1. Introduction
Metallurgical treatment of metallic sulfides is generally carried out by means of high temperature methods, usually pyrometallurgy. Conversion stage, there are two sequential stages—the elimination of iron and some other impurities in the so-called "slag blow" stage; and the subsequent stage to convert white metal (Cu
2S) to metallic copper (Cu), known as the "copper blow" stage. During the conversion process, highly uncontrolled oxidation occurs, causing chemical copper losses through direct contact of metallic copper with gaseous oxygen (reaction (1)), as well as through direct contact of the white metal with the main constituent of slag, iron oxide (FeO) (reaction (2)).
Thermodynamically, reaction (1) is very favorable; however and although reaction (2) should not occur because the activity of copper oxide in a slag is on the order of 4.25 × 10
−7, there is ample evidence of this oxidized phase. The concentration of copper in slag can vary between grades of 0.68 and 9.13 wt% [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11].
Slag can be disposed of in landfills as long as it contains less than 1% Cu. Due to the high concentration of useful metal in slag, a proper copper recovery pathway through the use of alternative treatments is attractive. Since this type of copper involves oxidation, one such treatment alternative is hydrometallurgy. Much research has been conducted on copper recovery from converter slags. In the chemical leaching pathway, Anand [
1] leached converter slag (4.03% Cu, 1.99% Ni, 0.48% Co) with ferric chloride and obtained extractions of 92%, 28% and 24% copper, nickel and cobalt, respectively, after 10,800 s (3 h). To increase extractions, the same author [
2] pre-treated slags with furnace oil (10 wt%) for 10,800 s (3 h). Extraction increased for all three metals, reaching values of 82%, 95% and 80% Cu, Ni and Co, respectively. Then, in a separate study, Anand [
3] applied a pressurized leaching solution of dilute sulfuric acid at temperature 403 K (130 °C) and 0.59 MPa O
2, yielding recovery of over 90% of the metals of interest (Cu, Ni and Co) and less than 0.8% of Fe (main impurity).
Research done since 2000; Cu, Co and Zn were recovered by leaching slags with diluted H
2SO
4; slags were first roasted [
5] with concentrated sulfuric acid, causing sulfation of slag constituents. Recoveries of 88% copper, 87% cobalt, 93% nickel and 83% iron were obtained after 7200 s (2 h) of roasting at 423 K (150 °C) and a 3:1 acid/slag ratio. Perederiy [
10] applied the High Pressure Oxidative Acid Leaching (HPOXAL) method to extract nickel, cobalt and copper. The work was carried out in a titanium autoclave with oxygen and acid injection. The results obtained at 523 K (250 °C), 90 psi
and times between 900 and 1200 s (15 to 20 min) yielded between 95–97% extraction of Ni, Co and Cu, each. In another approach, the Taguchi method was used to determine optimum conditions for dissolving copper from converter slags [
6] saturated with Cl
2 gas. The experimental work was performed in a 0.5 dm
3 (500 mL) capacity glass reactor. The results showed 98.35%, 8.79% and 25.17% extraction of Cu, Fe and Zn, respectively, at chlorine gas flow of 3.67 × 10
−3 dm
3/s (220 mL/min), solid/liquid ratio of 0.1, agitation of 550 rpm and reaction time 5400 s (90 min). Another study [
7] used potassium dichromate with sulfuric acid where they found that using the H
2SO
4-K
2Cr
2O
7 mixture was more advantageous for copper extraction than using H
2SO
4 alone—using only sulfuric acid, they obtained extractions of 20.5%, 66.6%, 62.1% and 65.7% of Cu, Co, Zn and Fe, respectively; with added potassium dichromate, extractions varied to 81.15%, 12.00%, 3.15% and 10.27% for the same order of metals. The amount of leached copper increased, while the other metals decreased their extractions.
In recent years, Turan [
11] leached a mixed slag (BS) generated during flash fusion and conversion using microwaves, hydrogen peroxide and acetic acid. Results showed that the use of microwaves gave shorter leaching times. High copper (95%) and zinc (30%) recoveries were achieved, while iron recoveries were minimal (1.6%). Beşe [
8] leached converter slag in a ferric sulfate-sulfuric acid system in the presence and absence of ultrasound. The results showed that ultrasound gave extractions of 89.28%, 51.32%, 69.87% and 13.73% of copper, zinc, cobalt and iron, correspondingly. The absence of ultrasound showed similar extractions.
A different kind of approach from biohydrometallurgy has also been used to recover copper from converter slags. For example, indirect bioleaching, Carranza [
9] leached 9 wt% Cu slag with ferric sulfate via the BRISA process (in Spanish—
Biolixiviación Rápida Indirecta con Separación de Acciones; in English-Fast Indirect Bioleaching with Actions Separation). That process gave recovery of nearly 93% copper over 14400 s (4 h) of work. In relation to direct bioleaching, Mehta [
4] used bacterium
Thiobacillus ferrooxidans and iron oxidizing bacteria to bioleach converter slags at room temperature. High recoveries of 99.0% Copper (over 6912 × 10
3 s (1920 h)), 22.0% nickel (over 6048 × 10
3 s (1680 h)) and 30.0% cobalt (over 4320 × 10
3 s (1200 h)) were obtained.
Of the above works on converter slags using acid systems, none look at alkaline system, that is, basic solutions at pH values above 6. Our research work, then, takes the novel approach to leaching converter slags in ammonia-based systems, with pH values of 6 to 14. Although different copper compounds have already been tested with this leaching alternative in different ammonia media such as malachite in ammonia/ammonium carbonate system [
12], oxidized copper ore in ammonium chloride solution [
13], complex copper in ammonia-ammonium chloride solution [
14], malachite ore in ammonium nitrate solutions [
15], tenorite and cuprite in ammonium media [
16,
17]. Ammonia leaching has not been carried out for converter slags.
Therefore, the main objective of this research work is to leach a slag from a Peirce-Smith converter using ammonium hydroxide (NH4OH) under different working conditions as an alternative to leaching with sulfuric acid (or any other acid).
2. Experimental Design
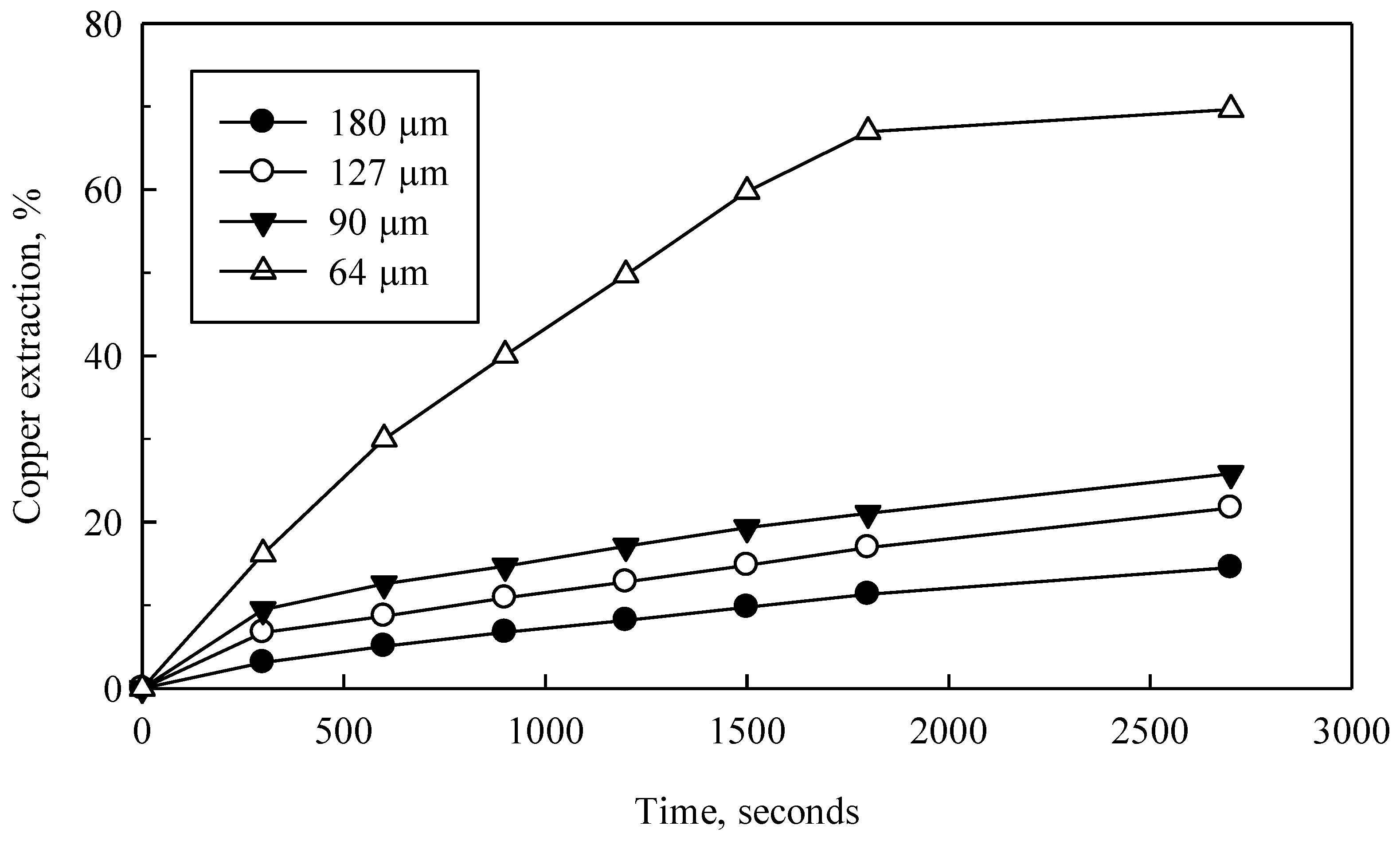
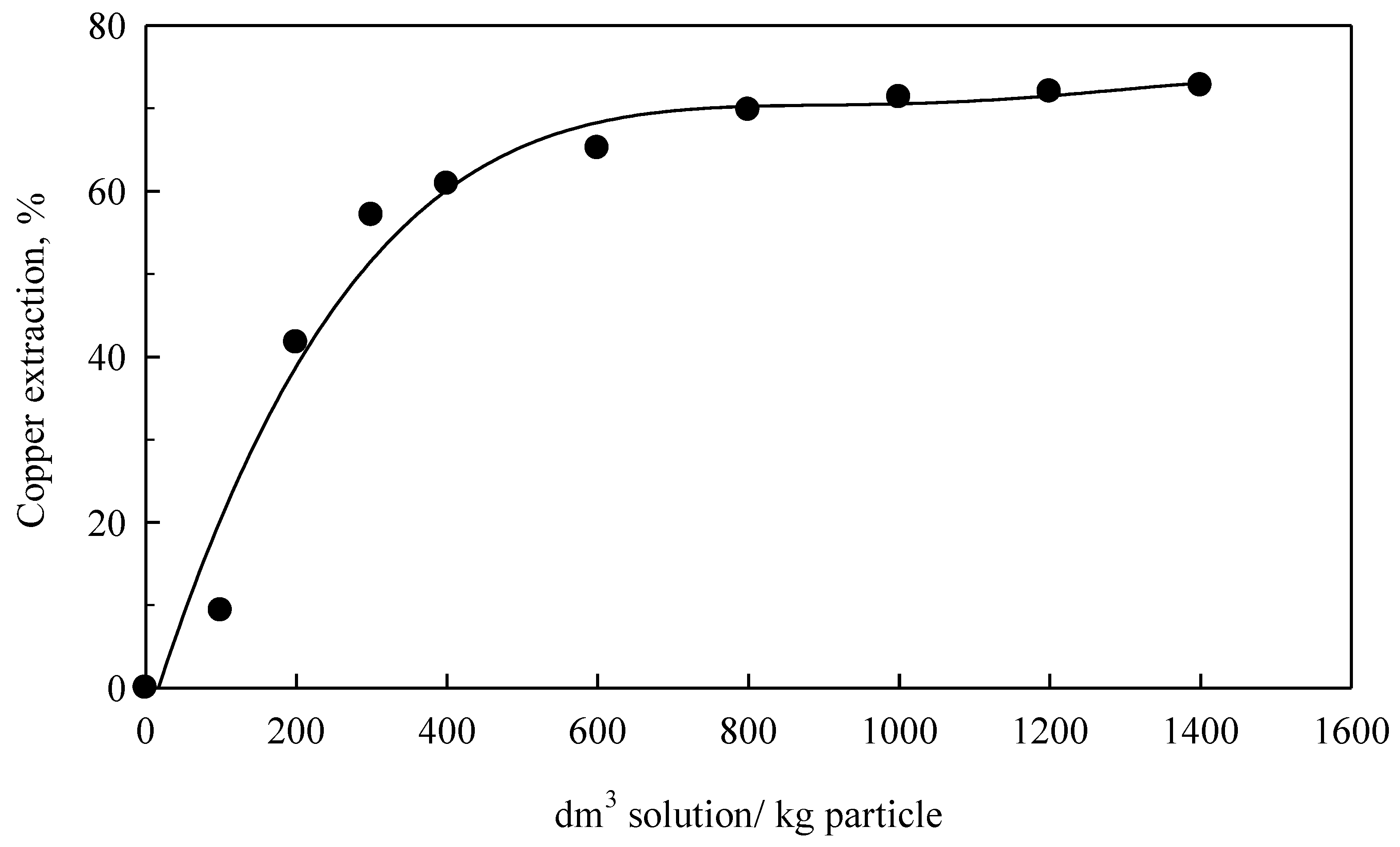
Codelco Chile’s Ventanas Division provided converter slag specifically from their conversion process in a Peirce-Smith Furnace. In brief, slag is cooled with industrial water to reduce temperature from 1373 K to 293 K, is then crushed, ground and classified into several size fractions, (most frequently −210 + 149, −149 + 105, −105 + 74 and −74 + 53 μm, with average particle sizes of 180, 127, 90 and 64 μm, respectively). Chemical analysis was performed on samples obtained at average sizes of 180 and 64 μm, showing 36% Cu, 19% Fe and the remaining insoluble parts. Converter slag here presented a very high concentration of copper, higher than bibliographically recorded (0.68–9.13% Cu). Therefore, hydrometallurgical treatment of waste (slag) was of interest.
Chemical analyses of the solutions were made by Atomic Absorption Spectroscopy (AAS) with a Hitachi Z-8100 Zeeman kit (Hitachi High-Technologies Corporation, Tokyo, Japan). For the slag characterization in scanning electron microscopy (SEM), the sample was covered with coal to be conductive. Chemical analysis (EDS) and compositional image capture (BSE) and morphological (SEM) were performed using a Tescan® Vega LSH electron microscope, equipped with an EDS Bruker® 6030 detector (TESCAN, Brno, Czech Republic). Solids were determined by X-ray diffraction (XRD), using a Bruker® D4 Endeavor kit, operated with Cu radiation and a Kβ Ni radiation filter (Bruker AXS Advance D8, Bruker, Billerica, MA, USA).
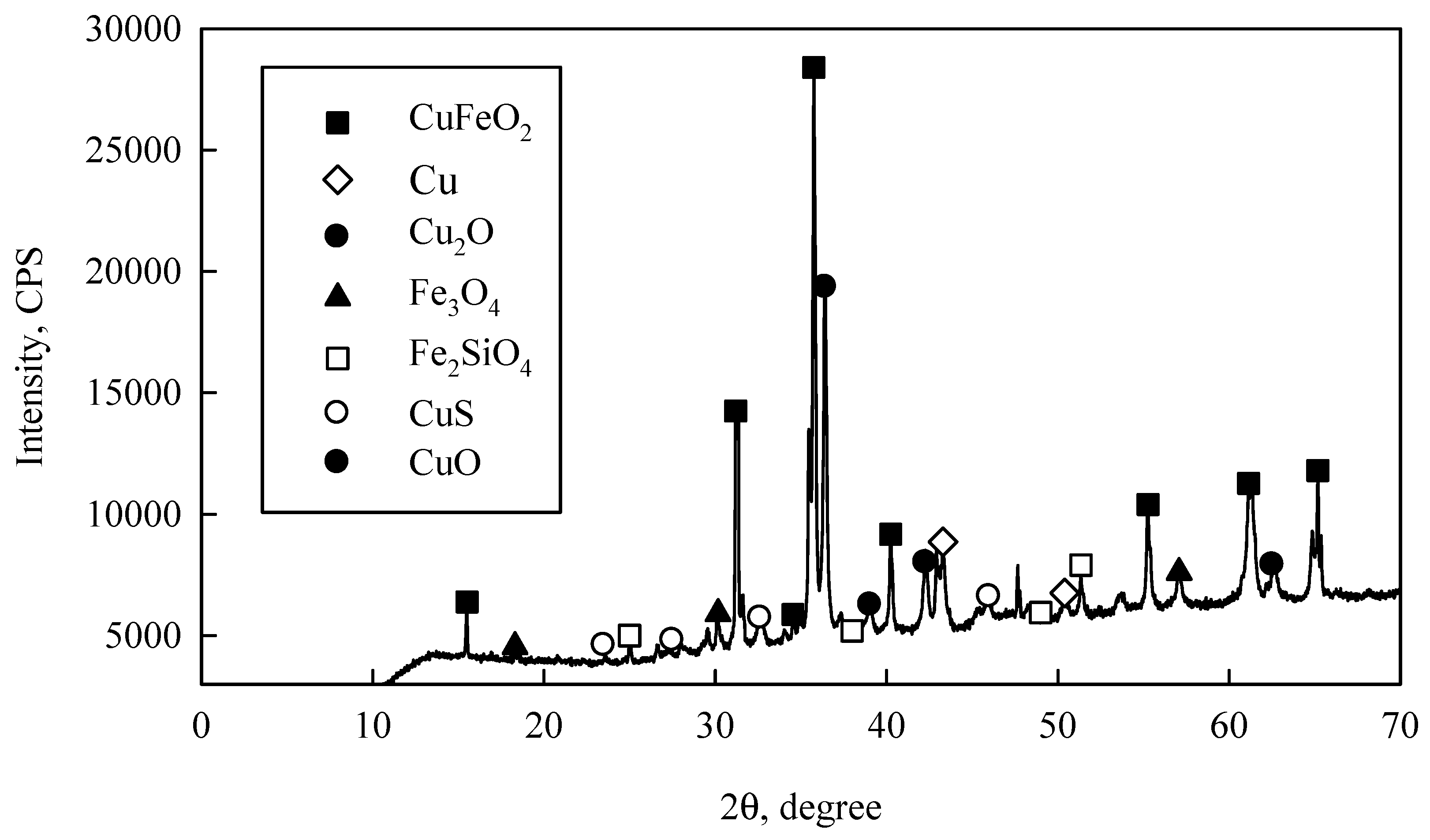
In order to know the chemical species and/or elements present in converter slag, an XRD of the original sample was performed, obtaining the results observed in
Figure 1. Here, peaks indicate delafossite (CuFeO
2), cuprite (Cu
2O), tenorite (CuO), metallic copper (Cu), chalcocite (CuS), magnetite (Fe
3O
4) and fayalite (Fe
2SiO
4) compounds-in short, copper present in the converter slag is mainly oxidized.
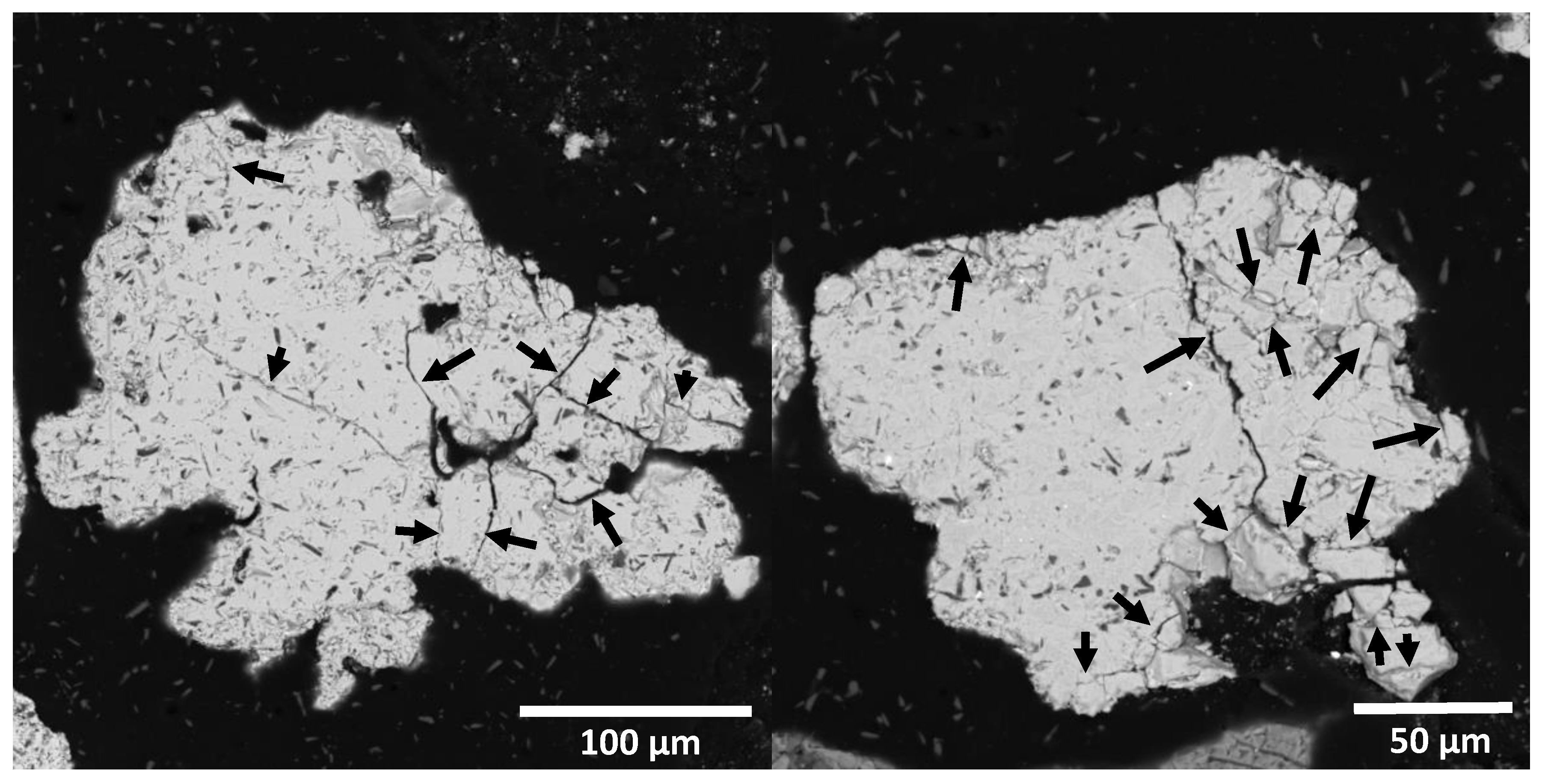
The 180 μm sample was analyzed by SEM to determine the degree of fracture and morphology of the converter slag particles (
Figure 2). The particles show an important degree of fracture or porosity (indicated by arrows in the images). This fracturing, called microcracks, could help diffuse leaching solutions through the particle.
Leaching experiments began by placing 1 dm3 of ammonium hydroxide leaching solution into a 2 dm3 capacity reactor. This reactor consisted of a heating blanket, condenser (to decrease the evaporation rate), mechanical agitator of the digital type (with a teflon impeller), thermocouple and a tube to extract the liquid samples. Then, 1 × 10−3 kg of converter slag sample was introduced. For experiments run at elevated temperature, the solution was first heated to the desired temperature. During the experimental time, liquid samples were extracted and analyzed by AAS for Cu and Fe analysis. In some cases, solid samples were obtained by washing and drying residues and they were analyzed by X-ray diffraction to get the qualitative phase analysis and BSE was used for cross sections of particle morphology.
3. Results and Discussion
Variables that directly affected converter slag dissolution rate, shown below, were NH4OH concentration (mol/dm3), temperature (K), solution pH, particle size (PS, μm), stirring speed (rpm) and liquid/solid ratio (dm3/kg).
3.4. Converter Slag Leach Mechanism
Ammonium hydroxide incorporated into the solution was immediately decomposed down to form an ammonium ion (NH
3) and a hydronium ion (H
3O
+), following reaction (3). In this and previous work [
16,
17], it was demonstrated that H
3O
+ is responsible for leaching copper oxides through physical and electrochemical processes, while NH
3 created copper complexes to maintain cupric ions in the alkaline solution system (constant dissociation of ammonia = 1.77 × 10
−5 to 298 K).
Therefore, the leaching mechanism for oxide slags (Cu
2O and CuO) in the presence of ammonium hydroxide is given by the following semi-reactions [
16,
17]:
In the case of the reaction (4), the hydronium ion provides protons necessary for the dissolution of CuO (the dissolution of this compound follows a physical process). Reaction (5), in contrast, has the hydronium ion in an electrochemical process to provide the necessary electrons to generate the oxide/reduction pair (using reaction (6)) and thus dissolve the Cu
2O.
With respect to the compound Cu(OH)
2 generated in reactions (4) and (5), this is leached with H
3O
+ according to reaction (7). The hydronium ion competes to be able to leach cuprite and tenorite (reactions (4) and (5)) and to dissolve Cu(OH)
2 (reaction (7)).
Copper ions released into the solution (reactions (4) and (5)) are complexed by the ammonium ion to generate copper tetra-amine (Cu(NH
3)
42+) according to reaction (8). It should be noted that the tetra-amine formation process is allowed through previous generation of Cu(NH
3)
22+; however, that ion is thermodynamically unstable in basic systems [
13,
15].
To corroborate the formation of copper tetra-amine, a Pourbaix stability diagram (E
H-pH) was constructed for the Cu-NH
4OH-H
2O system for temperature 293 K, copper concentration 0.00236 mol/dm
3 and ammonium hydroxide concentrations of 0.1, 0.35 and 0.7 mol/dm
3. The thermodynamic data used for the construction of the diagram were extracted from the HSC Chemistry program database [
19]. This diagram (
Figure 8) shows that solution pH was preponderant vs. potential in obtaining the copper tetra-amine. Thus, for NH
4OH concentration 0.1 mol/dm
3, Cu(NH
3)
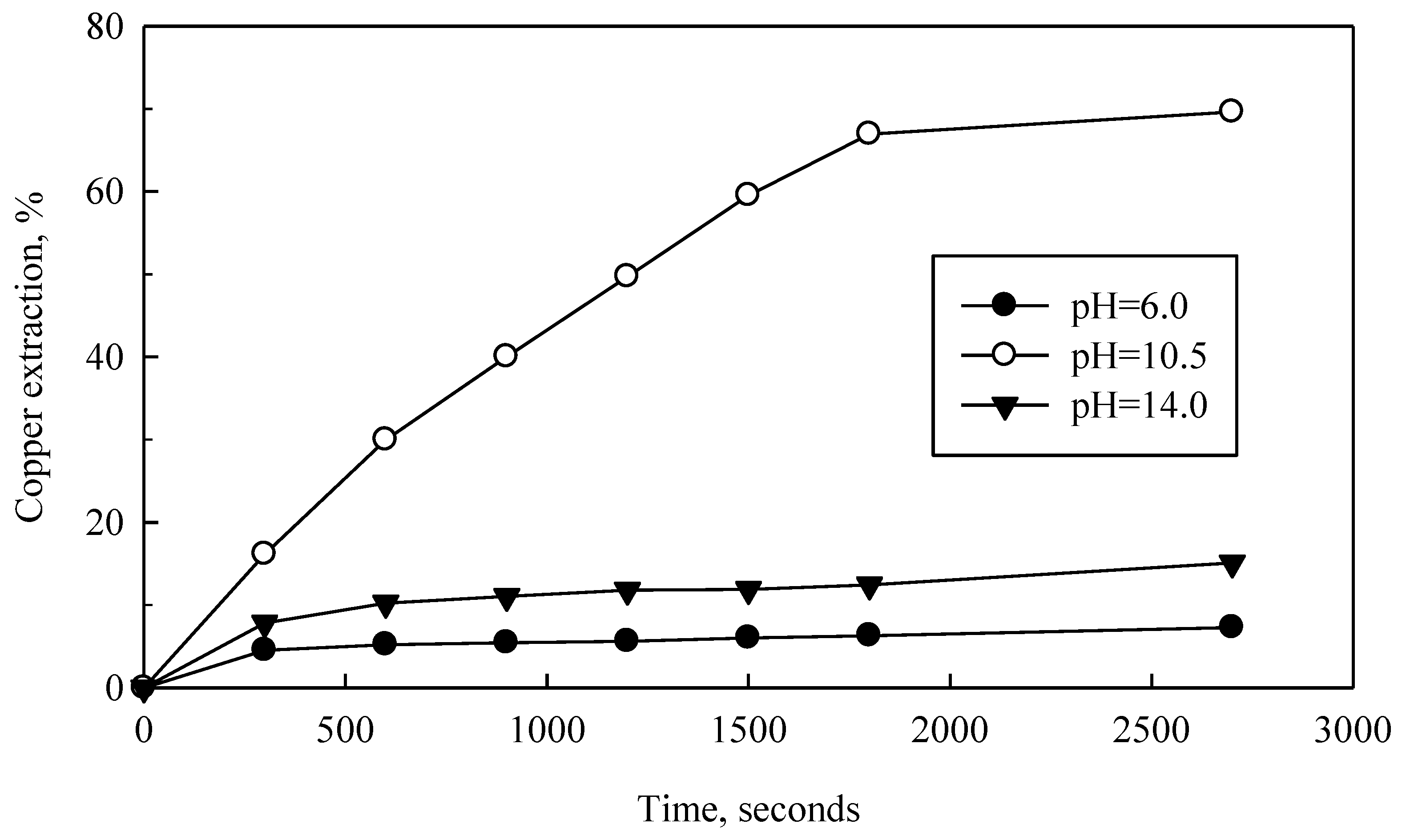
42+ was maintained in solution at pH ranging from 7 to 14 for the entire potential range. Outside those pH ranges, copper precipitated as an oxide (CuO), decreasing copper concentration in solution and inhibiting further purification/concentration of this metal. Thus pH effect is shown, here, as in
Figure 3. In addition, were copper oxide to form, NH
3 consumption (reaction (8)) would stop, NH
4OH would not decompose and H
3O
+ would not be generated (reaction (3)). In short, CuO production directly and negatively affects Cu
2O and CuO leaching from the slag.
Tests with NH4OH concentrations (0.35 and 0.70 mol/dm3) show that as concentration of ammonium hydroxide increases, the stability zone of the copper tetra-amine was reduced, yielding for NH4OH 0.7 mol/dm3 a pH range from 8.3 to 13.4.
The formation of the copper tetra-amine generated an imbalance between reactions (4) and (5) (i.e., Cu
2+ concentration decreased), thus causing a higher dissolution rate for the two copper oxides CuO and Cu
2O. These two compounds delivered a greater amount of copper ions to the basic solution, given by reactions (9) and (10).
It should be noted that if the concentration of ammonia in the solution is reduced, it could lead to the formation of copper oxide.
On the other hand, metallic copper can be dissolved by hydronium ions (coming from the disiation of NH
4OH) by an electrochemical process. Then, the dissolution mechanism would be given by the expression 11.
3.8. Converter Slag Leaching Kinetics
Although and as previously mentioned, converter slag particles have microcracks,
Figure 6 shows that slag leaching could also be described by the intraparticle diffusion model, that is, solution leaching through slag porosity. Assuming that constant reactant concentrations and diffusion rate through porous layer of radius r
o, the rate equation, described by the shrinking core model, is given by the following expression [
20]:
In Equation (11),
is the converted (reacted) fraction of the converter slag; apparent rate constant (k
app) is a function of temperature, NH
4OH concentration and particle size. Constant k
app is directly related to the intrinsic rate constant of the system, given by expression (13):
where k is the intrinsic rate constant,
, is the concentration of ammonium hydroxide and n is the reaction order of NH
4OH. Finally, r
o is the initial radius of the slag particle.
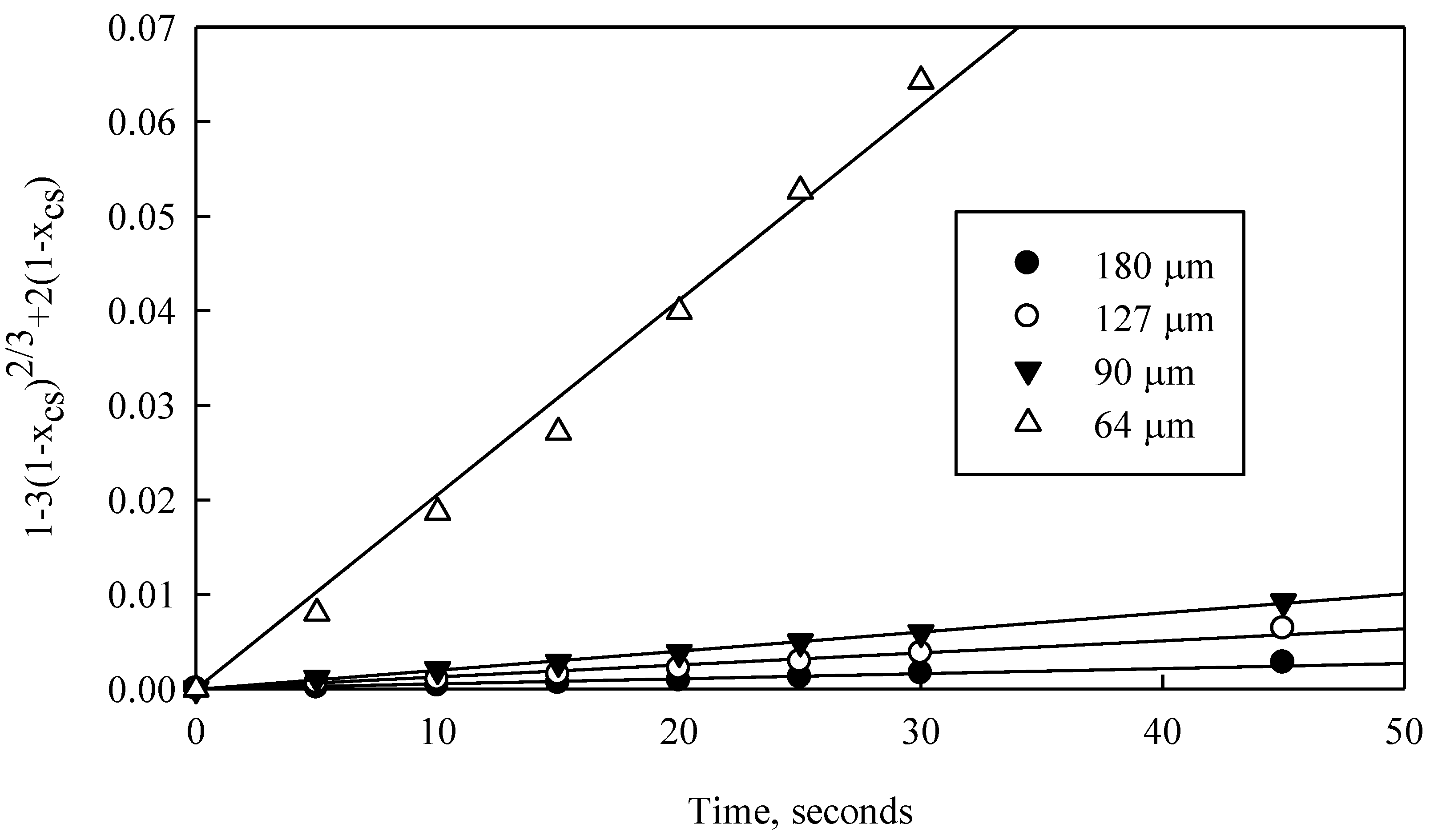
A graph of
versus time was constructed for the experimental data obtained in
Figure 6 for temperature range 283 to 333 K and for particle size 64 μm. Results are shown in
Figure 12. The same data treatment method was used for the results obtained on the effects of ammonium hydroxide concentration (
Figure 9) and particle size (
Figure 10).
The regression lines shown in
Figure 12 fit very well to experimental data over the entire temperature range (0.95 > R
2 > 0.99) These results demonstrate the applicability of this kinetic model for diffusion in a porous layer of radius r
o (Equation (12)).
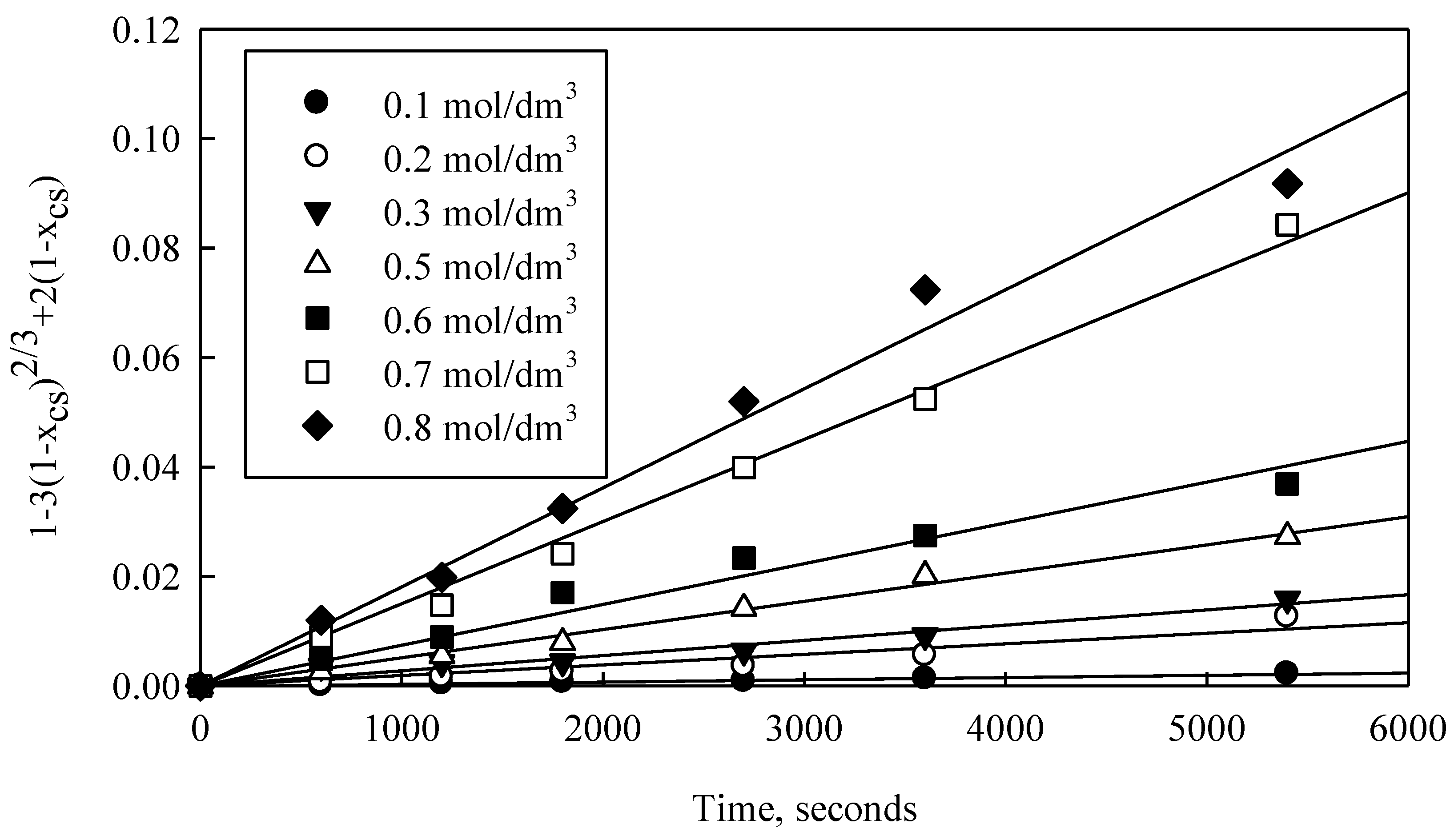
Similarly to the above, though to quantify reaction order n, a graph was constructed using kinetic data on the effect of NH
4OH concentrations. Results are shown in
Figure 13, where experimental data on all ammonium hydroxide concentrations measured fit very well to Equation (12). Afterwards, the slopes of each adjustment were used for Equation (13) and a graph of ln k
app versus ln
was constructed, with initial particle radius and temperature constant, as shown in
Figure 14.
This figure shows the linear adjustment of the seven kapp values (over the whole NH4OH concentration range) with R2 of 0.90. Reaction order n was then calculated using slope values, which resulted in a reaction order n of 1.2 for NH4OH concentration in the solution.
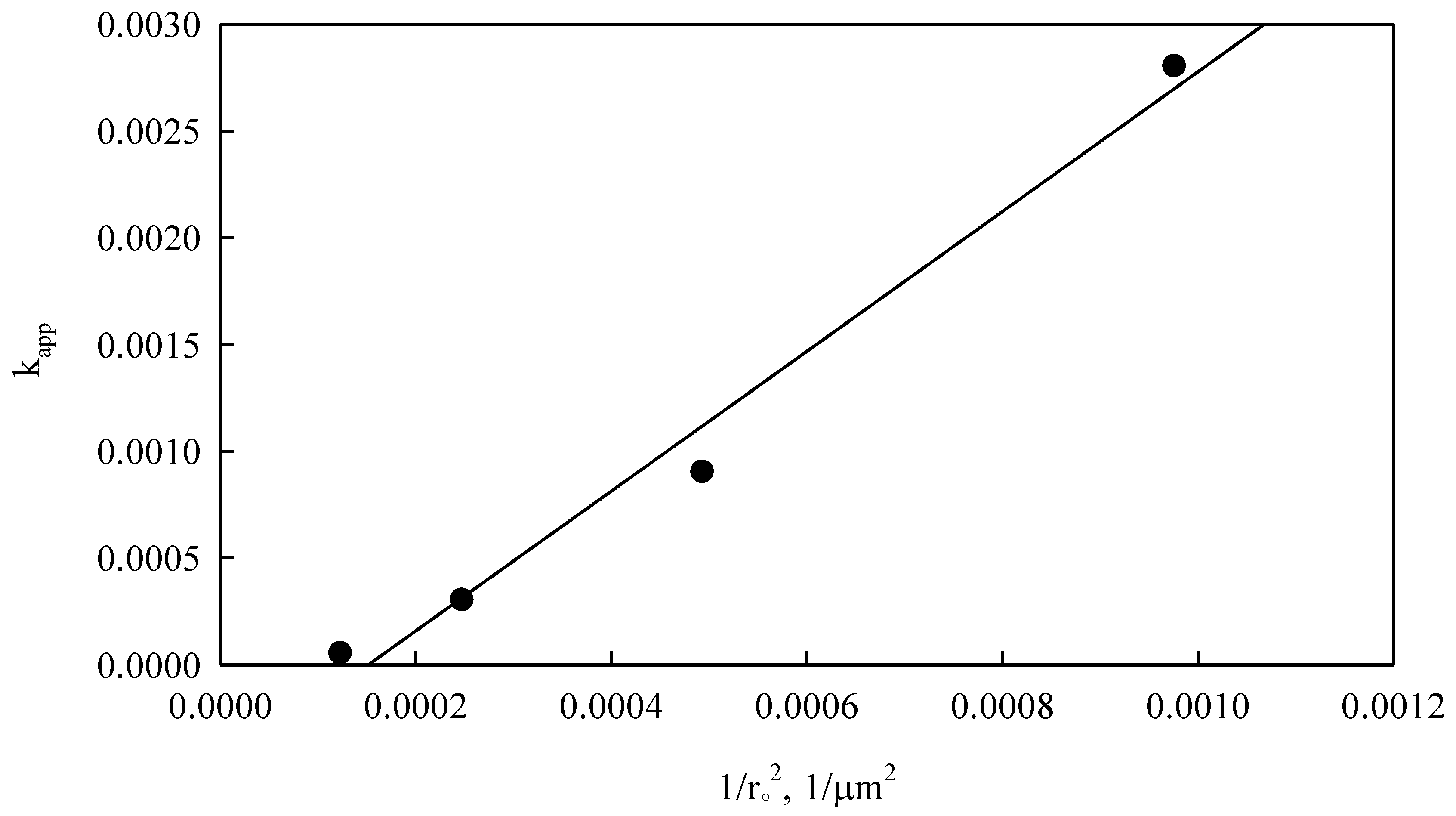
Looking at Equation (13), particle radius squared varied inversely with the apparent kinetic constant. To corroborate this relationship, experimental data obtained from
Figure 10 were used and adjusted according to Equation (12), yielding
Figure 15. Controlled diffusion through porous layer of radius r
o (Equation (13)), requires that 1/(r
o2) must vary linearly with ln k
app.
Figure 16 shows such variation, where it can be seen that the ln k
app data have a very good linear fit to experimental data, R
2 of 0.98, indicating that the heterogeneous kinetic model (Equation (12)) models converter slag dissolution very well.
To calculate the activation energy (E
a), the intrinsic rate constant (modifying Equation (13)) was first calculated using apparent rate constants (given in
Figure 11), at temperature range 283 to 333 K, NH
4OH concentration 0.7 mol/dm
3, reaction order n of 1.2 and particle size 67 μm (r
o = 33.5 μm).
Table 1 shows the k-values as a function of the temperature range used in this study.
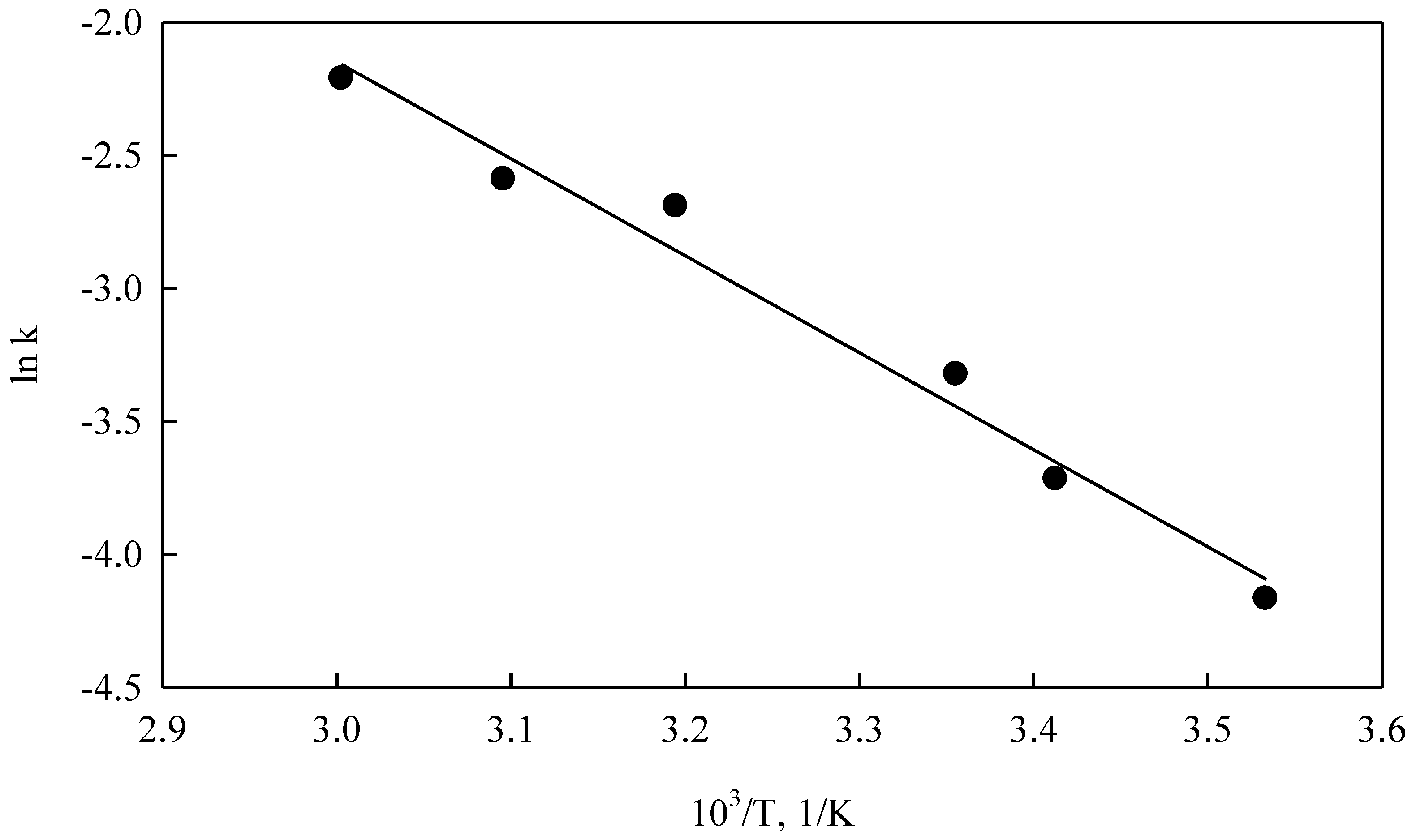
After the k-values had been obtained, an Arrhenius graph (ln k versus 1/T) was constructed (
Figure 17). A good linear adjustment (R
2 > 0.85) of the ln k data can be appreciated. The calculated activation energy was 42.3 kJ/mol for the temperature range 283 to 333 K, which is a typical value for a particle diffusion model (Equation (12)). Finally, then, the dissolution rate of converter slag in an ammonia system (NH
4OH) can be represented by the following expression:
Here, R = 8.314 J/mol/K, ro is given in microns, is in mol/dm3, t is s, T is Kelvin and k = 2.52 × 107 µm2·mol/(dm3·s).