Interfacial Reactions between Mg-40Al and Mg-30Y Master Alloys
Abstract
1. Introduction
2. Analytical Framework for Diffusion
2.1. Growth of Reaction Layers
2.2. Interdiffusion
3. Experimental
4. Results and Discussion
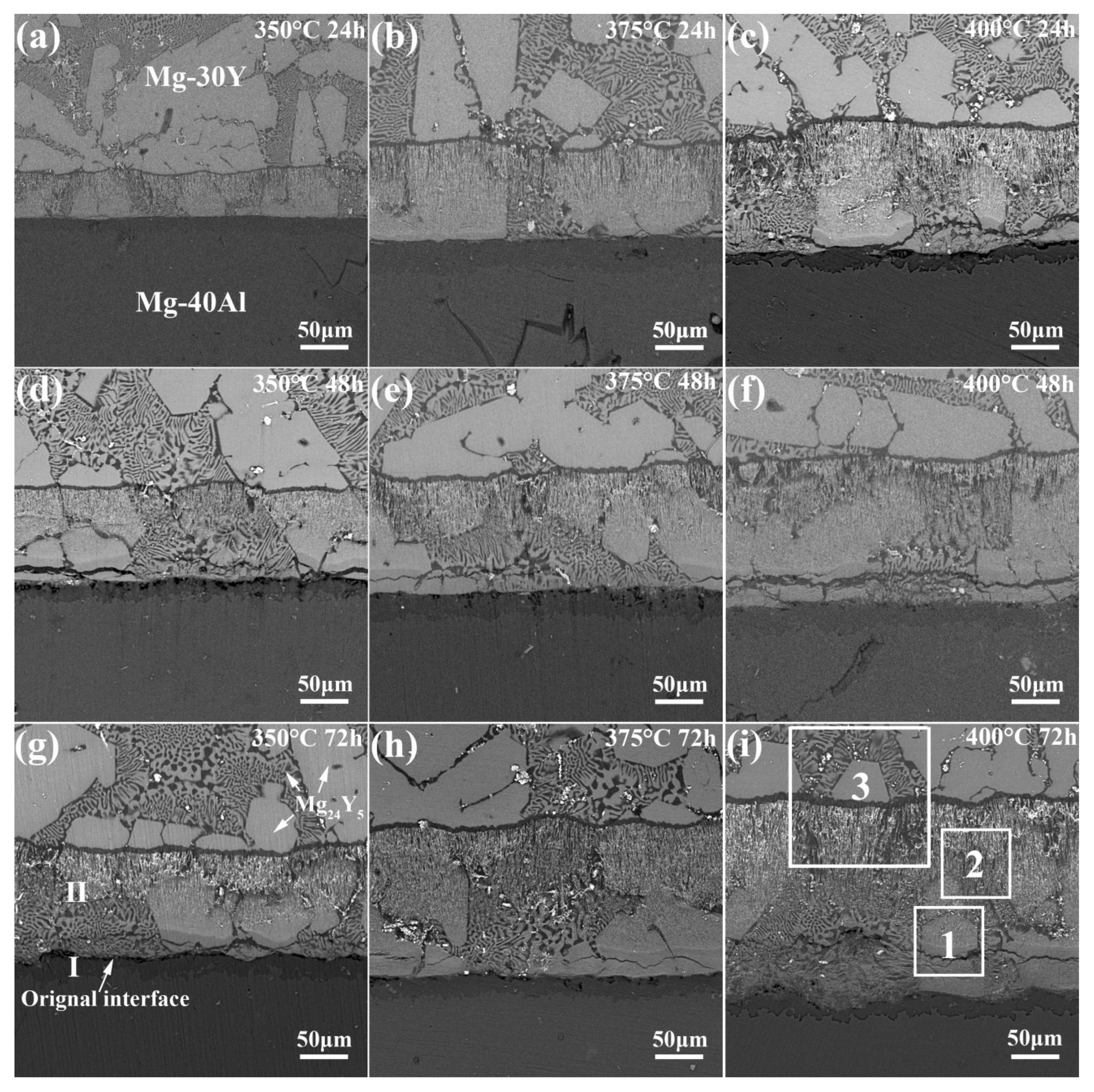
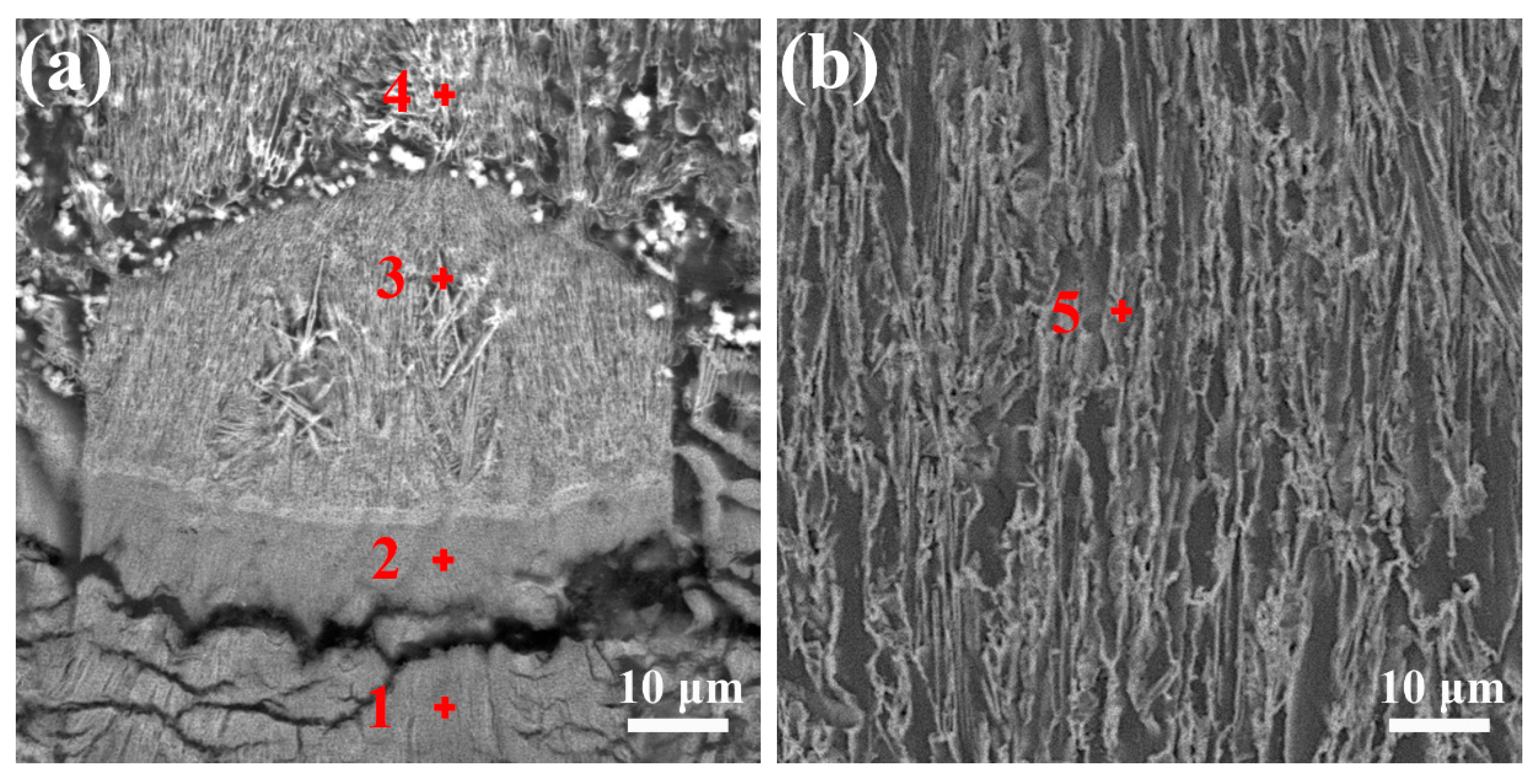
4.1. Interfaces Microstructure
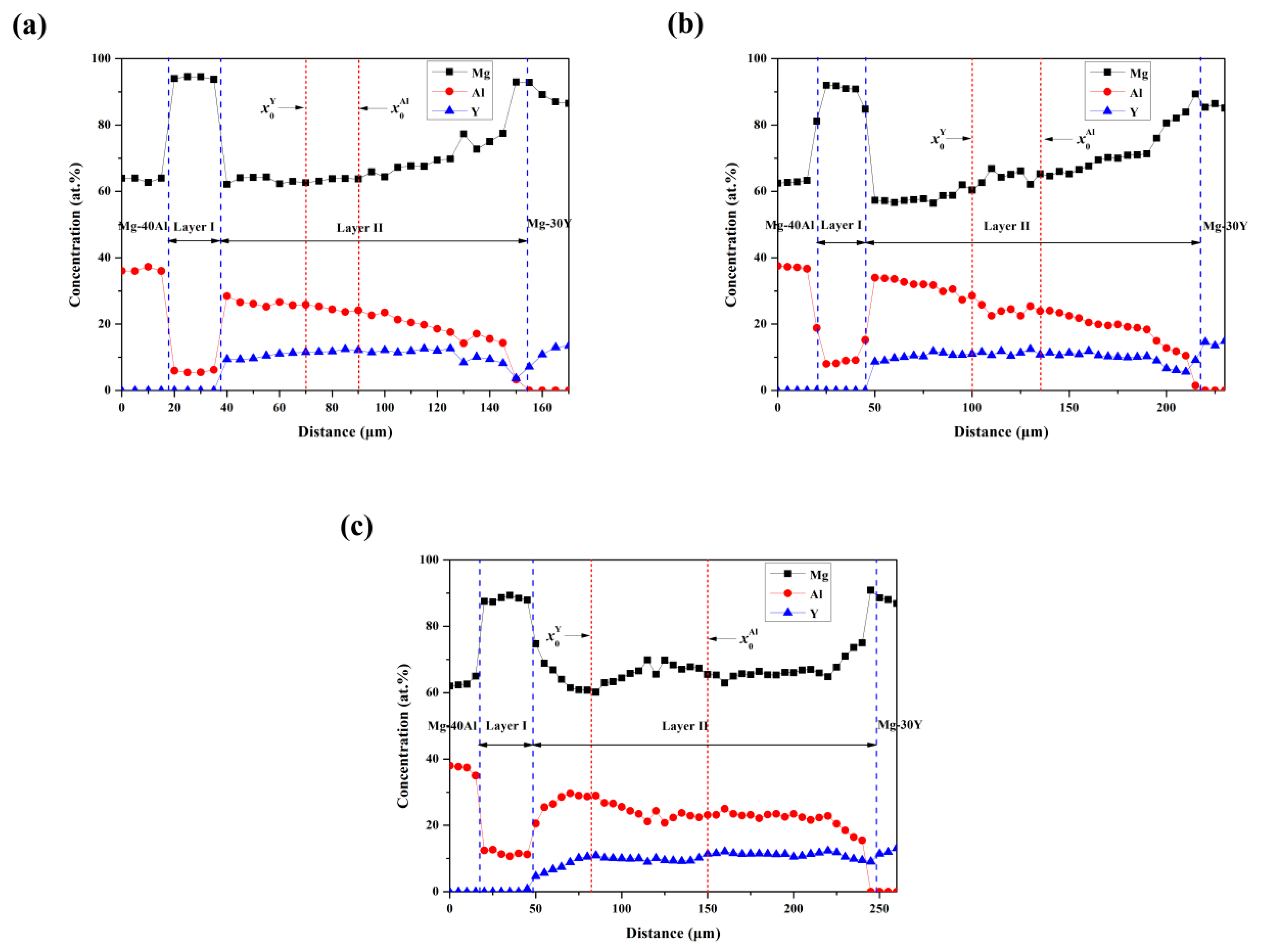
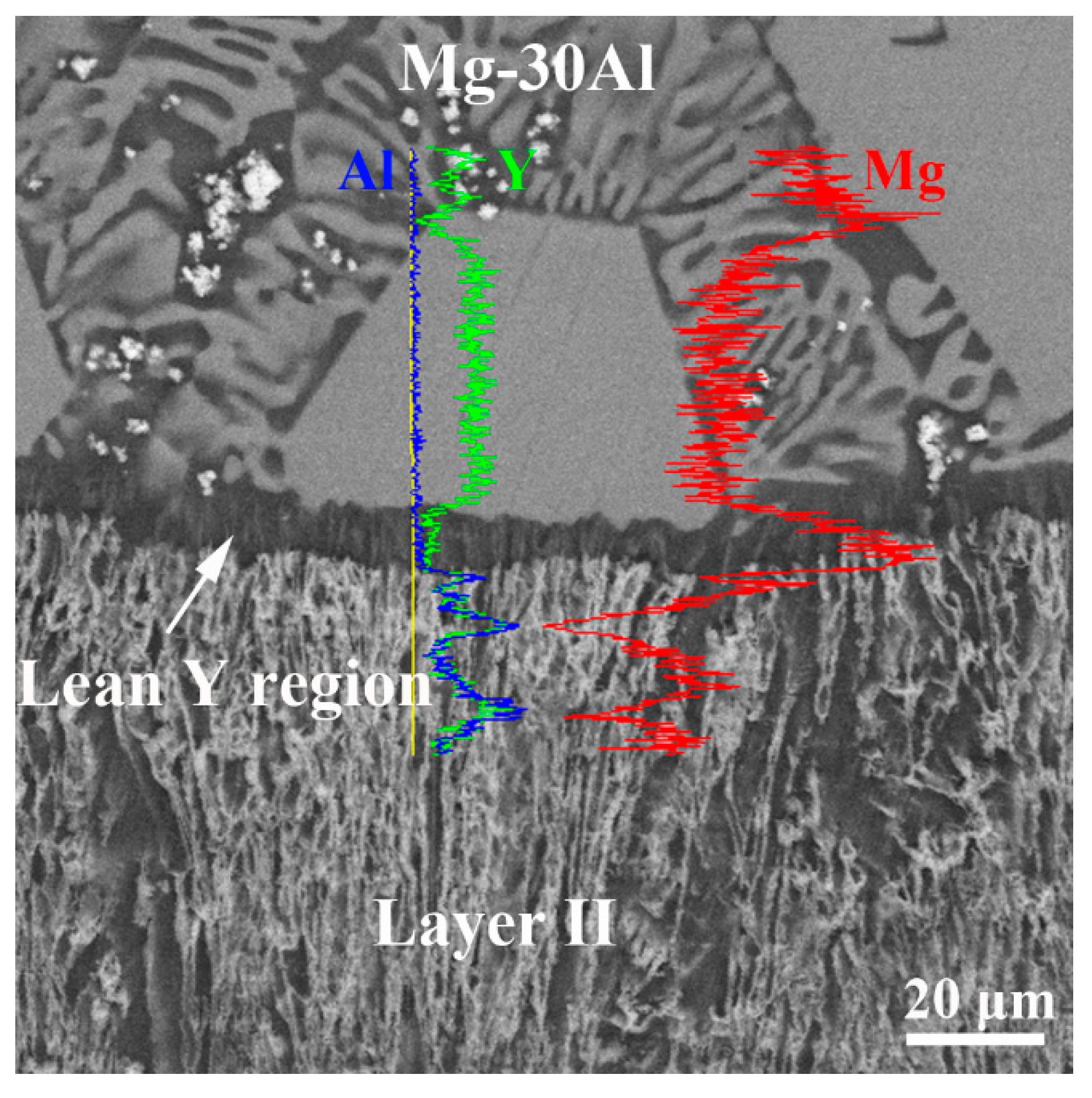
4.2. Concentration Profiles at Interfaces
4.3. Diffusion Path
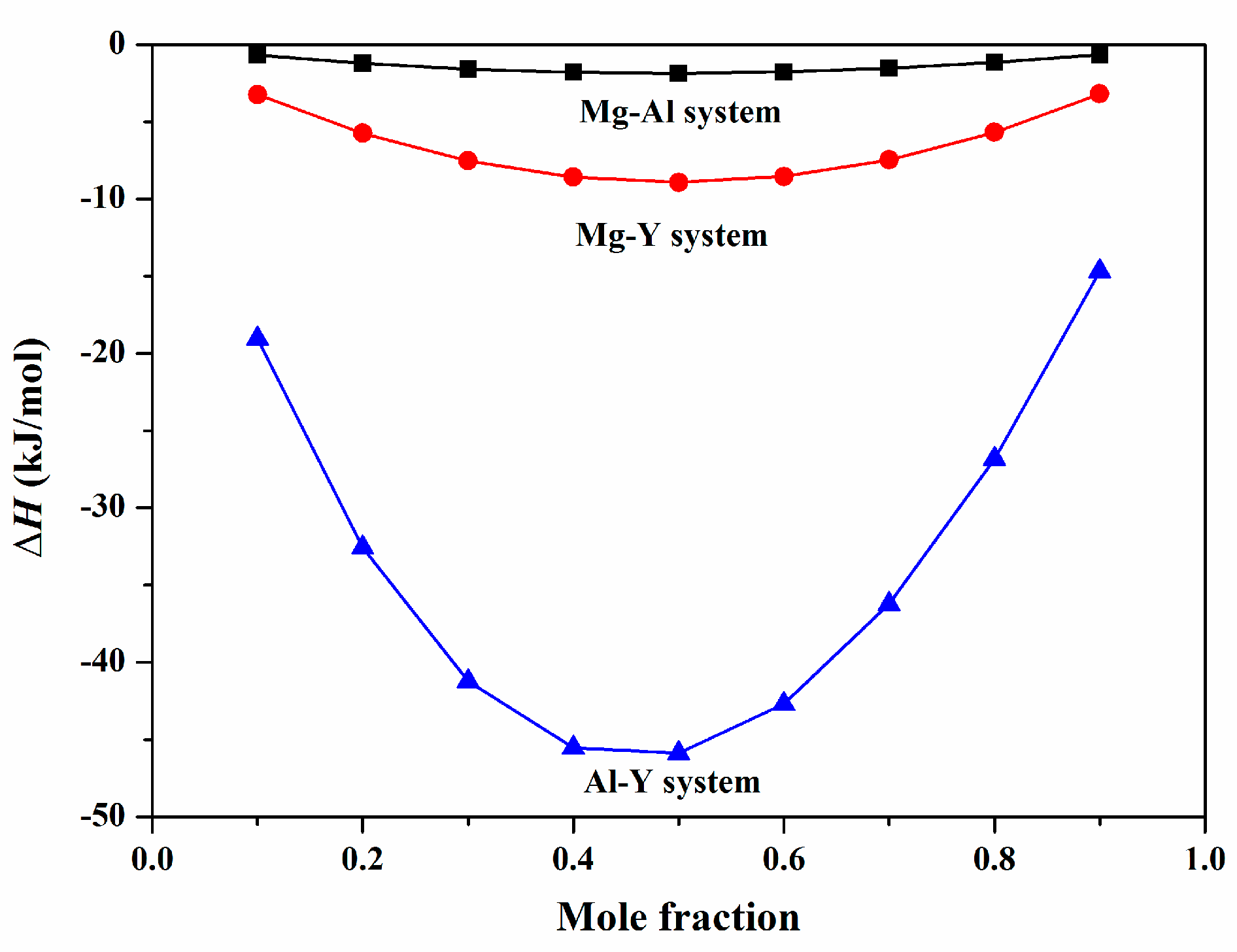
4.4. Thermodynamic Analysis
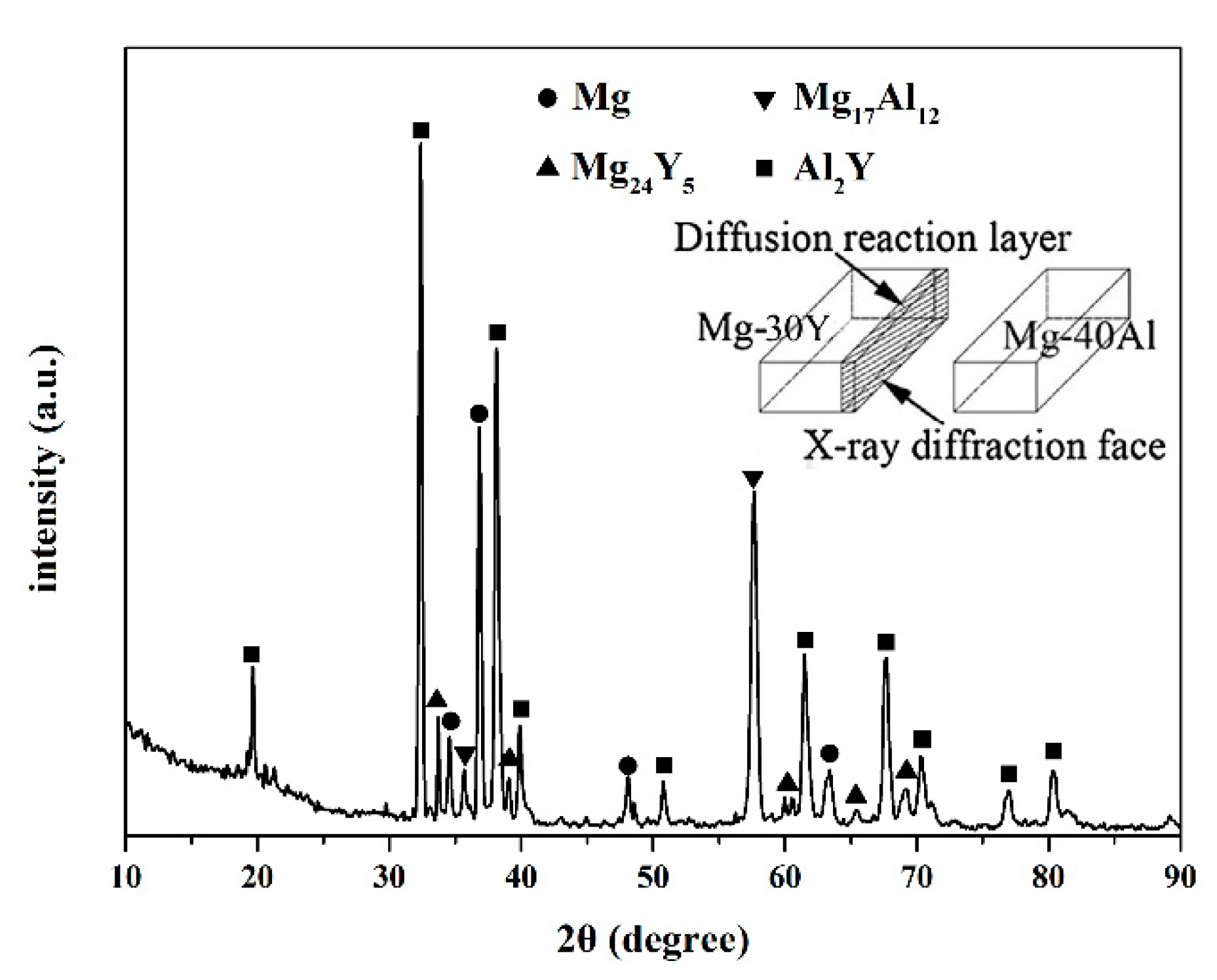
4.5. EDS Characterizing Intermetallic Phases
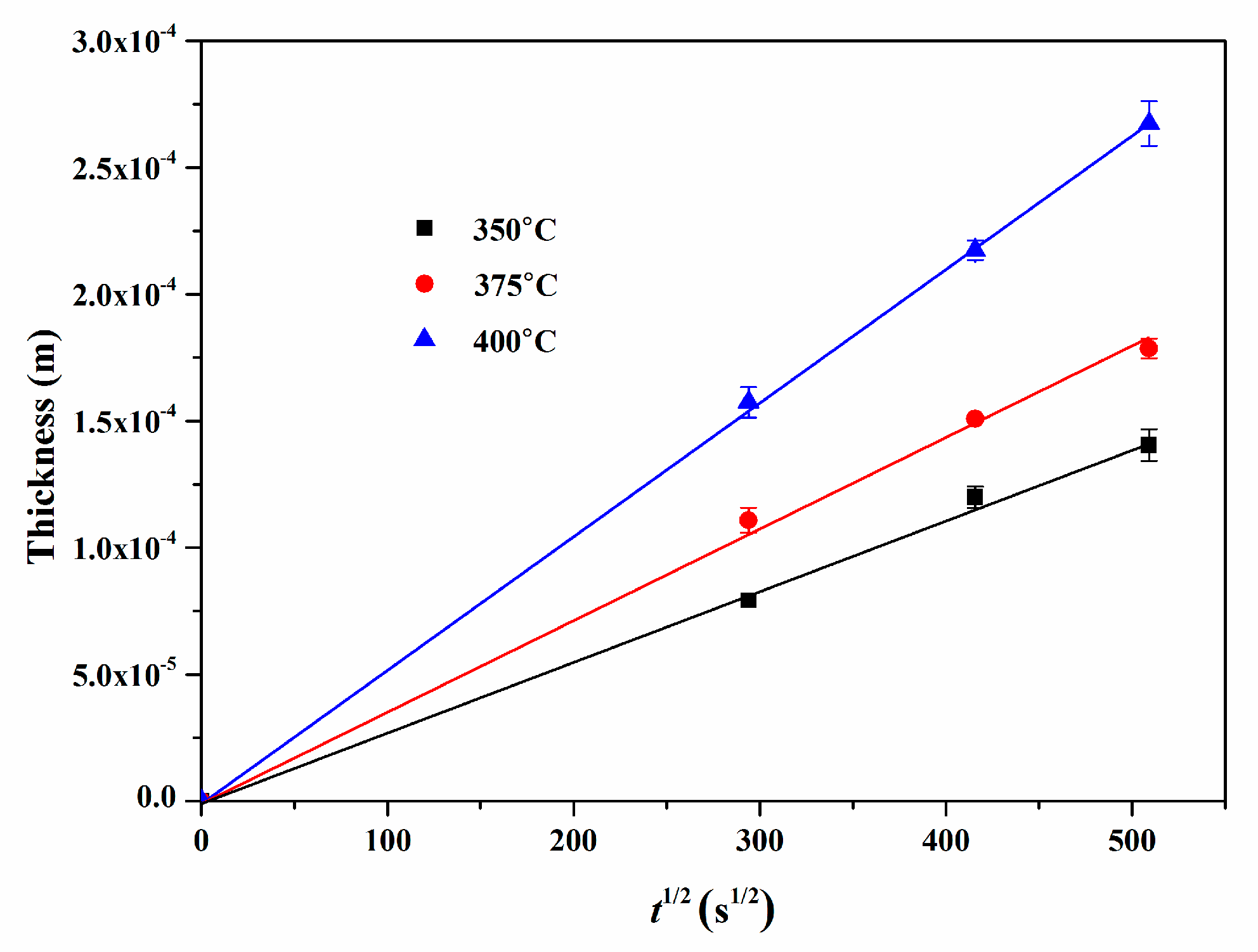
4.6. Growth Kinetics of Reaction Layers
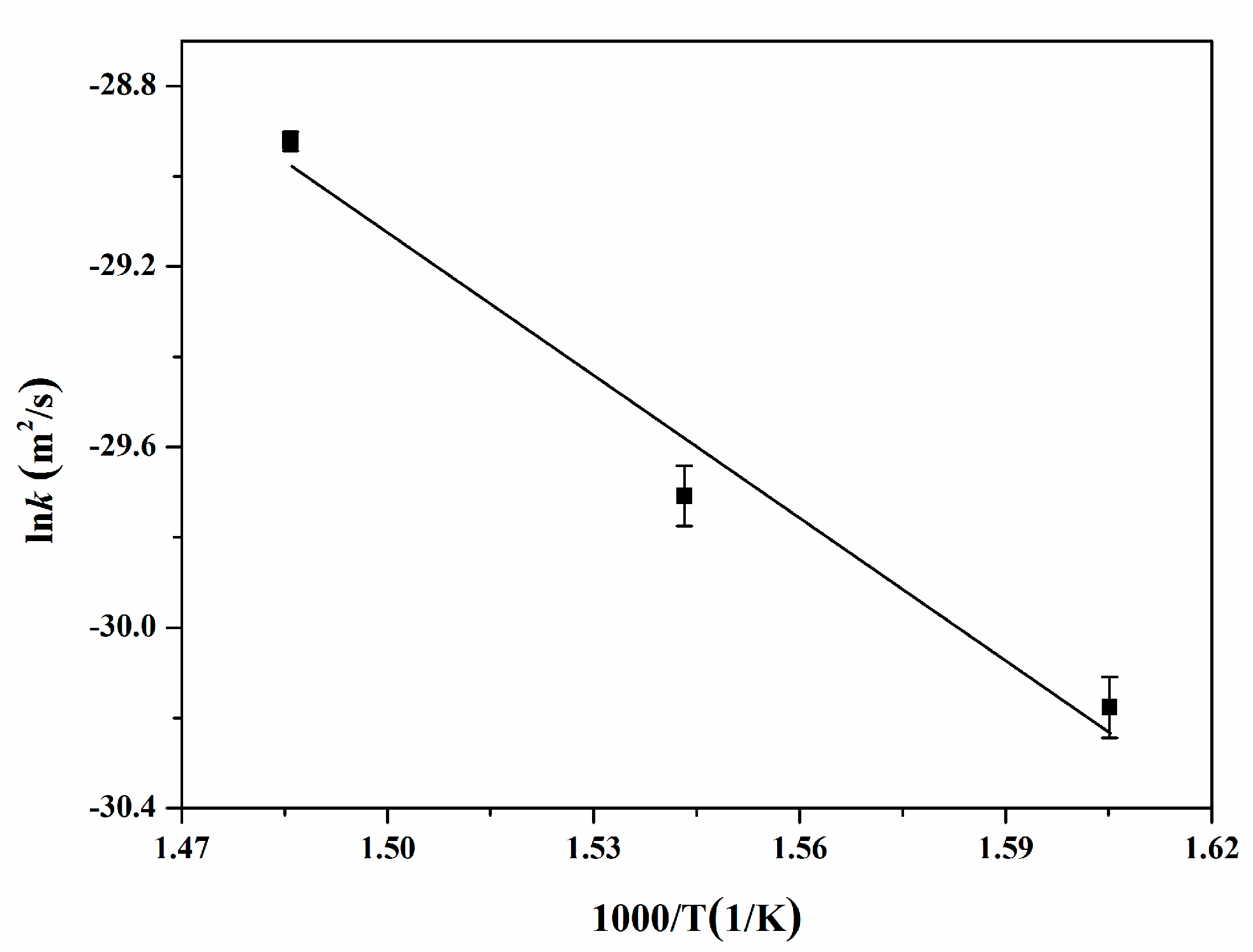
4.7. Interdiffusion Coefficients
5. Conclusions
- Two sub-layers were formed in the diffusion couples at 350–400 °C. Most of the newly formed intermetallic phases in the reaction layer distribute along the morphology of Mg24Y5 before diffusion.
- During the whole interfacial reactions process, Y in Mg-30Y matrix hardly diffused into Mg-40Al matrix, but Al from Mg-40Al matrix diffused into Mg-30Y matrix. The Al-Y intermetallic phases were formed when Al diffused into Mg-30Y matrix. Diffusion path, XRD, thermodynamics analysis and EDS results show that Al2Y is the only newly formed phase at the interface of diffusion couple.
- The growth of reaction layers is controlled by diffusion mechanisms. The diffusion activation energy for the reaction layers is calculated to be (87.09 ± 0.73) kJ/mol. The are higher than in the temperature range of 350–400 °C. The diffusion activation energy of Y is higher than that of Al.
Author Contributions
Funding
Conflicts of Interest
References
- Karakulak, E. A review: Past, present and future of grain refining of magnesium castings. J. Magnes. Alloy. 2019, 7, 355–369. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, M.-X. The nucleation crystallography and wettability of Mg grains on active Al2Y inoculants in an Mg-10 wt% Y Alloy. J. Alloys Compd. 2014, 586, 39–44. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, M.-X.; Kelly, P.M. Crystallography of heterogeneous nucleation of Mg grains on Al2Y nucleation particles in an Mg-10 wt.% Y alloy. Scr. Mater. 2009, 61, 312–315. [Google Scholar] [CrossRef]
- Chang, H.-W.; Qiu, D.; Taylor, J.; Easton, M.; Zhang, M.-X. The role of Al2Y in grain refinement in Mg-Al-Y alloy system. J. Magnes. Alloy. 2013, 1, 115–121. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, J.; Chen, Q.; Jiang, S.; Dai, J.; Jiang, B.; Pan, F. Preparation and Characterization of Magnesium Alloy Containing Al2Y Particles. Materials 2018, 11, 1748. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, H.; Zhou, M.; Quan, G. Effect of Y Addition on the Semi-Solid Microstructure Evolution and the Coarsening Kinetics of SIMA AZ80 Magnesium Alloy. Metals 2017, 7, 416. [Google Scholar] [CrossRef]
- Shakhshir, S.A.; Medraj, M. Computational thermodynamic model for the Mg-Al-Y system. J. Ph. Equilib. Diffus. 2006, 27, 231–244. [Google Scholar] [CrossRef]
- Huang, W.; Yan, H. Calculation of thermodynamic parameters of Mg-Al-Y alloy. J. Wuhan Univ. Technol. 2014, 29, 374–378. [Google Scholar] [CrossRef]
- Drits, M.E.; Padezhnova, E.M.; Dobatkina, T.V. Phase Equilibria in Magnesium-Yttrium-Aluminum Alloys. Metall 1979, 3, 223–227. [Google Scholar]
- Zarechnyuk, O.S.; Drits, M.E.; Rykhal, R.M.; Kinzhibalo, V.V. Examination of the Mg-Al-Y System (0-33 at% Y) at 400 °C. Metall 1980, 5, 242–244. [Google Scholar]
- Odinev, K.O.; Ganiev, I.N.; Kinzhibalo, V.V.; Kurbanov, K.K. Phase Equilibria in Aluminum-Magnesium-Yttrium and Aluminum-Magnesium-Cerium Systems at 673 K. Tsvetn. Metall 1989, 4, 75–77. [Google Scholar]
- Das, S.K.; Kim, Y.; Ha, T.; Gauvin, R.; Jung, I.-H. Anisotropic Diffusion Behavior of Al in Mg: Diffusion Couple Study Using Mg Single Crystal. Metall. Mater. Trans. A 2013, 44, 2539–2547. [Google Scholar] [CrossRef]
- Das, S.K.; Kang, Y.-B.; Ha, T.; Jung, I.-H. Thermodynamic modeling and diffusion kinetic experiments of binary Mg-Gd and Mg-Y systems. Acta Mater. 2014, 71, 164–175. [Google Scholar] [CrossRef]
- Dayananda, M.A. An analysis of concentration profiles for fluxes, diffusion depths, and zero-flux planes in multicomponent diffusion. Metall. Mater. Trans. A 1983, 14, 1851–1858. [Google Scholar] [CrossRef]
- Dayananda, M.A. Average effective interdiffusion coefficients and the Matano plane composition. Metall. Mater. Trans. A 1996, 27, 2504–2509. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, B.; Li, X.; Yang, Q.; Dong, H.; Xia, X.; Pan, F. The formation of intermetallic phases during interdiffusion of Mg-Al/Mg-Ce diffusion couples. J. Alloys Compd. 2015, 619, 411–416. [Google Scholar] [CrossRef]
- Dai, J.; Shen, S.; Jiang, B.; Zhang, J.; Yang, Q.; Jiang, Z.; Dong, H.; Pan, F. Interfacial reaction in (Mg-37.5Al)/(Mg-6.7Nd) diffusion couples. Met. Mater. Int. 2016, 22, 1–6. [Google Scholar] [CrossRef]
- Dai, J.; Xiao, H.; Jiang, B.; Xie, H.; Peng, C.; Jiang, Z.; Zou, Q.; Yang, Q.; Pan, F. Diffusion behavior and reactions between Al and Ca in Mg alloys by diffusion couples. J. Mater. Sci. Technol. 2018, 34, 291–298. [Google Scholar] [CrossRef]
- Kirkaldy, J.S.; Brown, L. Diffusion behaviour in ternary, multiphase systems. Can. Metall. Quart. 1963, 2, 89–115. [Google Scholar] [CrossRef]
- Morral, J.E. Diffusion path theorems for ternary diffusion couples. Metall. Mater. Trans. A 2012, 43, 3462–3470. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Mg-Y (aluminum-magnesium-yttrium). J. Ph. Equilib. Diff. 2007, 28, 477–479. [Google Scholar] [CrossRef]
- De Boer, F.R.; Boom, R.; Mattens, W.C.M.; Miedema, A.R.; Niessen, A.K. Cohesion in Metals: Transition Metal Alloys; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Miedema, A.; De Chatel, P.; De Boer, F. Cohesion in alloys-fundamentals of a semi-empirical model. Physica B + C 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Liu, S.; Du, Y.; Chen, H. A thermodynamic reassessment of the Al-Y system. Calphad 2006, 30, 334–340. [Google Scholar] [CrossRef]
- Ran, Q.; Lukas, H.L.; Effenberg, G.; Petzow, G. A thermodynamic optimization of the Al-Y system. J. Less Common Met. 1989, 146, 213–222. [Google Scholar] [CrossRef]
- Peng, Z.Z.; Shao, X.H.; Guo, X.W.; Wang, J.; Ma, X.L. Atomic-Scale Insight into Structure and Interface of Al2Y Phase in an Mg-Al-Y Alloy. Adv. Eng. Mater. 2018, 20. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, B.; Yang, H.; Yang, Q.; Xia, X.; Pan, F. High temperature oxidation behavior of Mg-Y-Sn, Mg-Y, Mg-Sn alloys and its effect on corrosion property. Appl. Surf. Sci. 2015, 353, 1013–1022. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, B.; He, J.; Liu, B.; Jiang, Z.; Pan, F. Effect of Zn addition on the oxidation property of Mg-Y alloy at high temperatures. J. Alloys Compd. 2016, 687, 252–262. [Google Scholar] [CrossRef]
- Brennan, S.; Bermudez, K.; Kulkarni, N.S.; Sohn, Y. Interdiffusion in the Mg-Al System and Intrinsic Diffusion in β-Mg2Al3. Metall. Mater. Trans. A 2012, 43, 4043–4052. [Google Scholar] [CrossRef]


| Intermetallic Phase | a (J mol−1) | b (J (mol K)−1) |
|---|---|---|
| Al3Y | −39,727.972 | 8.036 |
| Al2Y | −50,410.046 | 10.230 |
| AlY | −48,074.303 | 11.536 |
| Al2Y3 | −45,347.395 | 12.364 |
| AlY2 | −38,200.000 | 10.568 |
| Point | Mg (at.%) | Al (at.%) | Y (at.%) | Corresponding Phase |
|---|---|---|---|---|
| 1 | 74.71 | 20.56 | 4.72 | Al2Y + γ + (Mg) |
| 2 | 66.87 | 26.42 | 6.71 | Al2Y + γ + (Mg) |
| 3 | 60.78 | 28.68 | 10.54 | Al2Y + (Mg) |
| 4 | 62.91 | 25 | 12.09 | Al2Y + (Mg) |
| 5 | 65.7 | 22.95 | 11.35 | Al2Y + (Mg) |
| T (°C) | k (m2 s−1) | k0 (m2 s−1) | Q (kJ mol−1) |
|---|---|---|---|
| 350 | (7.84 ± 0.13) × 10−14 | (1.48 ± 0.10) × 10−6 | 87.09 ± 0.73 |
| 375 | (1.25 ± 0.08) × 10−13 | ||
| 400 | (2.75 ± 0.05) × 10−13 |
| i | T (°C) | (m2 s−1) | ||
|---|---|---|---|---|
| Al | 350 | 1.44 × 10−14 | 2.20 × 10−6 | 97.61 |
| 375 | 2.98 × 10−14 | |||
| 400 | 5.83 × 10−14 | |||
| Y | 350 | 5.13 × 10−15 | 2.40 × 10−5 | 115.29 |
| 375 | 1.24 × 10−14 | |||
| 400 | 2.68 × 10−14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Jiang, B.; Xie, H.; Yang, Q. Interfacial Reactions between Mg-40Al and Mg-30Y Master Alloys. Metals 2020, 10, 825. https://doi.org/10.3390/met10060825
Dai J, Jiang B, Xie H, Yang Q. Interfacial Reactions between Mg-40Al and Mg-30Y Master Alloys. Metals. 2020; 10(6):825. https://doi.org/10.3390/met10060825
Chicago/Turabian StyleDai, Jiahong, Bin Jiang, Hongmei Xie, and Qingshan Yang. 2020. "Interfacial Reactions between Mg-40Al and Mg-30Y Master Alloys" Metals 10, no. 6: 825. https://doi.org/10.3390/met10060825
APA StyleDai, J., Jiang, B., Xie, H., & Yang, Q. (2020). Interfacial Reactions between Mg-40Al and Mg-30Y Master Alloys. Metals, 10(6), 825. https://doi.org/10.3390/met10060825




