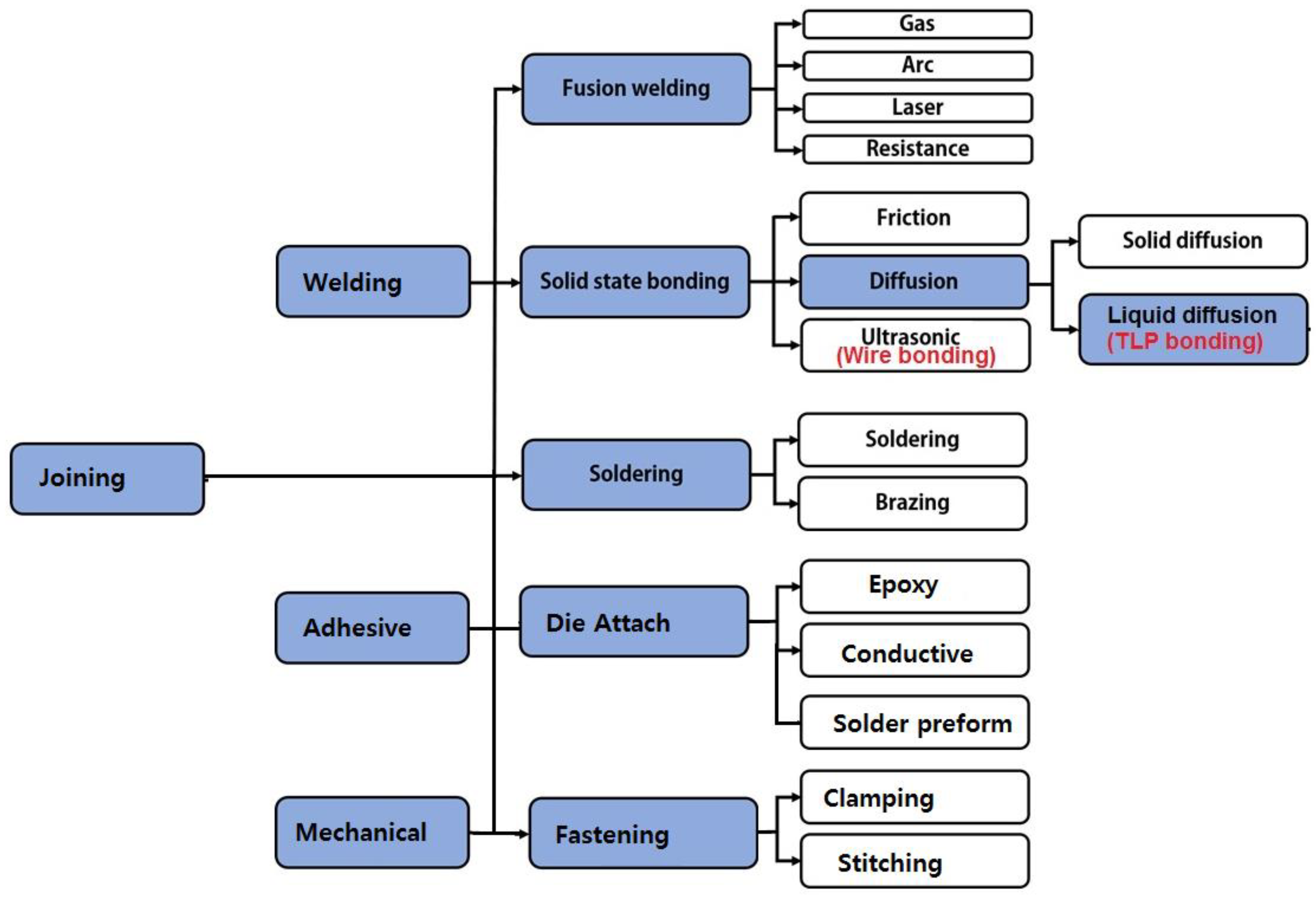
2. Joining Process Used for Power Modules
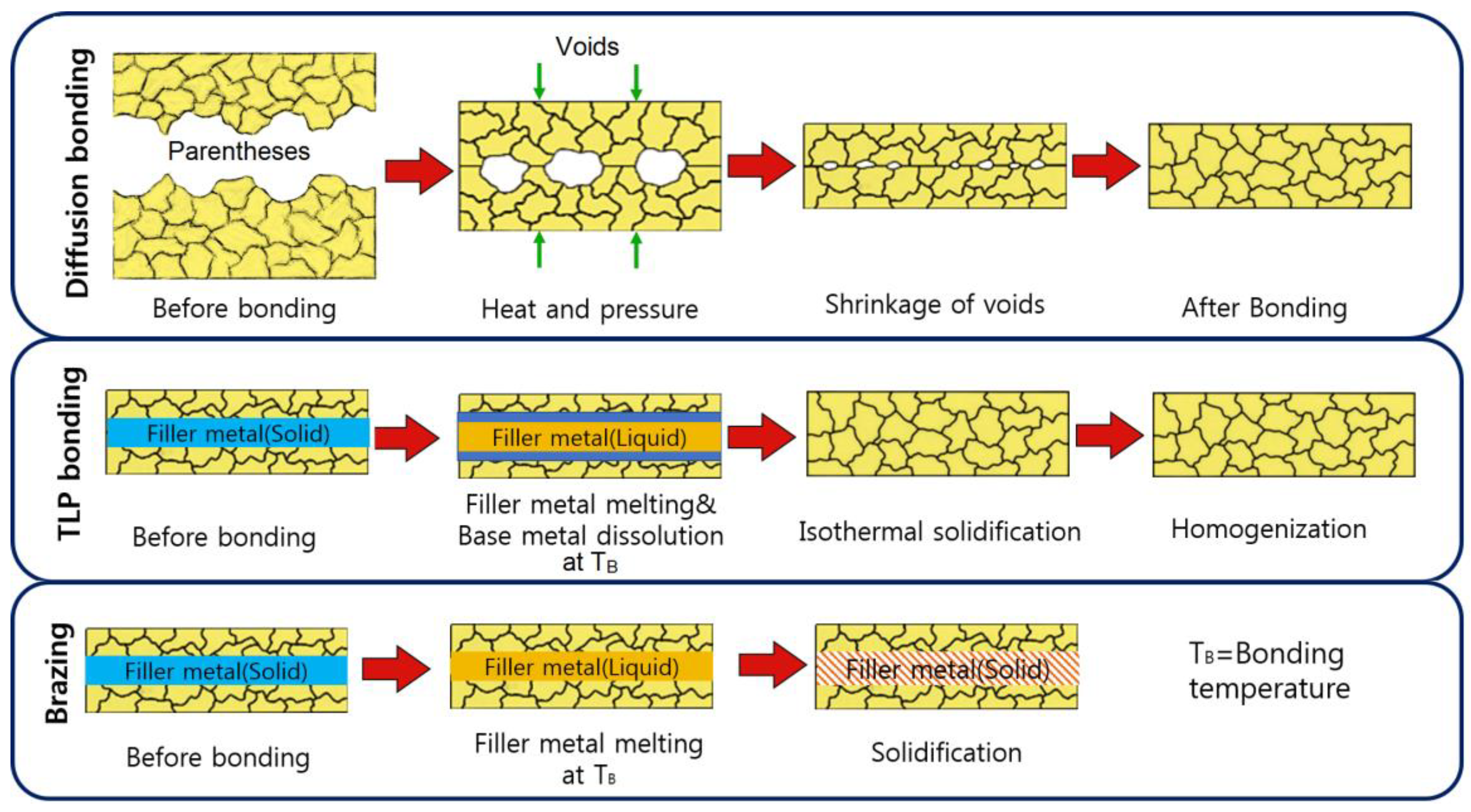
3. TLP Bonding Compared to Solid-State Diffusion Bonding and Brazing
4. Factors Affecting TLP Bonding
4.1. Alloying Elements in Sn-Based Solder
4.2. Bonding Time
4.3. Shear Strength
5. TLP Bonding with Different Filler Shapes
5.1. Foil Type Interlayers
5.2. Powder and Paste Fillers
5.3. Composite Fillers
5.4. Ag Die-Attached Materials
6. Ultrasonic Wire Bonding in Power Devices
6.1. Ball Bonding or Crescent Bonding
6.2. Wedge Bonding
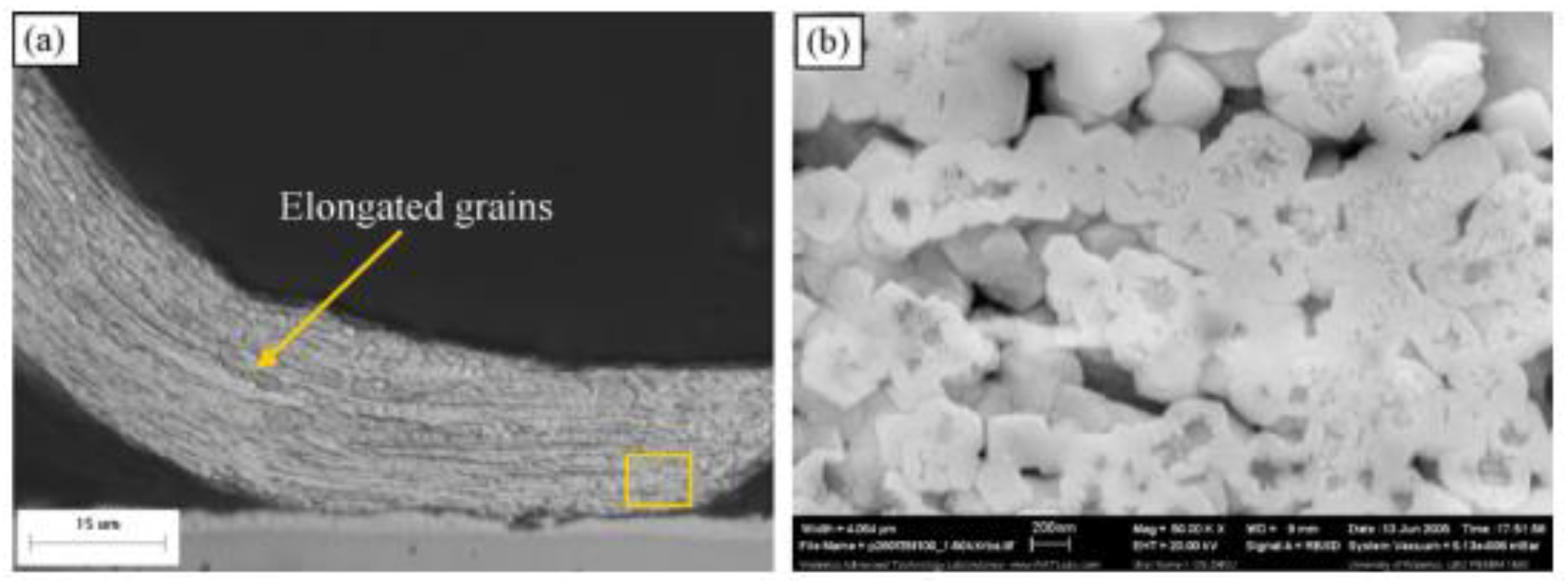
7. Recrystallization in Wedge Bonding
8. Reliability Studies
9. Conclusions
10. Future Directions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, A.; Lee, J.H.; Kim, K.H.; Jung, J.P. Recent advances in thermoelectric power generation technology. J. Microelectron. Packag. Soc. 2017, 24, 9–16. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Das, S.; Das, K. Pulse electrodeposition of lead-free tin-based composites for microelectronic packaging. In Electrodeposition of Composite Materials; Mohamed, A.A.A., Golden, T.D., Eds.; InTech: Rijeka, Croatia, 2016; Volume 3, pp. 253–274. [Google Scholar]
- Chen, C.; Luo, F.; Kang, Y. A review of SiC power module packaging: Layout material system and integration. CPSS TPEA 2017, 2, 170–186. [Google Scholar] [CrossRef]
- Brambilla, A.; Dallago, E.; Romano, R. Analysis of an IGBT power module. In Proceedings of the IEEE 20th International Conference on Industrial, Electronics, Control and Instrumentation, Bologna, Italy, 5–9 September 1994; IEEE: Bologna, Italy, 1994; pp. 129–134. [Google Scholar]
- Pithadia, S.; Kumar, N. Analysis of Power Supply Topologies for IGBT Gate Drivers in Industrial Drives(SLAA672); Application Report for Texas Instruments; Texas Instruments: Dallas, TX, USA, 2015. [Google Scholar]
- Cooper, J.A.; Melloch, M.R.; Singh, R.; Agarwal, A.; Palmour, J.W. Status and prospects for SiC power MOSFETs. IEEE Trans. Electron Devices 2002, 49, 658–664. [Google Scholar] [CrossRef]
- Roh, M.H.; Nishikawa, H.; Jung, J.P. A review of Ag paste bonding for automotive power device packaging. J. Microelectron. Packag. Soc. 2015, 22, 15–23. [Google Scholar] [CrossRef]
- Rajendran, S.H.; Jung, D.H.; Cheon, W.S.; Jung, J.P. Transient liquid phase bonding of copper using Sn coated Cu MWCNT composite powders for power electronics. Appl. Sci. 2019, 9, 529. [Google Scholar] [CrossRef]
- Roh, M.H.; Nishikawa, H.; Jung, J.P.; Kim, W.J. Transient liquid phase bonding for power module packaging. J. Microelectron. Packag. Soc. 2017, 24, 27–34. [Google Scholar] [CrossRef]
- Al-Hazaa, A.N.; Muhammad, A.S.; Shar, A.; Atieh, A.M.; Nishikawa, H. Transient liquid phase bonding of magnesium alloy AZ31 using Cu coatings and Cu coatings with Sn interlayers. Metals 2018, 8, 60. [Google Scholar] [CrossRef]
- Norrish, J. Advanced Welding Processes, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2006; p. 2. [Google Scholar]
- Choi, D.Y.; Sharma, A.; Uhm, S.H.; Jung, J.P. Liquid metal embrittlement of resistance spot welded 1180 TRIP steel: Effect of electrode force on cracking behavior. Met. Mater. Int. 2018, 25, 219. [Google Scholar] [CrossRef]
- Sharma, A.; Lim, D.U.; Jung, J.P. Microstructure and brazeability of SiC nanoparticles reinforced Al-9Si-20Cu produced by induction melting. Mater. Sci. Technol. 2016, 32, 773. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Pulse Electroplating of Ultrafine Grained Tin Coating. In Electroplating of Nanostructures; Aliofkhazraei, M., Ed.; Intechopen: Rijeka, Croatia, 2015. [Google Scholar]
- Sharma, A.; Mallik, S.; Ekere, N.N.; Jung, J.P. Printing morphology and rheological characteristics of lead-free Sn-3Ag-0.5 Cu (SAC) solder pastes. J. Microelectron. Packag. Soc. 2014, 21, 83. [Google Scholar] [CrossRef]
- Sharma, A.; Kee, S.H.; Jung, F.; Heo, Y.; Jung, J.P. Compressive strength evaluation in brazed ZrO2/Ti6Al4V joints using finite element analysis. J. Mater. Eng. Perform. 2016, 25, 1722. [Google Scholar] [CrossRef]
- Kolenak, R.; Kostolny, I.; Drapala, J.; Zackova, P.; Kuruc, M. Direct ultrasonic soldering of AlN ceramics with copper substrate using Zn-Al-Mg solder. Metals 2020, 10, 160. [Google Scholar] [CrossRef]
- Prach, M.; Kolenak, R. Soldering of copper with high-temperature Zn-based solders. Procedia Eng. 2015, 100, 1370. [Google Scholar] [CrossRef]
- Rettenmayr, M.; Lambracht, P.; Kempf, B.; Tschudin, C. Zn-Al based alloys as Pb-free solders for die attach. J. Electron. Mater. 2002, 31, 278–285. [Google Scholar] [CrossRef]
- Shimizu, T.; Ishikawa, H.; Ohnuma, I.; Ishida, K. Zn-Al-Mg-Ga Alloys as Pb-free solder for die-attaching use. J. Electron. Mater. 1999, 28, 1172–1175. [Google Scholar] [CrossRef]
- Cheng, F.; Gao, F.; Wang, Y.; Wu, Y.; Ma, Z.; Yang, J. Sn addition on the tensile properties of high temperature Zn-4Al-3Mg solder alloys. Microelectron. Reliab. 2012, 52, 579–584. [Google Scholar] [CrossRef]
- Cui, W.; Yan, J.; Dai, Y.; Li, D. Building a nano-crystalline-alumina layer at a liquid metal/sapphire interface by ultrasound. Ultrason. Sonochem. 2015, 22, 108–112. [Google Scholar] [CrossRef]
- Mishra, S.; Sharma, A.; Jung, D.H.; Jung, J.P. Recent advances in active metal brazing of ceramics and process. Met. Mater. Int. 2019, in press. [Google Scholar] [CrossRef]
- Sharma, A.; Ahn, B. Brazeability, microstructure and joint characteristics of ZrO2/Ti-6Al-4V brazed by Ag-CuTi filler reinforced with cerium oxide nanoparticles. AMSE 2019, 2019, 11. [Google Scholar] [CrossRef]
- Sharma, A. High-Entropy Alloys for Micro- and Nanojoining Applications. In Engineering Steels and High Entropy-Alloys; Sharma, A., Ed.; Intechopen: Rijeka, Croatia, 2020. [Google Scholar]
- Sharma, A.; Kumar, S.; Duriagina, Z. (Eds.) Engineering Steels and High Entropy-Alloys; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Srivastava, A.K.; Sharma, A. Advances in joining and welding technologies for automotive and electronic applications. Am. J. Mater. Eng. Technol. 2017, 5, 7–13. [Google Scholar] [CrossRef]
- Schwartz, M.M. Brazing, 2nd ed.; ASM International: Novelty, OH, USA, 2003. [Google Scholar]
- Gale, W.F.; Butts, D.A. Transient liquid phase bonding. Sci. Technol. Weld. Join. 2004, 9, 283–300. [Google Scholar] [CrossRef]
- Weyrich, N.; Leinenbach, C. Low temperature TLP bonding of Al2O3-ceramics using eutectic Au-(Ge, Si) alloys. J. Mater. Sci. 2013, 48, 7115–7124. [Google Scholar] [CrossRef]
- Hoppin, G.S.; Berry, T.F. Activated diffusion bonding. Weld. J. 1970, 49, 505–509. [Google Scholar]
- Duvall, D.S.; Owczarski, W.A.; Paulonis, D.F. Transient liquid phase bonding: A new method for joining heat resistant alloys. Weld. J. 1974, 53, 203–214. [Google Scholar]
- Jung, J.P.; Kang, C.S. Transient liquid phase process in Ni-B joining. Mater. Trans. 1997, 38, 886–891. [Google Scholar] [CrossRef][Green Version]
- Jung, J.P.; Kang, C.S. Liquid metal formation of Ni/B/Ni diffusion bonded joint. J. Korean. Inst. Met. Mater. 1995, 33, 1302–1308. [Google Scholar]
- Jung, J.P.; Kang, C.S. A study on the width of liquid layer of Ni/B/Ni diffusion bonding system. J. Korean. Weld. Soc. 1995, 13, 402–409. [Google Scholar]
- Jung, J.P.; Kang, C.S. Mechanical property of liquid phase diffusion bonded joint of Rene80/B/Rene80. J. Korean. Weld. Soc. 1995, 13, 125–133. [Google Scholar]
- Jung, J.P.; Kang, C.S. Liquid phase diffusion bonding procedure of Rene80/B/Rnene80 system. J. Korean. Weld. Soc. 1995, 13, 172–178. [Google Scholar]
- Jung, J.P.; Kang, C.S. Liquid phase diffusion bonding of Rene80 using pure boron. Mater. Trans. 1996, 37, 1008–1013. [Google Scholar] [CrossRef][Green Version]
- Jung, J.P.; Lee, C.D.; Kang, C.S. A study on the melting induced bonding of 304 stainless steel. J. Korean. Inst. Met. Mater. 1993, 31, 323–329. [Google Scholar]
- Cook III, G.O.; Sorensen, C.D. Overview of transient liquid phase and partial transient liquid phase bonding. J. Mater. Sci. 2011, 46, 5305. [Google Scholar] [CrossRef]
- Chang, S.Y.; Huang, Y.H.; Tsao, L.C. Active solders and active soldering. In Fillers-Synthesis, Characterization and Industrial Application; Patnaik, A., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Lis, A.; Leinenbach, C. Effect of process and service conditions on TLP-bonded components with (Ag,Ni–)Sn interlayer combinations. J. Electron. Mater. 2015, 44, 4576–4588. [Google Scholar] [CrossRef]
- Tollefsen, T.A.; Larsson, A.; Lřvvik, O.; Aasmundtveit, K. Au-Sn SLID bonding—Properties and possibilities. Metall. Mater. Trans. B 2012, 43, 397–405. [Google Scholar] [CrossRef]
- Nobeen, N.S.; Imade, R.; Lee, B.; Phua, E.; Wong, C.; Gan, C. Transient liquid phase (TLP) bonding using Sn/Ag multilayers for high temperature applications. In Proceedings of the IEEE 15th Electronics Packaging Technology Conference (EPTC 2013), Singapore, 11–13 December 2013; IEEE: Singapore, 2013; p. 647. [Google Scholar]
- Yoon, S.W.; Glover, M.D.; Shiozaki, K. Nickel-tin transient liquid phase bonding toward high-temperature operational power electronics in electrified vehicles. IEEE Trans. Power Electron. 2013, 28, 2448. [Google Scholar] [CrossRef]
- Honrao, C.; Huang, T.C.; Kobayashi, M.; Smet, V.; Raj, P.M.; Tummala, R. Accelerated SLID bonding using thin multi-layer copper-solder stack for fine-pitch interconnections. In Proceedings of the IEEE 64th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 27–30 May 2014; IEEE: Orlando, FL, USA, 2014; p. 1160. [Google Scholar]
- Bajwa, A.; Wilde, J. Reliability modeling of SnAg transient liquid phase die-bonds for high-power SiC devices. Microelectron. Reliab. 2016, 60, 116. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Qin, Y.Y.; Zeiser, R.; Wilde, J. Foil based transient liquid phase bonding as a die-attachment method for high temperature devices. In Proceedings of the CIPS 8th International Conference on Integrated Power Electronics Systems, Nuremberg, Germany, 25–27 February 2014; VDE Verlag Gmbh: Berlin, Germany, 2014; p. 1. [Google Scholar]
- Liu, B.; Tian, Y.; Feng, J.; Wang, C. Enhanced shear strength of Cu-Sn intermetallic interconnects with interlocking dendrites under fluxless electric current-assisted bonding process. J. Mater. Sci. 2017, 52, 1943. [Google Scholar] [CrossRef]
- Shao, H.; Wu, A.; Bao, Y.; Zhao, Y.; Zou, G. Interfacial reaction and mechanical properties for Cu/Sn/Ag system low temperature transient liquid phase bonding. J. Mater. Sci. 2016, 27, 4839–4848. [Google Scholar] [CrossRef]
- Mokhtari, O.; Nishikawa, H. The shear strength of transient liquid phase bonded Sn-Bi solder joint with added Cu particles. Adv. Powder Technol. 2016, 27, 1000–1005. [Google Scholar] [CrossRef]
- Greve, H.; Moeini, S.A.; McCluskey, F.P. Reliability of paste based transient liquid phase sintered interconnects. In Proceedings of the IEEE 64th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 27–30 May 2014; IEEE: Orlando, FL, USA, 2014; p. 1314. [Google Scholar]
- Zhao, H.Y.; Liu, J.H.; Li, Z.L.; Zhao, Y.X.; Niu, H.W.; Song, X.G.; Dong, H.J. Non-interfacial growth of Cu3Sn in Cu/Sn/Cu joints during ultrasonic-assisted transient liquid phase soldering process. Mater. Lett. 2017, 186, 283. [Google Scholar] [CrossRef]
- Ji, H.; Qiao, Y.; Li, M. Rapid formation of intermetallic joints through ultrasonic-assisted die bonding with Sn-0.7Cu solder for high temperature packaging application. Scr. Mater. 2016, 110, 19–23. [Google Scholar] [CrossRef]
- Ji, H.; Li, M.; Ma, S.; Li, M. Ni 3 Sn 4-composed die bonded interface rapidly formed by ultrasonic-assisted soldering of Sn/Ni solder paste for high-temperature power device packaging. Mater. Des. 2016, 108, 590–596. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Nishikawa, H. Thermally stable Cu3Sn/Cu composite joint for high-temperature power device. Scr. Mater. 2016, 110, 101–104. [Google Scholar] [CrossRef]
- Hu, T.; Chen, H.; Li, M. Die attach materials with high remelting temperatures created by bonding Cu@Sn microparticles at lower temperatures. Mater. Des. 2016, 108, 383–390. [Google Scholar] [CrossRef]
- Yang, T.L.; Aoki, T.; Matsumoto, K.; Toriyama, K.; Horibe, A.; Mori, H.; Orii, Y.; Wu, J.Y.; Kao, C.R. Full intermetallic joints for chip stacking by using thermal gradient bonding. Acta Mater. 2016, 113, 90–97. [Google Scholar] [CrossRef]
- Khaja, A.S.; Kaestle, C.; Reinhardt, A.; Franke, J. Optimized thin-film diffusion soldering for power-electronics production. In Proceedings of the 36th International Spring Seminar on Electronics Technology, Alba Iulia, Romania, 8–12 May 2013; IEEE: Alba Lulia, Romania, 2013; p. 11. [Google Scholar]
- Greve, H.; Chen, L.Y.; Fox, I.; McCluskey, F.P. Transient liquid phase sintered attach for power electronics. In Proceedings of the IEEE 63rd Electronic Components and Technology Conference, Las Vegas, NV, USA, 28–31 May 2013; IEEE: Las Vegas, NV, USA, 2013; p. 435. [Google Scholar]
- Ehrhardt, C.; Hutter, M.; Oppermann, H.; Lang, K.D. A lead free joining technology for high temperature interconnects using Transient Liquid Phase Soldering (TLPS). In Proceedings of the IEEE 64th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 27–30 May 2014; IEEE: Orlando, FL, USA, 2014; p. 1321. [Google Scholar]
- Hosseinzaei, B.; Ali, R.K.R. Transient liquid phase bonding in the Cu-Sn system. Solder. Surf. Mt. Technol. 2019, 31, 221–226. [Google Scholar] [CrossRef]
- Choudhury, S.F.; Ladani, L. Local shear stress strain response of Sn-3.5Ag/Cu solder joint with high fraction of intermetallic compounds: Experimental analysis. J. Alloys Compd. 2016, 680, 665–676. [Google Scholar] [CrossRef]
- Sohn, S.; Kim, D.; Kim, H.; Kang, N. Strategies to reduce transient liquid phase bonding time for the die attach of power semiconductors. JWJ 2020, 38, 158–165. [Google Scholar] [CrossRef]
- Jung, J.P.; Kang, C.S.; Lee, B.Y. Melting induced diffusion bonding of Rene80 superalloys using boron doping method. JWJ 1991, 9, 26–33. [Google Scholar]
- Shao, H.K.; Wu, A.; Bao, Y.; Zhao, Y.; Zou, G. Mechanism of Ag3Sn grain growth in Ag/Sn transient liquid phase soldering. Trans. Nonferrous Met. Soc. China 2017, 27, 722–732. [Google Scholar] [CrossRef]
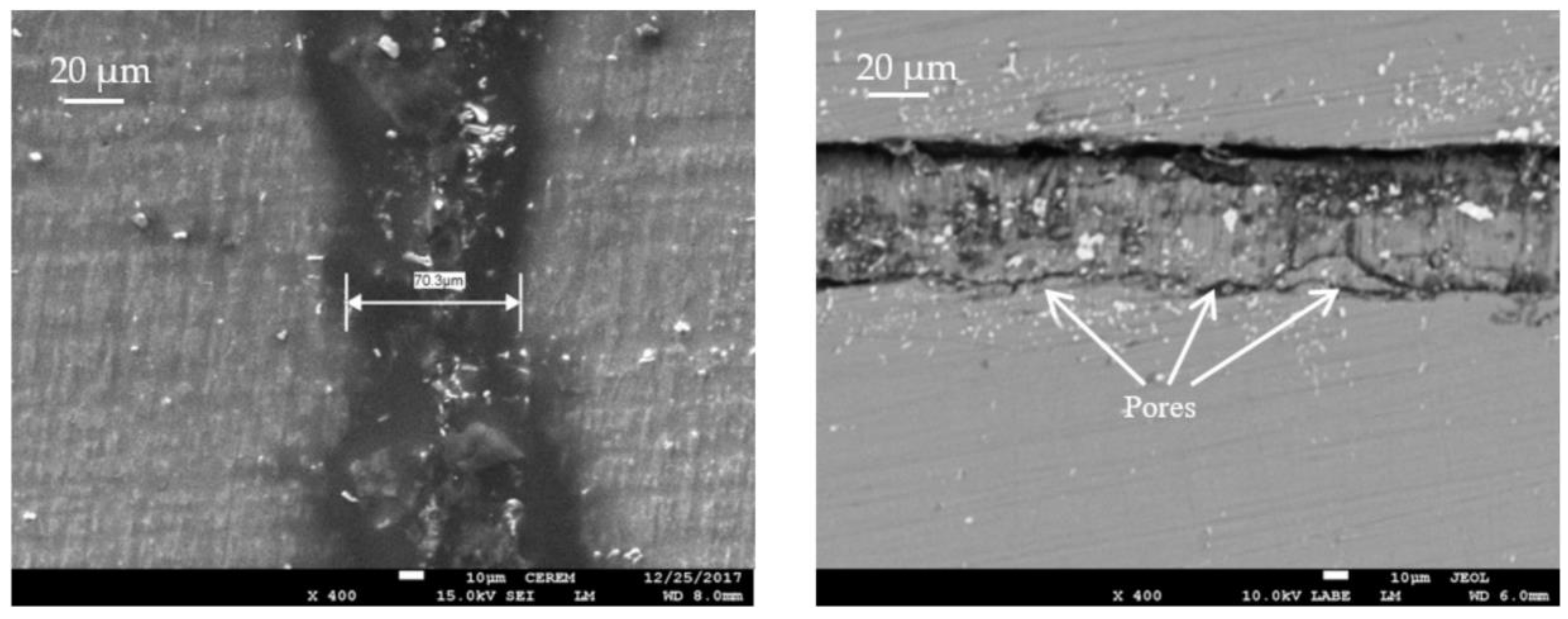
- Mokhtari, O. A review: Formation of voids in solder joint during the transient liquid phase bonding process—Causes and solutions. Microelectron. Reliab. 2019, 98, 95–105. [Google Scholar] [CrossRef]
- Jung, D.H.; Sharma, A.; Mayer, M.; Jung, J.P. A review on recent advances in transient liquid phase (TLP) bonding for thermoelectric power module. Rev. Adv. Mater. Sci. 2018, 53, 147–160. [Google Scholar] [CrossRef]
- Chu, K.; Sohn, Y.; Moon, C. A comparative study of Cn/Sn/Cu and Ni/Sn/Ni solder joints for low temperature stable transient liquid phase bonding. Scr. Mater. 2015, 109, 113–117. [Google Scholar] [CrossRef]
- Shao, H.; Wu, A.; Bao, Y.; Zhao, Y.; Zou, G.; Liu, L. Novel transient liquid phase bonding through capillary action for high temperature power devices packaging. Mater. Sci. Eng. A 2018, 724, 231–238. [Google Scholar] [CrossRef]
- Brincker, M.; Sohl, S.; Eisele, R.; Popok, V.N. Strength and reliability of low temperature transient liquid phase bonded Cu-Sn-Cu interconnects. Microelectron. Reliab. 2017, 76, 378–382. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, J.H.; Li, Z.L.; Song, X.G.; Zhao, Y.X.; Niu, H.W.; Tian, H.; Dong, H.J.; Feng, J.C. A Comparative Study on the Microstructure and Mechanical Properties of Cu6Sn5 and Cu3Sn Joints Formed by TLP Soldering With/Without the Assistance of Ultrasonic Waves. Metall. Mater. Trans. A 2018, 49, 2739–2749. [Google Scholar] [CrossRef]
- Elmer, J.W.; Mulay, R.P.; Elmer, J. Superplastic creep of AuSn eutectic solder alloy. Scr. Mater. 2016, 120, 14–18. [Google Scholar]
- Zhu, Z.X.; Li, C.C.; Liao, L.L.; Liu, C.K.; Kao, C.R. Au-Sn bonding material for the assembly of power integrated circuit module. J. Alloys Compd. 2016, 671, 340–345. [Google Scholar] [CrossRef]
- Peng, J.; Wang, R.C.; Wang, M.; Liu, H.S. Interfacial Microstructure Evolution and Shear Behavior of Au-Sn/Ni-xCu Joints at 350C. J. Electron. Mater. 2017, 46, 2021–2029. [Google Scholar] [CrossRef]
- Sharma, A.; Jung, D.H.; Cheon, J.S.; Jung, J.P. Epoxy polymer solder pastes for micro-electronic packaging applications. JWJ 2019, 37, 7–14. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Son, J.H.; Ko, Y.H.; Lee, C.W.; Dongjin, B.; Bang, J. A Study on Joint Properties of Sn-Cu-(X)Al-(Y)Si/Cu Solder by Multiple Reflow. JWJ 2020, 38, 131–137. [Google Scholar]
- Corbin, S.F. High-temperature variable melting point Sn-Sb lead-free solder pastes using transient liquid-phase powder processing. J. Electron. Mater. 2005, 34, 1016–1025. [Google Scholar] [CrossRef]
- Chidambaram, V.; Hattel, J. Hald, High-temperature lead-free solder alternatives. Microelectron. Eng. 2011, 88, 981–989. [Google Scholar] [CrossRef]
- Sharif, A.; Gan, C.L.; Chen, Z. Transient liquid phase Ag-based solder technology for high-temperature packaging applications. J. Alloys Compd. 2014, 587, 365–368. [Google Scholar] [CrossRef]
- Fujino, M.; Narusawa, H.; Kuramochi, Y.; Higurashi, E.; Suga, T.; Shiratori, T.; Mizukoshi, M. Transient liquid-phase sintering using tin and silver powder mixture for die bonding. In Proceedings of the International Conference on Solid State Devices and Materials, Sapporo, Japan, 27–30 September 2015; The Japan Society of Applied Physics: Tokyo, Japan, 2015; pp. 60–61. [Google Scholar]
- Bao, Y.D.; Wu, A.P.; Shao, H.K.; Zhao, Y.; Liu, L.; Zou, G.S. Microstructural evolution and mechanical reliability of transient liquid phase sintered joint during thermal aging. J. Mater. Sci. 2019, 54, 765–776. [Google Scholar] [CrossRef]
- Saud, N.; Said, R.M. Transient liquid phase bonding for solder-a short review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012050. [Google Scholar] [CrossRef]
- Greve, H.; Moeini, S.A.; McCluskey, P.; Joshi, S. Prediction and mitigation of vertical cracking in high-temperature transient liquid phase sintered joints by thermomechanical simulation. J. Electron. Packag. 2018, 140, 020903. [Google Scholar] [CrossRef]
- Lang, F.; Yamaguchi, H.; Nakagawa, H.; Sato, H. Thermally stable bonding of SiC devices with ceramic substrates: Transient liquid phase sintering using Cu/Sn powders. J. Electrochem. Soc. 2013, 160, 315–319. [Google Scholar] [CrossRef]
- Sun, L.; Chen, M.H.; Zhang, L. Microstructure evolution and grain orientation of IMC in Cu-Sn TLP bonding solder joints. J. Alloys Compd. 2019, 786, 677–687. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Nishikawa, H. Low temperature solid-state bonding using Sn-coated Cu particles for high temperature die attach. J. Alloys Compd. 2017, 695, 2165–2172. [Google Scholar] [CrossRef]
- Tatsumi, H.; Lis, A.; Yamaguchi, H.; Matsuda, T.; Sano, T.; Kashiba, Y.; Hirose, A. Evolution of transient liquid-phase sintered Cu–Sn skeleton microstructure during thermal aging. Appl. Sci. 2019, 9, 157. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Zhang, Y.; Xu, F.; Jian, Q.; Lu, W. Microstructure evolution and mechanical properties of the In-Sn-20Cu composite particles TLP bonding solder joints. Appl. Phys. A 2020, 126, 343. [Google Scholar] [CrossRef]
- Li, J.F.; Agyakwa, J.F.; Johnson, C.M. Kinetics of Ag3Sn growth in Ag-Sn-Ag system during transient liquid phase soldering process. Acta Mater. 2010, 58, 3429–3443. [Google Scholar] [CrossRef]
- Deng, X.; Chawla, N. A study on the bonding process of Cu bump/Sn/Cu bump bonding structure for 3D packaging applications. Acta Mater. 2004, 52, 4291–4330. [Google Scholar] [CrossRef]
- Nai, S.M.L.; Wei, J.; Gupta, M. Interfacial intermetallic growth and shear strength of lead-free composite solder joints. J. Alloys Compd. 2009, 473, 100–106. [Google Scholar] [CrossRef]
- Yim, B.S.; Lee, J.I.; Kim, J.M. Reliability properties of carbon nanotube-filled solderable anisotropic conductive adhesives. JWJ 2017, 35, 15–20. [Google Scholar] [CrossRef]
- Yim, B.S.; Youn, H.J.; Lee, J.I.; Kim, J.M. Influence of carbon nanotube concentration on the interconnection properties of solderable isotropic and anisotropic conductive adhesive. JWJ 2020, 38, 152–157. [Google Scholar] [CrossRef]
- Liu, B.; Tian, Y.; Wang, C.; An, R.; Liu, Y. Extremely fast formation of Cu3Sn intermetallic compounds in Cu/Sn/Cu system via a micro-resistance spot welding process. J. Alloys Compd. 2016, 687, 667–673. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Xiao, Y.; Wang, C. Rapid formation of Cu/Cu3Sn/Cu joints using ultrasonic bonding processes at ambient temperature. Appl. Phys. Lett. 2013, 102, 094104. [Google Scholar] [CrossRef]
- Rautiainan, A.; Xu, H.; Österlund, E.; Li, J.; Vuorinen, V.; Krockel, M.P. Microstructural characterization and mechanical performance of Wafer-Level SLID bonded Au-Sn and Cu-Sn seal rings for MEMS encapsulation. J. Electron. Mater. 2015, 44, 4533–4548. [Google Scholar] [CrossRef]
- Morozumi, A.; Hokazono, H.; Nishimura, Y.; Mochizuki, E.; Takahashi, Y. Influence of Antimony on Reliability of Solder Joints Using Sn-Sb Binar y Alloy for Power Semiconductor Modules. Trans. Japan Inst. Electron. Packag. 2015, 8, 8–16. [Google Scholar]
- Harman, G.G. Wire Bonding in Microelectronics., 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- Lum, I.; Jung, J.P.; Zhou, Y. Bonding mechanism in ultrasonic gold ball bonds on copper substrate. Metall. Mater. Trans. A. 2005, 36, 1279. [Google Scholar] [CrossRef]
- Mindlin, R.D. Compliance of elastic bodies in contact. J. Appl. Mech. ASME 1949, 16, 259. [Google Scholar]
- Kolenak, R.; Kostolny, I.; Drapala, J.; Drienovsky, M.; Sahul, M. Research on joining metal-ceramics composite Al/Al2O3 with Cu substrate using solder type Zn-In-Mg. J. Compos. Mater. 2019, 53, 1411–1422. [Google Scholar] [CrossRef]
- Kolenak, R.; Kostolny, I.; Drapala, J.; Babincova, P.; Pasak, M. Study of Zn6Al6Ag Alloy Application in Ultrasonic Soldering of Al2O3-(Al/Al2O3) Joints. Metals 2020, 10, 343. [Google Scholar] [CrossRef]
- Kolenak, R.; Kostolny, I.; Drapala, J.; Sahul, M.; Urminský, J. Characterizing the soldering alloy Type In-Ag-Ti and the study of direct soldering of SiC ceramics and copper. Metals 2018, 8, 274. [Google Scholar] [CrossRef]
- Lum, I.; Mayer, M.; Zhou, Y. Footprint study of ultrasonic wedge bonding with aluminum wire on copper substrate. J. Electron. Mater. 2006, 35(3), 433. [Google Scholar] [CrossRef]
- Lee, L. Wire Bonding. EDFAAO 2016, 1, 22–28. [Google Scholar]
- Ozaki, K.; Kurosu, T.; Onuki, J. Development of damage free thick Al-Cu wire bonding process and reliability of the wire bonds. Electrochem. 2014, 82, 100. [Google Scholar] [CrossRef][Green Version]
- Kurosu, T.; Khoo, K.; Nakamura, Y.; Ozaki, K.; Ishikawa, N.; Onuki, J. Reliability enhancement of Thick Al Cu Wire Bonds in IGBT Modules Using Al2Cu Precipitates. Mater. Trans. 2012, 52, 453. [Google Scholar] [CrossRef]
- Geissler, U.; Martin, S.-R.; Lang, K.-D.; Reichl, H. Investigation of microstructural processes during ultrasonic wedge/wedge bonding of AlSi1 wires. J. Electron. Mater. 2006, 35, 173–180. [Google Scholar] [CrossRef]
- Kotani, K.; Jung, J.P.; Ikeuchi, K.; Matsuda, F. Effects of oxide morphology on bond strength of diffusion bonded interfaces of Al-alloys. Trans. JWRL 2000, 28, 27. [Google Scholar]
- Hazlett, T.H.; Ambekar, S.M. Additional studies on interface temperatures and bonding mechanisms of ultrasonic welds. Weld. J. 1970, 50, 196–200. [Google Scholar]
- Patel, V.K.; Bhole, S.D.; Chen, D.L. Improving weld strength of magnesium to aluminium dissimilar joints via tin interlayer during ultrasonic spot welding. Sci. Technol. Weld. Join. 2012, 17, 342–347. [Google Scholar] [CrossRef]
- Koellhoffer, S.; Gillespie, J.W.; Advani, S.G.; Bogetti, T.A. Role of friction on the thermal development in ultrasonically consolidated aluminum foils and composites. J. Mater. Process. Technol. 2011, 211, 1164–1877. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, C.; Lumb, I.; Mayer, M.; Jung, J.P.; Zhou, Y. Investigation of ultrasonic copper wire wedge bonding on Au/Ni plated Cu substrates at ambient temperature. J. Mater. Proc. Technol. 2008, 208, 179–186. [Google Scholar] [CrossRef]
- Park, J.Y.; Oh, C.; Won, D.H.; Hong, W.S. Ultrasonic bonding property of aluminum wire for power conversion module of automotive. JWJ 2018, 36, 57–64. [Google Scholar] [CrossRef]
- Cahn, R.W.; Haasen, P. Physical Metallurgy, 4th ed.; Elsevier: North Holland, The Netherlands, 1996. [Google Scholar]
- Jedrasiak, P.; Shercliff, H.R.; Chen, Y.C.; Wang, L.; Prangnell, P.; Robson, J. Modeling of the thermal field in dissimilar alloy ultrasonic welding. J. Mater. Eng. Perform. 2015, 24, 799–807. [Google Scholar] [CrossRef]
- Chen, K.K.; Zhang, Y.S. Numerical analysis of temperature distribution during ultrasonic welding process for dissimilar automotive alloys. Sci. Technol. Weld. Join. 2015, 20, 522–531. [Google Scholar] [CrossRef]
- Bakavos, D.; Prangnell, P.B. Mechanisms of joint and microstructure formation in high power ultrasonic spot welding 6111 aluminium automotive sheet. Mater. Sci. Eng. A 2010, 527, 6320–6334. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Li, D.; Zhang, Y.S. Effect of welding energy on interface zone of Al–Cu ultrasonic welded joint. Sci. Technol. Weld. Join. 2013, 18, 354–360. [Google Scholar] [CrossRef]
- Shin, H.S.; Leon, M. Parametric study in similar ultrasonic spot welding of A5052-H32 alloy sheets. J. Mater. Process. Technol. 2015, 224, 222–232. [Google Scholar] [CrossRef]
- Kim, J.M.; Jung, J.P.; Zhou, Y.N.; Kim, J.Y. Ambient temperature ultrasonic bonding of Si-dice using Sn-3.5 wt.%Ag. J. Electron. Mater. 2008, 37, 324–330. [Google Scholar] [CrossRef]

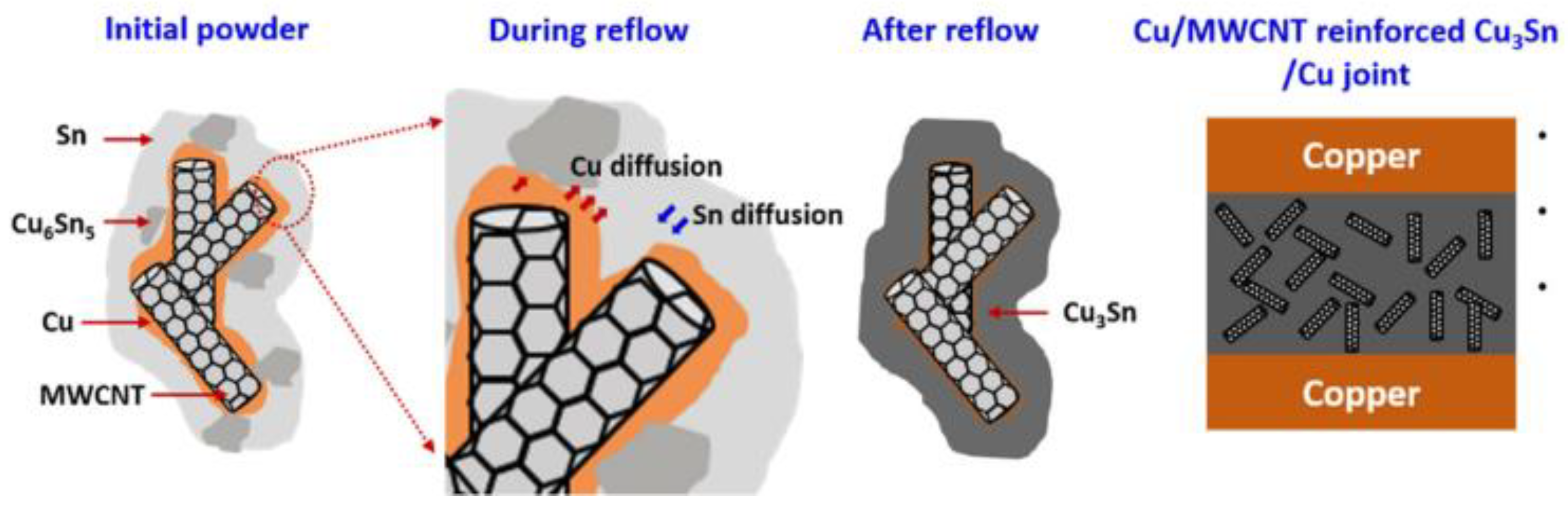

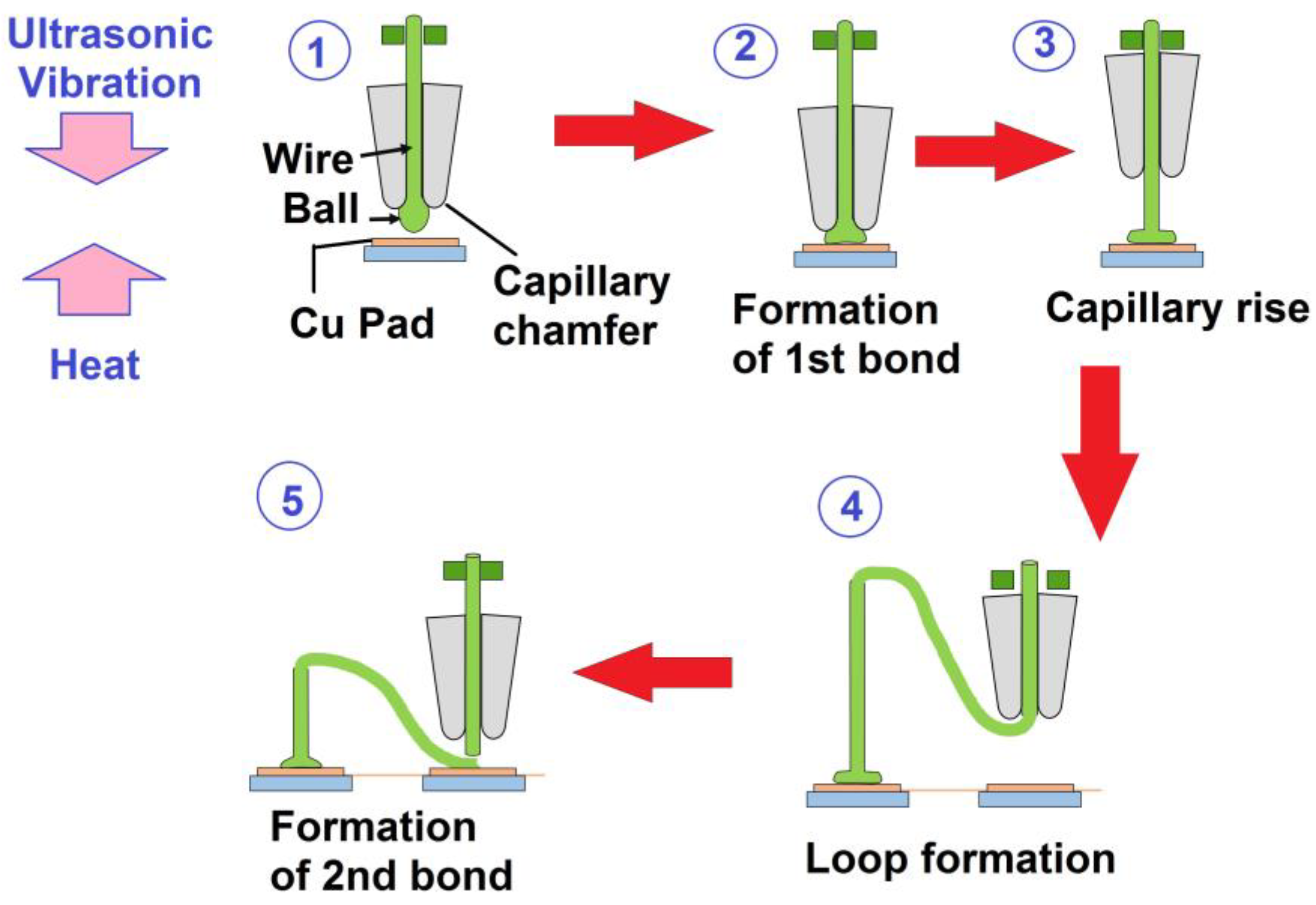
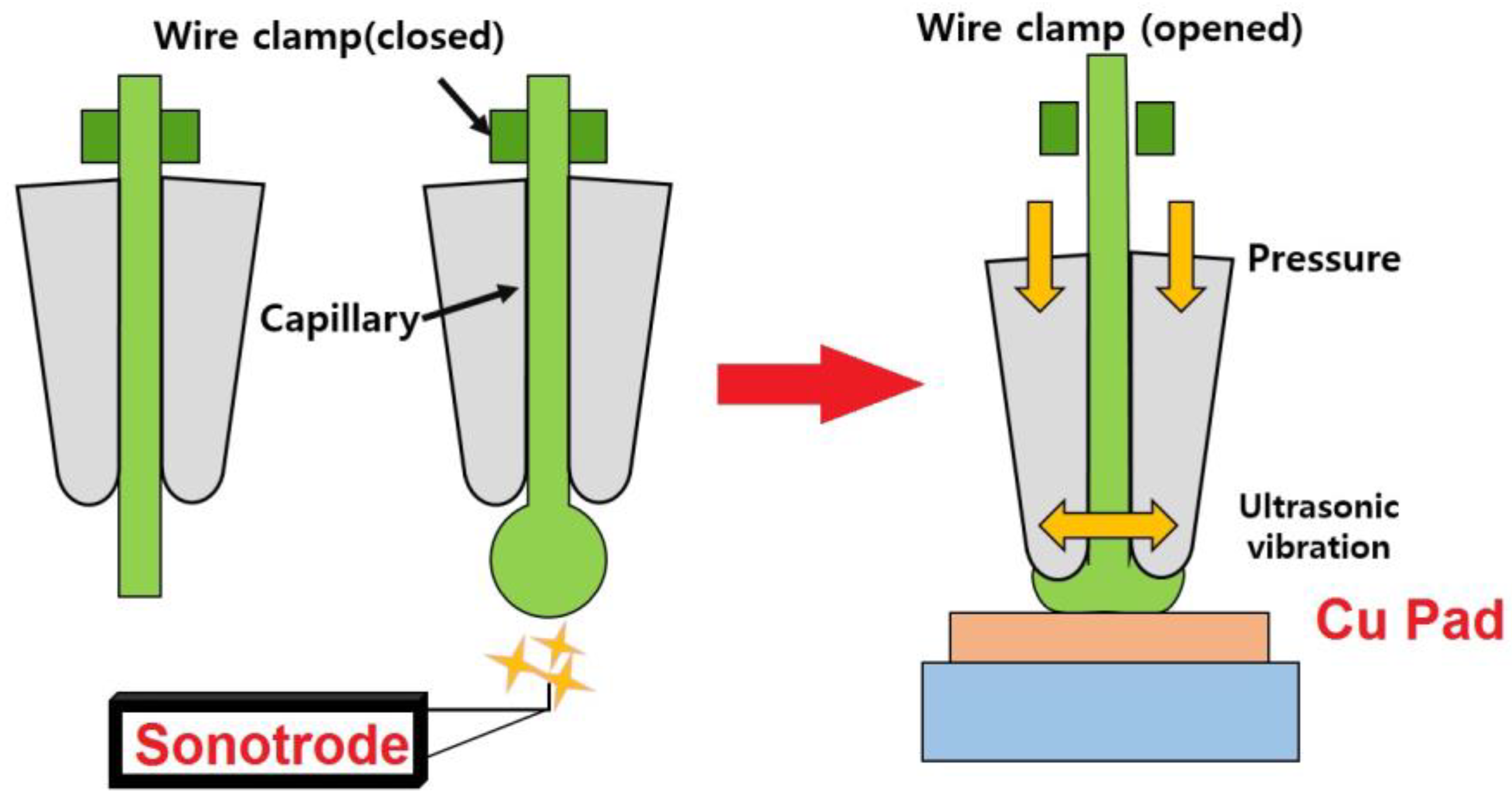


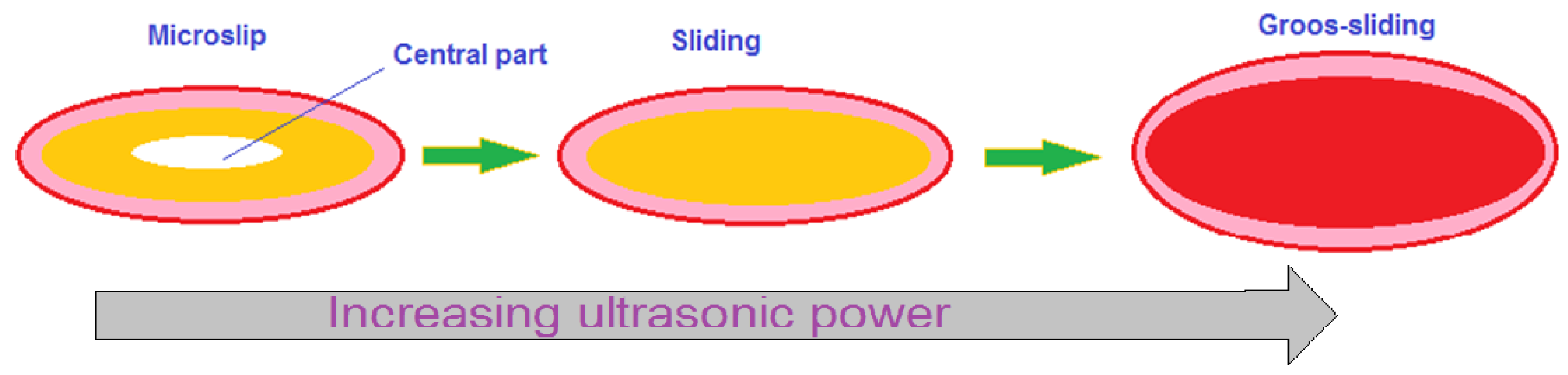
| TLP Process | Filler Type | Couple | Filler Component | Ref. |
|---|---|---|---|---|
| Conventional TLP | Coating | Cu-Sn | Au/Ti/Ag | [42] |
| Conventional TLP | Coating | Ni-Sn | Au/Ti/Ag | [42] |
| Conventional TLP | Coating | Au-Sn | Au/Ti/Ag | [43] |
| Conventional TLP | Coating | Ag-Sn | Au/Ti/Ag | [44] |
| Inserted foil TLP | Foil | Ni-Sn | Sn foil | [45] |
| Improved TLP | Foil | Cu-Sn | Cu/Sn/Cu/Sn | [46] |
| Improved foil TLP | Foil | Ag-Sn | Ag/Sn/Ag/Sn/Ag/Sn/Ag/Sn/Ag | [47] |
| Improved foil TLP | Foil | Ag-Sn | Ag/Sn/Ag/Sn/Ag/Sn/In/Ag/In/Ag | [48] |
| Current assisted TLP | Foil | Cu-Sn | Sn foil, 250 ms | [49] |
| Ternary TLP | Foil | Ag-Sn-Cu | Ag/Sn foil/Cu | [50] |
| Ternary TLP | Paste | Sn-Bi-Cu | SnBi, Cu powder paste | [51] |
| Ternary TLP | Paste | Ni-Cu-Sn | Ni, Cu, Sn paste | [52] |
| Ultrasonic assisted TLP | Foil | Cu-Sn | Sn foil, 8 s | [53] |
| Ultrasonic assisted TLP | Foil | Cu-Sn | Sn-0.7Cu foil/Ni barrier | [54] |
| Ultrasonic assisted TLP | Powder | Ni-Sn | Ni, Sn powder mixture | [55] |
| Plating assisted TLP | Plated balls | Cu-Sn | Sn plated Cu balls, >60 s | [56] |
| Plating assisted TLP | Plated balls | Cu-Sn | Sn plated Cu, pressure less | [57] |
| Thermal gradient TLP | IMC layer | Cu-Sn | IMC layer | [58] |
| Solder paste TLP | Paste | Cu-Sn | Solder paste | [59] |
| Sintering assisted TLP | Residue | Cu-Sn | Sn-residue | [60] |
| Sintering assisted TLP | Activated paste | Cu-Sn | Formic acid-solder paste | [61] |
| Filler type | Data Point in Figure 7 | Bonding conditions | Reference |
|---|---|---|---|
| Foil | 1 | 280 °C–20 min–50 kN*Cu/Sn(0.3)/Cu | [69] |
| 2 | 280 °C–20 min–50 kN*Ni/Sn(0.3)/Ni | [69] | |
| 3 | 255 °C–5 min–25 MPa*Cu/Sn(5)/Cu | [71] | |
| 4 | 280 °C–8 sec–0.5 MPa–Ultrasonic*Cu/Sn(20)/Cu | [72] | |
| 5 | 280 °C–8 sec–0.5 MPa*Cu/Sn(20)/Cu | [72] | |
| 6 | 250 °C–20 min–0.3 MPa*Cu/Sn/Cu | [70] | |
| 7 | 250 °C–5 min–0.3 MPa*Cu/Sn/Cu | [70] | |
| 8 | 250 °C–20 min–0.3 MPa*Cu/Sn/Cu | [70] | |
| 9 | 250 °C–5 min–0.3 MPa*Cu/Sn/Cu | [70] | |
| Powder | 1 | 250 °C–8 min*Cu/Cu@Sn/Cu | [57] |
| 2 | 250 °C–40 min*Cu/Cu@Sn/Cu | [57] | |
| 3 | 200 °C–20 min–20 MPa*Cu/Cu@Sn/Cu | [87] | |
| 4 | 200 °C–5 min–20 MPa*Cu/Cu@Sn/Cu | [87] | |
| 5 | 200 °C–20 min–5 MPa*Cu/Cu@Sn/Cu | [87] | |
| 6 | 200 °C–5 min–5 MPa*Cu/Cu@Sn/Cu | [87] | |
| 7 | 260 °C–20 min–40 MPa*Cu/Cu3Sn/Cu | [85] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Sharma, A.; Jung, J.P. Recent Progress in Transient Liquid Phase and Wire Bonding Technologies for Power Electronics. Metals 2020, 10, 934. https://doi.org/10.3390/met10070934
Kang H, Sharma A, Jung JP. Recent Progress in Transient Liquid Phase and Wire Bonding Technologies for Power Electronics. Metals. 2020; 10(7):934. https://doi.org/10.3390/met10070934
Chicago/Turabian StyleKang, Hyejun, Ashutosh Sharma, and Jae Pil Jung. 2020. "Recent Progress in Transient Liquid Phase and Wire Bonding Technologies for Power Electronics" Metals 10, no. 7: 934. https://doi.org/10.3390/met10070934
APA StyleKang, H., Sharma, A., & Jung, J. P. (2020). Recent Progress in Transient Liquid Phase and Wire Bonding Technologies for Power Electronics. Metals, 10(7), 934. https://doi.org/10.3390/met10070934






