Stress Corrosion and Corrosion Fatigue of Biodegradable Mg-Zn-Nd-Y-Zr Alloy in In-Vitro Conditions
Abstract
1. Introduction
2. Materials and Methods
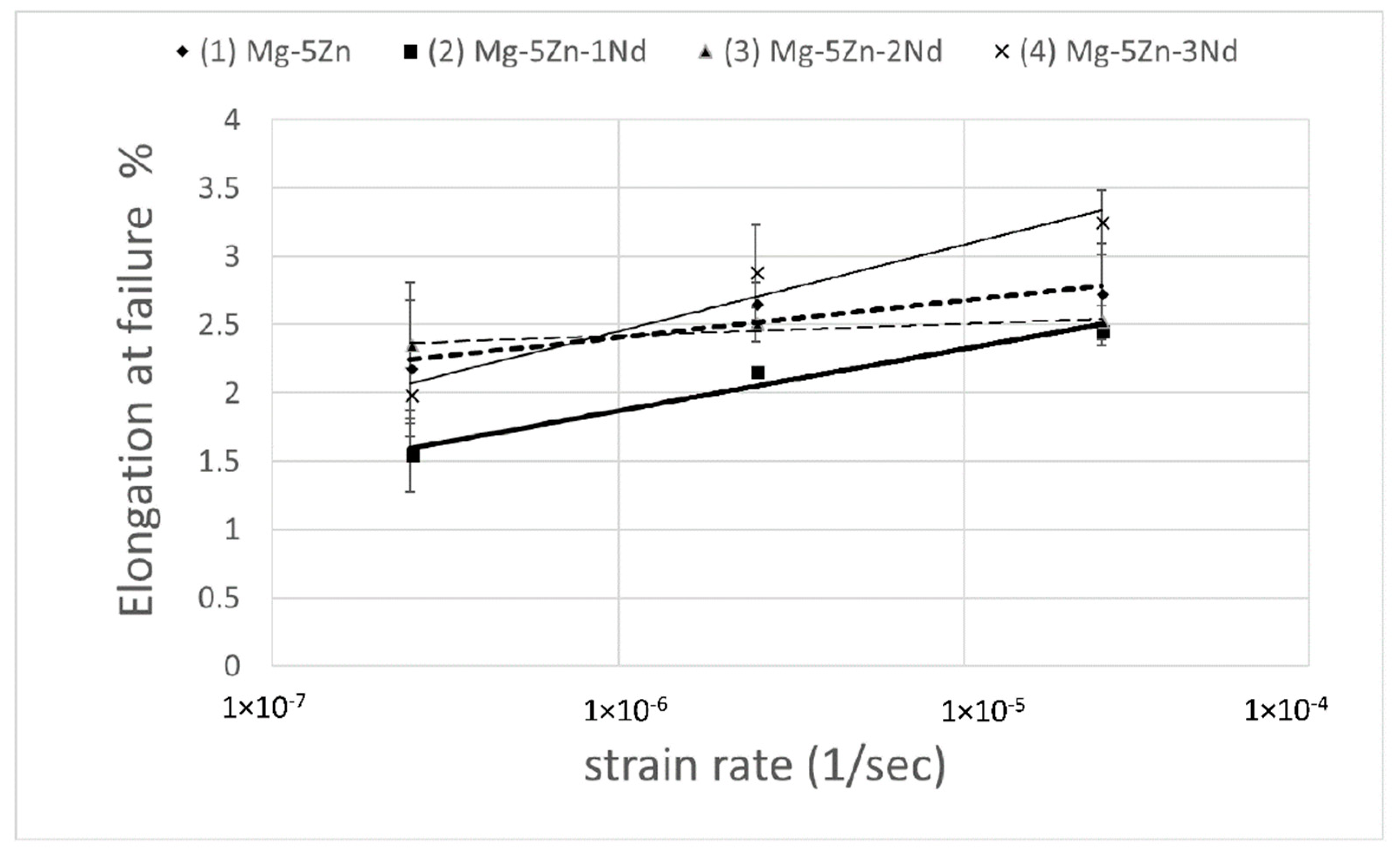
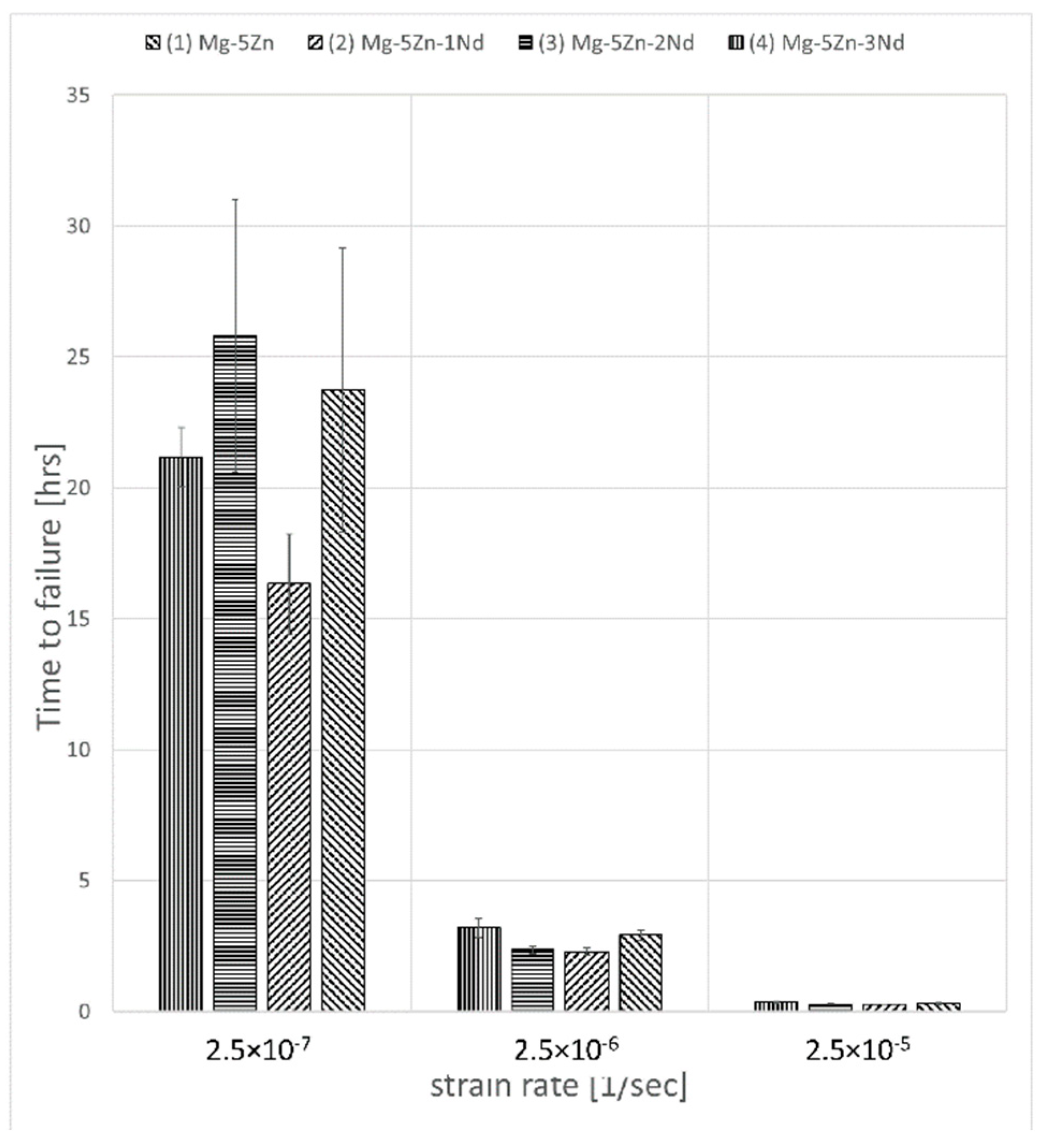
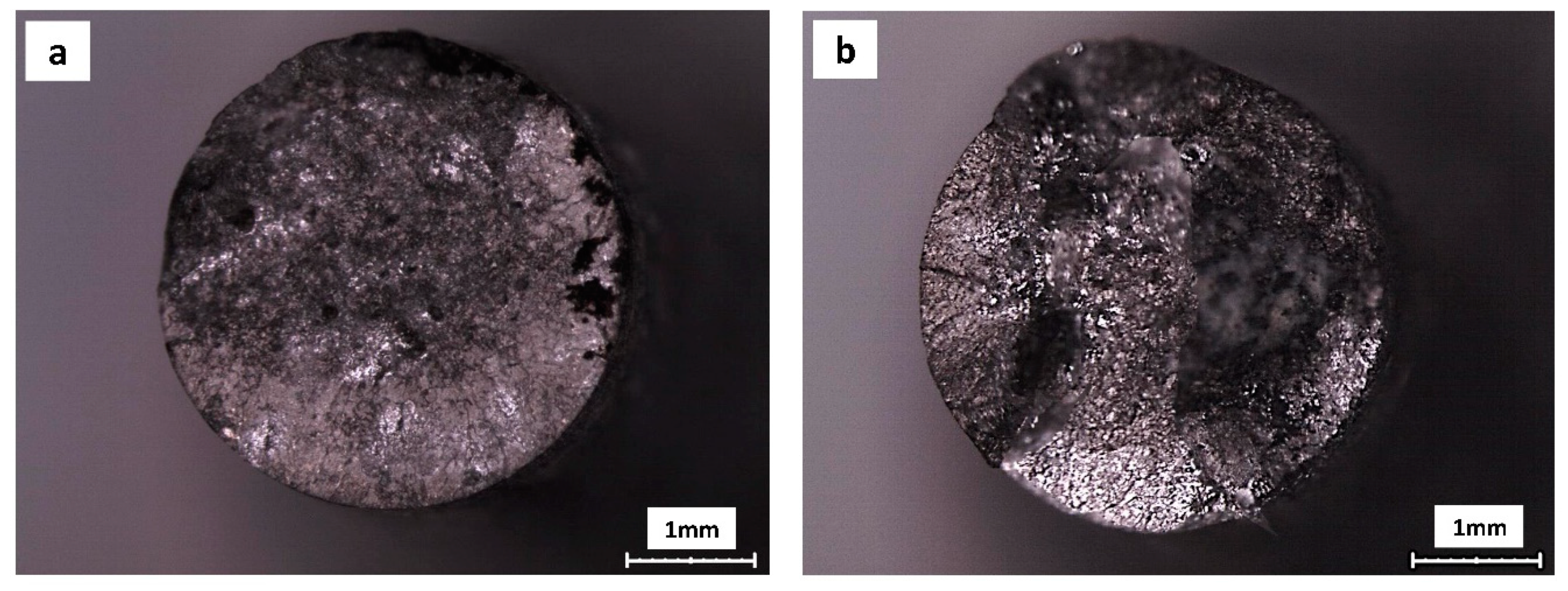
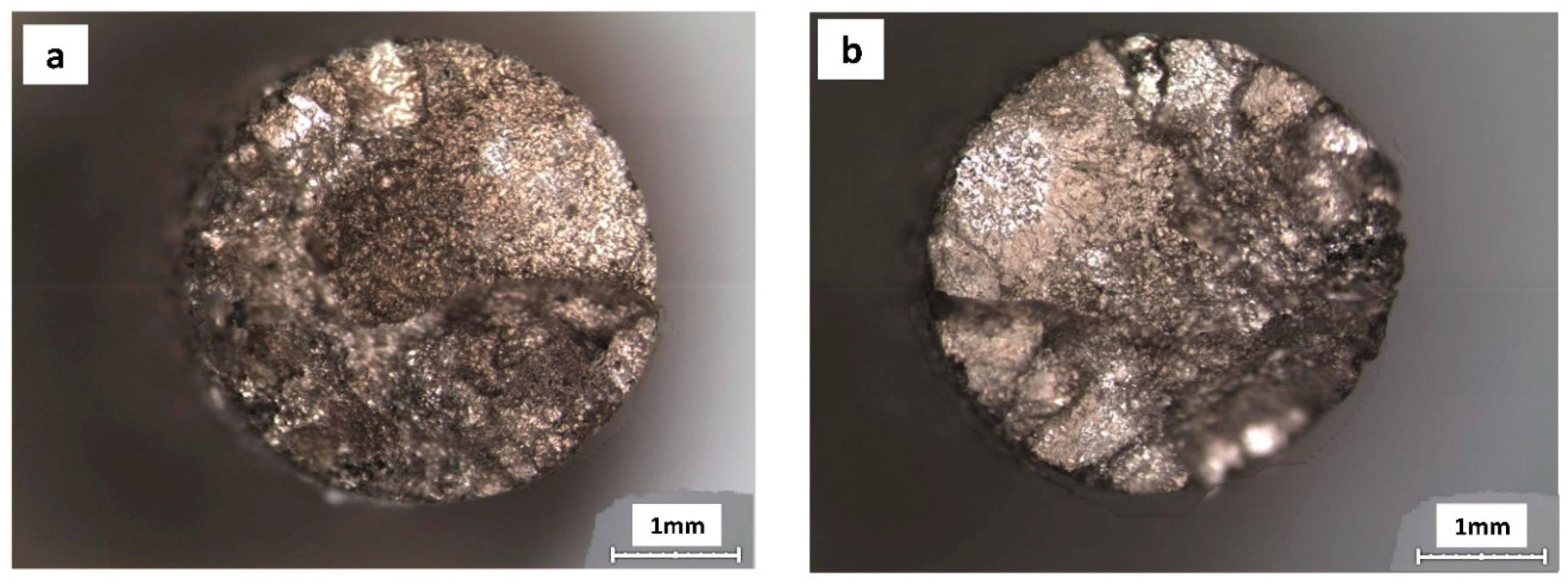
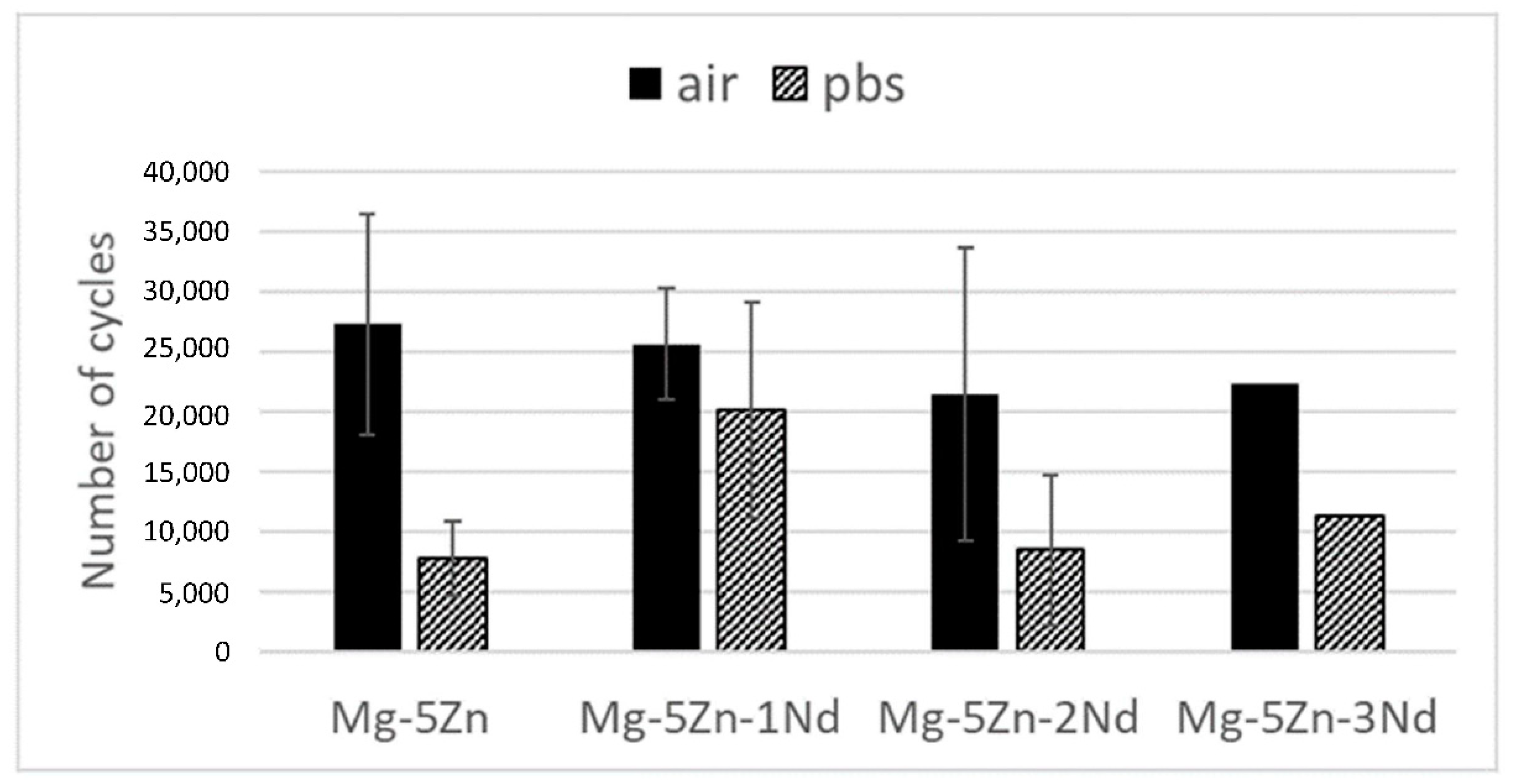
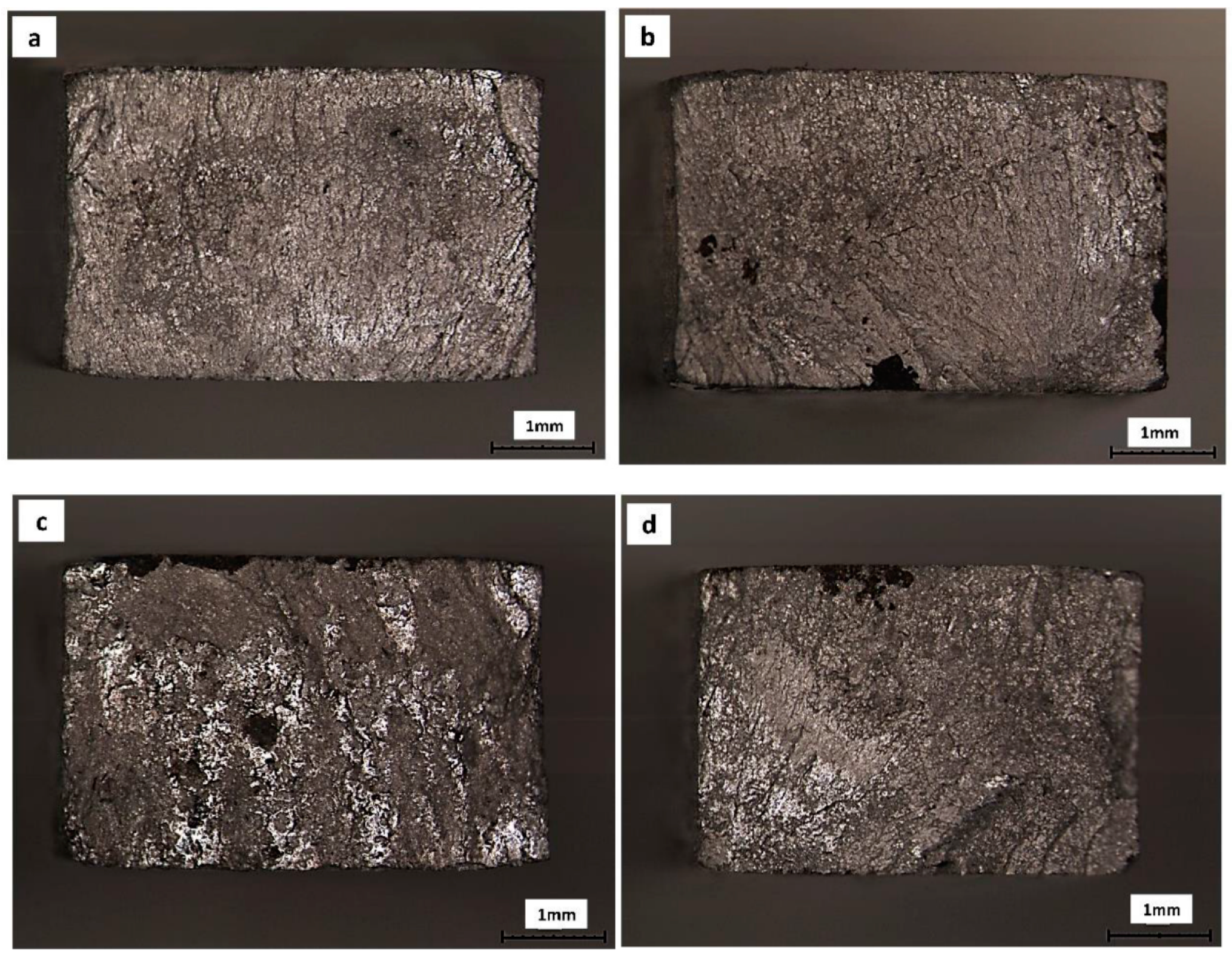
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Waizy, H.; Seitz, J.M.; Reifenrath, J.; Weizbauer, A.; Bach, F.W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2013, 48, 39–50. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Aghion, E.; Levy, G. The effect of Ca on the in vitro corrosion performance of biodegradable Mg-Nd-Y-Zr alloy. J. Mater. Sci. 2010, 45, 3096–3101. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatible magnesium alloy. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Ulrich Kainer, K.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid. State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Zhang, L.N.; Hou, Z.T.; Ye, X.; Xu, Z.B.; Bai, X.L.; Shang, P. The effect of selected alloying element additions on properties of Mg-based alloy as bioimplants: A literature review. Mater. Sci. 2013, 7, 227–236. [Google Scholar] [CrossRef]
- Kamrani, S.; Fleck, C. Biodgradable magnesium alloys as temporary orthopeadic implants: A review. BioMetals 2019, 32, 185–193. [Google Scholar] [CrossRef]
- Kannan, M.B.; Dietzel, W.; Blawert, C.; Alterns, A.; Lyon, P. Stress-Corrosion Cracking of Rare-earth Containing Magnesium Alloys ZE41, QE22 and Elektron 21 (WV31A) Compared with AZ80. Mater. Sci. Eng. A 2008, 480, 529–539. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Song, G.L.; Liu, M.; Dargusch, M.S.; Atrens, A. Stress corrosion cracking of several solutions heat-treated Mg–X alloys. Corros. Sci. 2015, 96, 121–132. [Google Scholar] [CrossRef]
- Arnon, A.; Aghion, E. Stress Corrosion Cracking of nano/sub-micron E906 magnesium alloy. Adv. Eng. Mater. 2008, 8, 742–745. [Google Scholar] [CrossRef]
- Katarivas-Levy, G.; Leon, A.; Kafri, A.; Ventura, Y.; Drelich, J.W.; Goldman, J.; Vago, R.; Aghion, E. Evaluation of biodegradable Zn-1%Mg and Zn-1%Mg-0.5%Ca alloys for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 174. [Google Scholar] [CrossRef] [PubMed]
- Elkaiam, L.; Hakimi, O.; Yosafovich-Doitch, G.; Ovadia, S.; Aghion, E. In Vivo Evaluation of Mg–5%Zn–2%Nd Alloy as an Innovative Biodegradable Implant Material. Ann. Biomed. Eng. 2020, 48, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hofstetter, J.; Cao, F.; Uggowitzer, P.J.; Dargusch, M.S.; Atrens, A. Corrosion and Stress Corrosion Cracking of Ultra-high-purity Mg5Zn. Corros. Sci. 2015, 93, 330–335. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Goldman, J.; Drelich, J.; Aghion, E. The suitability of Zn-1.3%Fe alloy as a biodegradable implant material. Metals 2018, 8, 153. [Google Scholar] [CrossRef]
- Jafaria, S.; Raman, R.K.S.; Davies, C.H.J. Stress corrosion cracking of an extruded magnesium alloy (ZK21) in a simulated body fluid. Eng. Fract. Mech. 2018, 201, 47–55. [Google Scholar] [CrossRef]
- Vasilev, E.; Linderov, M.; Nugmanov, D.; Sitdikov, O.; Markushev, M.; Vinogradov, A. Fatigue Performance of Mg-Zn-Zr Alloy Processed by Hot Severe Plastic Deformation. Metals 2015, 5, 2316–2327. [Google Scholar] [CrossRef]
- Li, H.; Peng, Q.; Li, X.; Li, K.; Han, Z.; Fang, D. Microstructures, mechanical and cytocompatibility of degradable Mg–Zn based orthopedic biomaterials. Mater. Des. 2014, 58, 43–51. [Google Scholar] [CrossRef]
- Rokhlin, L.L. Magnesium Alloys Containing RE Metals; Taylor and Francis: London, UK, 2003. [Google Scholar]
- Ben-Hamu, G.; Eliezer, D.; Dietzel, W.; Shin, K.S. Stress corrosion cracking of new Mg–Zn–Mn wrought alloys containing Si. Corros. Sci. 2008, 50, 1505–1517. [Google Scholar] [CrossRef]
- Wang, B.J.; Wang, S.D.; Xu, D.K.; Han, E.H. Recent progress in fatigue behavior of Mg alloys in air and aqueous media: A review. J. Mater. Sci. Tech. 2017, 33, 1075–1086. [Google Scholar] [CrossRef]
- Hakimi, O.; Aghion, E.; Goldman, J. Improved Stress Corrosion Cracking of a Novel Biodegradable EW62 Magnesium Alloy by Rapid Solidification, in Simulated Electrolytes. Mater. Sci. Eng. C 2015, 51, 226–232. [Google Scholar] [CrossRef]
- Bronfin, B.; Aghion, E.; Von-Buch, F.; Schumann, S.; Katsir, M. Die casting Magnesium alloys for elevateol temperature applications. In Proceeding of the TMS Annual meeting Magnesium Technology, New Orleans, LA, USA, 11–15 February 2001; pp. 127–130. [Google Scholar]
- Aghion, E.; Gueta, Y.; Moscovitch, N.; Bronfin, B. Effect of yttrium additions on the properties of grain-refined Mg-3%Nd alloy. J. Mater. Sci. 2008, 43, 4870–4875. [Google Scholar] [CrossRef]
- Aghion, E.; Bronfin, B.; Eliezer, D.; Von Buch, F.; Schumann, S.; Friedrich, H. The art of developing new Magnesium alloys for high temperature applications. Mater. Sci. Forum 2003, 419–422, 407–418. [Google Scholar] [CrossRef]
- Elkaiam, L.; Hakimi, O.; Goldman, J.; Aghion, E. The Effect of Nd on Mechanical Properties and Corrosion Performance of Biodegradable Mg-5%Zn Alloy. Metals 2018, 8, 438. [Google Scholar] [CrossRef]
- Kaya, A.; Uzan, P.; Eliezer, D.; Aghion, E. Electron microscopical investigation of as cast AZ 91 D alloy. Mater. Sci. Tech. 2000, 16, 1001–1006. [Google Scholar] [CrossRef]
- Unigovski, Y.B.; Lothongkum, G.; Gutman, E.M.; Alush, D.; Cohen, R. Low-cycle Fatigue Behavior of 316L-type Stainless Steel in Chloride Solutions. Corros. Sci. 2009, 51, 3014–3020. [Google Scholar] [CrossRef]
- Feng, H.; Yang, Y.; Chang, H. Influence of W phase on mechanical properties and damping capacity of Mg-Zn-Y-Nd-Zr alloys. Mater. Sci. Eng. A 2014, 609, 7–15. [Google Scholar] [CrossRef]
- Padezhnova, E.M.; Mel’nik, E.V.; Dobatkina, T.V. Study of phase equilibriums in magnesium-zinc-yttrium system. Russ. Metall. Eng. Trans. 1979, 1, 179. [Google Scholar]
- Padezhnova, E.M.; Mel’nik, E.V.; Miliyevskiy, R.A.; Dobatkina, T.V.; Kinzhibalo, V.V. Magnesium-zincyttrium system. Russ. Metall. Eng. Trans. 1982, 4, 185. [Google Scholar]
- Yang, J.; Wang, J.; Wang, L.; Wu, Y.; Wang, L.; Zhang, H. Microstructure and mechanical properties of Mg–4.5Zn–xNd (x = 0, 1 and 2, wt %) alloys. Mater. Sci. Eng. A 2008, 479, 339–344. [Google Scholar] [CrossRef]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion Fatigue Behavior of Two Biomedical Mg Alloys–AZ91D and WE43–In Simulated Body Fluid. Acta Mater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef]
- Merson, D.; Vasilev, E.; Markushev, M.; Vinogradov, A. On the corrosion of ZK60 magnesium alloy after severe plastic deformation. Lett. Mater. 2017, 7, 421–427. [Google Scholar] [CrossRef]
- Chen, L.; Sheng, Y.; Wang, X.; Zhao, X.; Liu, H.; Li, W. Effect of Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biodegradable Mg-Zn-Zr-xSr Alloys. Materials 2018, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Xu, D.K.; Sun, J.; Han, E.H. Effect of grain structure on the stress corrosion cracking (SCC) behavior of an as-extruded Mg-Zn-Zr alloy. Corr. Sci. 2019, 157, 347–356. [Google Scholar] [CrossRef]
- Prabhu, D.B.; Nampoothiri, I.; Elakkiya, V.; Narmadha, R.; Selvakumar, R.; Sivasubramanian, R.; Gopalakrishnan, P.; Ravi, K.R. Elucidating the role microstructural modification on stress corrosion cracking of biodegradable Mg-4Zn alloy in simulated body fluid. Mater. Sci. Eng. C 2020, 106. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.U.; Hort, N.; Blawert, C. A Critical Review of the Stress Corrosion Cracking (SCC) of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar]
- Winzer, N.; Atrensa, A.; Dietzel, W.; Raja, V.S.; Song, G.; Kainer, K.U. Characterization of stress corrosion cracking (SCC) of Mg-Al alloys. Mater. Sci. Eng. A 2008, 488, 339–351. [Google Scholar]
- Zhou, L.F.; Liu, Z.Y.; Wu, W.; Li, X.G.; Du, C.W.; Jian, B. Stress corrosion cracking behavior of ZK60 magnesium alloy under different conditions. Inter. J. Hydrog. Energy 2017, 42, 26162–26174. [Google Scholar] [CrossRef]
- Choudhary, L.; Raman, R.K.S.; Hofstetter, J.; Uggowitzer, P.J. In-vitro Characterization of Stress Corrosion Cracking of Aluminium-free Magnesium Alloys for Temporary Bio-implant Application. Mater. Sci. Eng. C 2014, 42, 629–636. [Google Scholar] [CrossRef]
- Prabhu, D.B.; Dhamotharan, S.; Sathishkumar, G.; Gopalakrishnan, P.; Raavi, K.R. Stress-Corrosion Cracking of Biodegaradable Mg-4Zn Alloy in Simulated Body Fluid at Different Strain Rates—A Fractography Investigation. Mater. Sci. Eng. A 2018, 730, 223–231. [Google Scholar] [CrossRef]
- Jafari, S.; Harandi, S.H.; Reman, R.K.S. A Review of Stress-Corrosion Cracking and Corrosion Fatigue of Magnesium Alloys for Biodegradable Implant Application. JOM 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, D.; Sun, J.; Luo, Y.; Xu, J.; Zhang, H.; Pan, F. High Cycle Fatigue behavior of Extruded and Double-aged Mg-6Zn-1Mn Alloy. Mater. Sci. Eng. A 2016, 662, 1–8. [Google Scholar] [CrossRef]
- Xu, D.K.; Liu, L.; Xu, Y.B.; Han, E.H. The Fatigue Behavior of I-Phase Containing As-cast Mg-Zn-Y-Zr Alloy. Acta Mater. 2008, 56, 985–994. [Google Scholar] [CrossRef]
- Bian, D.; Zhou, W.; Liu, Y.; Li, N.; Zheng, Y.; Sun, Z. Fatigue Behaviors of HP-Mg, Mg-Ca and Mg-Zn-Ca Biodegradable Metals in air and Simulated Body Fluid. Acta Biomater. 2016, 41, 351–360. [Google Scholar] [CrossRef] [PubMed]
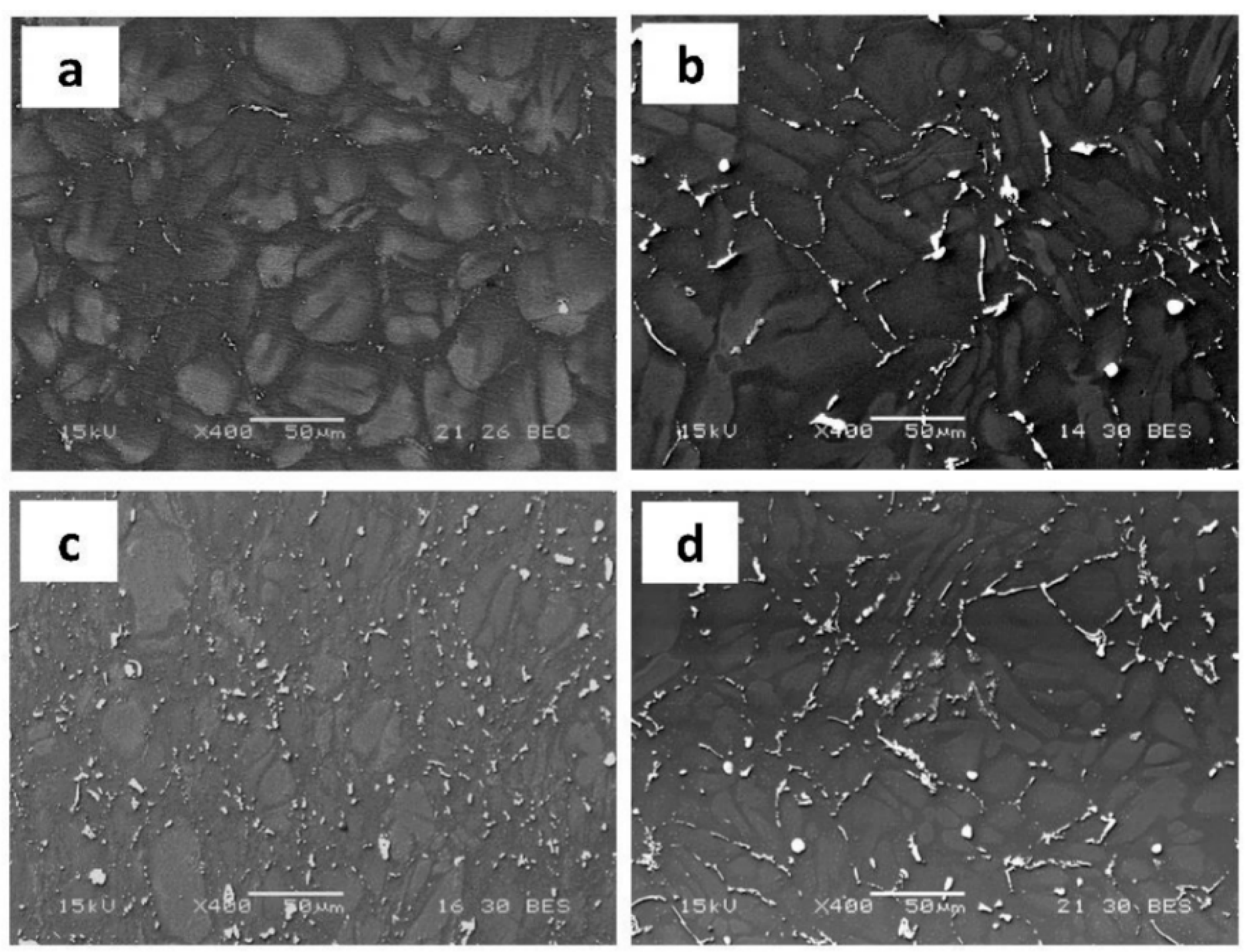
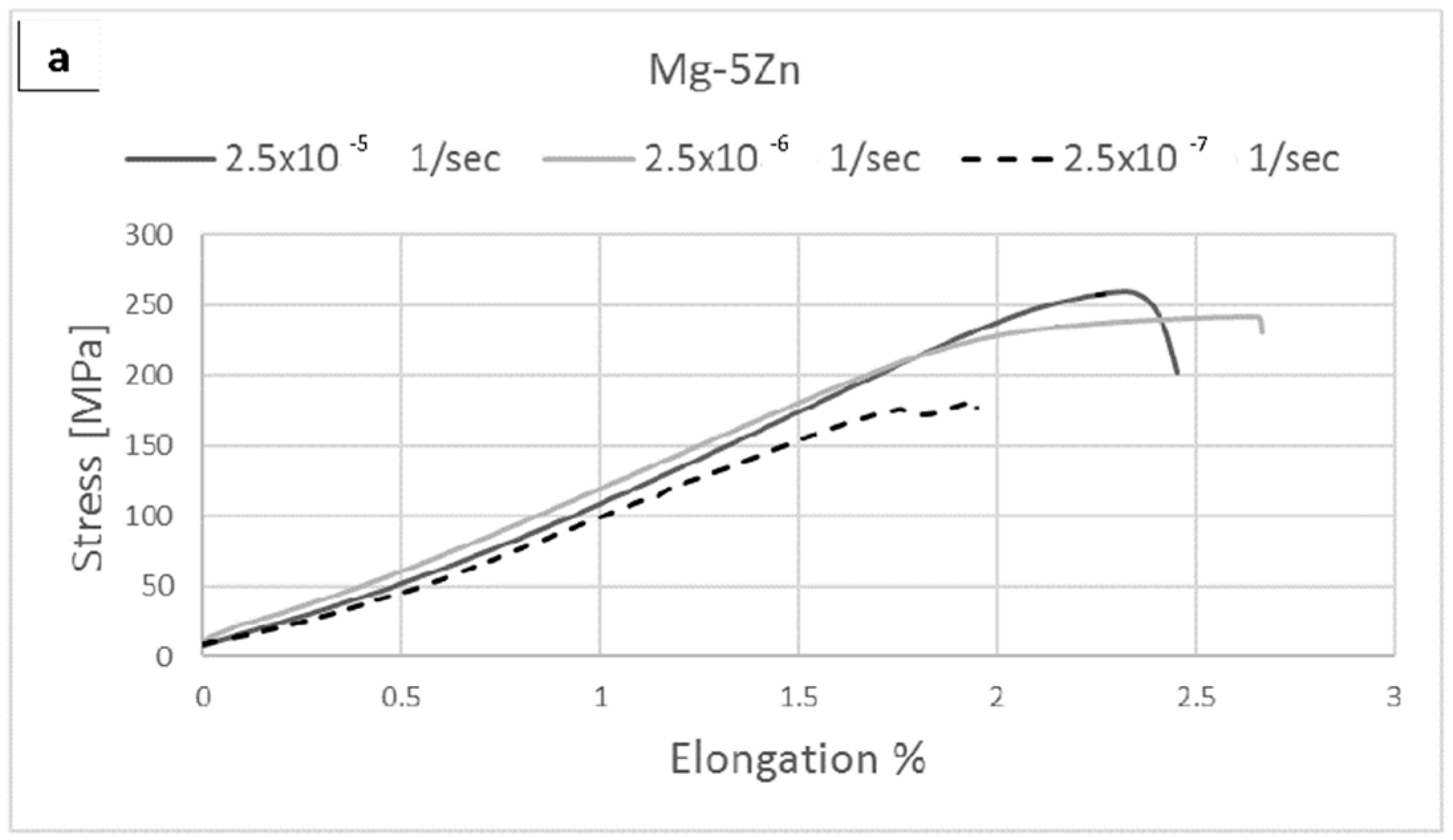
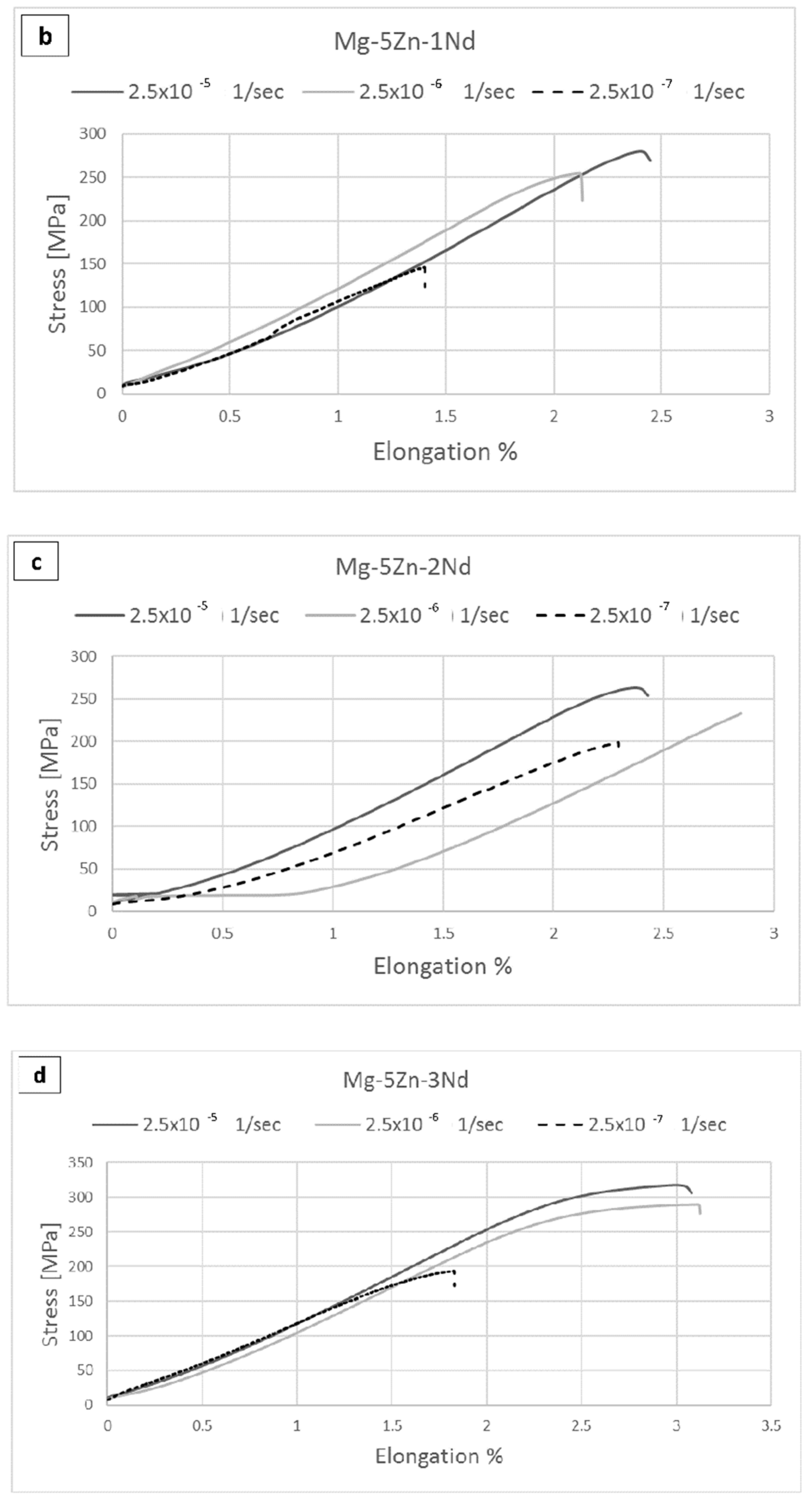
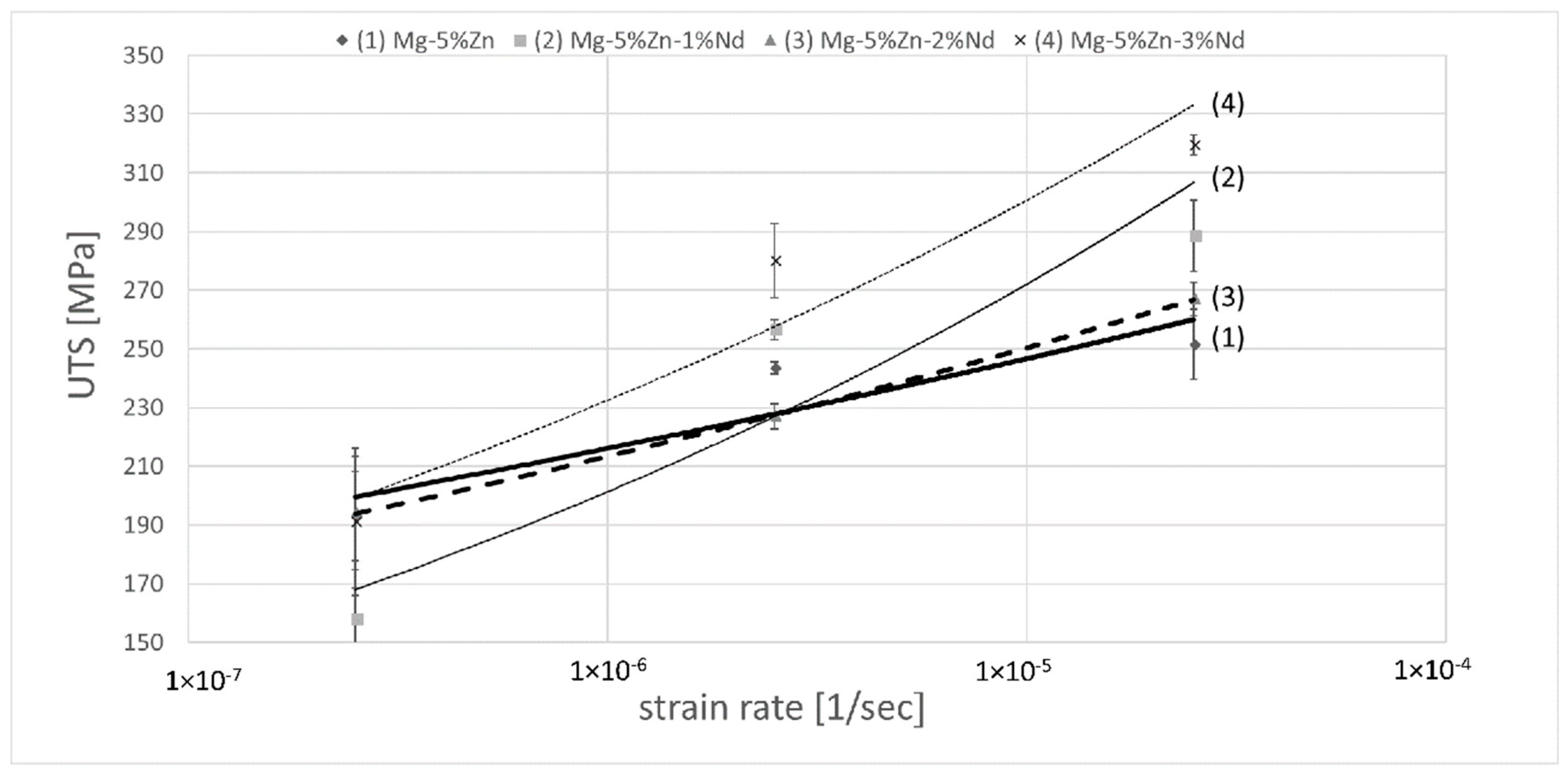

| Alloy Compositions | Zn | Nd | Zr | Y | Fe | Si | Ni | Cu | Mg |
|---|---|---|---|---|---|---|---|---|---|
| Mg-5% Zn | 5.1 | 0.0 | 0.38 | 0.15 | 0.005 | 0.01 | 0.001 | 0.001 | Balanced |
| Mg-5% Zn-1% Nd | 5.0 | 1.2 | 0.36 | 0.14 | 0.008 | 0.01 | 0.001 | 0.001 | Balanced |
| Mg-5% Zn-2% Nd | 5.1 | 2.1 | 0.33 | 0.12 | 0.006 | 0.01 | 0.001 | 0.001 | Balanced |
| Mg-5% Zn-3% Nd | 5.2 | 3.2 | 0.35 | 0.12 | 0.006 | 0.01 | 0.001 | 0.001 | Balanced |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkaiam, L.; Hakimi, O.; Aghion, E. Stress Corrosion and Corrosion Fatigue of Biodegradable Mg-Zn-Nd-Y-Zr Alloy in In-Vitro Conditions. Metals 2020, 10, 791. https://doi.org/10.3390/met10060791
Elkaiam L, Hakimi O, Aghion E. Stress Corrosion and Corrosion Fatigue of Biodegradable Mg-Zn-Nd-Y-Zr Alloy in In-Vitro Conditions. Metals. 2020; 10(6):791. https://doi.org/10.3390/met10060791
Chicago/Turabian StyleElkaiam, Lilach, Orly Hakimi, and Eli Aghion. 2020. "Stress Corrosion and Corrosion Fatigue of Biodegradable Mg-Zn-Nd-Y-Zr Alloy in In-Vitro Conditions" Metals 10, no. 6: 791. https://doi.org/10.3390/met10060791
APA StyleElkaiam, L., Hakimi, O., & Aghion, E. (2020). Stress Corrosion and Corrosion Fatigue of Biodegradable Mg-Zn-Nd-Y-Zr Alloy in In-Vitro Conditions. Metals, 10(6), 791. https://doi.org/10.3390/met10060791






