Precipitation Versus Partitioning Kinetics during the Quenching of Low-Carbon Martensitic Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Material Characterization
3.2. Carbon Kinetics Simulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kömi, J.; Karjalainen, P.; Porter, D. Direct-Quenched Structural Steels. Encycl. Iron Steel Alloy 2016, 1109–1125. [Google Scholar]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels: Microstructure and Properties, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Krauss, G. Martensite in steel: Strength and structure. Mater. Sci. Eng. A 2002, 273–275, 40–57. [Google Scholar] [CrossRef]
- Pereloma, E.; Edmonds, D. Phase Transformations in Steels; Elsevier Science & Technology: London, UK, 2012; ISBN 9781845699710. [Google Scholar]
- Babu, S.R.; Nyyssönen, T.; Jaskari, M.; Järvenpää, A.; Davis, T.P.; Pallaspuro, S.; Kömi, J.; Porter, D. Observations on the relationship between crystal orientation and the level of auto-tempering in an as-quenched martensitic steel. Metals 2019, 9, 1255. [Google Scholar] [CrossRef]
- Hutchinson, B.; Hagström, J.; Karlsson, O.; Lindell, D.; Tornberg, M.; Lindberg, F.; Thuvander, M. Microstructures and hardness of as-quenched martensites (0.1–0.5%C). Acta Mater. 2011, 59, 5845–5858. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Matsuda, H.; Mizuno, R.; Funakawa, Y.; Seto, K.; Matsuoka, S.; Tanaka, Y. Effects of auto-tempering behaviour of martensite on mechanical properties of ultra high strength steel sheets. J. Alloy. Compd. 2013, 577, S661–S667. [Google Scholar] [CrossRef]
- Takaki, S.; Akama, D.; Tsuchiyama, T. Quantitative Evaluation of Auto-Tempering During Quenching. NETSUSHORI 2017, 56, 340–344. [Google Scholar]
- Babu, S.R.; Jaskari, M.; Järvenpää, A.; Porter, D. The effect of hot-mounting on the microstructure of an As-Quenched auto-tempered low-carbon martensitic steel. Metals 2019, 9, 550. [Google Scholar] [CrossRef]
- Babu, S.R.; Davis, T.P.; Haas, T.; Jarvenpää, A.; Kömi, J.; Porter, D. Image Processing Tool Quantifying Auto-Tempered Carbides in As-Quenched Low Carbon Martensitic Steels. Metals 2020, 10, 171. [Google Scholar] [CrossRef]
- Borgenstam, A.; Höglund, L.; Ågren, J.; Engström, A. DICTRA, a tool for simulation of diffusional transformations in alloys. J. Phase Equilibria 2000, 21, 269–280. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, K.; Sterner, G.; Mason, P. Modeling Precipitation Kinetics During Heat Treatment with Calphad-Based Tools. J. Mater. Eng. Perform. 2014, 23, 4193–4196. [Google Scholar] [CrossRef]
- Ramesh Babu, S.; Ivanov, D.; Porter, D. Influence of Microsegregation on the Onset of the Martensitic Transformation. ISIJ Int. 2018, 59, 169–175. [Google Scholar] [CrossRef]
- Klinger, M.; Jäger, A. Crystallographic Tool Box (CrysTBox): Automated tools for transmission electron microscopists and crystallographers. J. Appl. Cryst. 2015, 48, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad Comput. Coupling Phase Diagr. 2002, 26, 273–312. [Google Scholar]
- Garcia, J.; Lindwall, G.; Prat, O.; Frisk, K. Kinetics of formation of graded layers on cemented carbides: Experimental investigations and DICTRA simulations. Int. J. Refract. Met. Hard Mater. 2011, 29, 256–259. [Google Scholar] [CrossRef]
- Kashchiev, D. Nucleation: Basic Theory with Applications; Butterworth-Heinemann: Oxford, UK, 2000. [Google Scholar]
- Russell, K.C. Nucleation in solids: The induction and steady state effects. Adv. Colloid Interface Sci. 1980, 13, 205–318. [Google Scholar] [CrossRef]
- Thermo-Calc, Version 2019b. In Precipitation Module (TC-PRISMA) User Guide; Thermo-Calc Software AB: Solna, Sweden, 2019.
- Chen, Q.; Jeppsson, J.; Ågren, J. Analytical treatment of diffusion during precipitate growth in multicomponent systems. Acta Mater. 2008, 56, 1890–1896. [Google Scholar] [CrossRef]
- Prat, O.; García, J.; Rojas, D.; Sanhueza, J.P.; Camurri, C. Study of nucleation, growth and coarsening of precipitates in a novel 9%Cr heat resistant steel: Experimental and modeling. Mater. Chem. Phys. 2014, 143, 754–764. [Google Scholar] [CrossRef]
- Morsdorf, L.; Tasan, C.C.; Ponge, D.; Raabe, D. Acta Materialia 3D structural and atomic-scale analysis of lath martensite: Effect of the transformation sequence. Acta Mater. 2015, 95, 366–377. [Google Scholar] [CrossRef]
- Morsdorf, L.; Jeannin, O.; Barbier, D.; Mitsuhara, M.; Raabe, D.; Tasan, C.C. Acta Materialia Multiple mechanisms of lath martensite plasticity. Acta Mater. 2016, 121, 202–214. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Carbon content of retained austenite in quenched steels. Met. Sci. 1983, 17, 151–152. [Google Scholar] [CrossRef]
- Sarikaya, M.; Thomas, G.; Steeds, J.W.; Barnard, S.J.; Smith, G.D.W. Solute Element Partitioning and Austenite Stabilization in Steels. In Proceedings of the International Conference on Solid to Solid Phase Transformations, H.I Aaronson, TMS, Warrendale, PA, USA, 1982; pp. 1421–1425. [Google Scholar]
- Santofimia, M.J.; Speer, J.G.; Clarke, A.J.; Zhao, L.; Sietsma, J. Influence of interface mobility on the evolution of austenite–martensite grain assemblies during annealing. Acta Mater. 2009, 57, 4548–4557. [Google Scholar] [CrossRef]
- Hillert, M.; Höglund, L.; Ågren, J. Escape of carbon from ferrite plates in austenite. Acta Met. Mater. 1993, 41, 1951–1957. [Google Scholar] [CrossRef]
- Jang, J.H.; Bhadeshia, H.K.D.H.; Suh, D.-W. Solubility of carbon in tetragonal ferrite in equilibrium with austenite. Scr. Mater. 2013, 68, 195–198. [Google Scholar] [CrossRef]
- Zener, C. Kinetics of the decomposition of austenite. Trans. Met. Soc. Aime 1946, 167, 550–595. [Google Scholar]
- Morito, S.; Nishikawa, J.; Maki, T. Dislocation Density within Lath Martensite in Fe-C and Fe-Ni Alloys. ISIJ Int. 2003, 43, 1475–1477. [Google Scholar] [CrossRef]
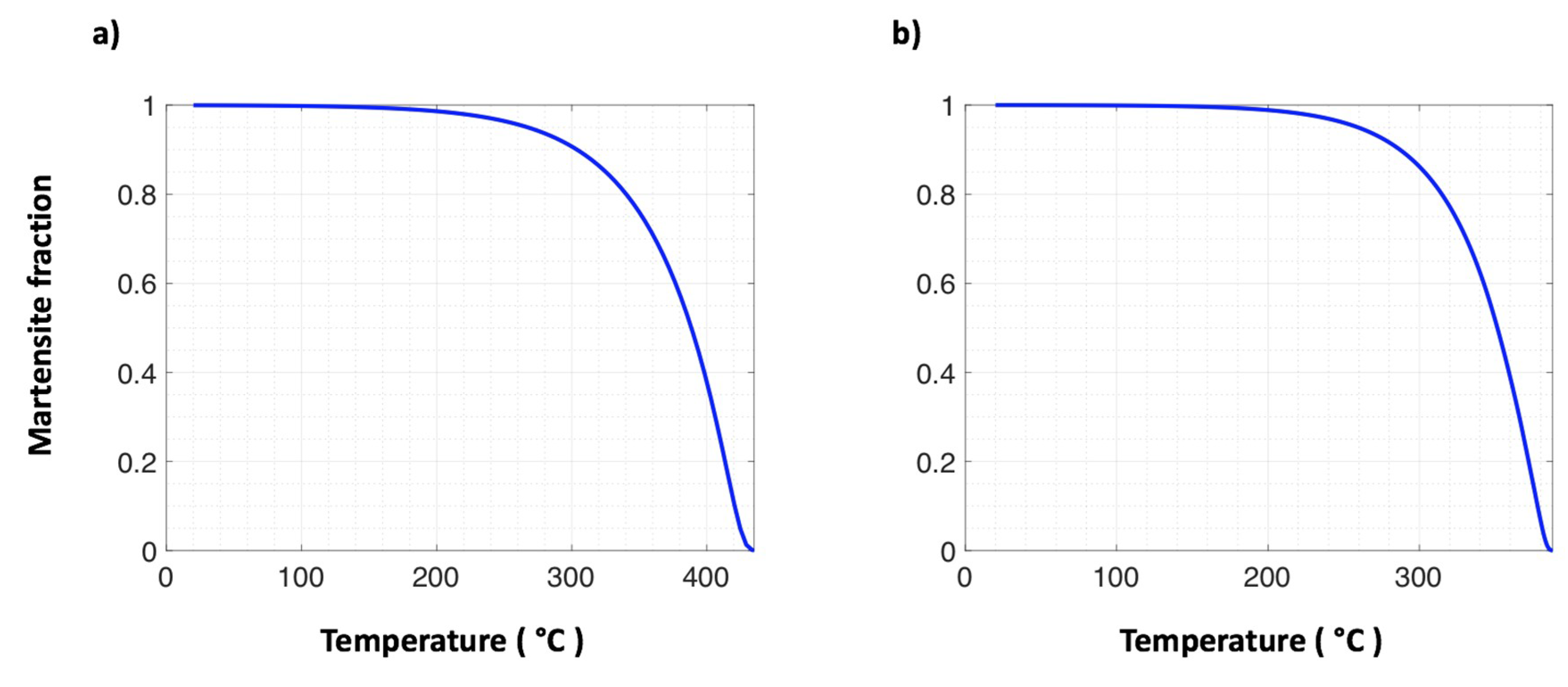
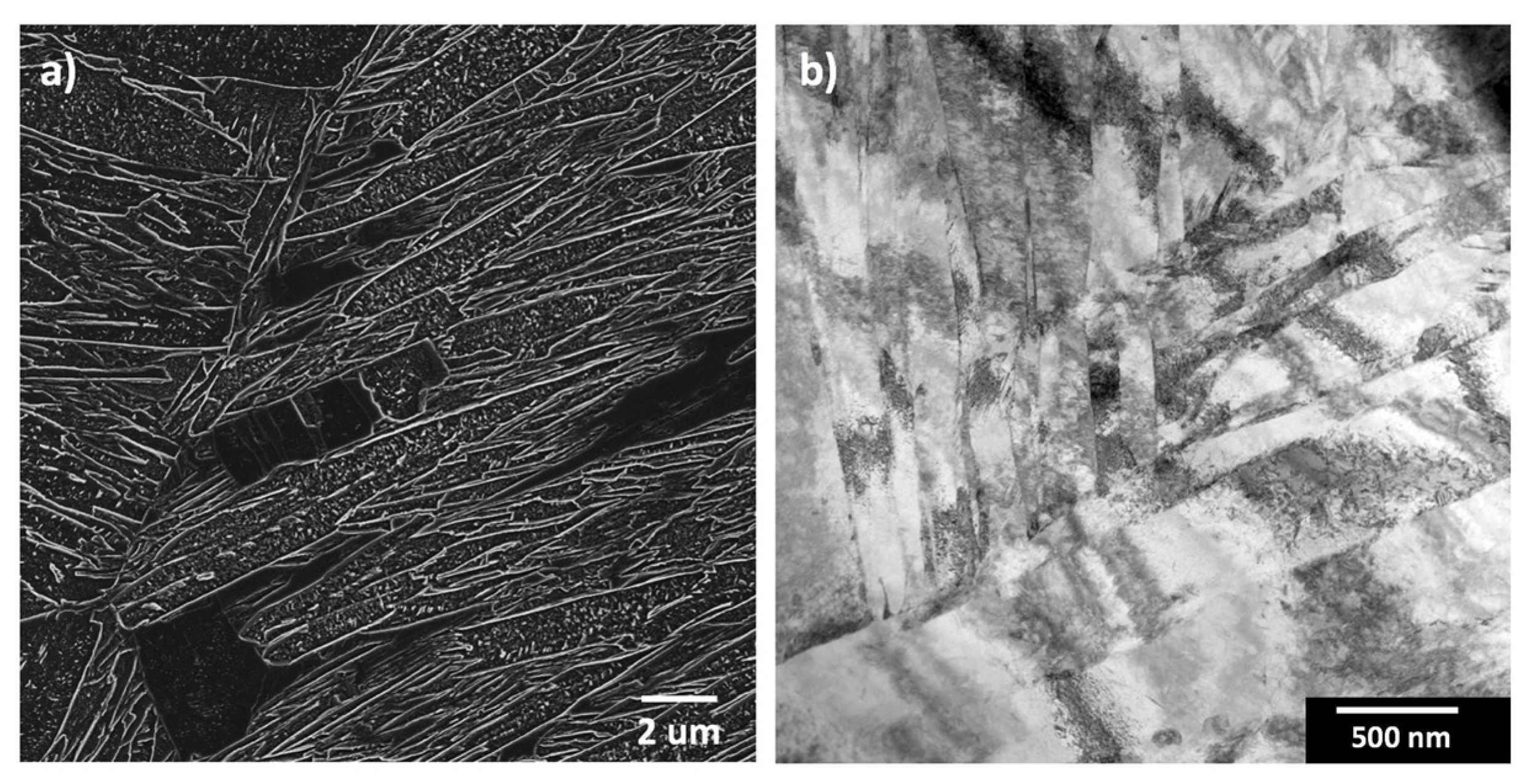
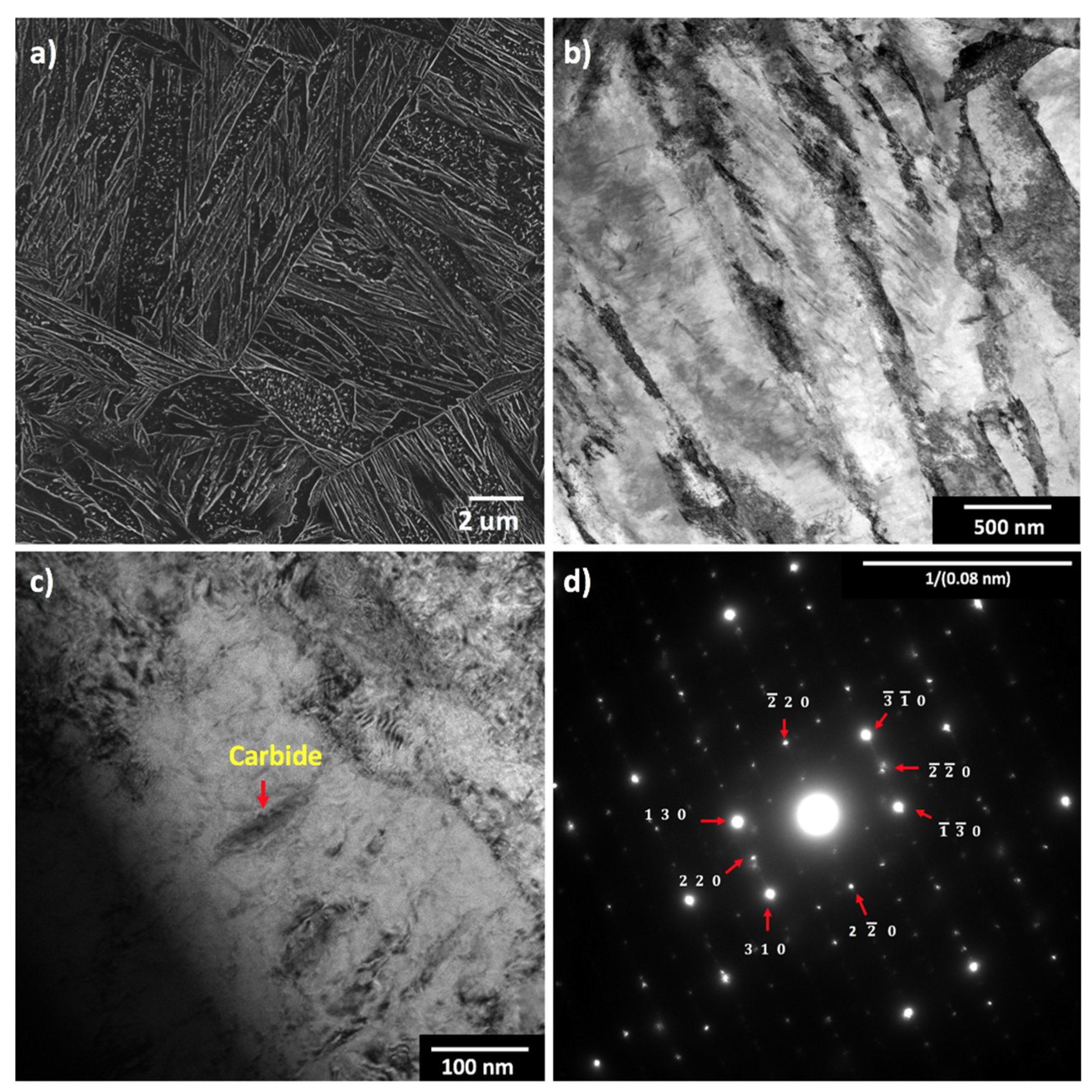
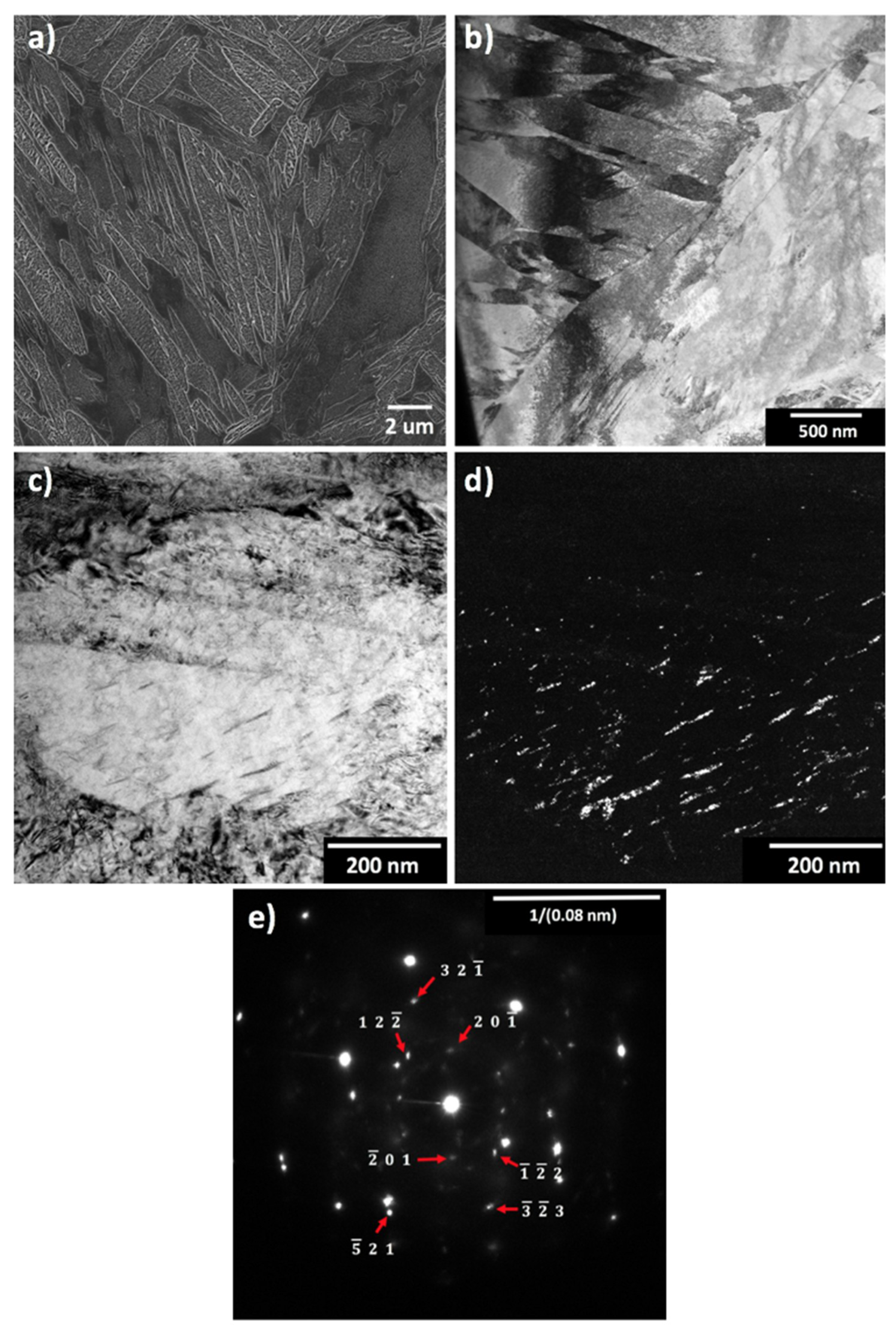
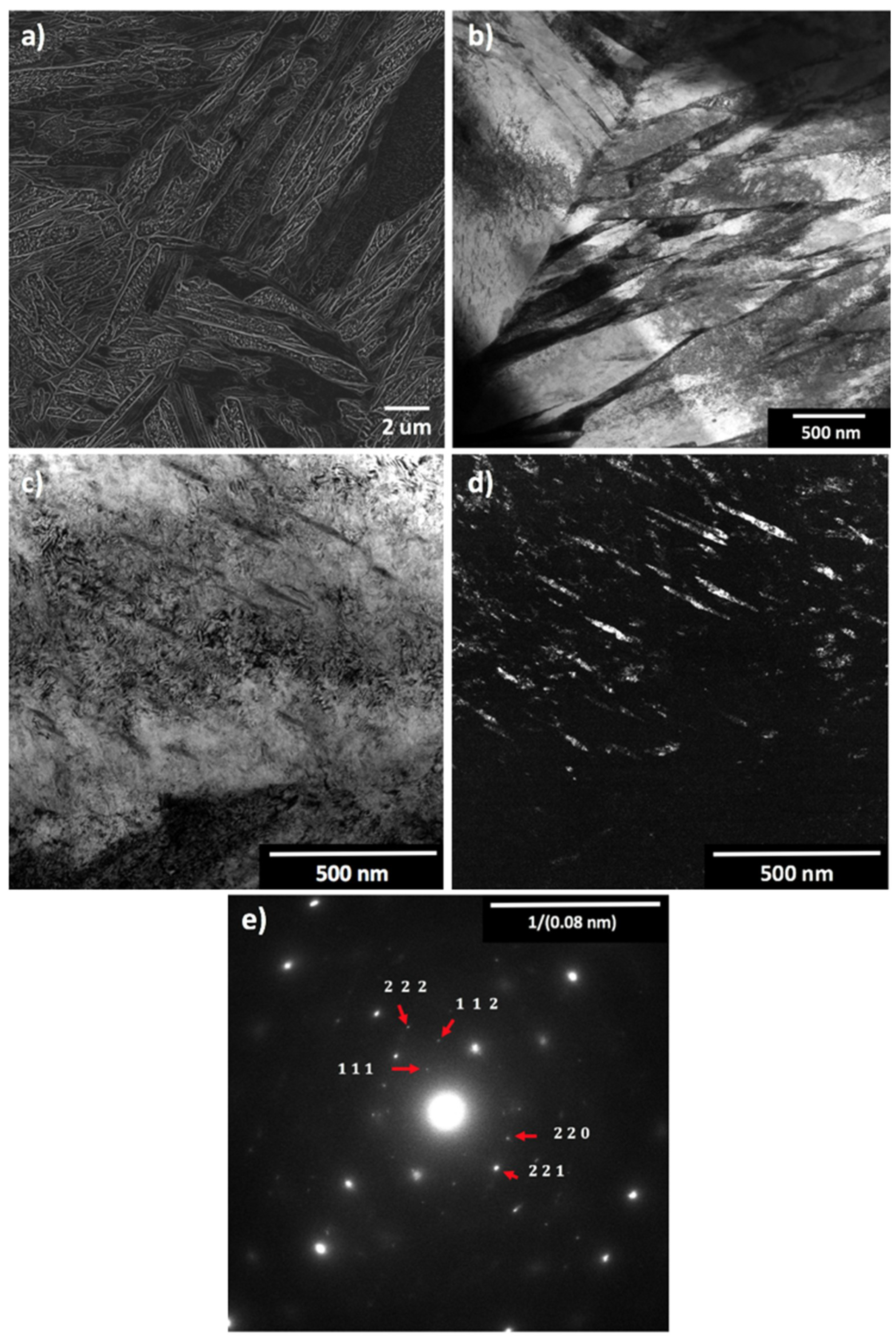
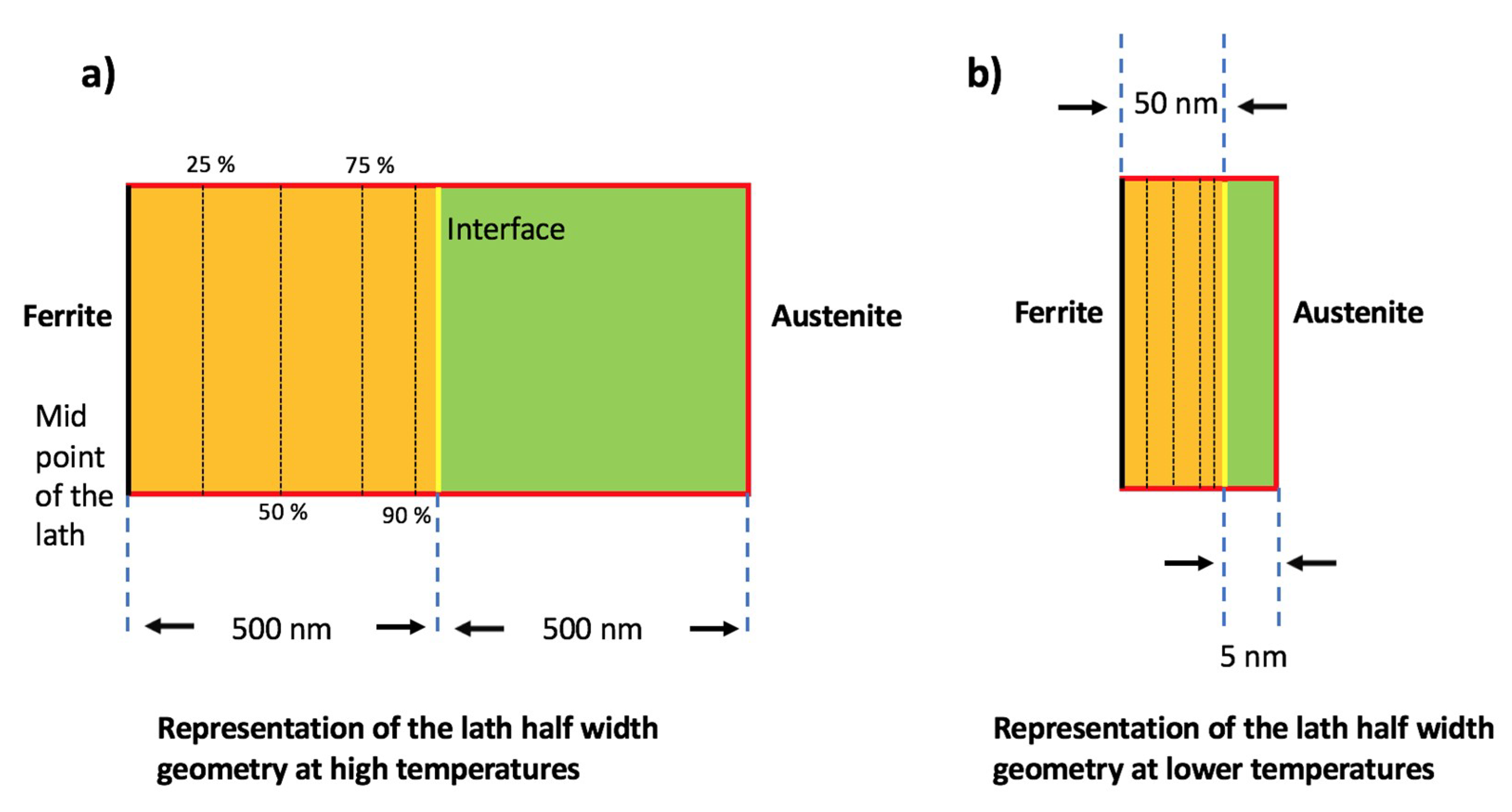
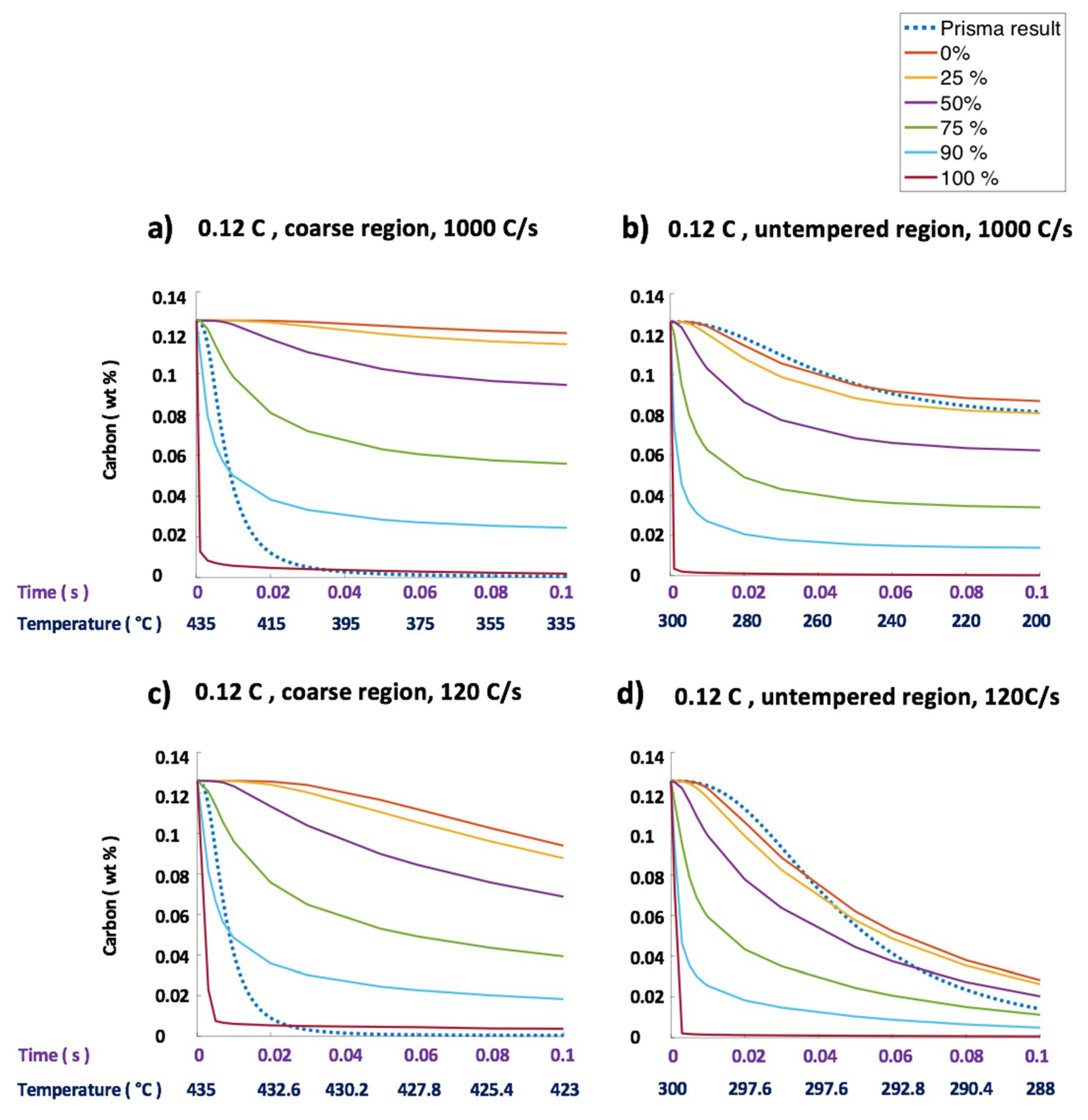

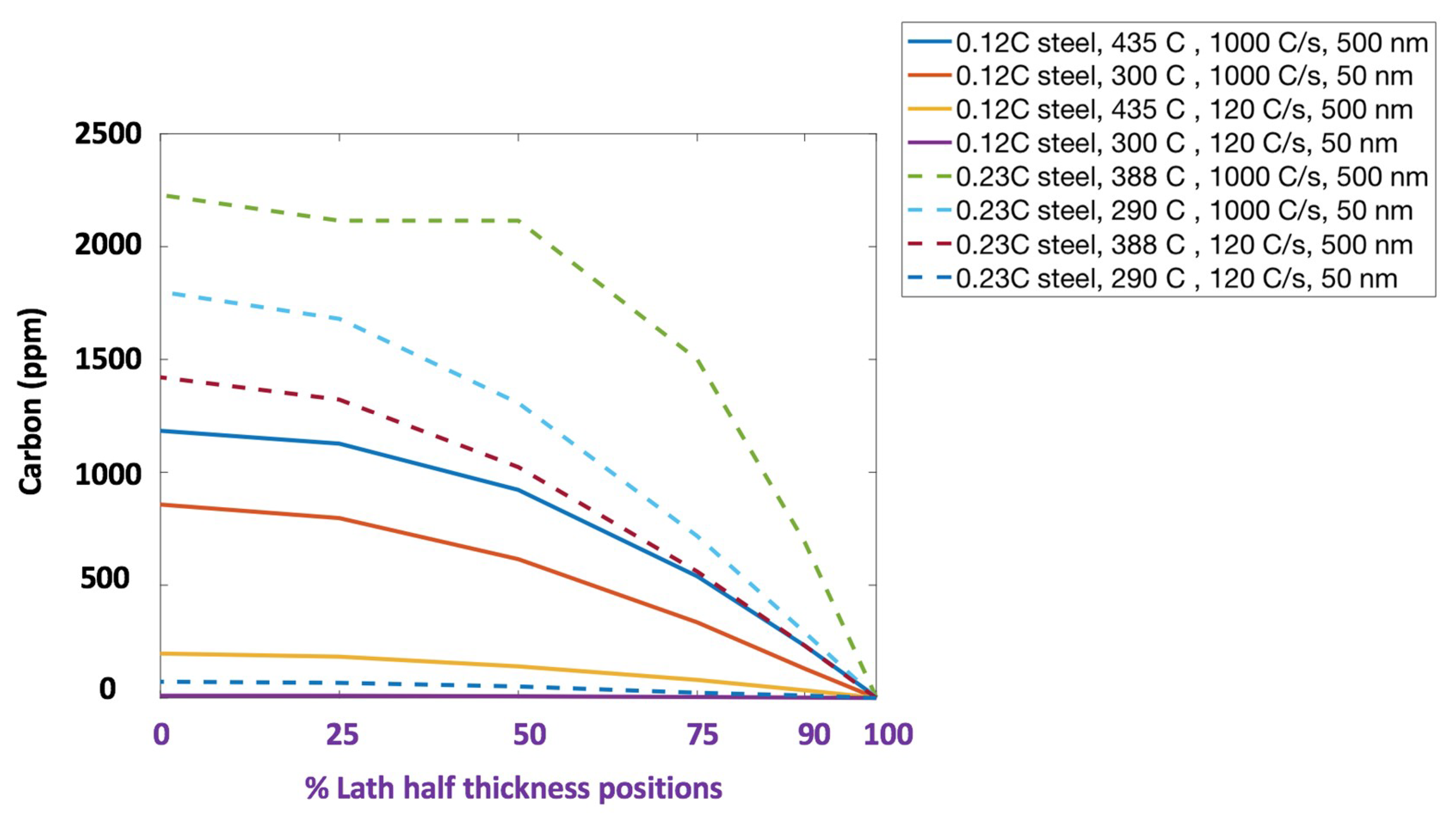
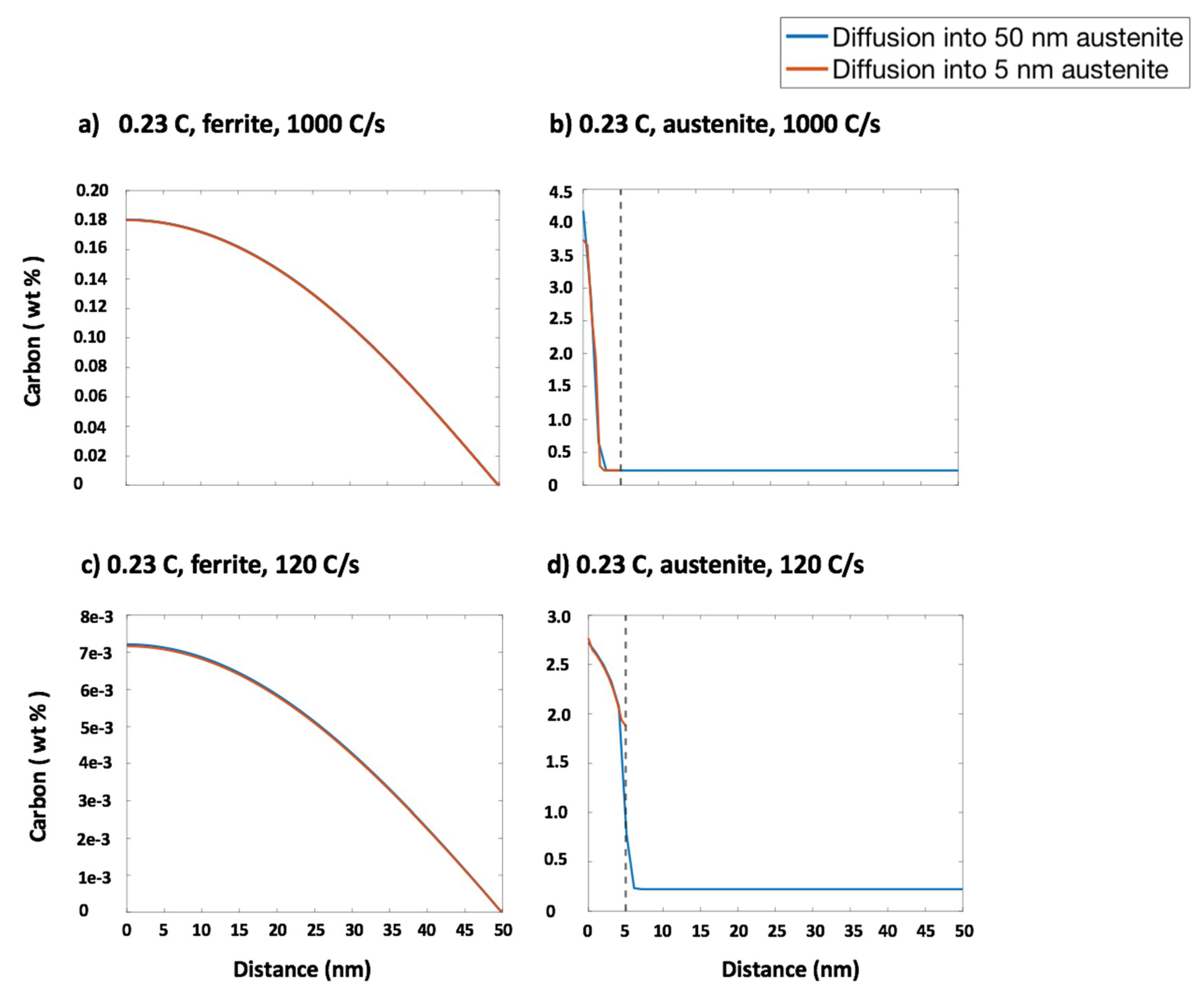
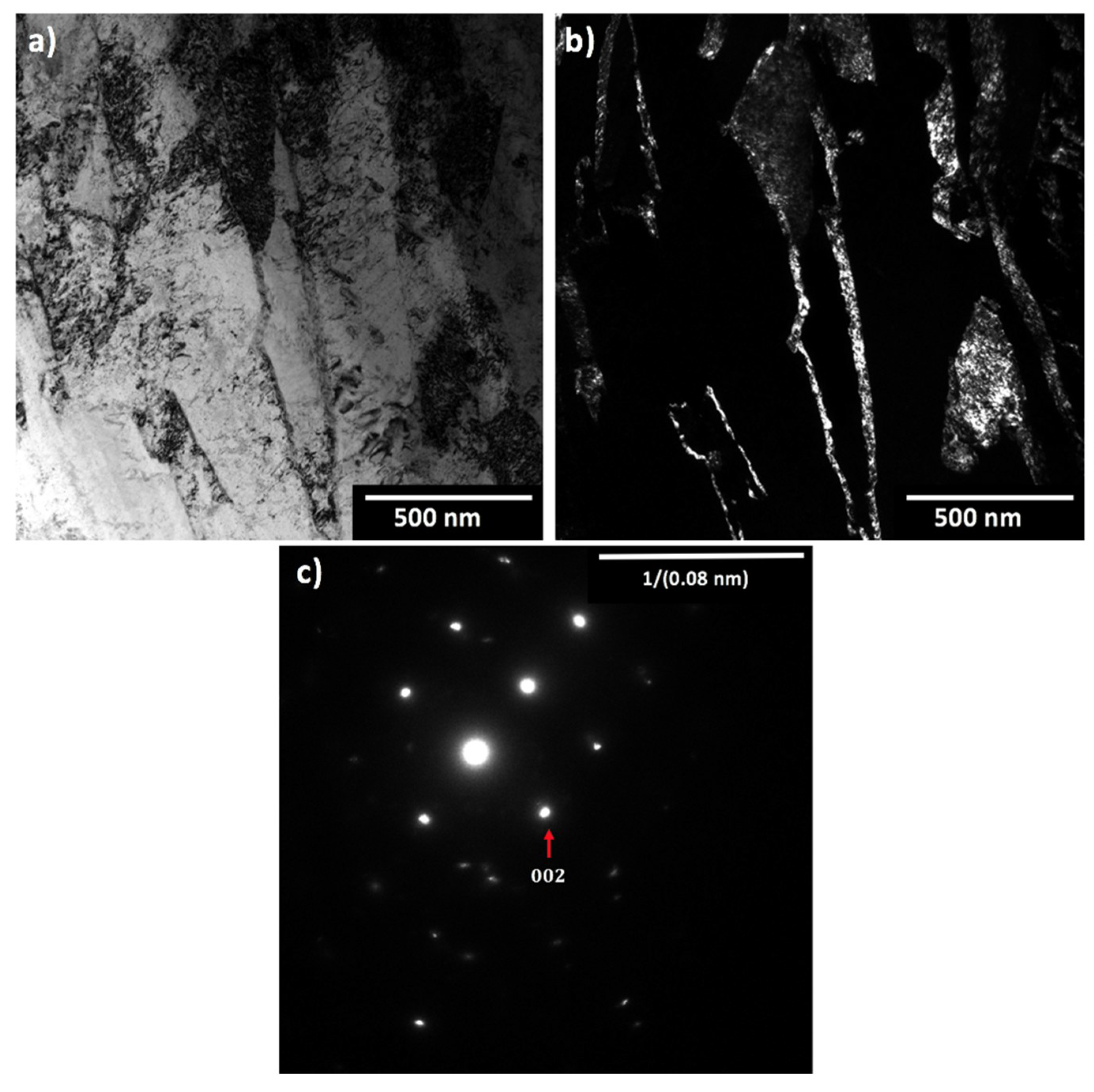

| Steel Code | C | Mn | Si | Cr | Ni | Ti | V | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.12C | 0.126 | 1.66 | 0.72 | 0.27 | 0.038 | 0.027 | 0.047 | 0.054 | Bal. |
| 0.23C | 0.223 | 1.26 | 0.42 | 0.25 | 0.044 | 0.019 | 0.043 | 0.058 | Bal. |
| Alloy | 500 nm Ferrite Half-Thickness | 50 nm Ferrite Half-Thickness |
|---|---|---|
| Lath Formation Temperature (°C) | Lath Formation Temperature (°C) | |
| 0.12C steel | 435 | 300 |
| 0.23C steel | 388 | 290 |
| Steel Code | 0.12C | 0.23C | ||
| Lath Formation Temperature, °C | 434 °C | 300 °C | 388 °C | 290 °C |
| 1000 °C/s | 4.3 | n.a. | 0 | 343.5 |
| 120 °C/s | 0 | n.a. | 0 | 0 |
| Steel Code | 0.12C | 0.23C | ||||||
| Cooling rate (C/s) | 120 | 1000 | 120 | 1000 | ||||
| Lath formation temperature (°C) | 435 | 300 | 435 | 300 | 388 | 290 | 388 | 290 |
| Minimum austenite half-width (nm) | 70 | 5 | 35 | 5 | 55 | 5 | 20 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh Babu, S.; Jaskari, M.; Jarvenpää, A.; Davis, T.P.; Kömi, J.; Porter, D. Precipitation Versus Partitioning Kinetics during the Quenching of Low-Carbon Martensitic Steels. Metals 2020, 10, 850. https://doi.org/10.3390/met10070850
Ramesh Babu S, Jaskari M, Jarvenpää A, Davis TP, Kömi J, Porter D. Precipitation Versus Partitioning Kinetics during the Quenching of Low-Carbon Martensitic Steels. Metals. 2020; 10(7):850. https://doi.org/10.3390/met10070850
Chicago/Turabian StyleRamesh Babu, Shashank, Matias Jaskari, Antti Jarvenpää, Thomas Paul Davis, Jukka Kömi, and David Porter. 2020. "Precipitation Versus Partitioning Kinetics during the Quenching of Low-Carbon Martensitic Steels" Metals 10, no. 7: 850. https://doi.org/10.3390/met10070850
APA StyleRamesh Babu, S., Jaskari, M., Jarvenpää, A., Davis, T. P., Kömi, J., & Porter, D. (2020). Precipitation Versus Partitioning Kinetics during the Quenching of Low-Carbon Martensitic Steels. Metals, 10(7), 850. https://doi.org/10.3390/met10070850





