Recent Advances in the Control of the Degradation Rate of PEO Treated Magnesium and Its Alloys for Biomedical Applications
Abstract
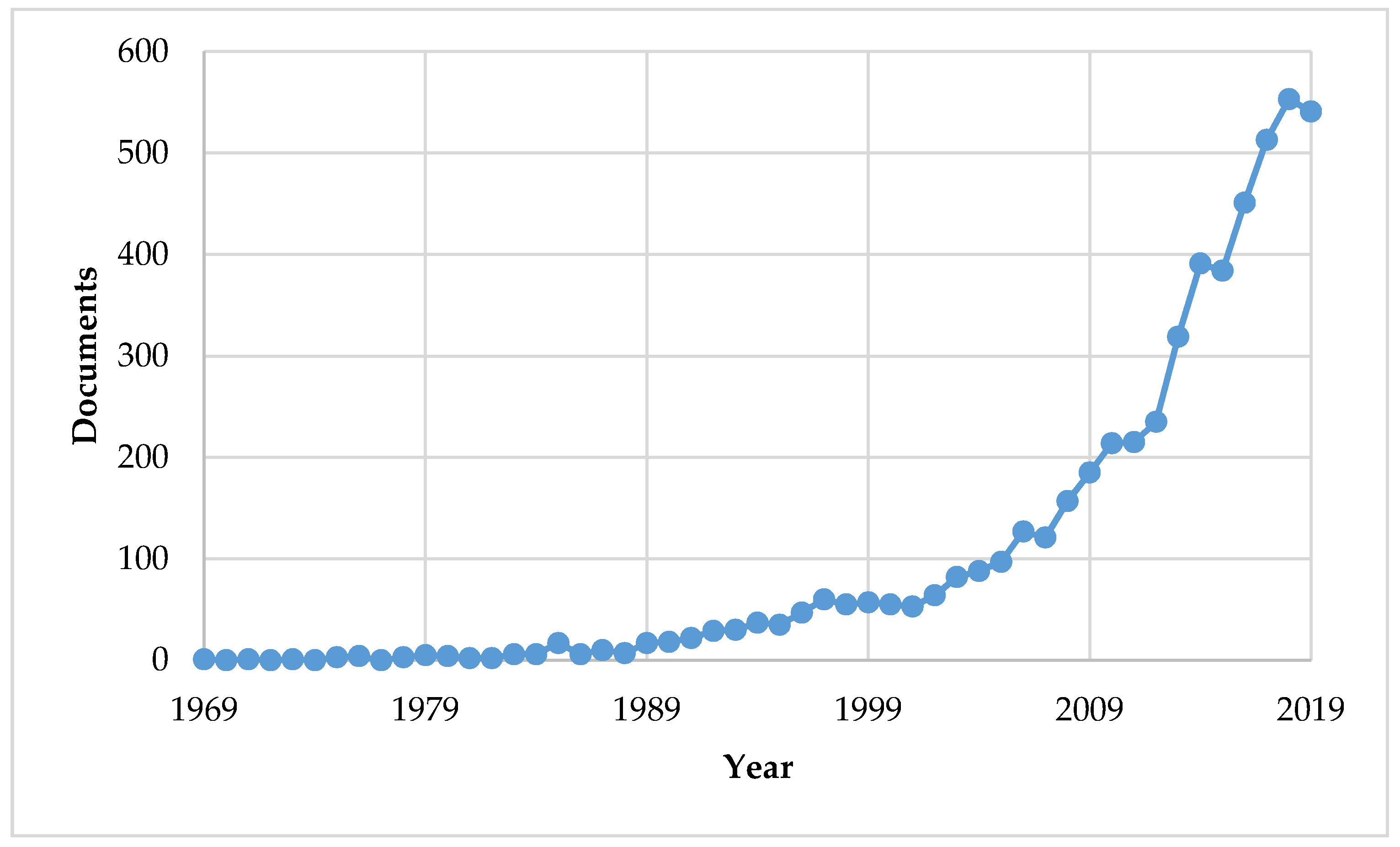
1. Introduction
- (i)
- (ii)
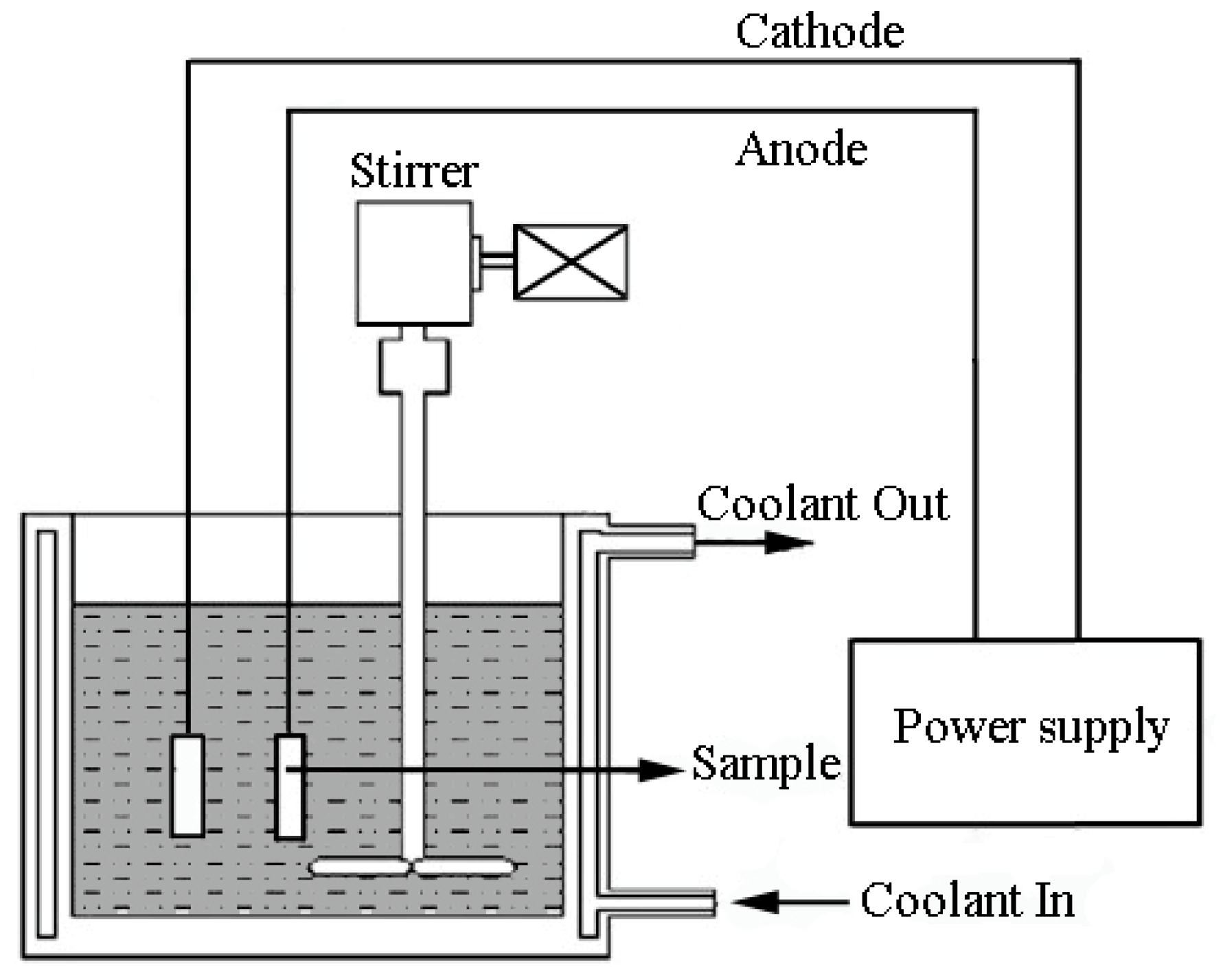
2. The PEO Process
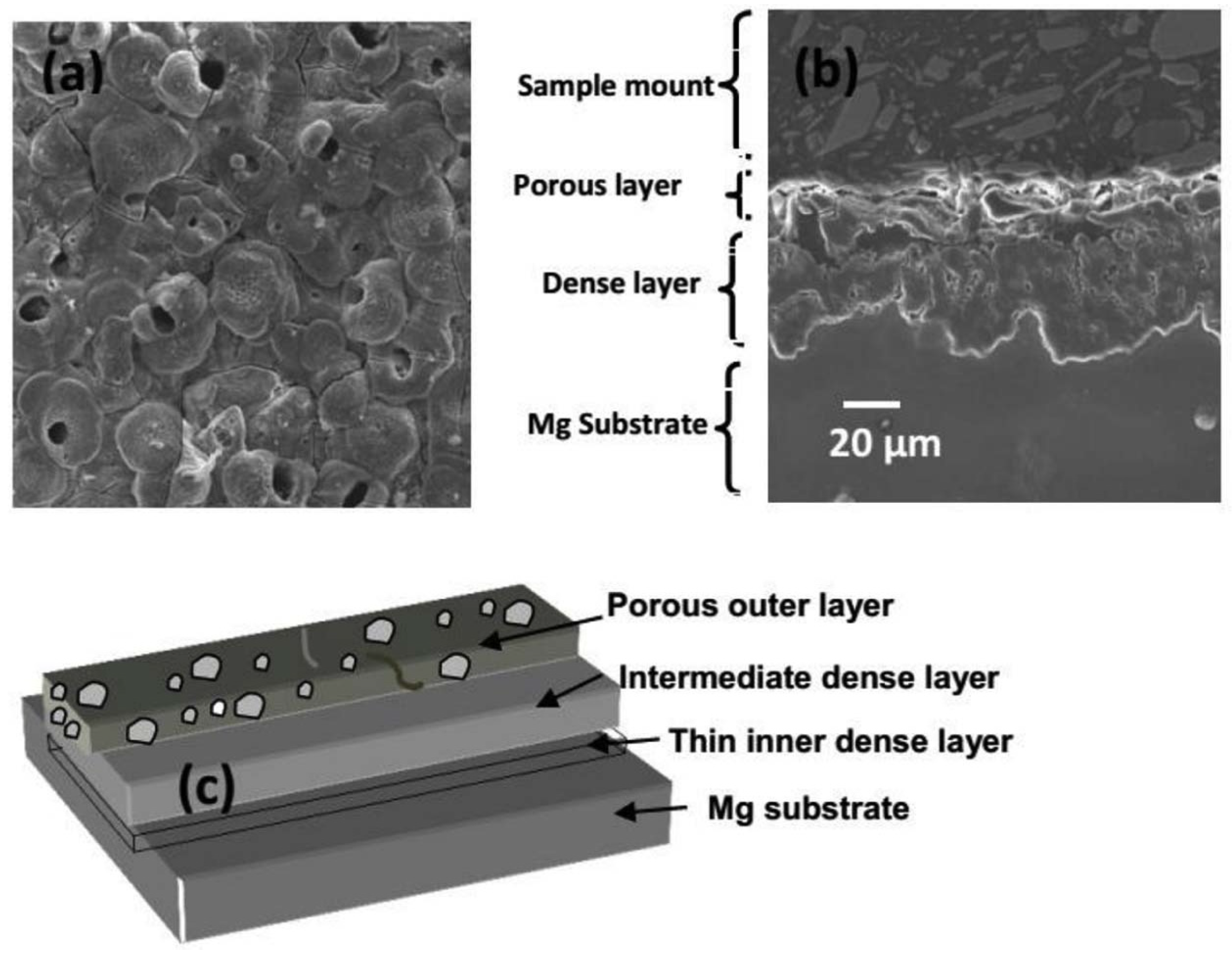
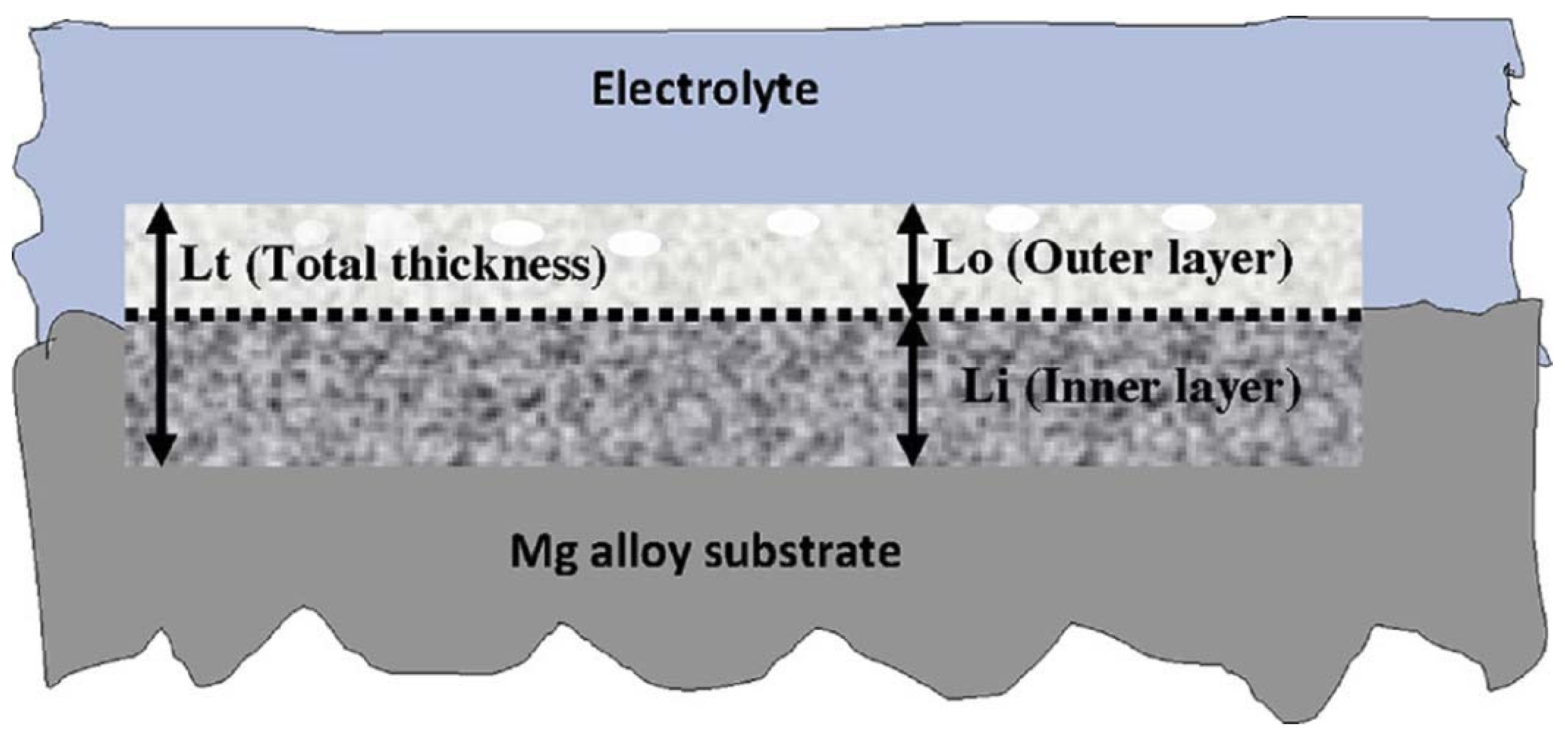
2.1. PEO Layer Structure
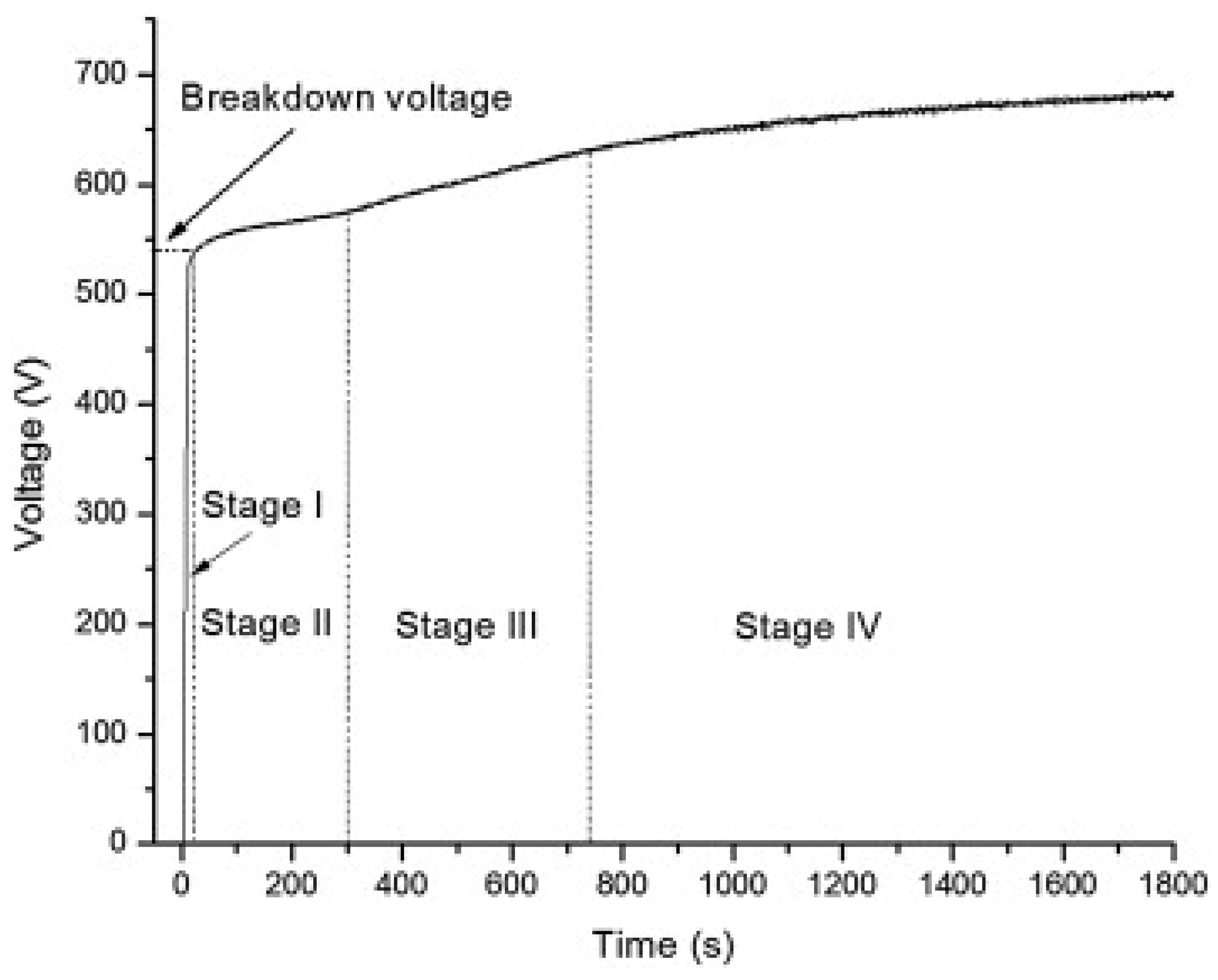
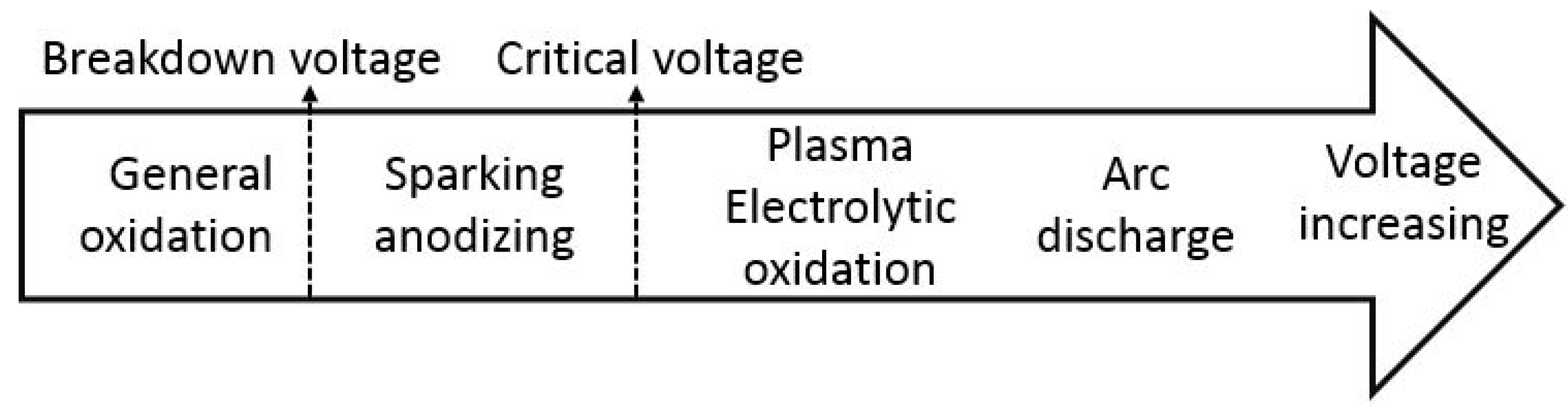
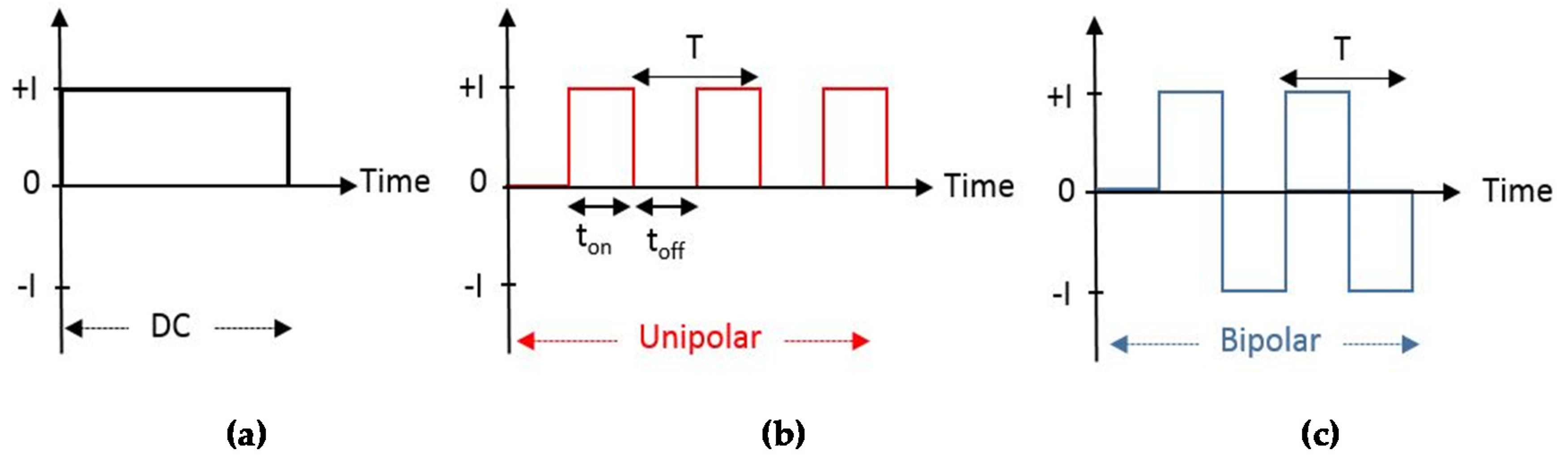
2.2. The Electrical Regime
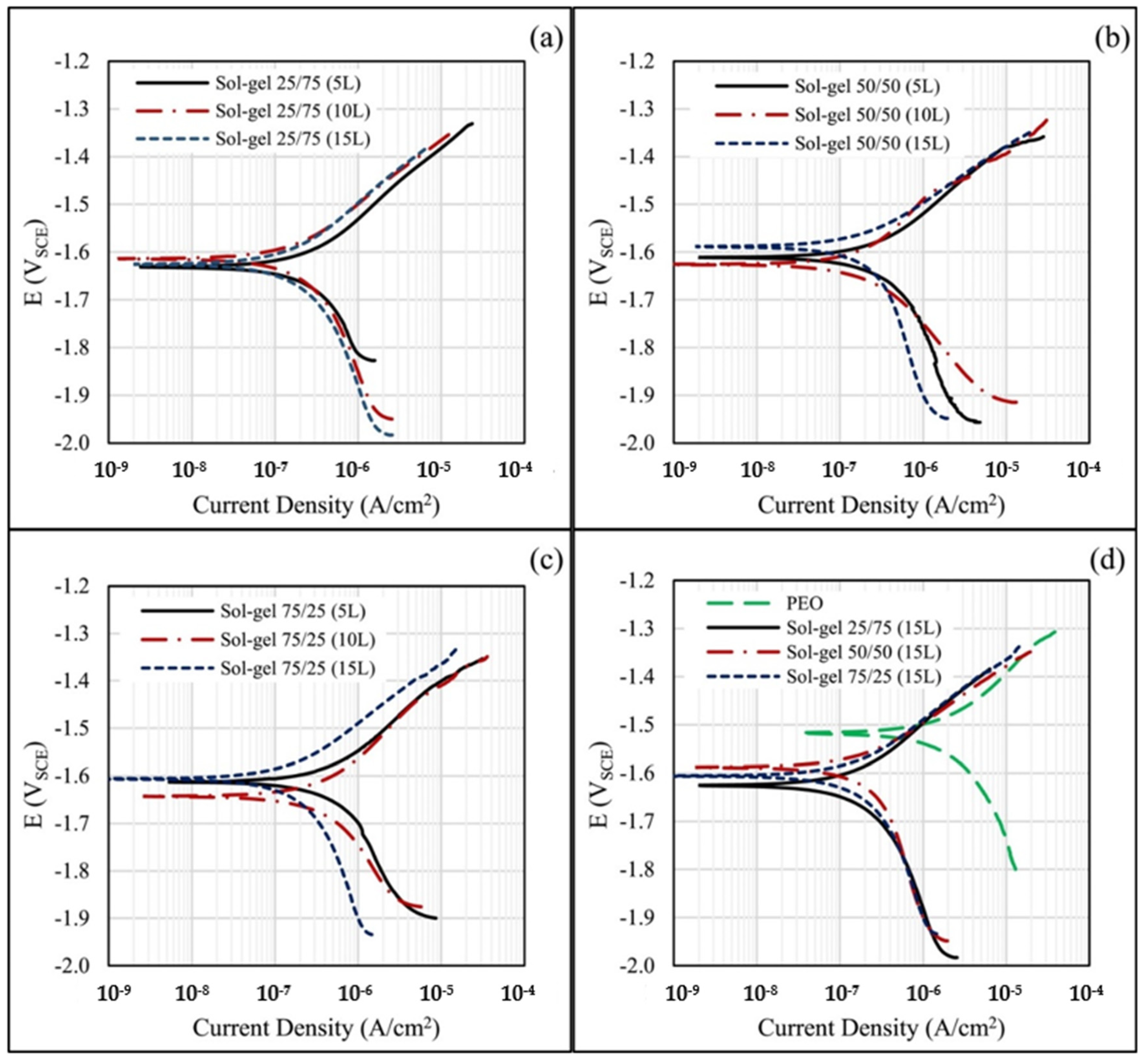
3. Coating PEO Modified Mg and Its Alloys
- Producing a ceramic or biodegradable polymer coating on the PEO layer to seal its porosities and crack;
- Utilizing biocompatible nanoparticles (e.g., tricalcium phosphate, hydroxyapatite, etc.) to seal the pores and cracks of the PEO layer.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Toorani, M.; Aliofkhazraei, M. Review of electrochemical properties of hybrid coating systems on Mg with plasma electrolytic oxidation process as pretreatment. Surf. Interfaces 2019, 14, 262–295. [Google Scholar] [CrossRef]
- Seiler, H.G.; Sigel, H. Handbook on Toxicity of Inorganic Compounds; Marcel Dekker: New York, NY, US, 1988. [Google Scholar]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef]
- Thamaraiselvi, T.; Rajeswari, S. Biological Evaluation of Bioceramic Materials—A Review Trends. Trends Biomater. Artif. Organs 2003, 18, 172. [Google Scholar]
- Huse, E.C. A new ligature. Chic. Med. J. Exam 1878, 172. [Google Scholar]
- Porter, R.; Kaplan, J.; Homeier, B.; Beers, M. Merk Manual of Diagnosis and Therapy (Chapter 12) Endocrine & Metabolic Disorders (Section 2): Water, Electrolyte Mineral and Acid/Base Metabolism; Merck & Co. Inc.: Whitehouse Station, NJ, USA, 1995. [Google Scholar]
- Song, G. Recent Progress in Corrosion and Protection of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Song, G.-L.; Sz, S. Corrosion behaviour of pure magnesium in a simulated body fluid. Acta Phys. Chim. Sin. 2006, 22, 1222–1226. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Windhugen, H.; Witte, F. Medical Implant for The Human or Animal Body. Available online: http://www.freepatentsonline.com/y2004/0241036.html (accessed on 3 July 2020).
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; You, C.; Chen, M. Effects of grain size on the corrosion resistance of pure magnesium by cooling rate-controlled solidification. Front. Mater. Sci. 2015, 9, 247–253. [Google Scholar] [CrossRef]
- Op’t Hoog, C.; Birbilis, N.; Zhang, M.X.; Estrin, Y. Surface Grain Size Effects on the Corrosion of Magnesium. Key Eng. Mater. 2008, 384, 229–240. [Google Scholar] [CrossRef]
- Wang, H.; Estrin, Y.; Zúberová, Z. Bio-corrosion of a magnesium alloy with different processing histories. Mater. Lett. 2008, 62, 2476–2479. [Google Scholar] [CrossRef]
- Xin, R.L.; Wang, M.Y.; Gao, J.C.; Liu, P.; Liu, Q. Effect of Microstructure and Texture on Corrosion Resistance of Magnesium Alloy. Mater. Sci. Forum 2009, 610, 1160–1163. [Google Scholar] [CrossRef]
- Kaesel, V.; Tai, P.T.; Bach, F.W.; Haferkamp, H.; Witte, F.; Windhagen, H. Approach to control the corrosion of magnesium by alloying. In Magnesium: Proceedings of the 6th International Conference Magnesium Alloys and Their Applications, Weinheim, Germany, 2 September 2002; Wiley‐VCH Verlag GmbH & Co.: Geestacht, Germany, 2003; pp. 534–539. [Google Scholar]
- Chen, J.; Tan, L.; Yu, X.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: A review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Donato, N.; Bellucci, F. Durability of AZ31 magnesium biodegradable alloys polydopamine aided: Part 1. J. Magnes. Alloys 2017, 5, 412–422. [Google Scholar] [CrossRef]
- Carangelo, A.; Acquesta, A.; Monetta, T. Durability of AZ31 magnesium biodegradable alloys polydopamine aided. Part 2: Ageing in Hank’s solution. J. Magnes. Alloys 2019, 7, 218–226. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Allain, J.P.; Robledo, S.M.; Echeverria, F.; Harmsen, M.C. Coatings for biodegradable magnesium-based supports for therapy of vascular disease: A general view. Mater. Sci. Eng. C 2019, 102, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Tan, L.; Yang, K. Surface Modification on Biodegradable Magnesium Alloys as Orthopedic Implant Materials to Improve the Bio-adaptability: A Review. J. Mater. Sci. Technol. 2016, 32, 827–834. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.; Lee, I.S. Surface Modifications of Magnesium Alloys for Biomedical Applications. Ann. Biomed. Eng. 2011, 39, 1857–1871. [Google Scholar] [CrossRef]
- Blawert, C.; Srinivasan, P.B. Plasma electrolytic oxidation treatment of magnesium alloys. In Surface Engineering of Light Alloys; Woodhead Publishing Elsevier: Sawstone, Cambridge, UK, 2010; pp. 155–183. [Google Scholar]
- Echeverry-Rendon, M.; Duque, V.; Quintero, D.; Harmsen, M.C.; Echeverria, F. Novel coatings obtained by plasma electrolytic oxidation to improve the corrosion resistance of magnesium-based biodegradable implants. Surf. Coat. Technol. 2018, 354, 28–37. [Google Scholar] [CrossRef]
- Bender, S.; Göllner, J.; Heyn, A.; Blawert, C.; Srinivasan, P. Corrosion and Surface Finishing of Magnesium and Its Alloys; Woodhead Publishing Elsevier: Sawstone, Cambridge, UK, 2013; pp. 232–265. [Google Scholar] [CrossRef]
- Shim, G. Factors Influencing Plasma Electrolytic Oxidation(PEO) Coatings on Magnesium Alloys: A Review. Korean J. Met. Mater. 2017, 55, 296–307. [Google Scholar] [CrossRef]
- Xu, J.L.; Xiao, Q.F.; Mei, D.D.; Tong, Y.X.; Zheng, Y.F.; Li, L.; Zhong, Z.C. Microstructure, corrosion resistance and formation mechanism of alumina micro-arc oxidation coatings on sintered NdFeB permanent magnets. Surf. Coat. Technol. 2017, 309, 621–627. [Google Scholar] [CrossRef]
- Ghasemi, A.; Raja, V.S.; Blawert, C.; Dietzel, W.; Kainer, K.U. The role of anions in the formation and corrosion resistance of the plasma electrolytic oxidation coatings. Surf. Coat. Technol. 2010, 204, 1469–1478. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Chen, C.-f.; Gu, Y. Advances in microarc oxidation coated AZ31 Mg alloys for biomedical applications. Corros. Sci. 2015, 91, 7–28. [Google Scholar] [CrossRef]
- Hussein, R.O. Plasma Process Control for Improved PEO Coatings on Magnesium Alloys. Ph.D. Thesis, University of Windsor, Windsor, ON, Canada, 2015. [Google Scholar]
- Tsn, S.N.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Durdu, S.; Aytaç, A.; Usta, M. Characterization and corrosion behavior of ceramic coating on magnesium by micro-arc oxidation. J. Alloys Compd. 2011, 509, 8601–8606. [Google Scholar] [CrossRef]
- Xue, W.; Jin, Q.; Zhu, Q.; Hua, M.; Ma, Y. Anti-corrosion microarc oxidation coatings on SiCP/AZ31 magnesium matrix composite. J. Alloys Compd. 2009, 482, 208–212. [Google Scholar] [CrossRef]
- Langmuir, I. Oscillations in Ionized Gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Dehnavi, V.; Shoesmith, D.; Luan, B.; Yari, M.; Liu, X.Y.; Rohani, S. Corrosion properties of plasma electrolytic oxidation coatings on an aluminium alloy—The effect of the PEO process stage. Mater. Chem. Phys. 2015, 161, 49–58. [Google Scholar] [CrossRef]
- Chang, L. Growth regularity of ceramic coating on magnesium alloy by plasma electrolytic oxidation. J. Alloys Compd. 2009, 468, 462–465. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Nie, X. The influence of pulse timing and current mode on the microstructure and corrosion behaviour of a plasma electrolytic oxidation (PEO) coated AM60B magnesium alloy. J. Alloys Compd. 2012, 541, 41–48. [Google Scholar] [CrossRef]
- Jin, F.; Chu, P.K.; Xu, G.; Zhao, J.; Tang, D.; Tong, H. Structure and mechanical properties of magnesium alloy treated by micro-arc discharge oxidation using direct current and high-frequency bipolar pulsing modes. Mater. Sci. Eng. A 2006, 435, 123–126. [Google Scholar] [CrossRef]
- Ibrahim, H.; Dehghanghadikolaei, A.; Advincula, R.; Dean, D.; Luo, A.; Elahinia, M. Ceramic coating for delayed degradation of Mg-1.2Zn-0.5Ca-0.5Mn bone fixation and instrumentation. Thin Solid Film 2019, 687, 137456. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Jang, Y.-S.; Kim, S.-Y.; Lee, M.-H. Functions achieved by the hyaluronic acid derivatives coating and hydroxide film on bio-absorbed Mg. Appl. Surf. Sci. 2019, 473, 31–39. [Google Scholar] [CrossRef]
- Hariprasad, S.; Gowtham, S.; Arun, S.; Ashok, M.; Rameshbabu, N. Fabrication of duplex coatings on biodegradable AZ31 magnesium alloy by integrating cerium conversion (CC) and plasma electrolytic oxidation (PEO) processes. J. Alloys Compd. 2017, 722, 698–715. [Google Scholar] [CrossRef]
- Castano, C.E.; O’Keefe, M.J.; Fahrenholtz, W.G. Cerium-based oxide coatings. Curr. Opin. Solid State Mater. Sci. 2015, 19, 69–76. [Google Scholar] [CrossRef]
- Mu, S.; Du, J.; Jiang, H.; Li, W. Composition analysis and corrosion performance of a Mo–Ce conversion coating on AZ91 magnesium alloy. Surf. Coat. Technol. 2014, 254, 364–370. [Google Scholar] [CrossRef]
- Ng, W.F.; Wong, M.; Cheng, F. Cerium-based coating for enhancing the corrosion resistance of bio-degradable Mg implants. Mater. Chem. Phys. 2010, 119, 384–388. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Z.; Tang, S.; Dong, W.; Tong, Q.; Li, W. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 aluminum alloy and the coating properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Zhang, C. Effect of the graphene oxide additive on the corrosion resistance of the plasma electrolytic oxidation coating of the AZ31 magnesium alloy. Corros. Sci. 2017, 114, 146–155. [Google Scholar] [CrossRef]
- Wen, C.; Zhan, X.; Huang, X.; Xu, F.; Luo, L.; Xia, C. Characterization and corrosion properties of hydroxyapatite/graphene oxide bio-composite coating on magnesium alloy by one-step micro-arc oxidation method. Surf. Coat. Technol. 2017, 317, 125–133. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved corrosion performance of biodegradable magnesium in simulated inflammatory condition via drug-loaded plasma electrolytic oxidation coatings. Mater. Chem. Phys. 2020, 239, 122003. [Google Scholar] [CrossRef]
- Singh, S. Principles of Regenerative Medicine. Ann. Biomed. Eng. 2009, 2658–2659. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Vargas-Alfredo, N.; Arrabal, R.; Rodríguez-Hernández, J.; Matykina, E. Hierarchical Functionalized Polymeric-Ceramic Coatings on Mg-Ca Alloys for Biodegradable Implant Applications. Macromol. Biosci. 2019, 19, 1900179. [Google Scholar] [CrossRef] [PubMed]
- Calejo, M.T.; Ilmarinen, T.; Skottman, H.; Kellomäki, M. Breath figures in tissue engineering and drug delivery: State-of-the-art and future perspectives. Acta Biomater. 2018, 66, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Cui, B.; Bai, K.; Guan, S.; Wang, L.; Zhu, S. In vitro degradation of AZ31 magnesium alloy coated with nano TiO2 film by sol–gel method. Appl. Surf. Sci. 2011, 257, 8772–8777. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Controlling the degradation rate of AZ91 magnesium alloy via sol–gel derived nanostructured hydroxyapatite coating. Mater. Sci. Eng. C 2013, 33, 3817–3825. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, K.; Fu, Z.; Zhang, C.; Zhou, H.; Wang, L.; Zhu, S.; Guan, S.; Li, D.; Hu, J. Composite coating prepared by micro-arc oxidation followed by sol–gel process and in vitro degradation properties. Appl. Surf. Sci. 2012, 258, 2939–2943. [Google Scholar] [CrossRef]
- Wang, S.; Wen, S.; Shen, M.; Guo, R.; Cao, X.; Wang, J.; Shi, X. Aminopropyltriethoxysilane-mediated surface functionalization of hydroxyapatite nanoparticles: Synthesis, characterization, and in vitro toxicity assay. Int. J. Nanomed. 2011, 6, 3449–3459. [Google Scholar] [CrossRef]
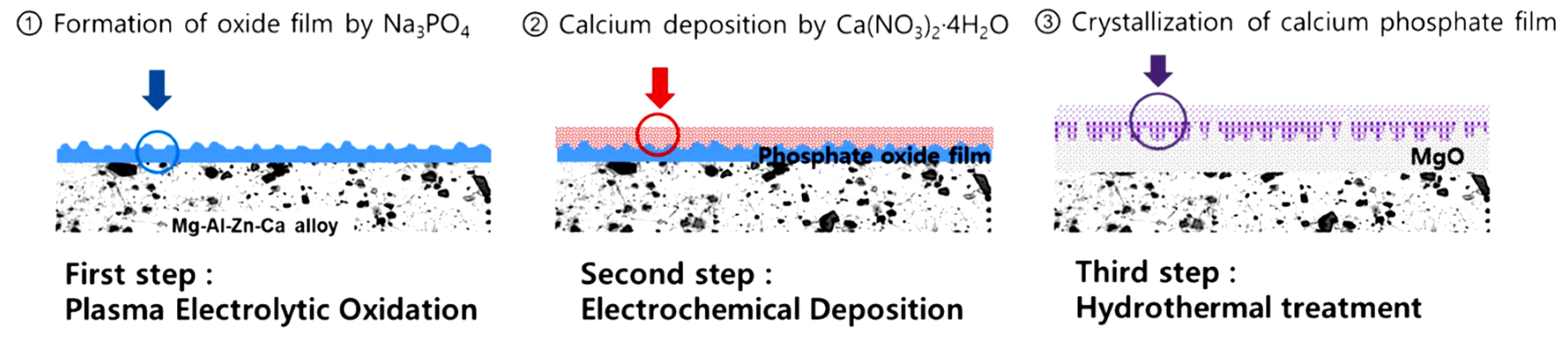
- Kim, Y.K.; Jang, Y.S.; Lee, Y.H.; Yi, H.K.; Bae, T.S.; Lee, M.H. Effect of Ca-P compound formed by hydrothermal treatment on biodegradation and biocompatibility of Mg-3Al-1Zn-1.5Ca alloy; in vitro and in vivo evaluation. Sci. Rep. 2017, 7, 712. [Google Scholar] [CrossRef]

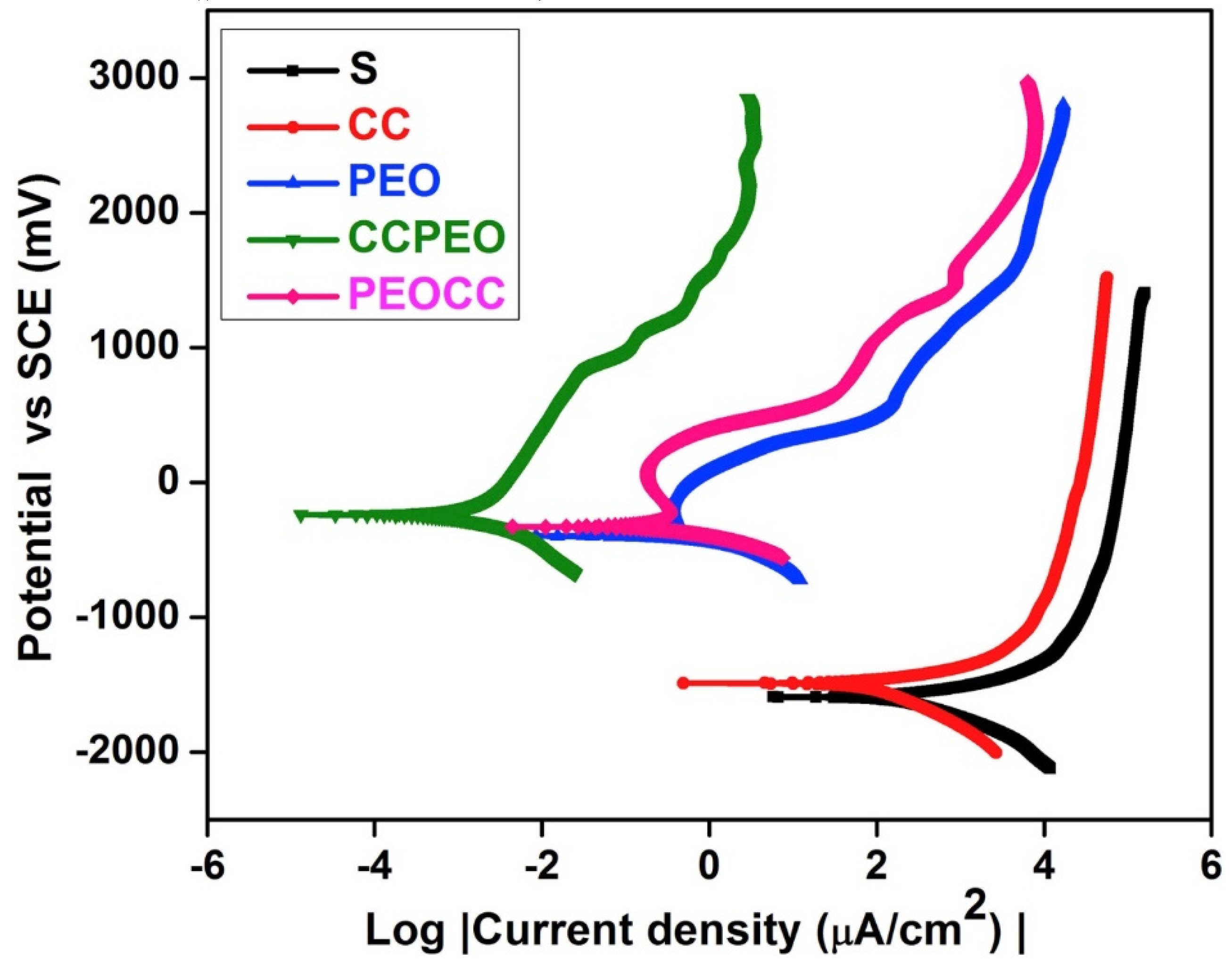
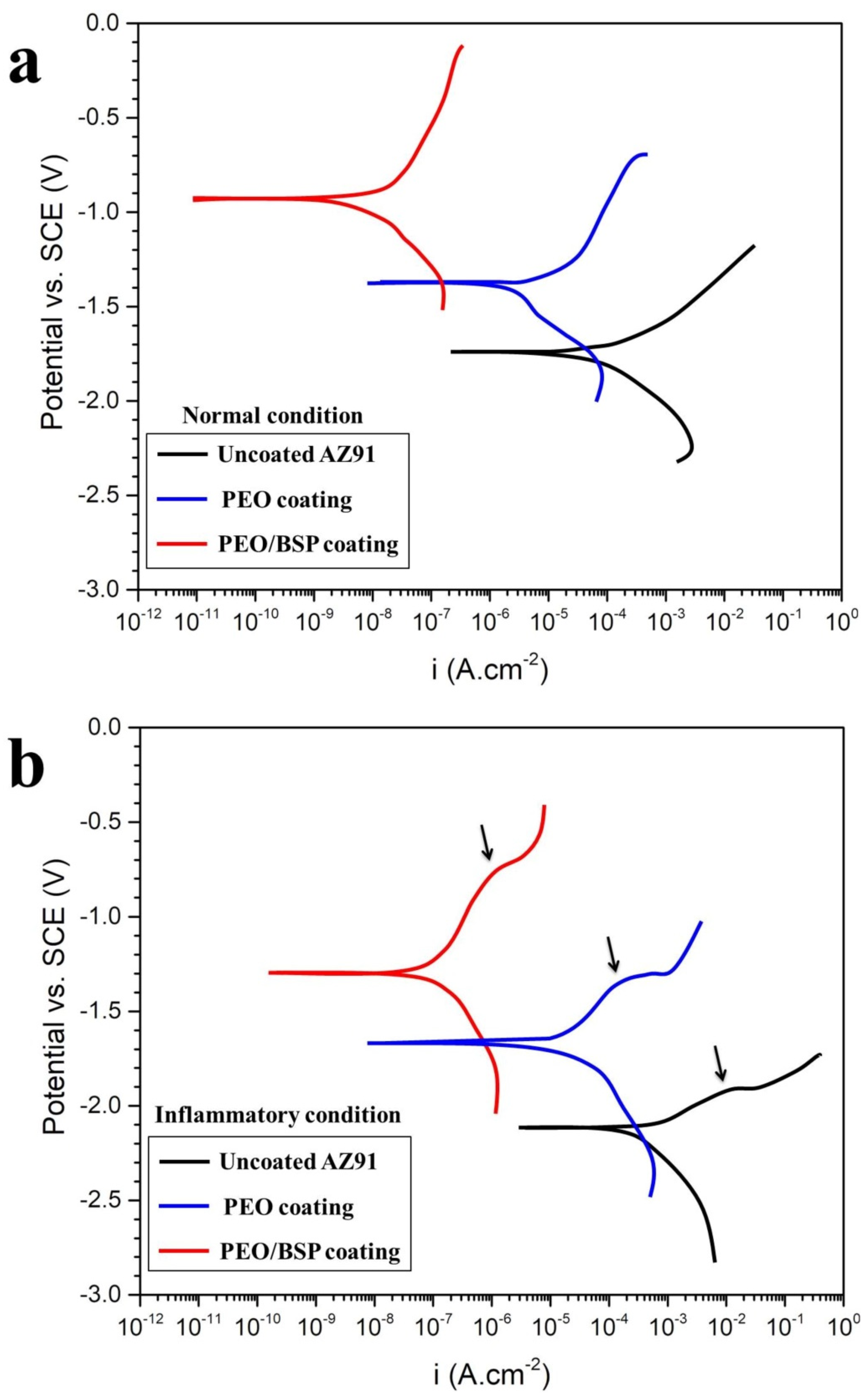
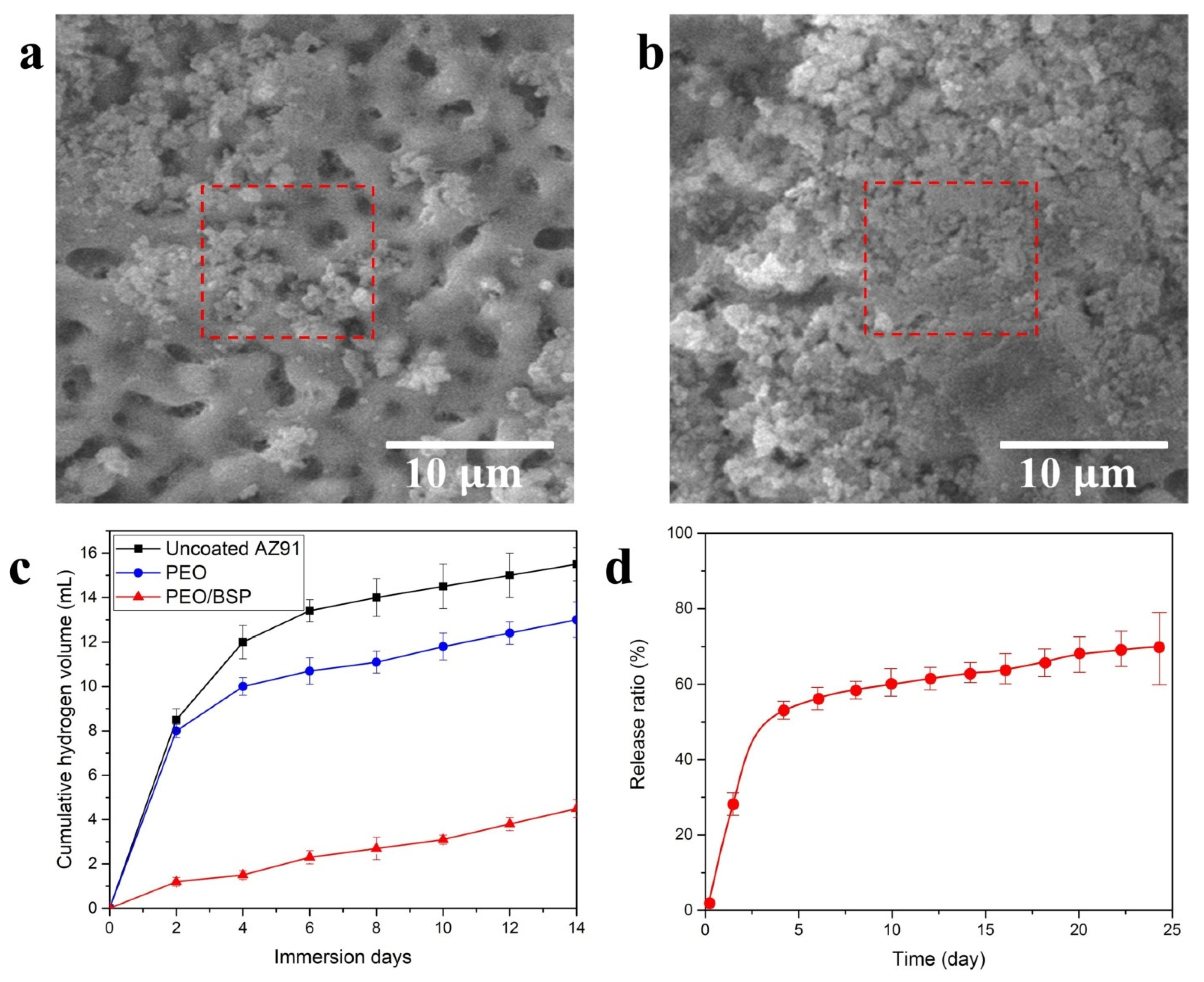
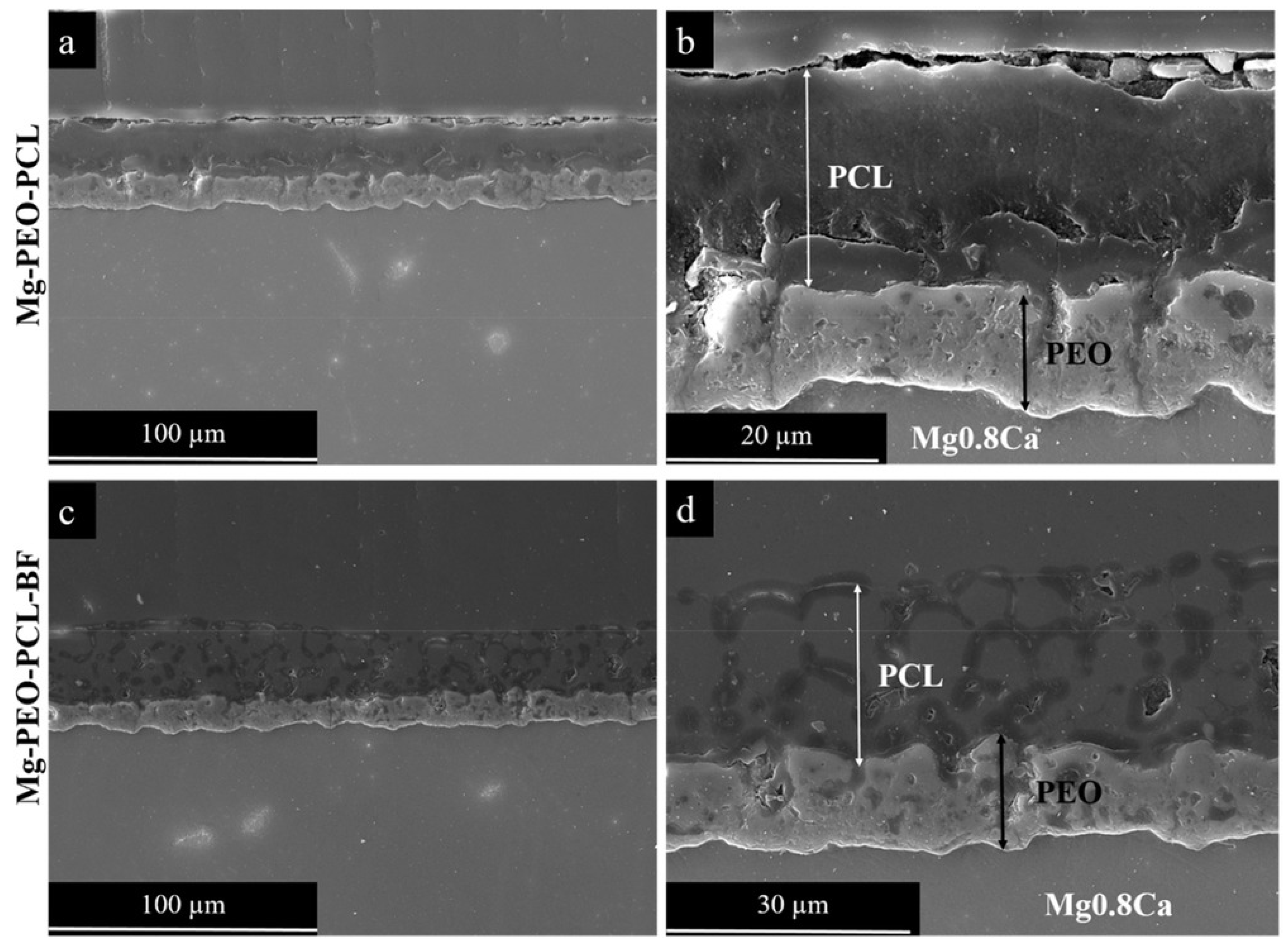
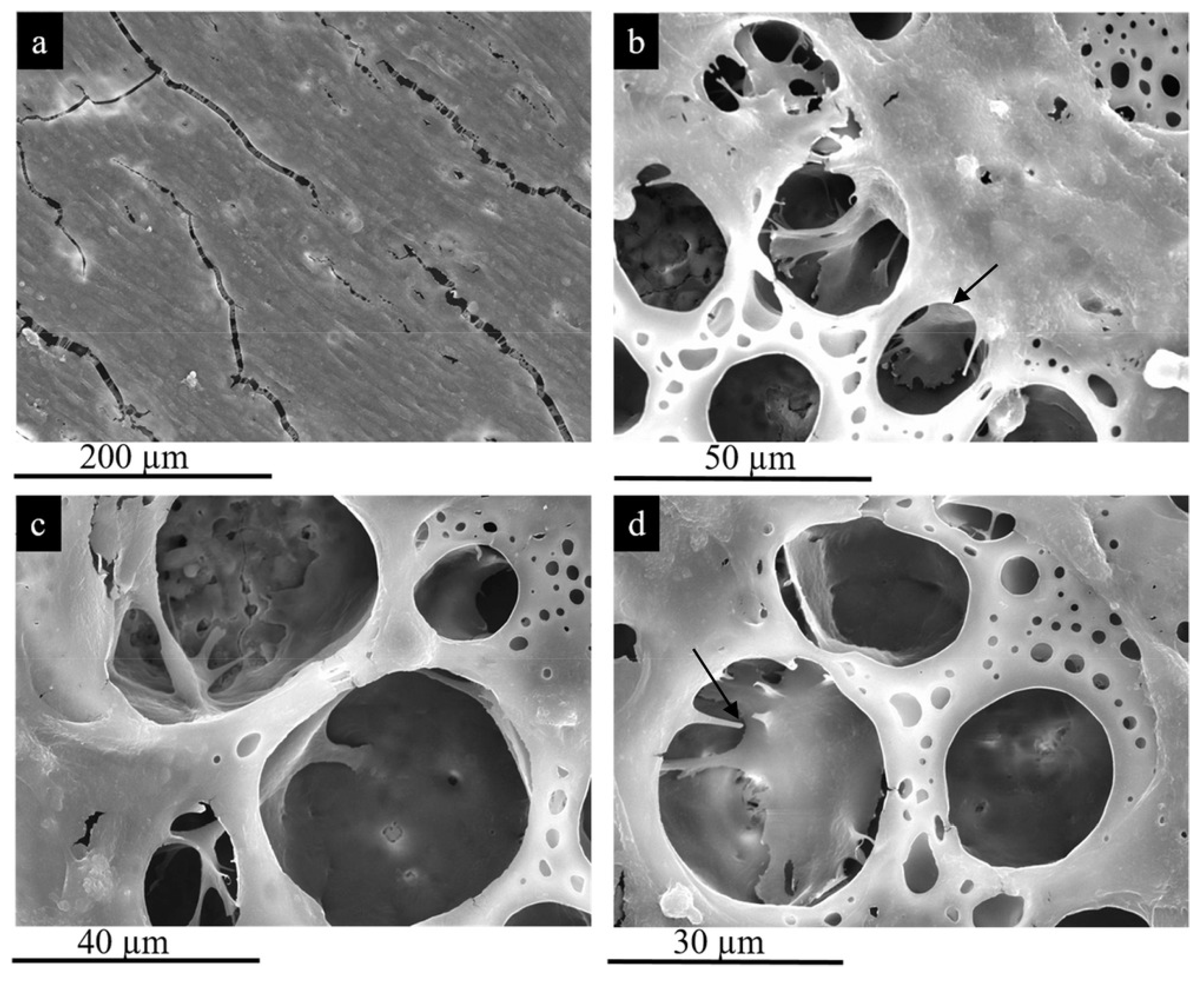
| Sl.No | Sample Code | Electrolyte Composition | pH | k (mS/cm) | Vb (±2 V) | Vf (±2 V) | Process Time (min) |
|---|---|---|---|---|---|---|---|
| 1 | CC | 10 g/L Ce(NO3)3·6H2O + 2 mL/L H2O2 | 3 | 8.3 | – | – | 240 |
| 2 | PEO | 7 g/L Na2SiO3·10H2O + 3 g/L KOH | 12.4 | 18 | 245 | 512 | 15 |
| 3 | CCPEO | 10 g/L Ce(NO3)3·6H2O + 2 mL/L H2O2; | 3 | 8.3 | – | – | 240 |
| 7 g/L Na2SiO3·10H2O + 3 g/L KOH | 12.4 | 18 | 220 | 502 | 15 | ||
| 4 | PEOCC | 7 g/L Na2SiO3·10H2O + 3 g/L KOH; | 12.4 | 18 | 245 | 512 | 15 |
| 10 g/L Ce(NO3)3·6H2O + 2 mL/L H2O2 | 3 | 8.3 | – | – | 240 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monetta, T.; Parnian, P.; Acquesta, A. Recent Advances in the Control of the Degradation Rate of PEO Treated Magnesium and Its Alloys for Biomedical Applications. Metals 2020, 10, 907. https://doi.org/10.3390/met10070907
Monetta T, Parnian P, Acquesta A. Recent Advances in the Control of the Degradation Rate of PEO Treated Magnesium and Its Alloys for Biomedical Applications. Metals. 2020; 10(7):907. https://doi.org/10.3390/met10070907
Chicago/Turabian StyleMonetta, Tullio, Pooyan Parnian, and Annalisa Acquesta. 2020. "Recent Advances in the Control of the Degradation Rate of PEO Treated Magnesium and Its Alloys for Biomedical Applications" Metals 10, no. 7: 907. https://doi.org/10.3390/met10070907
APA StyleMonetta, T., Parnian, P., & Acquesta, A. (2020). Recent Advances in the Control of the Degradation Rate of PEO Treated Magnesium and Its Alloys for Biomedical Applications. Metals, 10(7), 907. https://doi.org/10.3390/met10070907







