A New Method for Plasticization of Inclusions in Saw-Wire Steel by NaF Addition
Abstract
1. Introduction
2. Experimental Aspects
2.1. Material Preparation
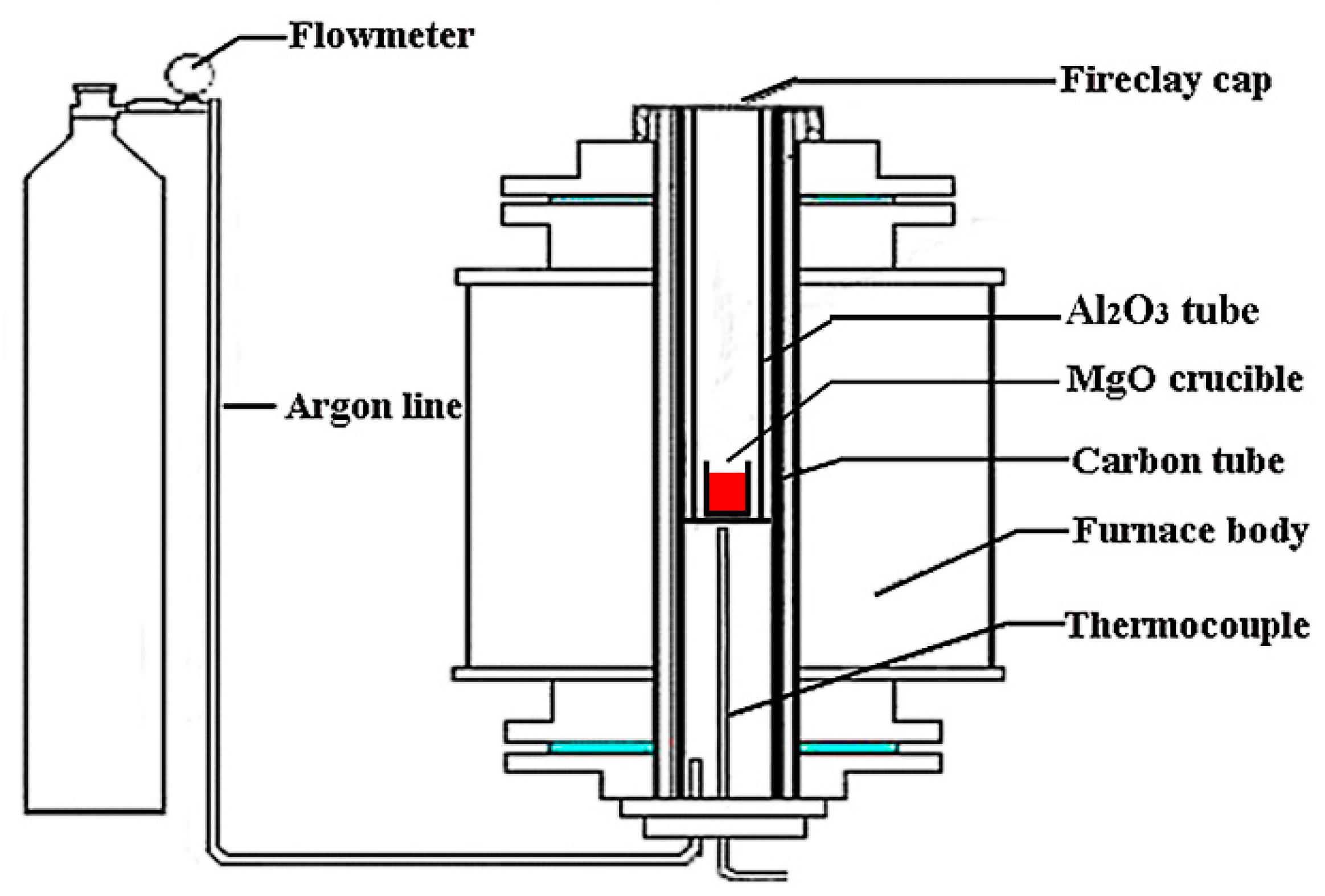
2.2. Experimental Equipment
2.3. Experimental Procedure
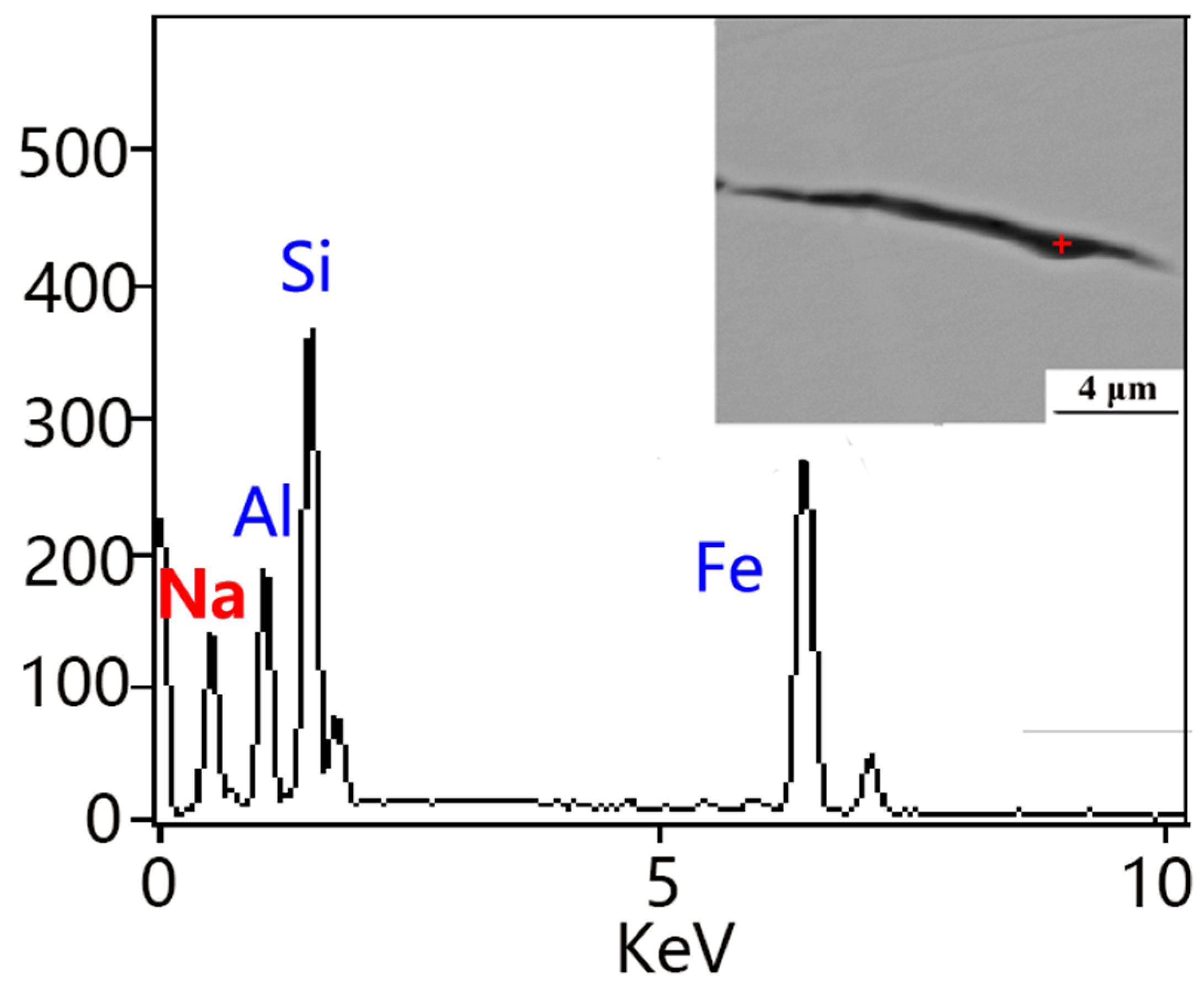
2.4. Analysis
3. Results and Discussion
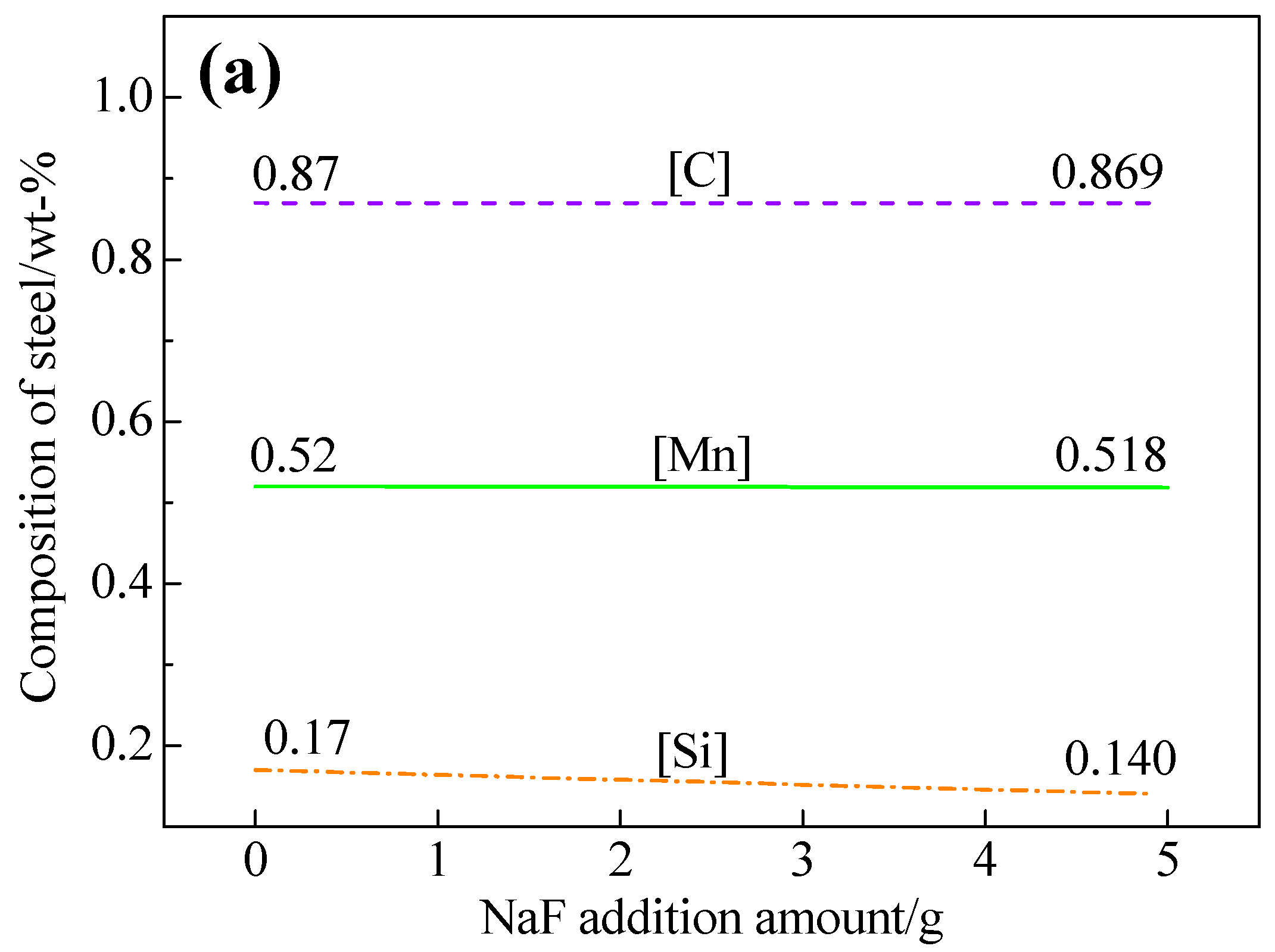
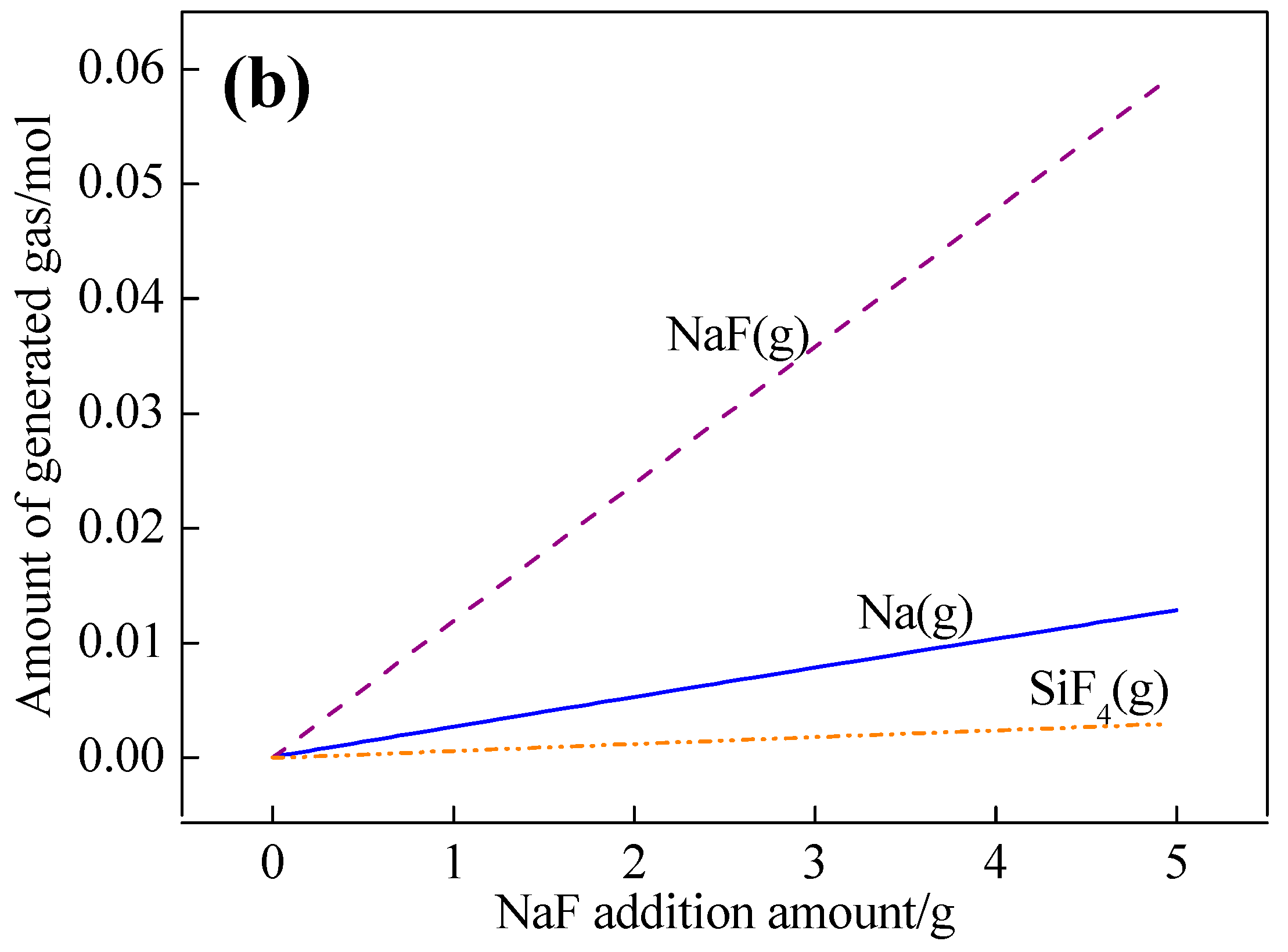
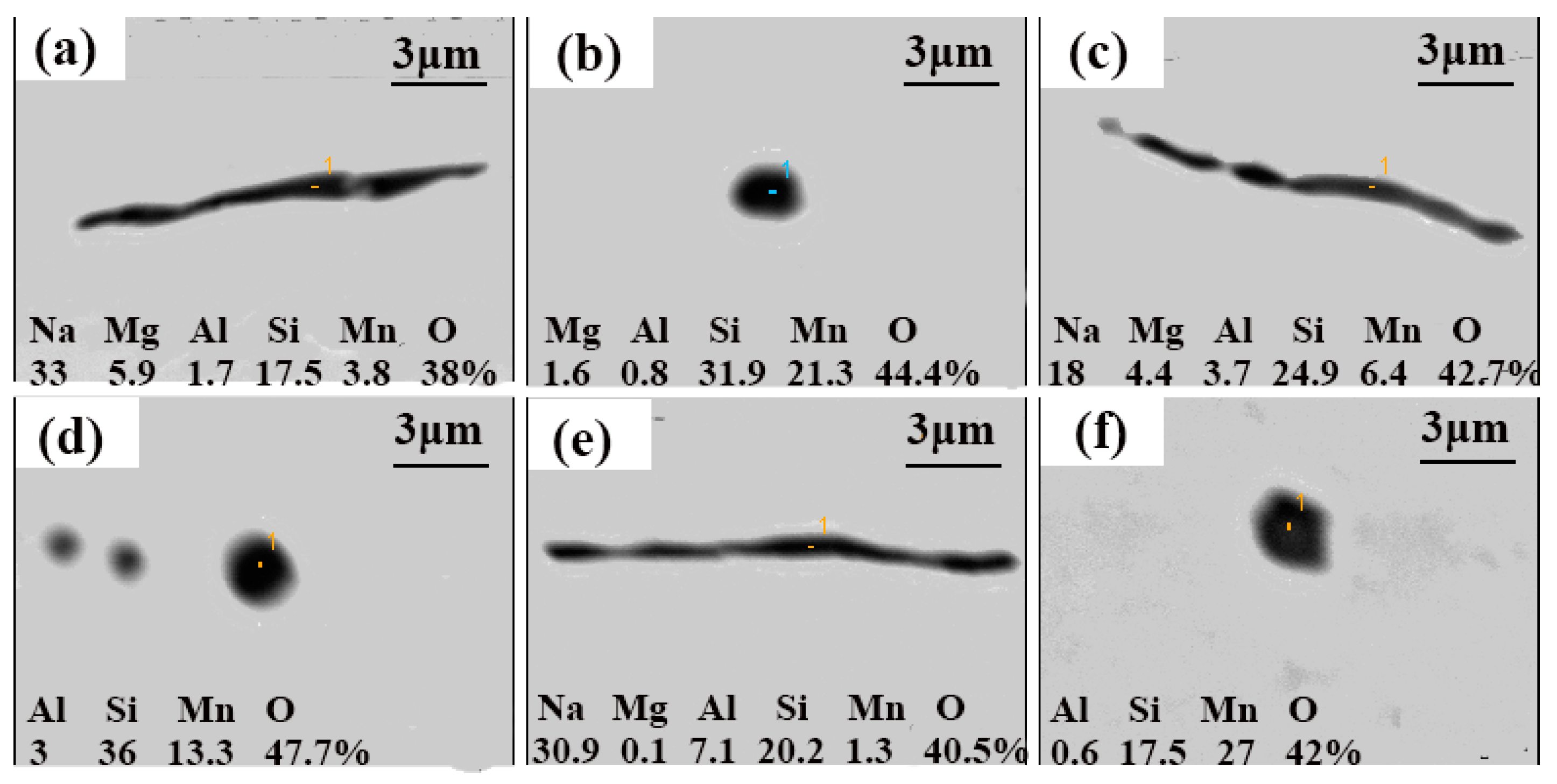
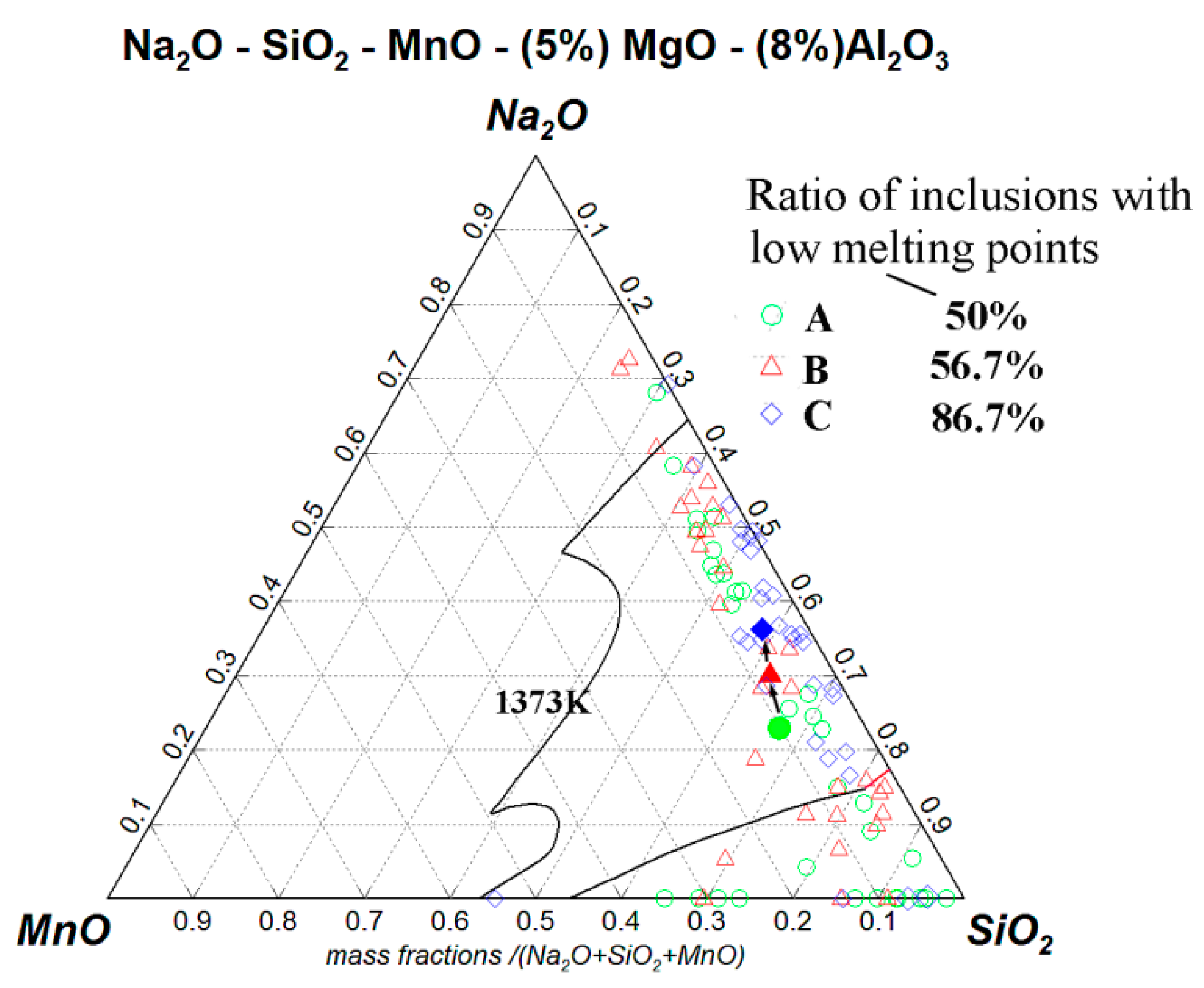
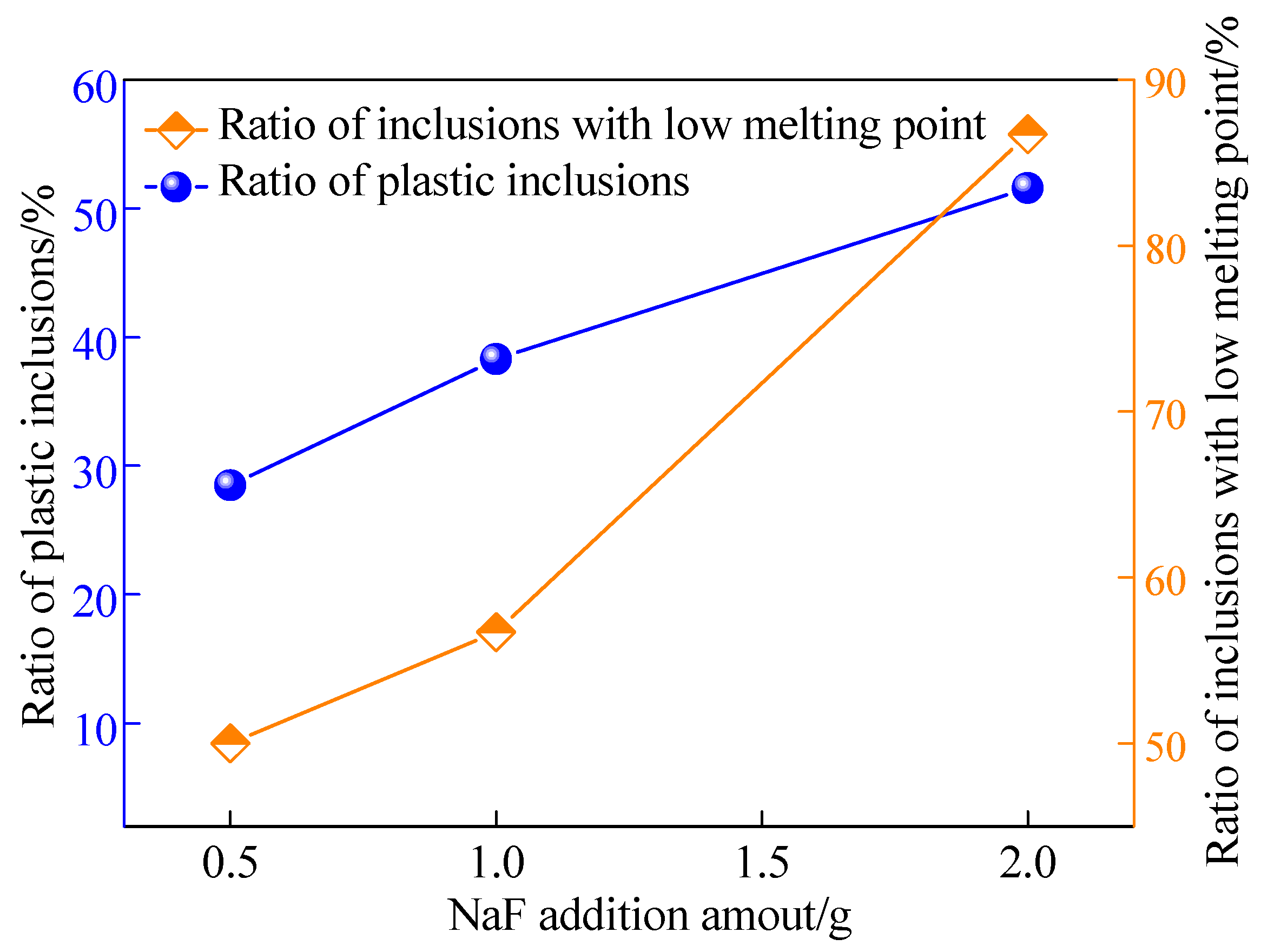
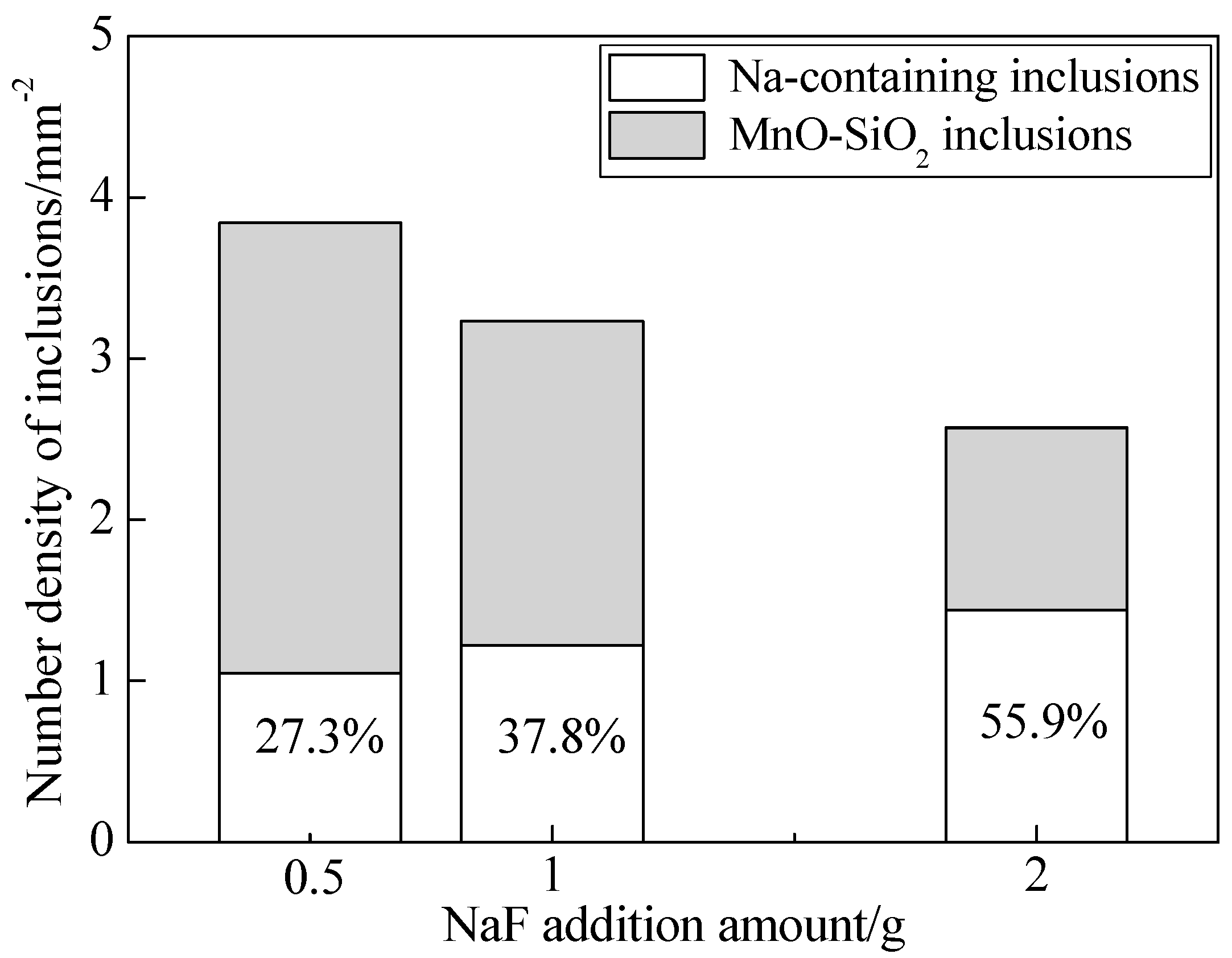
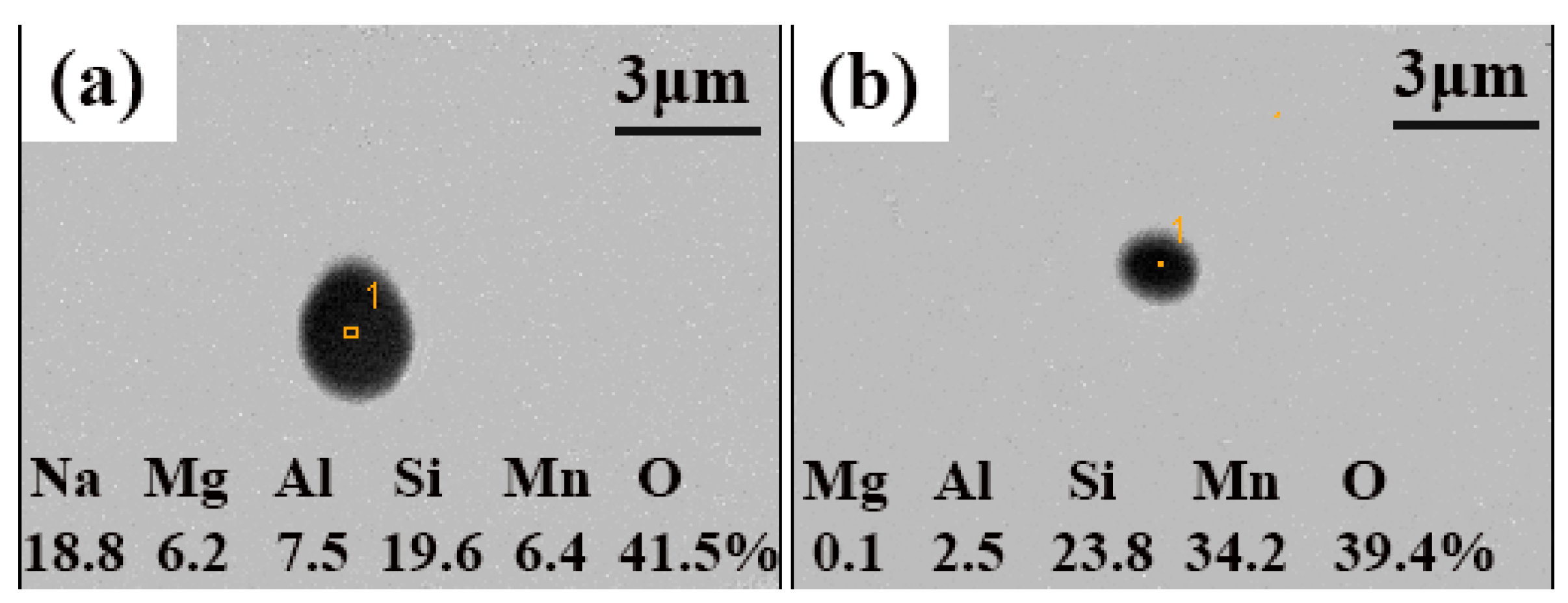
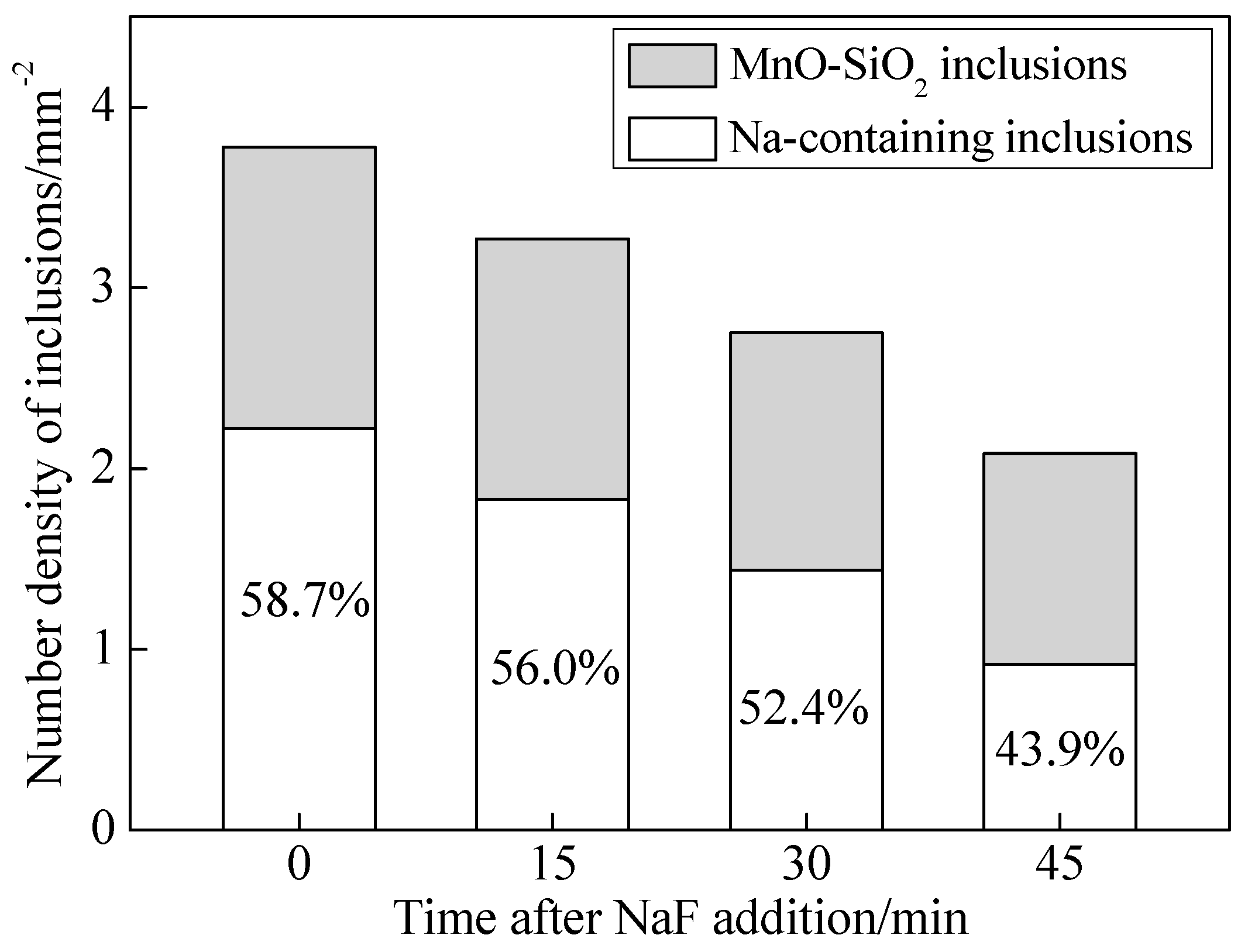
3.1. Effects of NaF Addition
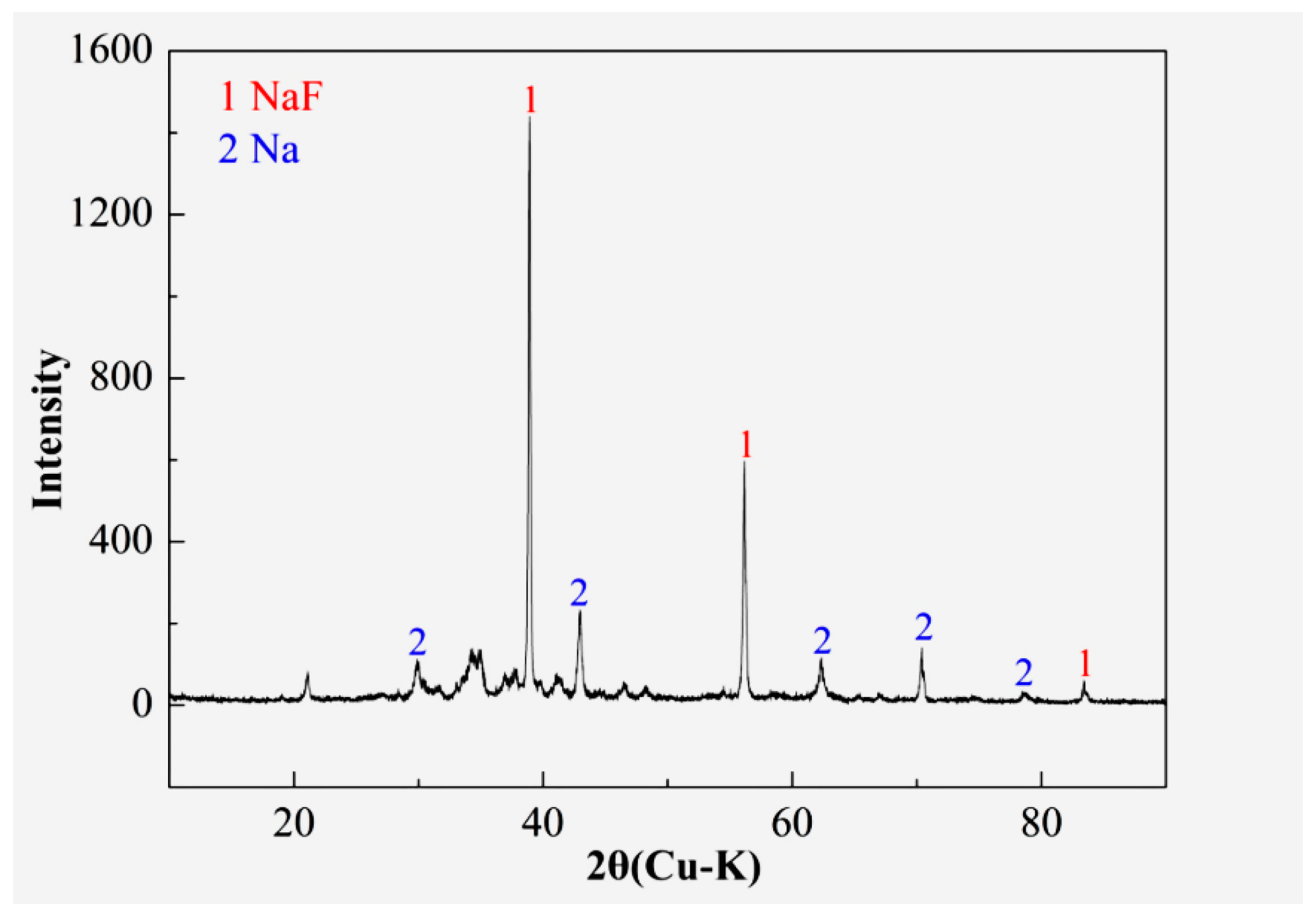
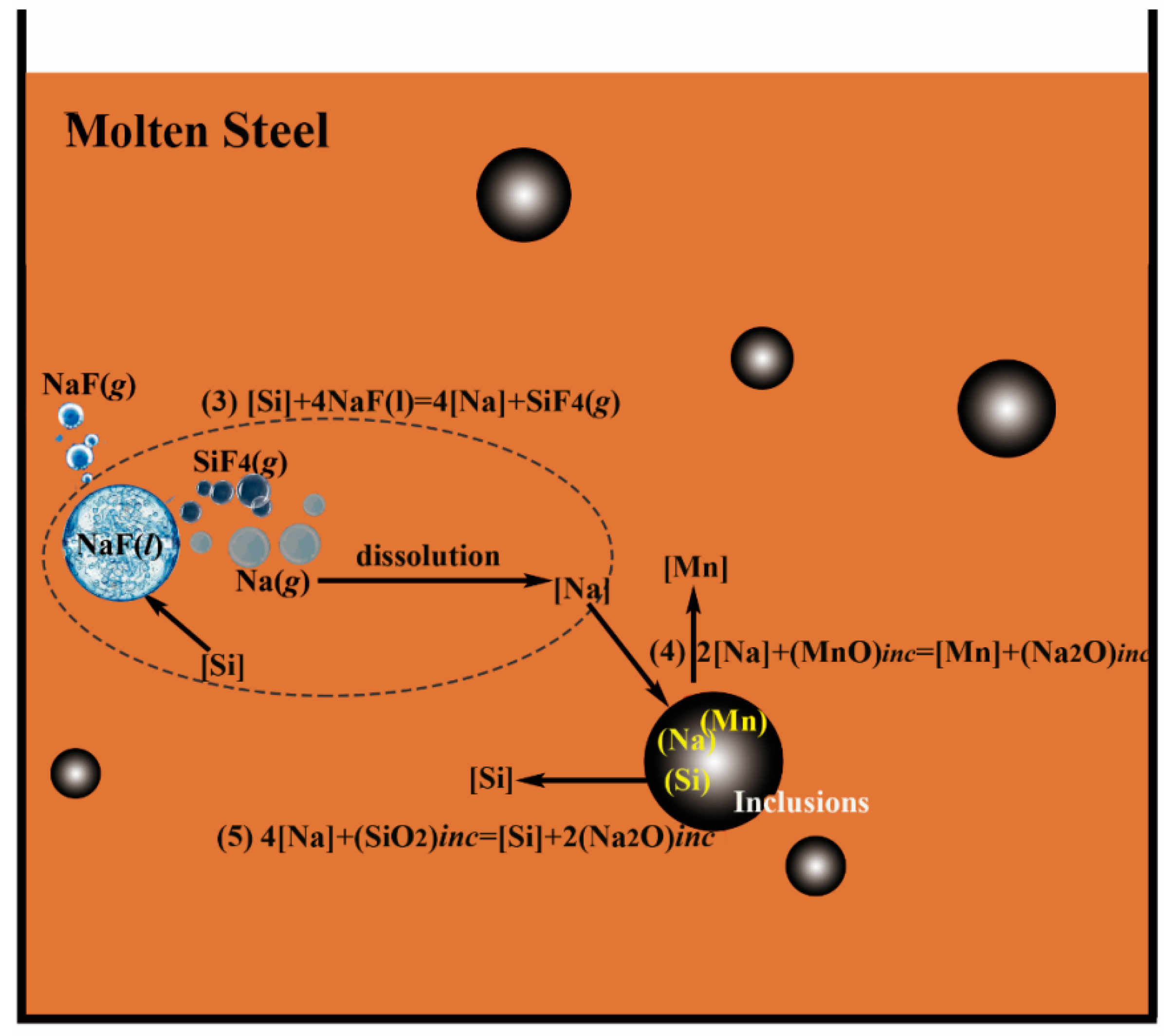
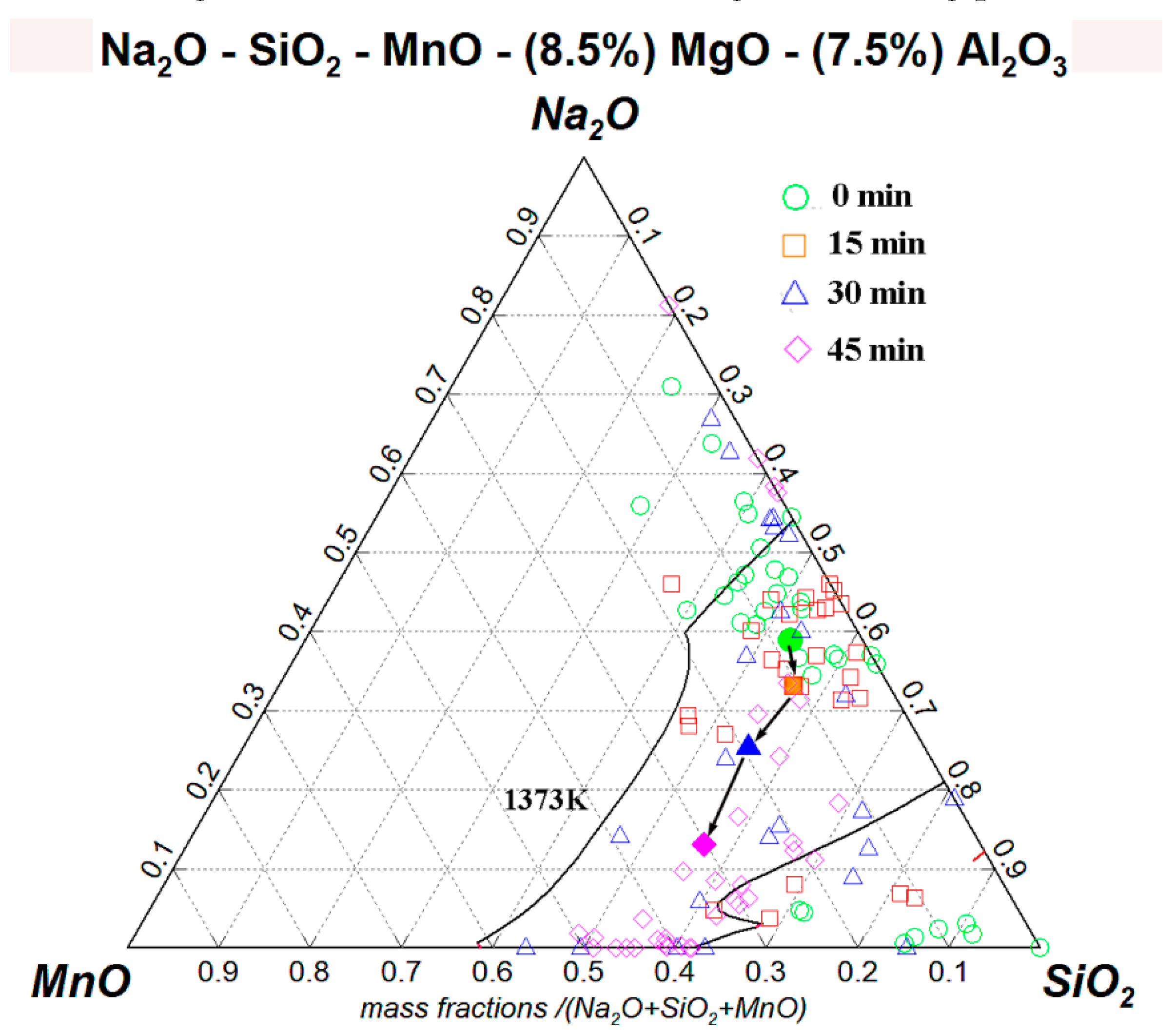
3.2. Mechanism of Inclusion Modification
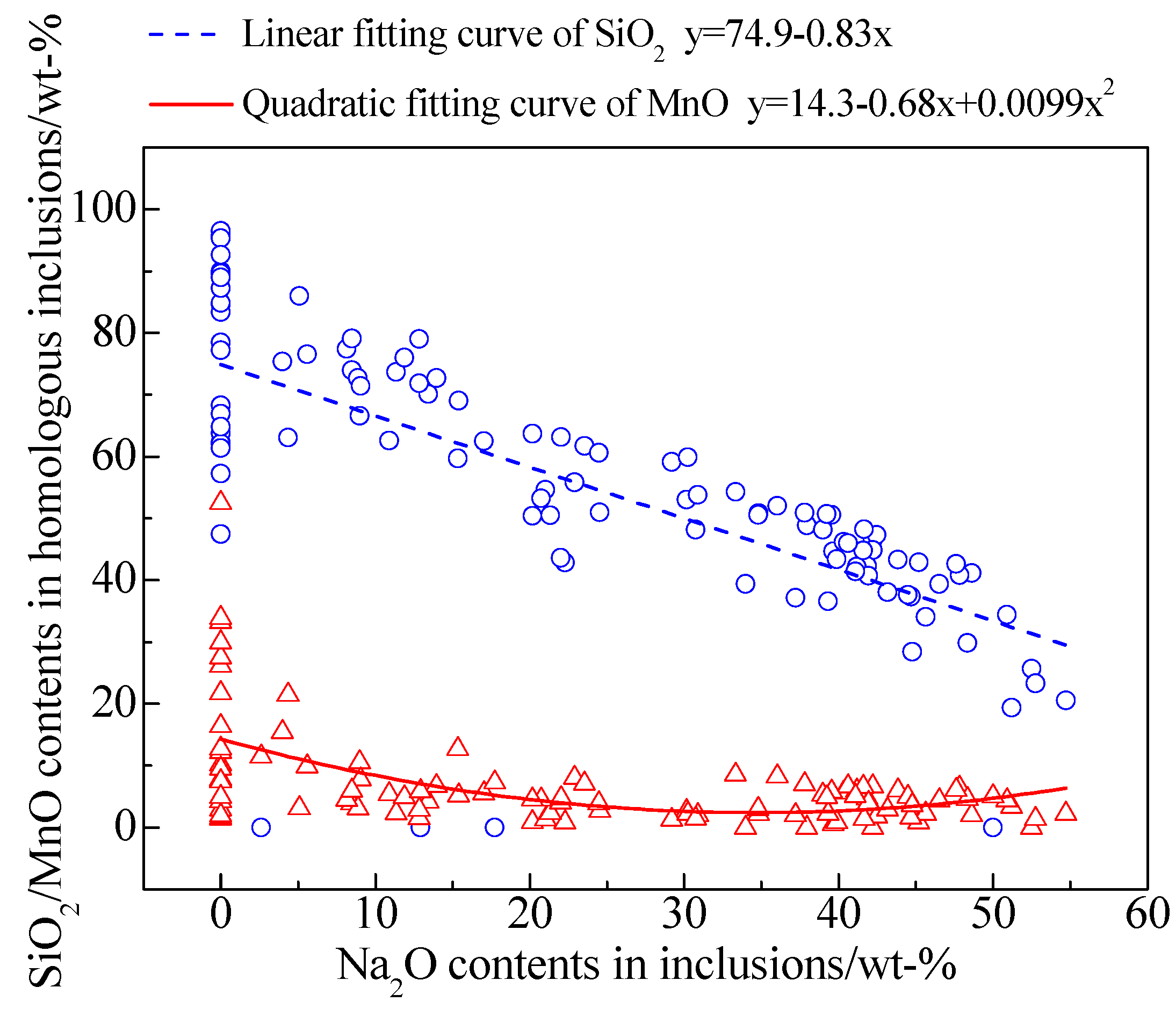
3.3. Evolution of Inclusions
4. Conclusions
- (1)
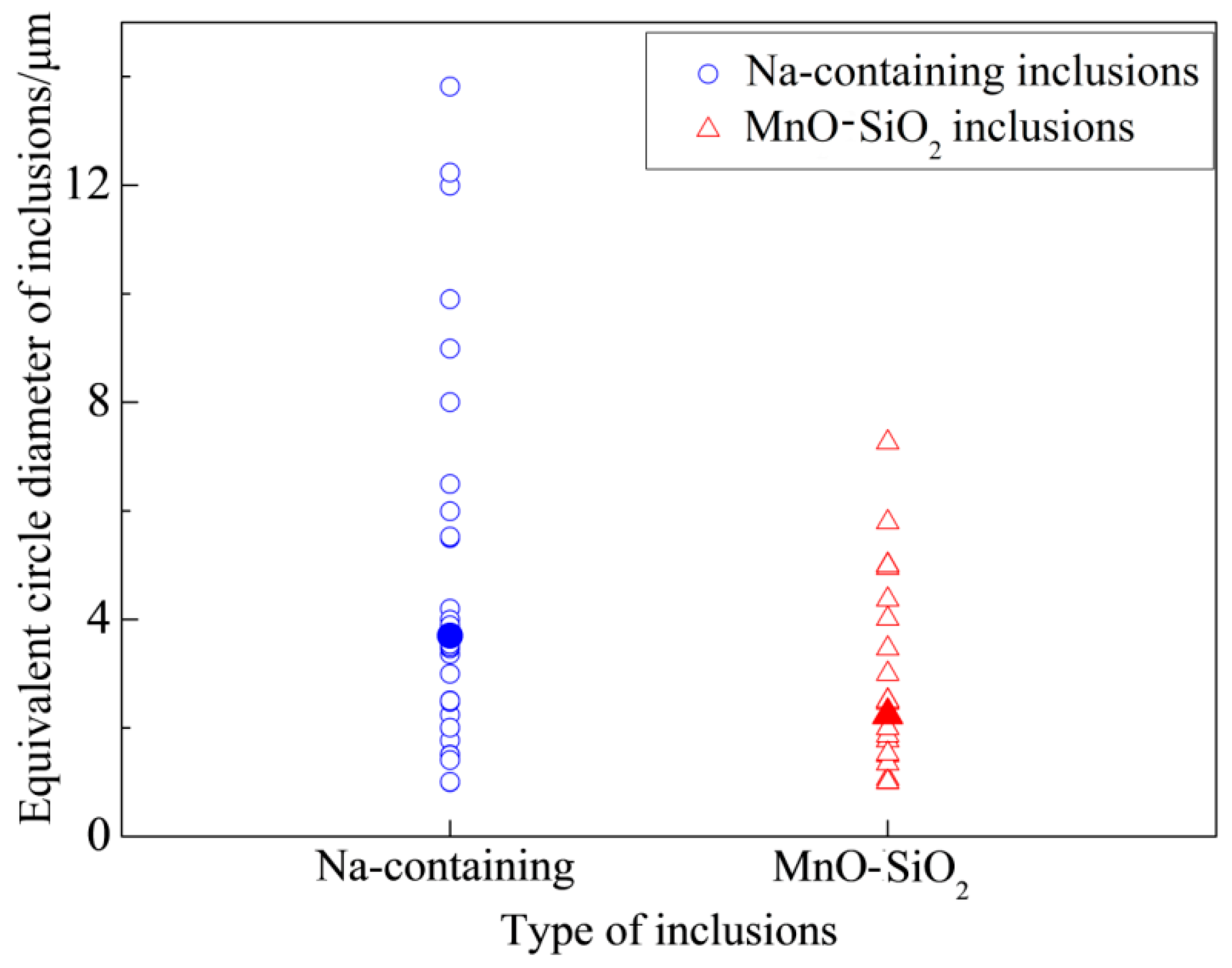
- The Na-containing inclusions were generally larger than the MnO-SiO2 inclusions and were therefore more likely to be removed by flotation. Thus, NaF addition promoted inclusion removal and improved cleanliness of the steel by modifying MnO-SiO2 inclusions into Na-containing inclusions;
- (2)
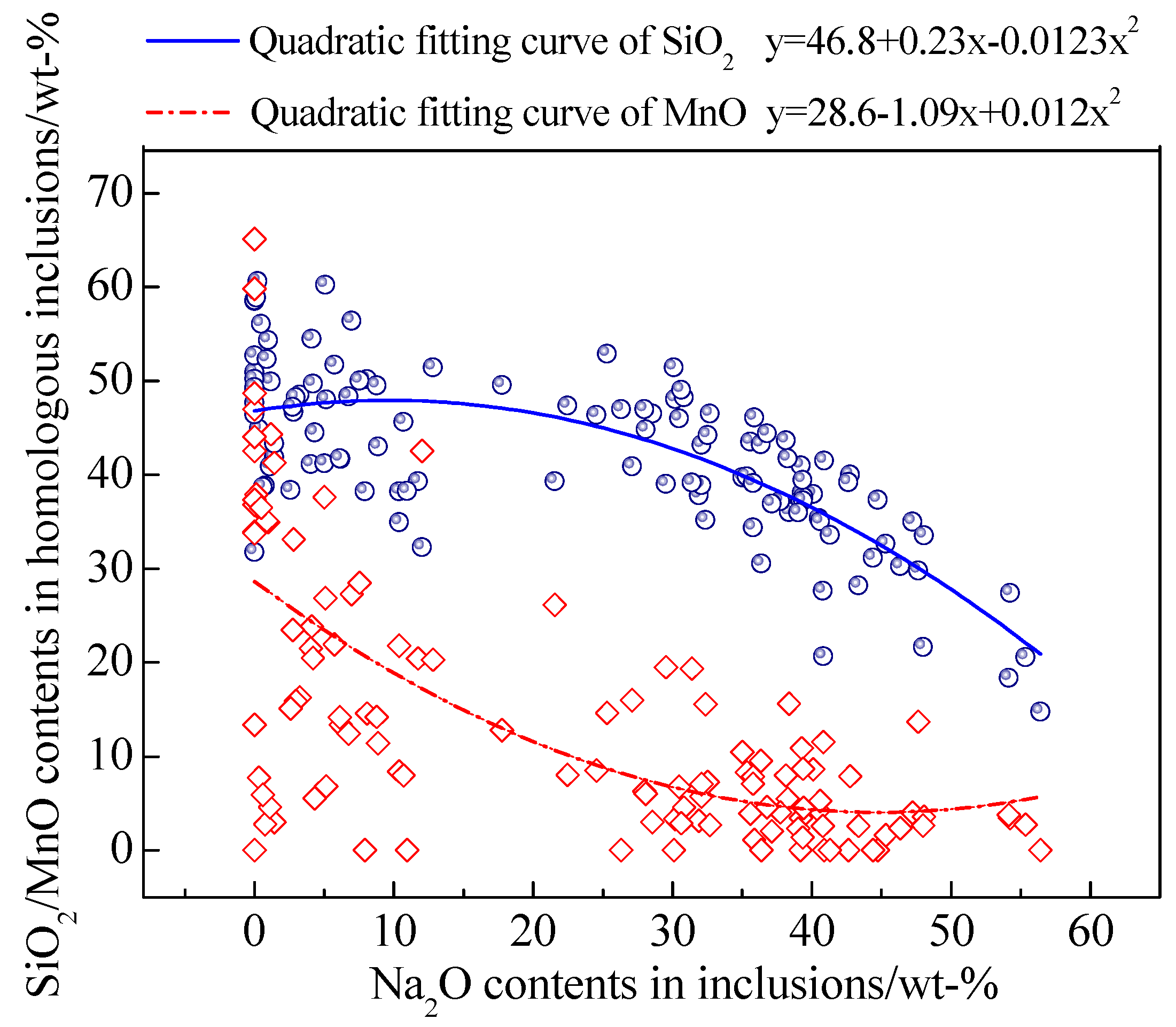
- With the increase of NaF addition, the proportion of Na-containing inclusions with low melting point increased and the proportion of plastic inclusions showed a relevant increasing tendency.
- (3)
- The modification mechanism of inclusions by NaF is generalized as two steps:
- Step 1:
- Silicon in the molten steel reacts with NaF droplets through Reaction [Si] + 4NaF (l) = SiF4 (g) + 4[Na] to generate [Na] in steel.
- Step 2:
- The generated [Na] then transfered into inclusions by reducing the MnO or SiO2 in inclusions through Reaction 2[Na] + (MnO)inc = [Mn] + (Na2O)inc and 4[Na] + (SiO2)inc = [Si] + 2(Na2O)inc, thus modifying the inclusions.
Author Contributions
Funding
Conflicts of Interest
References
- Kirihara, K. Production technology of wire rod for high tensile strength steel cord. Kobelco Technol. Rev. 2011, 30, 62–65. [Google Scholar]
- Son, S.; Lee, Y.K.; Kang, S.H.; Chung, H.; Cho, J.S.; Moon, J.; Oh, K.H. A numerical approach on the inclusion effects in ultrafine gold wire drawing process. Eng. Fail. Anal. 2011, 18, 1272–1278. [Google Scholar] [CrossRef]
- Norasethasopon, S.; Yoshida, K. Prediction of chevron crack initiation in inclusion copper shaped-wire drawing. Eng. Fail. Anal. 2008, 15, 378–393. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Z.; Li, Y.; Sun, M.Y.; Chen, K.; Wang, Q.; Li, H. Effect of Na2O and Rb2O on inclusion removal in C96 V saw-wire steels using low-basicity LF (Ladle Furnace) refining slags. Metals 2018, 8, 691. [Google Scholar] [CrossRef]
- Bertrand, C.; Molinero, J.; Landa, S.; Elvira, R.; Wild, M.; Barthold, G.; Valentin, P.; Schifferl, H. Metallurgy of plastic inclusions to improve fatigue life of engineering steels. Ironmak. Steelmak. 2003, 30, 165–169. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.S.; Chen, Z.J. Mechanism of non-metallic inclusion formation and modification and their deformation during compact strip production (CSP) process for aluminum-killed steel. ISIJ Int. 2013, 53, 2142–2151. [Google Scholar]
- Kanaun, S.; Martinez, R. Numerical solution of the integral equations of elasto-plasticity for a homogeneous medium with several heterogeneous inclusions. Comput. Mater. Sci. 2012, 55, 147–156. [Google Scholar] [CrossRef]
- Qin, J.S.; Qu, T.P.; Wang, D.Y.; Hu, M.; Tian, J. Mg treatment process for high carbon steel 82B. Steelmaking 2019, 35, 18–23. [Google Scholar]
- Li, C.R.; Yang, H.; Wen, H. Thermodynamic analysis of modification treatment of rare earth elements on inclusions in hard wire steel. Hot Work. Technol. 2010, 39, 12–14. [Google Scholar]
- Cui, H.Z.; Chen, W.Q. Effect of boron on morphology of inclusions in tire cord steel. J. Iron Steel Res. Int. 2012, 19, 22–27. [Google Scholar] [CrossRef]
- Wang, W.; Sun, K.; Liu, H. Effects of different aluminum sources on morphologies and properties of ceramic floor tiles from red mud. Constr. Build. Mater. 2020, 241, 118119. [Google Scholar] [CrossRef]
- Sakamoto, K.; Sugimura, N.; Yoshida, N. A Method of Producing High-Purity Steel with High Fatigue Strength and High Cold Processing. China Patent Application No. CN1836052A, 20 September 2006. [Google Scholar]
- Sakamoto, K.; Sugimura, T.; Yoshida, A.; Fukuzaki, Y.; Suda, S. Method for Producing High Cleanness Steel Excellent in Fatigue Strength or Cold Workability. U.S. Patent Application No. 7,608,130 B2, 27 October 2009. [Google Scholar]
- Chen, L.; Chen, W.J.; Hu, Y.; Chen, Z.; Xu, Y.; Yan, W. Effect of Na2CO3 addition on inclusions in high-carbon steel for saw wire. Trans. Indian Inst. Met. 2018, 71, 383–391. [Google Scholar] [CrossRef]
- Kang, Y.B.; Jung, I.H.; Decterov, S.A.; Pelton, A.D.; Lee, H.G. Critical thermodynamic evaluation and optimization of the CaO-MnO–SiO2 and CaO-MnO-Al2O3 systems. ISIJ Int. 2004, 44, 965–974. [Google Scholar] [CrossRef]
- Tomioka, K.; Ogawa, K.; Matsumoto, H. Thermodynamics of reaction between trace amount of Al and inclusion in Mn-Si killed steel. ISIJ Int. 1996, 36, S101–S104. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Chandra, S.; Ghosh, A. Prediction of deoxidation and inclusion precipitation in semi-killed steel. Metall. Mater. Trans. B 2005, 36, 59–66. [Google Scholar] [CrossRef]
- Bernard, G.; Ribound, P.V.; Urbain, G. Oxide inclusions plasticity. Rev. Metallurgie-CTT 1981, 78, 421–433. [Google Scholar] [CrossRef]
- Mandal, G.K.; Kamaraj, A.; Humane, M.M.; Minj, R.K.; Das, S.K.; Ramana, R.V.; Venugopalan, T. Development of specialty grade wire by controlling the inclusions in high-carbon steel using synthetic slag treatment. Trans. Indian Inst. Met. 2019, 72, 369–381. [Google Scholar] [CrossRef]
- Jha, P.K.; Rao, P.S.; Dewan, A. Effect of height and position of dams on inclusion removal in a six strand tundish. ISIJ Int. 2008, 48, 154–160. [Google Scholar] [CrossRef]
| Heats No. | NaF Amount/g | Fe Amount/g |
|---|---|---|
| A | 0.5 | 0.5 |
| B | 1 | 1 |
| C | 2 | 2 |
| D | 1.5 | 1.5 |
| [C] | [Si] | [Mn] | [Al] | [Ca] | [Mg] | [Na] | T.O. |
|---|---|---|---|---|---|---|---|
| 0.87 | 0.17 | 0.52 | 0.007 | <0.0003 | 0.0002 | <0.005 | 0.001 |
| Heats No. | [C] | [Si] | [Mn] | [Na] |
|---|---|---|---|---|
| A | 0.86 | 0.15 | 0.50 | <0.005 |
| B | 0.84 | 0.14 | 0.51 | <0.005 |
| C | 0.82 | 0.12 | 0.51 | <0.005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wan, Y.; Li, J.; Chen, W.; Yang, Y.; McLean, A. A New Method for Plasticization of Inclusions in Saw-Wire Steel by NaF Addition. Metals 2020, 10, 704. https://doi.org/10.3390/met10060704
Chen L, Wan Y, Li J, Chen W, Yang Y, McLean A. A New Method for Plasticization of Inclusions in Saw-Wire Steel by NaF Addition. Metals. 2020; 10(6):704. https://doi.org/10.3390/met10060704
Chicago/Turabian StyleChen, Liangjun, Yong Wan, Jie Li, Weiqing Chen, Yindong Yang, and Alexander McLean. 2020. "A New Method for Plasticization of Inclusions in Saw-Wire Steel by NaF Addition" Metals 10, no. 6: 704. https://doi.org/10.3390/met10060704
APA StyleChen, L., Wan, Y., Li, J., Chen, W., Yang, Y., & McLean, A. (2020). A New Method for Plasticization of Inclusions in Saw-Wire Steel by NaF Addition. Metals, 10(6), 704. https://doi.org/10.3390/met10060704





